Translate this page into:
Role of Th1/Th2 cytokine balance in predicting treatment outcomes and disease severity in tuberculosis
⁎Corresponding author. selvarajj.sdc@saveetha.com (Selvaraj Jayaraman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Tuberculosis (TB) is an age-old disease that remains a significant global public health issue. The protective response to Mycobacterium tuberculosis (MTB) is a complex and multifaceted process involving several components of the immune system, primarily driven by the cooperation between macrophages and T-cell populations. Various animal and human studies have well established the influential roles that cytokines and chemokines play in determining the outcome of MTB infection. The study focused on assessing the influence of Th1 and Th2 responses in tuberculosis by examining the current cytokine profiles in TB patients, emphasizing Th1 and Th2 cytokines, and comparing these profiles with those of patients undergoing treatment and a control group. Additionally, the relationship between cytokine status and the patients’ sex and age was assessed. The analysis of Th1/Th2 cytokines revealed a dichotomy between untreated and treated conditions. The results showed that untreated individuals suffered from a Th1 cytokine deficiency. However, this condition was reversed following the administration of anti-TB antibiotics, with patients who received these drugs showing a shift towards a protective Th1 cytokine profile. Cytokines play a decisive role in various infectious diseases, and this study confirms that TB is among them. The findings from this research could pave the way for novel diagnostic and therapeutic approaches in tuberculosis research.
Keywords
Mycobacterium tuberculosis
Cytokines
Chemokines
ELISA
Therapeutic regimen
1 Introduction
Drug-resistant forms of tuberculosis (TB), such as extensively drug-resistant (XDR-TB) and multidrug-resistant (MDR-TB), pose a significant threat to global health, particularly in developing nations (Gupta et al., 2024). The complex management of TB heavily relies on prompt diagnosis and effective treatment plans (Iacobino et al., 2020). However, drug-resistant TB strains complicate treatment outcomes, often requiring longer treatment durations with more expensive and potent medications (Vanino et al., 2023). Understanding the impact of these strains on treatment outcomes is crucial, especially when considering the role of cytokine dynamics, which are pivotal in immune responses against tuberculosis. The study noted that Th2 cytokines were more prevalent than Th1 cytokines in untreated TB patients, suggesting a potentially inefficient immune response (Shen and Chen, 2018). The challenge of drug-resistant tuberculosis, where traditional treatments may be less effective, underscores the need for novel strategies, such as cytokine-based therapeutics. Modifying cytokine profiles could potentially enhance immune responses and improve treatment outcomes, particularly in drug-resistant TB cases where conventional therapies often prove ineffective (Domingo-Gonzalez et al., 2016). When immune cells come into contact with Mycobacterium tuberculosis (MTB) bacilli, it initiates a series of immune responses (Chandra et al., 2022). This interaction leads to the up-regulation or down-regulation of genes associated with various immune molecules, including cytokines and chemokines. The coordinated activity of these molecules determines whether the infection progresses to pathological conditions or leads to a cure for tuberculosis (TB). Thus, the initial cytokine milieu plays a vital role in determining the prognosis or cure of TB. However, a single cytokine may not have a causal role; rather, the outcome could be due to a combination of multiple cytokines (Gideon et al., 2015). Early immune events initiated when MTB encounters alveolar type II pneumocytes, airway epithelial cells, dendritic cells, alveolar macrophages, and neutrophils (Corleis and Dorhoi, 2020). Among these, the first two cell types are responsible for producing immune mediators such as cytokines and chemokines, as well as directly eliminating MTB. In addition to these cells, T lymphocytes and B lymphocytes are also crucial for the large-scale production of cytokines (Reinhardt et al., 2009). Besides cytokine production, B cells produce antibodies that restrict the movement of MTB. T cells are further classified into CD4 T cells (also known as T-helper (Th) cells) and CD8 T cells (also known as cytotoxic T lymphocytes (CTL)). T-helper cells are divided into Th1 and Th2 cells based on the cytokines they produce. Generally, Th1 cells produce IFN-γ, TNF-α, IL-2, and other cytokines responsible for cell-mediated immunity (CMI) (Tan et al., 2012). Similarly, Th2 cells produce IL-4, IL-5, IL-10, IL-6, IL-13, and other cytokines that govern humoral (antibody-mediated) immunity. An important measure of the Th1/Th2 cytokine balance in immune responses is the IFN-γ/IL-10 ratio. Th1 cells produce IFN-γ, which activates macrophages and strengthens cell-mediated immunity against intracellular infections such as MTB. Conversely, IL-10, produced by macrophages and regulatory T cells, reduces immune responses and inflammation. In pathogen control, a dominant Th1 response is indicated by a higher IFN-γ/IL-10 ratio, whereas a shift towards Th2 responses, indicated by a lower ratio, may be associated with immune suppression or disease progression (Khan et al., 2016). Monitoring this ratio can help assess the effectiveness of treatment for diseases like tuberculosis and evaluate immunological status.
The management of TB is a complex process that primarily depends on timely diagnosis. It becomes even more complicated in cases of latent TB and pediatric TB. In the vast majority of patients, TB is manageable with appropriate drugs. However, the situation can worsen if the causal agent is MDR or XDR. The management of TB, particularly in the context of drug resistance, has not been extensively studied concerning the cytokine status of the host (Allué-Guardia et al., 2021). As discussed above, these cytokines act as a double-edged sword, as they can determine the outcome of a disease, leading either to a cure or to pathogenesis. Understanding cytokine levels could assist in developing novel cytokine-therapy-assisted anti-MTB treatment regimens (Cao et al., 2024). Therefore, while this study provides vital insights into cytokine dynamics during tuberculosis and treatment, future research examining cytokine-based therapy techniques could significantly impact the management of drug-resistant tuberculosis and improve patient outcomes. The aim of this study is to examine the cytokine profiles of tuberculosis patients, with a specific emphasis on the ratio of Th1 to Th2 cytokines and the changes in these profiles throughout the therapeutic process. We also seek to investigate any connections between patient variables, such as age and sex, and these cytokine profiles by comparing them to those of healthy controls. Our study aims to shed light on the immunopathogenesis of tuberculosis and suggest methods for improving treatment regimens by clarifying these cytokine dynamics.
2 Materials and methods
2.1 Study Cohort
The current research included 300 tuberculosis (TB) patients, comprising 156 treatment-naïve individuals and 144 patients undergoing therapy. Additionally, a control group of 100 age- and sex-matched individuals was included in the study. The study samples were from Government Hospital, Tambaram Sanatorium, Chennai, India (Approval No: UM/IHEC/16–2013-I).
2.2 Samples
5 mL of peripheral blood was taken from each patient with Pulmonary TB and serum was separated for the study.
2.3 Inclusion criteria
-
Pulmonary Tuberculosis cases
-
AFB positive
2.4 Exclusion criteria
-
HIV positive cases
-
Extra Pulmonary TB cases
2.5 Cytokine assay
The cytokines tested were IFN-γ and TNF-α (Th1 cytokines), and IL-10 and IL-6 (Th2 cytokines), using ELISA kits. IL-6, TNF-α, and IL-10 were detected using kits obtained from PeproTech (900-K16, 900-K25, 900-K21), and IFN-γ was measured using EIA kits from Thermo Scientific (Cat. No KHIFNG). The following protocol was employed for the EIA kits manufactured by PeproTech.
2.6 ELISA protocol used for the detection of IL-6, TNF-α and IL-10
PeproTech ELISA kits were used to quantify the cytokines IL-6, TNF-α, and IL-10. The protocol was as follows: A 96-well microtiter plate was incorporated with a coating antibody at a concentration of 1 μg/mL, diluted in PBS (pH 7.4). The plate was sealed and incubated overnight at room temperature. The next day, the seal was removed, and the wells were blocked with 300 µL of 1 % BSA, followed by a one-hour incubation at room temperature (RT). After blocking, the content was decanted, and the test sample was added. The plate was then incubated for 2 h at room temperature. Following incubation, the plates were washed three times with washing buffer (PBS with 0.05 % Tween-20). Subsequently, 100 µL of detecting antibody at a concentration of 0.25 μg/mL was added, and the plate was incubated for an additional two hours. After incubation, washing step was followed by the addition of 100 µL of avidin peroxidase conjugate at a concentration of 0.6 mg/mL was added and incubated for 45 min at room temperature. The plates were then washed again, and 100 µL of ABTS substrate solution was added for color development. The absorbance was read at a wavelength of 405 nm using an ELISA equipment. Standards were included on each ELISA plate, and a standard curve was plotted. A linear regression analysis method was used to calculate the cytokine concentrations in the unknown samples (Yildirim et al., 2020). The coefficient of variation (CV) was < 10 %.
2.7 ELISA protocols used for the detection of IFN –γ
For this ELISA, 96-well polystyrene microtiter plates (Sigma-Aldrich Cat. No. M9410) were coated with anti-human IFN-γ antibody (coating antibody). After the addition of one hundred microliter and followed by incubation, the plate was kept overnight in the optimum temperature. The coating antibody was then decanted, and the plates were washed three times with PBS (pH 7.4) containing 0.05 % Tween 20 (wash buffer). Following this, 300 µL of blocking buffer was added to each well and incubated for 2 h, after which the blocking buffer was aspirated. One hundred microliters of test samples, positive control, and negative control samples were added to the wells. The plates were then covered and incubated for 60 min, followed by three washes. Next, 100 µL streptavidin-HRP conjugate was added. The plate was then kept for 30 min in the optimum temperature and washed.
Then, 100 µL of substrate solution was incubated in all the wells and kept for 20 min in optimum temperature at dark. After, stop solution 50 µL was added in each well and measured the color intensity using ELISA equipment (BioTek Instruments, Vermont, USA). A standard curve was plotted for each ELISA plate, and linear regression analysis was used to calculate the cytokine concentrations in the unknown samples (Maruthamuthu et al., 2020; Davidson et al., 2021; Narenkumar et al., 2021). After obtaining the ELISA values, the concentration of each cytokine was calculated and represented as pg/mL. The experimental groups included active TB patients, patients undergoing treatment, and negative controls. Experiments were performed in triplicate, and the mean and standard deviation of each cytokine were calculated. The Th1/Th2 ratio was determined from the ratio of IFN-γ to IL-10. The values were then analyzed based on sex and age group.
2.8 Statistical analysis
Standard curves were utilized to determine the cytokine concentrations (pg/mL), with linear regression was applied for result analysis. Statistical analysis was conducted using IBM SPSS Statistics v21, and chi-square tests were employed to assess categorical data (IBM Corp., IBM SPSS Statistics v21). The chi-square test was also used to calculate the p-value.
3 Results
3.1 Clinical characteristics and demographic study
A total of 300 TB patients (156 treatment-naïve and 144 undergoing treatment) and 100 TB-negative individuals were enrolled. Serum samples obtained from these participants were screened for Th1 and Th2 cytokines by ELISA (Fig. 1a). The age groups were subdivided into pediatric (<12 years old), adolescents (12–18 years), young adults (19–40 years), middle-aged adults (41–60 years), and older adults (>60 years). The samples were sex- and age-matched (Fig. 1b).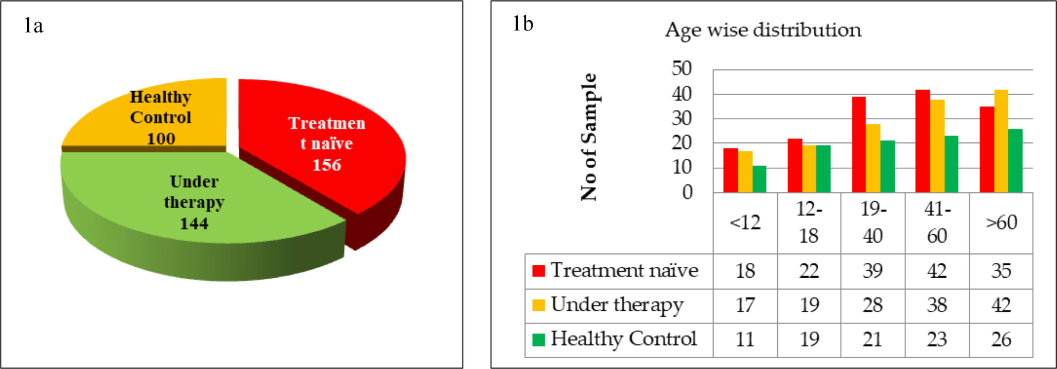
(a) Number and proportion of population enrolled. The demography consisted of 300 were TB positive individuals (156 treatment naïve and 144 under therapy) and 100 were healthy controls, (b) The age wise classification of TB positive patients and control groups.
3.2 Serum cytokine levels among TB positive and control individuals
In this study, Th1 cytokines, namely IFN-γ and TNF-α, and Th2 cytokines, namely IL-10 and IL-6, were evaluated in the serum of TB patients and controls. A striking observation was that among the untreated TB patients, there was lower level of Th1 while extremely high levels of Th2 (Fig. 2a & 2b). Another important finding was that among the TB patients undergoing treatment, the situation was reversed: Th1 cytokines were upregulated, and Th2 cytokines were downregulated. Experiments were repeated three times, and the figure shows the mean ± SD of the three experiments. When analyzing Th1 cytokines separately, untreated TB patients had an IFN-γ level of 45.5 ± 3.8 pg/mL, which increased remarkably by 13.4-fold to 608.5 ± 7.6 pg/mL among treated individuals. Similarly, TNF-α levels increased by 12.7-fold among treated TB patients. Analysis of Th2 cytokines showed that treatment-naïve TB patients had significantly high serum IL-10 levels, i.e., 372.4 ± 4.9 pg/mL, which were downregulated by 8-fold to 42.3 ± 5.1 pg/mL upon treatment. Likewise, a 9.5-fold reduction was observed in another Th2 cytokine, IL-6. These serum cytokine levels were also reflected in the Th1/Th2 ratio, which often indicates disease progression versus improvement. The overall Th1/Th2 ratio for untreated TB patients was 0.1, which increased dramatically to 14.4 among patients under treatment (Fig. 3a). Negative control subjects did not show a preference for either Th1 or Th2 cytokines, and their Th1/Th2 ratio remained around 1.1. Overall, this study revealed a positive correlation with Th1 cytokines during treatment, whereas there was a negative correlation with Th2 cytokines. It appears that TB pathogenesis initially skews immune cells towards a non-protective Th2 phenotype, but TB treatment restores the Th1 cytokine profile. In this context, we selected only a few cytokines from each Th1 and Th2 paradigm for this study, and the observed differences were discussed. However, other Th1 and Th2 cytokines were not included, and their inclusion may provide further insights into the overall role of each cytokine during TB.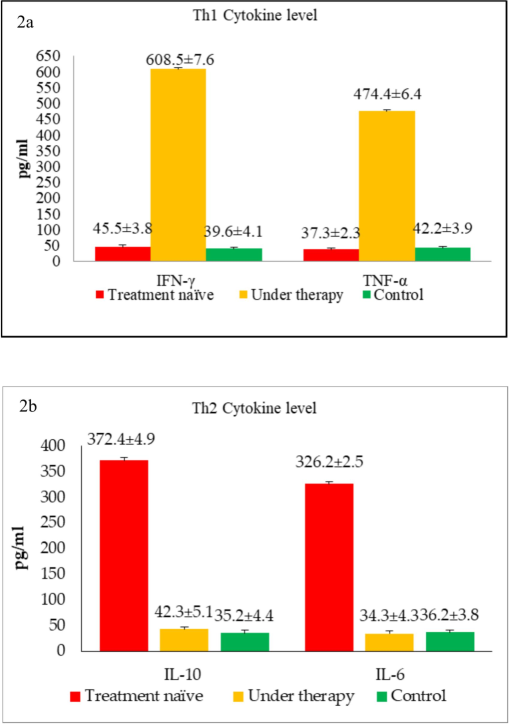
(a) The overall Th1 cytokine levels of TB patients and healthy controls, (b) The overall Th2 cytokine levels of TB patients and healthy controls.
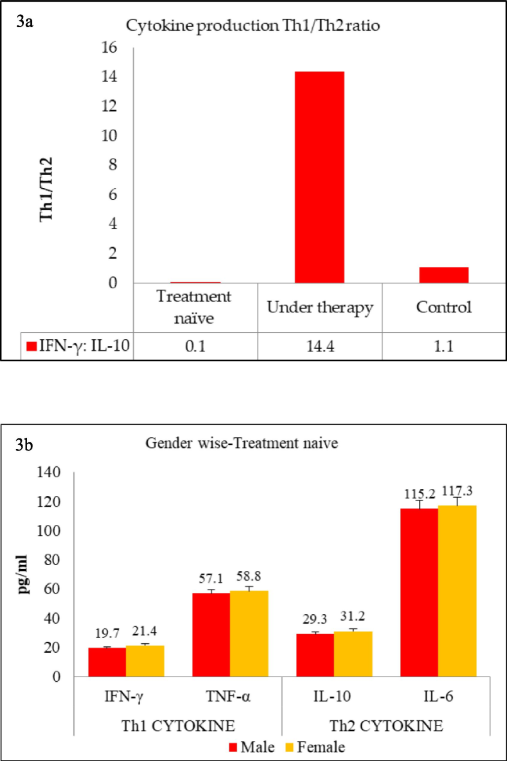
(a)Th1/Th2 cytokine ratio among TB-positive groups and control individuals which was calculated by dividing Th1 values (mean pg/mL) by Th2 values, (b) Study group was divided into various groups namely treatment naïve, patients under therapy and controls.
3.3 Correlation of gender or age of Th-1 and Th-2 cytokine secretion among TB patients
As described above, the untreated TB population exhibited a preponderance of the Th2 T-cell phenotype, which shifted back to the Th1 type upon treatment. The data were then analyzed to determine whether these cytokine patterns differed between genders and age groups. Fig. 3b shows the cytokine patterns among TB treatment-naïve patients. As illustrated, both males and females showed low level of Th1 and significantly higher levels of Th2 (p < 0.001) among untreated patients. However, there was no difference between genders in terms of cytokine quantity or Th1/Th2 ratio. This trend was reversed when the cytokine spectrum was evaluated among patients undergoing treatment, as shown in Fig. 4a. TB treatment shifted the trend towards the Th1 phenotype, as evidenced by increased secretion of Th1 cytokines and simultaneous downregulation of Th2 cytokines. Even among the treated TB cases, there was no difference between genders regarding cytokine secretion patterns or the Th1/Th2 ratio. It is important to note that none of the control samples exhibited any shift towards Th1 or Th2 phenotypes (Fig. 4b). As shown in Fig. 4c, both males and females had comparable levels of the Th1/Th2 ratio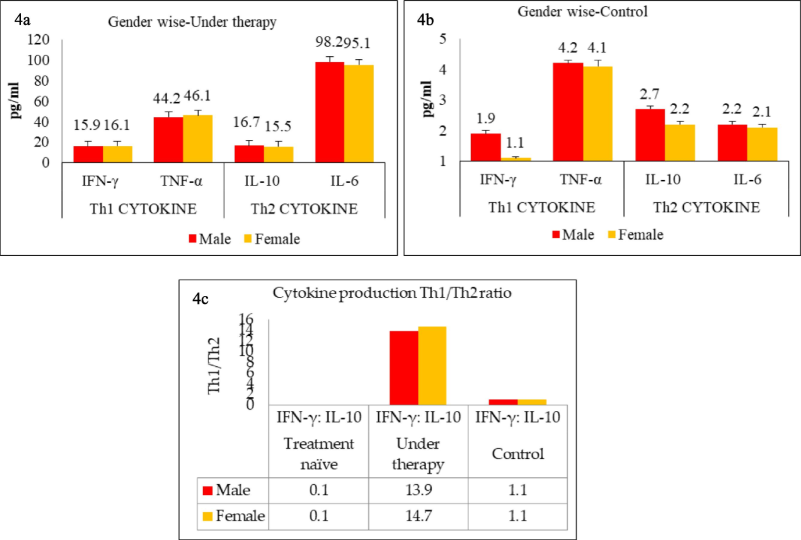
(a) Study group divided into various groups namely treatment naïve, patients under therapy, (b) Study group divided into various groups namely treatment naïve, patients under therapy and controls, (c) Th1/Th2 cytokine ratio among TB-positive groups and control individuals which was calculated by dividing Th1 values (mean pg/mL) by Th2 values.
The data were further analyzed to determine whether cytokine patterns differed between various age groups. Age subgroups were formed as mentioned above, and the results for treatment-naïve (untreated) patients are shown in Fig. 5a. As illustrated, all age groups showed low level of Th1 cytokines and significantly higher levels of Th2 (p < 0.001) among the treatment-naïve group. However, there was no difference between different age groups in terms of cytokine quantity or Th1/Th2 ratio. This trend was reversed when the cytokine spectrum was evaluated among patients undergoing treatment, as shown in Fig. 5b. TB treatment shifted the trend towards the Th1 phenotype, as evidenced by increased secretion of Th1 cytokines and simultaneous downregulation of Th2 cytokines. Even among the treated TB cases, there was no difference among the different age groups regarding cytokine secretion patterns or the Th1/Th2 ratio. As shown above, none of the control samples exhibited any shift towards Th1 or Th2 phenotypes (Fig. 6a). As shown in Fig. 6b, all age groups had comparable levels of the Th1/Th2 ratio.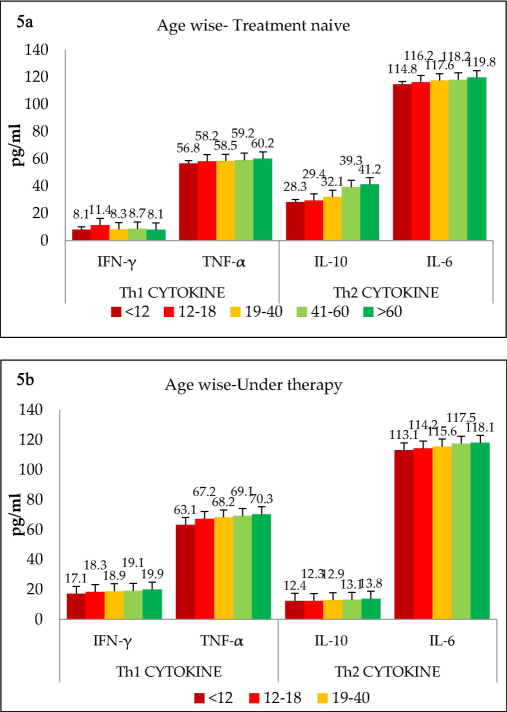
(a) Age wise classification of various groups namely treatment naïve, (b) Age wise classification of various groups patients under therapy and controls.
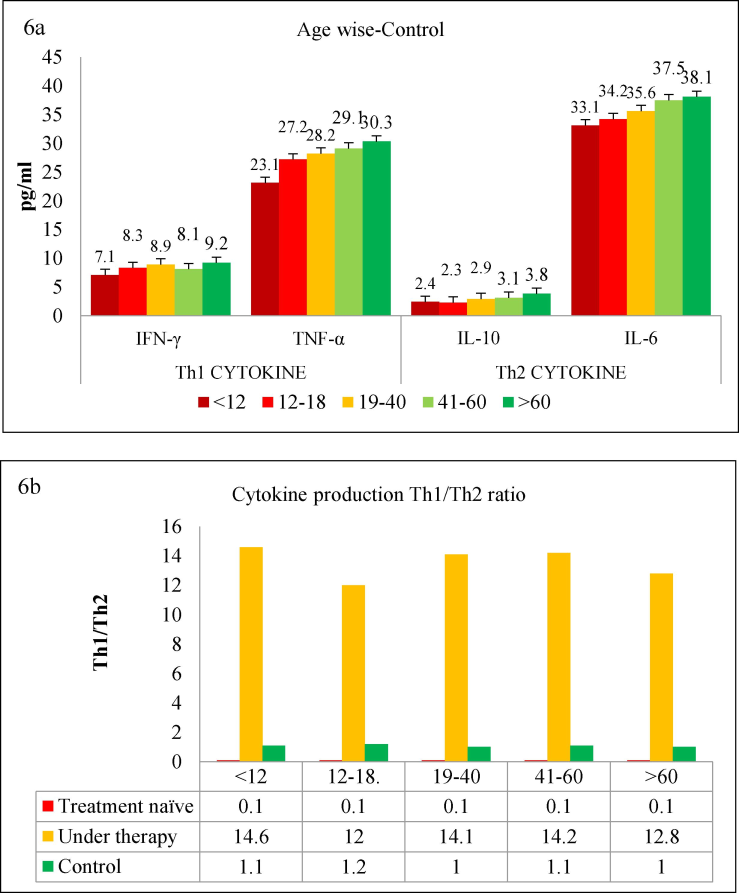
(a) Age wise classification of various groups namely treatment naïve, patients under therapy and controls, (b) Th1/Th2 cytokine ratio among TB-positive groups and control individuals was calculated by dividing Th1 values (mean pg/mL) by Th2 values.
4 Discussion
The cytokine profiles of tuberculosis (TB) patients and their implications for disease management are significantly illuminated by our work. The results highlight important ramifications for TB pathogenesis and treatment approaches by revealing notable disparities in cytokine balances among untreated TB patients, those receiving treatment, and healthy controls. One of the most concerning findings of our study is the prevalence of Th2 cytokines, such as IL-10 and IL-6, in untreated TB patients. Th2 cytokines are generally associated with immunological suppression and the modulation of antibody-mediated responses, rather than with cell-mediated immunity, which is crucial for combating intracellular infections like Mycobacterium tuberculosis (MTB) (Rijnink et al., 2021; Ramalingam et al., 2023). Cytokines are low molecular weight (∼5–20 kDa) messengers that play a vital role in all aspects of immunity and inflammation (Ibelgaufts, 2013; O'Garra et al., 2013). Interleukins (IL) are complex immune modulatory proteins involved in cell proliferation, maturation, migration, and adhesion (Brocker et al., 2010; Day et al., 2011). The Th1 and Th2 paradigm was introduced by Mosmann and Coffman in 1989, and subsequently, Th1 and Th2 cytokines have been reported as causal factors in several microbial diseases. With this background, the current study was conducted on MTB patients and controls, examining various serum Th1 and Th2 cytokines before and after treatment, and observed three major findings.
First, during active TB before the initiation of treatment (untreated group), patients had a preponderance of Th2 serum cytokines over Th1 cytokines. Second, TB patients undergoing active treatment showed a predilection toward Th1 serum cytokines. Third, these disparities in cytokine levels were not biased by gender or age. Among Th1 cytokines, IFN-γ plays a critical role during inflammation caused by MTB. It is important for the migratory and functional properties of various cell types, including T cells, natural killer (NK) cells, and antigen-presenting cells such as macrophages (Agger et al., 2008; Wong et al., 2020). The importance of IFN-γ during MTB infections has been well documented. Filipe-Santos et al. (2006) and Zhang et al. (2008) reported that individuals with a genetic deficiency of IFN-γ were more prone to MTB infection. Similarly, patients with defective IFN-γ receptors, such as autosomal complete recessive IFN-γ R1 (Sologuren et al., 2011) or IFN-γ R2 (Vogt et al., 2005), also exhibited greater susceptibility to MTB infections. It has also been documented that IFN-γ knockout mice were susceptible to infection even with a low dose of MTB (Sologuren et al., 2011) and these mice had poor macrophage activation and increased granulocyte inflammation (Dorman and Holland, 1998; Vogt et al., 2005).
In humans, it has been shown that during MTB infections, there is an impairment of IFN-γ secretion (Safak and Risvanli, 2022). This observation corroborates our findings. However, our observation is more significant in that while they observed the impairment in macrophages isolated from MTB patients, we conducted our experiments using serum collected from MTB patients, where the impairment was observed in the serum. In addition, this impairment was not limited to IFN-γ secretion but also included a general downregulation of Th1 cytokines. Flynn et al. (1993) showed that mice deficient in TNF-α had enhanced susceptibility to MTB infections, supporting our findings of diminished TNF-α secretion in our patients. Our study also provides additional insight into the dynamics of Th2 cytokines in MTB patients. We found that Th2, specifically IL – 10 and I L – 6, were abundantly higher during active TB infections.
Another notable observation in our study was that both genders and all age groups exhibited similar cytokine production patterns during TB infections, with no apparent bias. It has been documented that females generally respond better to treatment or vaccination than males, which is attributed to sex hormones that have a profound effect on immune cells (Safak et al., 2022). For instance, the female hormone estrogen can stimulate the secretion of IFN – γ, TNF – α, and IL – 12 while inhibiting the production of IL-10 (Fish, 2008). Conversely, the male sex hormone testosterone can stimulate IL-10 production and suppress IFN – γ secretion (Lotter et al., 2013), which could contribute to the higher incidence of TB among males, with a male-to-female ratio of 1.9:1 (Pinzan et al., 2010).
Age-related changes in Th1 and Th2 cytokine profiles often show a shift from Th1 to Th2 with increasing age (Gardner and Murasko, 2002; Neyrolles and Quintana-Murci, 2009; Amelio et al., 2017; Maruthamuthu et al., 2020; Yildirim et al., 2020; Davidson et al., 2021; Narenkumar et al., 2021). However, the influence of sex or age on TB has not been extensively studied. In our study, we did not find any differences in Th1 or Th2 cytokine profiles related to sex or age among patients or controls, except for the differences associated with the untreated versus treated status of the TB patients, as discussed above.
5 Conclusions
Cytokines are crucial in various infectious diseases, including tuberculosis (TB), as highlighted by our study. Our analysis of Th1/Th2 cytokines revealed a significant difference between untreated and treated conditions. Untreated individuals showed a deficiency in Th1 cytokines, which may exacerbate the severity of TB. This deficiency was reversed with the administration of anti-TB antibiotics, resulting in a shift towards a Th1-dominant cytokine profile that is typically protective. However, the widespread use of anti-TB drugs carries the risk of developing drug-resistant strains, such as multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB, which are difficult to treat. To address this, cytokine therapy could be considered. By administering the deficient cytokines to enhance Th1 levels, it might be possible to prevent the emergence of MDR or XDR TB or improve management if such strains occur. Additionally, exploring host genetics and developing immunotherapy based on cytokine modulation may offer new avenues for improving TB treatment and reducing the global disease burden. Thus, a thorough understanding of cytokine dynamics in TB is essential for advancing clinical management and developing innovative treatments to improve patient outcomes and achieve global TB eradication goals.
5.1 Future directions of this study
The findings of this study on the cytokine profiles of TB patients have significant potential to enhance our understanding of and approach to treating tuberculosis in the future. Further investigation into the molecular interactions between Th1 and Th2 cytokines during tuberculosis pathogenesis is necessary, with a focus on their roles in immune regulation and bacterial clearance. Integrating systems biology techniques could offer a holistic view of host-pathogen interactions, potentially uncovering new biomarkers and treatment targets. Additionally, investigating the roles of host genetics, epigenetics, and non-cytokine immunological mediators on cytokine responses could improve the customization of TB therapy regimens. Longitudinal studies are essential for monitoring cytokine dynamics throughout various stages of tuberculosis infection and treatment, which will inform the development of cytokine-based diagnostics and immunotherapies. By translating these findings into clinical trials and global health policies, we can work towards optimizing TB management techniques and ultimately contribute to global efforts aimed at reducing the burden of tuberculosis.
Institutional Review Board Declaration
The current research included 300 tuberculosis (TB) patients, comprising 156 treatment-naïve individuals and 144 patients undergoing therapy. Additionally, a control group of 100 age- and sex-matched individuals was included in the study. The study samples were from Government Hospital, Tambaram Sanatorium, Chennai, India (Approval No: UM/IHEC/16–2013-I).
CRediT authorship contribution statement
Gopinath Ramalingam: Writing – original draft, Methodology, Conceptualization. Javed Masood Khan: Writing – review & editing. Sharmila Jasmine: Writing – review & editing. Gowsalya Saminathan: Methodology. Elanchezhiyan Manickan: Supervision. Ponnulakhmi Rajagopal: Writing – original draft, Formal analysis. Vishnu Priya Veeraraghavan: Writing – review & editing, Formal analysis. Selvaraj Jayaraman: Writing – review & editing, Data curation, Conceptualization.
Acknowledgements
The authors are grateful to the Researchers Supporting Project number (RSP2024R360), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adjuvant modulation of the cytokine balance in Mycobacterium tuberculosis subunit vaccines; immunity, pathology and protection. Immunology. 2008;124(2):175-185.
- [CrossRef] [Google Scholar]
- Evolution of drug-resistant Mycobacterium tuberculosis strains and their adaptation to the human lung environment. Front. Microbiol.. 2021;12:612675
- [CrossRef] [Google Scholar]
- Mixed Th1 and Th2 Mycobacterium tuberculosis-specific CD4 T cell responses in patients with active pulmonary tuberculosis from Tanzania. PLOS Negl. Trop. Dis.. 2017;11(7):e0005817.
- [Google Scholar]
- Evolutionary divergence and functions of the human interleukin (IL) gene family. Hum Genomics.. 2010;5(1):30-55.
- [CrossRef] [Google Scholar]
- Promising cytokine adjuvants for enhancing tuberculosis vaccine immunity. Vaccines. 2024;12(5):477.
- [CrossRef] [Google Scholar]
- Immune evasion and provocation by Mycobacterium tuberculosis. Nat. Rev. Microbiol.. 2022;20(12):750-766.
- [CrossRef] [Google Scholar]
- Early dynamics of innate immunity during pulmonary tuberculosis. Immunol. Lett.. 2020;221:56-60.
- [CrossRef] [Google Scholar]
- A paper-based colorimetric molecular test for SARS-CoV-2 in saliva. Biosens. Bioelectron.. 2021;9:100076
- [CrossRef] [Google Scholar]
- Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J. Immun. Balt.. 2011;187(5):2222-2232.
- [CrossRef] [Google Scholar]
- Cytokines and chemokines in Mycobacterium tuberculosis infection. Microbiol. Spectr.. 2016;4(5):10-1128.
- [CrossRef] [Google Scholar]
- Mutation in the signal-transducing chain of the interferon-gamma receptor and susceptibility to mycobacterial infection. J. Clin. Invest.. 1998;101(11):2364-2369.
- [CrossRef] [Google Scholar]
- Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin. Immunol.. 2006;18(6):347-361.
- [CrossRef] [Google Scholar]
- The X-files in immunity: sex-based differences predispose immune responses. Nat. Rev. Immunol.. 2008;8(9):737-744.
- [CrossRef] [Google Scholar]
- An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med.. 1993;178(6):2249-2254.
- [CrossRef] [Google Scholar]
- Age-related changes in Type 1 and Type 2 cytokine production in humans. Biogerontology. 2002;3(5):271-290.
- [CrossRef] [Google Scholar]
- Variability in tuberculosis granuloma T cell responses exists, but a balance of pro- and anti-inflammatory cytokines is associated with sterilization. PLoSPathog.. 2015;11(1):e1004603.
- [Google Scholar]
- Global adoption of 6-month drug-resistant TB regimens: Projected uptake by 2026. Plos One. 2024;19(1):e0296448.
- [Google Scholar]
- Drug-resistant tuberculosis 2020: where we stand. Appl. Sci.. 2020;10(6):2153.
- [CrossRef] [Google Scholar]
- Ibelgaufts, H. Cytokines in Cytokines & Cells Online Pathfinder Encyclopedia Version 31.4 (Spring/Summer, 2013 Edition).
- Emerging role of mesenchymal stem cells during tuberculosis: The fifth element in cell mediated immunity. Tuberculosis. 2016;101:S45-S52.
- [CrossRef] [Google Scholar]
- Testosterone increases susceptibility to amebic liver abscess in mice and mediates inhibition of IFNγ secretion in natural killer T cells. PloS One.. 2013;8(2):e55694.
- [Google Scholar]
- Raman spectra‐based deep learning: A tool to identify microbial contamination. Open J. Med. Microbiol.. 2020;9:e1122.
- [Google Scholar]
- TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol.. 1989;7:145-173.
- [CrossRef] [Google Scholar]
- Effect of crude methanolic extract of Lawsoniainermis for anti-biofilm on mild steel 1010 and its effect on corrosion in a re-circulating wastewater system. J. King Saud Univ.sci.. 2021;33:101611
- [CrossRef] [Google Scholar]
- The immune response in tuberculosis. Annu. Rev. Immunol.. 2013;31:475-527.
- [CrossRef] [Google Scholar]
- Immunological basis for the gender differences in murine Paracoccidioidesbrasiliensis infection. PloS One. 2010;5(5):e10757.
- [Google Scholar]
- Exploring recombinant secretory proteins from Mycobacterium tuberculosis to develop a serological platform for tuberculosis diagnosis. Int. J. Biol. Macromol.. 2023;249:126769
- [CrossRef] [Google Scholar]
- Cytokine-secreting follicular T cells shape the antibody repertoire. Nat. Immunol.. 2009;10(4):385-393.
- [CrossRef] [Google Scholar]
- B-Cells and Antibodies as Contributors to Effector Immune Responses in Tuberculosis. Front. Immunol.. 2021;12:640168
- [CrossRef] [Google Scholar]
- Udder defense system: Effect of milk SCC level on Th1/Th2 cytokine balance. J. Hellenic Vet. Med. Soc.. 2022;73(2):4135-4140.
- [CrossRef] [Google Scholar]
- Th1/Th2 Cytokine Polarization in Milk According to Different Pathogens Causing Subclinical Mastitis in Cows. Mljekarstvo: Časopis Za Unaprjeđenje Proizvodnje i Prerade Mlijeka. 2022;72(2):105-113.
- [CrossRef] [Google Scholar]
- The crucial roles of Th17-related cytokines/signal pathways in M. tuberculosis infection. Cell. Mol. Immunol.. 2018;15(3):216-225.
- [CrossRef] [Google Scholar]
- Partial recessive IFN-γR1 deficiency: genetic, immunological and clinical features of 14 patients from 11 kindreds. Hum. Mol. Genet.. 2011;20(8):1509-1523.
- [CrossRef] [Google Scholar]
- Characterization of Th1-and Th2-type immune response in human multidrug-resistant tuberculosis. Eur. J. Clin. Microbiol. Infect. Dis.. 2012;31:1233-1242.
- [CrossRef] [Google Scholar]
- Update of drug-resistant tuberculosis treatment guidelines: A turning point. Int. J. Infect. Dis.. 2023;130:S12-S15.
- [CrossRef] [Google Scholar]
- Gains of glycosylation comprise an unexpectedly large group of pathogenic mutations. Nat. Genet.. 2005;37(7):692-700.
- [CrossRef] [Google Scholar]
- IL-10 impairs local immune response in lung granulomas and lymph nodes during early Mycobacterium tuberculosis infection. J. Immunol.. 2020;204(3):644-659.
- [CrossRef] [Google Scholar]
- Roll‐to‐Roll (R2R) Production of Large‐Area High‐Performance Piezoelectric Films Based on Vertically Aligned Nanocolumn Forests. Adv. Mater. Technol.. 2020;5:2000553.
- [CrossRef] [Google Scholar]
- Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunol. Rev.. 2008;226:29-40.
- [CrossRef] [Google Scholar]







