Translate this page into:
Role of Phoenix dactylifera (Ajwa) on lipid profile; a randomized controlled trial
⁎Corresponding author at: Department of Pharmacology, Rehman College of Dentistry/Rehman Medical Institute, Peshawar, Pakistan. dr.naseer99@gmail.com (Naseer Ahmed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Date palm is a significant crop mainly of hot regions and used as a traditional remedy for various ailments. Ajwa is a well-known functional food with an abundance of phytochemicals that possess different beneficial and medicinal properties. The study's objective was to calculate the impact of Ajwa consumption in a daily routine on lipid profile and HDL in the general population.
Methods
Randomized clinical trial was conducted for six weeks on participants, including both genders, with HDL levels below 40 mg/dl. The intervention group was advised to take Ajwa (70 gm) on an empty stomach in the morning for five days a week for six consecutive weeks with their routine diet and physical activity. The control group was advised not to make any changes in their diet and everyday life. Body weight, BMI, blood glucose, and lipid levels were recorded before and after the trial to evaluate the effect of Ajwa on these markers.
Result
The Ajwa intervention group showed a significant effect for total cholesterol, triglycerides, LDL, and HDL (p = 0.001), representing that the Ajwa group showed more alterations than the control group. However, no significant interaction effect was identified for body weight, BMI, and blood glucose level for both Ajwa and the control group.
Conclusion
The study findings recommend that intake of Ajwa is well tolerated, and its inclusion in diet and routine life provides cardioprotection by improving the lipid profile and raising the sub-optimal HDL levels.
Keywords
Ajwa date
Lipid profile
Cardioprotection
Antioxidant
Healthy diet
1 Introduction
Phoenix dactylifera (Date palm) fruit has been used as natural food in the Middle East and Northern Africa for the last 5000 years. It is one of the ancient trees planted by humankind (Shams Ardekani, 2010). Date tree has been used traditionally for various purposes and is known as a holy and blessed tree in different religions, including Islam, Christianity, and Judaism (Ghnimi et al., 2017). The Food and Agricultural Organization measured that Egypt, Iran, Saudi Arabia, Iraq, Pakistan, Oman, United Arab Emirates, Tunisia, and Libya are the most significant date palm cultivators (Samad et al., 2016). Different variations of the date palm are available worldwide, including Khodry, Khalas, Ruthana, Sukkary, Sefri, Segae, Ajwa, Hilali, and Munifi. Among them, Ajwa is a very popular variety that belongs only to the holy city of Al Madinah Al Munawara and its adjoining areas in Saudi Arabia (Mallhi, 2014). Ajwa dates have significant importance in Islam and its benefits have been mentioned in Quran verses and hadiths.
Ajwa date is a rich energy source with nutrients like sugar, mineral vitamin, carbohydrates, potassium, calcium, vitamin A, B3, folic acid, and dietary fibers. Date flesh comprises 85% to 90% of date fruit weight, while date seed or pit constitutes about 6 to 12% of the total weight of the mature date (Baliga et al., 2011). Ajwa date has been recognized to contain the most nutritional and medicinal properties, mainly due to several bioactive compounds such as phenolic and antioxidants. The literature revealed that date fruits and pits are used abundantly in various forms, including whole fruit, powder, pulp, and infusion. In traditional and folk medicine, dates are used to treat a variety of health conditions. Dates are extensively used in atherosclerosis, cancer, pulmonary diseases, and diarrhea. Moreover, the date is commonly used as a hypoglycemic agent, expectorant, and tonic (Hasan and Mohieldein, 2016). Several animal experimental studies have proven that dates exhibit hypolipidemic, anti-oxidant (Rahmani, 2014; Yasin et al., 2015); hepatoprotective (El Arem et al), nephroprotective (El Arem, 2014), antimicrobial (Mahdhi et al., 2013), neuroprotective (Pujari et al., 2011) and anti-cancer (Khan et al., 2017) effects. Research studies confirmed that date fruit act as an effective radical scavenger (Al-Farsi et al., 2005; Abbès et al., 2013). The anti-inflammatory effect of dates is attributed to polyphenol compounds that scavenge free radicals produced during the inflammatory process and prevent unwanted biochemical reactions. Date fruit prevents the production of nitric oxide and TNF-α (Schauss et al., 2013). Date fruit promotes the action of superoxide dismutase and catalase enzymes (Taleb, 2016). In the clinical trial study, dates have been found to increase normal labor progress and decrease the chances of induction during pregnancy (Razali et al., 2017).
In this current study trial, the hypolipidemic effect of Ajwa date was investigated. It was assumed that it is more appropriate to investigate any effect of dates on serum HDL levels in a population that is inherently prevalent with low HDL (Gupta et al., 2006), like the South Asian population. The current study trial was designed to explore the effects of Ajwa on lipid profile in adult population. It was hypothesized that Ajwa would improve the lipid profile, particularly HDL, and prevent the risk of ischemic heart diseases.
2 Research methodology
2.1 Design of the study
The study design was a randomized controlled trial (RCT). The trial was conducted at setting of Aga Khan University Hospital (AKUH), Karachi, Pakistan.
2.2 Sample size
The sample size for the study was calculated with consideration of earlier conducted clinical studies on Ajwa (Kordi, 2014; Al-Kuran, 2011). The power of the study for a significant increase in HDL level was 80%, with 5% of the significance level.
2.3 Participants
Study participants were selected from employees (faculty and staff) of the AKUH. Employees were informed by emails, phone calls, departmental visits, and individual contacts. The study was conducted according to the “ Declaration of Helsinki and Good Clinical Practice guidelines”. After enrolling the participants, the protocol was clearly explained, and written informed consent was taken from each participant. Participants were not offered any inducement, money, and favors for their participation in the study.
2.4 Inclusion and exclusion criteria
The inclusion parameter of the study trial included both healthy males and females of age 18–70 years. The participants with HDL serum levels <40 mg/dl for both men and women were included in the trial. Individuals regularly taking Ajwa in their diet or individuals with dates or Ajwa allergy were excluded during the screening process. Pregnancy, individuals with pre-diabetes and diabetics, metabolic syndrome or any other co-morbidity were excluded from the study.
Ethical approval
The clinical trial approval was taken from the Ethical Review Committee (ERC) of AKUH (ERC # 2019-0633-2318). The trial was registered at NIH, US National Library of Medicine as NCT03805139. The study protocol was described in detail to all the participants, the privacy of participants was maintained, and the secrecy of data was preserved by using codes for each participant.
2.5 Screening and enrolment
The screening was conducted for all the employees interested in participating in the trial. Employees were asked if they have tested for lipid profile in the previous four weeks; if yes, they were requested to show their lipid profile report. Individuals who did not have lipid profile reports were asked to have a lipid profile test with 10–12 hrs of fasting. After screening, individuals with low HDL levels (<40 mg/dl) were enrolled in the study. The whole process of trial participation invitation, screening, and enrolment of participation took around 4–6 weeks. The study protocol was clearly explained, and all the participants signed the informed consent.
2.6 Randomization
After the enrolment of the participants, randomization was performed, and the participants were divided into control and the Ajwa group.
2.7 Ajwa doses preparation
Ajwa doses were prepared in the research pharmacy under the supervision of the Clinical trial unit of Aga Khan University, and the amounts were properly labeled with the study name, protocol number, name of PI, subject ID, week number, quantity dispensed, direction for use, storage conditions, and date distributed. Doses were prepared every week. Both pharmacists and study personnel cross-checked the log and labels for quality assurance.
2.8 Ajwa and control groups
Ajwa group was instructed to take Ajwa (70gm) on an empty stomach in the morning for five days a week for six weeks, while the control group was told to continue their regular dietary pattern and were recommended not to make any particular changes in their diet and lifestyle. Messages were sent to Ajwa group participants in the early morning as a reminder to take Ajwa. Compliance with the protocol was observed through phone calls to participants every week during the six weeks trial period. However, no side effects of Ajwa were reported, but still, for any emergency or concern, the contact number of registered medical practictioner and principal investigator was shared with the participants.
2.9 Baseline and post-study data collection
Then enrolled participants (both groups) were requested to visit the multidisciplinary laboratory (MDL) at AKU with fasting of 10–12 h. A specified room was allocated for data collection, where a participant's filled questionnaires about their eating routine and physical activity. The parameters including body weight, body fat, and water content were taken with the means of an impedance scale. Participant's height and blood pressure were also recorded. The blood sample was taken from each participant for lipid profile test and glucose approximation. They were asked to visit after 6-weeks again with fasting of 10–12 h, and the same procedure of measurement of vitals and blood sampling was conducted.
2.10 Sample analysis
Blood samples were centrifuged on the same collection day at 2500 RPM at 4 °C for 15 min. Serum was separated into various aliquots and was stored at −20 °C for lipid analysis. The Cobas c 111 kits (Roche Diagnostics, Germany) was used for conducting lipid profile test with Cobas c 111 automated analyzers (Roche Cobas).
2.11 Data analysis
IBM SPSS Statistics 20 software was used for data analysis. Data are presented as mean ± standard deviation (SD) in Tables 1 and 2. While data in Table 3 is offered as mean difference ± standard error means (SEM). The significance level was measured as α <0.05 for all two-sided tests. To identify changes over time and differences between the groups, repeated-measures ANOVA with factors time (pre, post) × group (Control, Ajwa) was performed to check the interaction effects. Bonferroni corrected Student's t-tests was performed to recognize any pre to post-differences. Results are presented as mean ± SD; *p < 0.05, **p < 0.001.
PARAMETERS
CONTROL
AJWA
Age (Year)
43.77 ± 11.73
38.05 ± 10.03
Gender
Male
12
15
Female
14
11
BMI (kg/m2)
Underweight
0
2
Normal
14
10
Overweight
8
10
Obese
4
4
Blood Pressure (mmHg)
Systolic
125.90 ± 13.88
119.67 ± 16.73
Diastolic
85.65 ± 12.34
79.91 ± 10.51
Health Condition (Ratio)
Yes: No
Yes: No
Hypertension
5:21
2:24
Diabetes mellitus
3:23
1:25
Cigarette Smoking
2:24
1:25
Family History of IHD/CVD
11:15
9:17
PARAMETERS
GROUPS
BASELINE
POST-STUDY
INTERACTION EFFECT
TIME EFFECT
(Time × group)
Body Weight (kg)
Control
67.65 ± 11.69
67.46 ± 11.74
0.282
0.006**
Ajwa
69.65 ± 17.43
69.23 ± 17.23
BMI (kg/m2)
Control
26.23 ± 4.95
26.14 ± 4.94
0.606
0.004**
Ajwa
25.00 ± 4.16
24.88 ± 4.12
Total cholesterol (mg/dl)
Control
211.19 ± 45.27
209.57 ± 44.23
0.0001**
0.0001**
Ajwa
216.42 ± 36.49
194.42 ± 32.70
Triglycerides (mg/dl)
Control
132.96 ± 25.17
131.92 ± 26.02
0.0001**
0.0001**
Ajwa
136.42 ± 25.10
124.30 ± 25.93
LDL (mg/dl)
Control
119.85 ± 19.64
119.91 ± 18.76
0.003*
0.009*
Ajwa
120.63 ± 20.95
109.25 ± 26.9
HDL (mg/dl)
Control
35.84 ± 2.64
36.01 ± 2.57
0.0001**
0.0001**
Ajwa
36.58 ± 2.16
39.78 ± 3.02
Blood Glucose (mg/dl)
Control
88.11 ± 6.27
85.80 ± 8.28
0.395
0.235
Ajwa
84.88 ± 7.51
85.26 ± 8.55
3 Results
Screenings of 80 individuals were performed; out of them, 52 were selected for the study and were equally distributed in two groups, with 26 participants in each group. All of the 52 participants included in the trial completed the study. The flow of the clinical research has been mentioned in Fig. 1.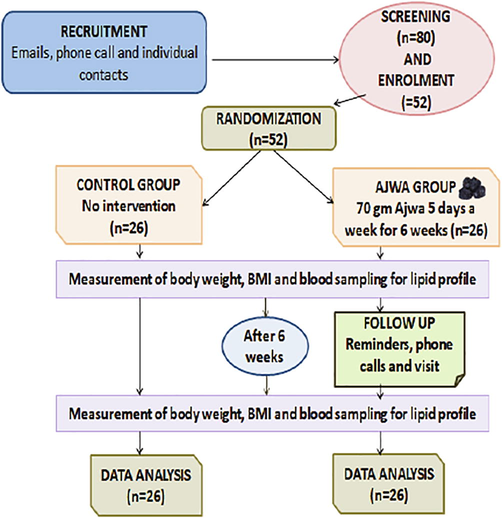
Flow Diagram of the Trial.
Table 1 summarizes the baseline details of study participants, including their age, gender, blood pressure, BMI, and record of prevailing medical illness. The comprehensive questionnaire was filled at pre and post-study levels, including details on participants eating practices and physical movement. Baseline and post-study comparisons showed no difference, and all the participants maintained their eating habits and daily routines as advised.
The changes in different parameters, including vitals and lipids level at baseline and post-six-week study, are mentioned in Table 2. Bodyweight and BMI did not show any significant interaction effect; however, both showed a time effect with a p-value of 0.006 and 0.004, respectively. Regarding lipid levels, total cholesterol, triglycerides, and HDL displayed significant interaction and time effects with a p-value of 0.0001, while LDL exhibited a significant interaction effect (p = 0.003) and time effect (p = 0.009). Moreover, blood glucose did not demonstrate any meaningful interaction or time effect.
The mean changes in body weight, BMI, lipids levels, and blood glucose levels from baseline to post-study for the control and Ajwa groups and outcomes of post-hoc analysis of within-group change are mentioned in Fig. 2. The Ajwa group exhibited significant reduction in body weight (-0.41 ± 0.14 kg; p = 0.007) and BMI (-0.11 ± 0.04 kg/m2; p = 0.015). The mean changes in Ajwa group exhibited significant modification for total cholesterol (–22.00 ± 4.23 mg/dl; p = 0.0001), triglycerides (-15.57 ± 4.02 mg/dl; p = 0.0001), LDL (-11.38 ± 1.79 mg/dl; p = 0.0001) and HDL (3.19 ± 0.80 mg/dl (p = 0.0001). No significant changes were found for any of the parameters for the control group.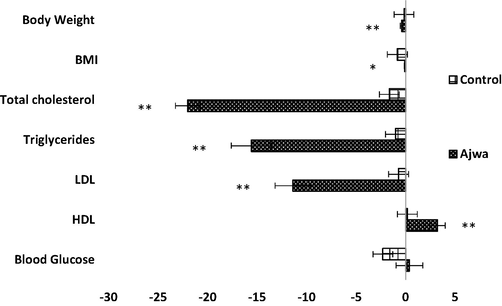
Mean difference in parameters among control (n = 26) and Ajwa (n = 26) groups at baseline and post-study.
During the six-week trial, during weekly away dispensing, participants were also asked how they feel after taking Ajwa on an empty stomach. They were asked to mention one word for their weekly experience of taking Ajwa. It was found that most of the participants found the inclusion of Ajwa normal; intake of Ajwa did not bring any change in their daily routine, while some participants felt heaviness in starting weeks of the study. The participants also felt energetic and active during the study period. Fig. 3 represents various words used by participants to express their experiences.
Word cloud of patients expressions.
4 Discussion
The result of the clinical trial performed on the general population indicated that the use of Ajwa produced significant improvement in lipid profile. The data showed a significant increase in HDL level and a significant decrease in total cholesterol, triglycerides, and LDL levels. The study result confirmed the outcome of various experimental animal studies that suggested that Ajwa possesses hypolipidemic properties.
Khalid et al. conducted experiments on albino mice with high cholesterol using Ajwa pulp and pits as treatment and found that Ajwa decreased total cholesterol, triglycerides, and LDL levels. In addition, Ajwa also led to improvement in HDL level (Sumaira Khalid, 2017). At the same time, another study evaluated the hypolipidemic effect of extracts of date leaves and flax seeds in alloxan diabetic rats. It was found that both the extracts considerably decrease the total cholesterol level. A significant decrease was also noticed in LDL levels. However, both extracts did not show any improvement in HDL levels (Abuelgassim, 2010). Evaluation of the antihyperlipidemic outcome of Ajwa date seeds was conducted on diet-made hyperlipidemic rabbits. The study found that HDL level was improved while a significant decrease was noticed in total cholesterol, triglyceride, and LDL. Moreover, Ajwa leads to the reduction of the atherogenic index of plasma and LDL/HDL proportion. (Mushtaq, 2017; Alqarni et al., 2019). However, the sciencetific literature has very limited clinical studies on short term Ajwa use and lacks long term clinical studies on Ajwa use in regular diet.
Various studies on dates' therapeutic and pharmacological properties suggested that the mechanism of improving lipid profile is the antioxidant effect. The antioxidant effect is due to active constituents, including phenolic compounds, that are rich in Ajwa compared to any other date variation. In addition, Ajwa date increases antioxidant enzymes activation, including glutathione peroxidase, catalase, and superoxide dismutase. Moreover, the fiber present in date seed might also improve lipid profile with the absorption of dietary fats (Mushtaq, 2017). It was also suggested that Ajwa limits the weakening of endogenous antioxidants superoxide dismutase and catalase, leading to inhibition of lipid peroxidation. Al-Yahya evaluated that Ajwa acts as an antioxidant, hypolipidemic, anti-inflammatory, and anti-apoptotic in myocardial injury by reducing the numbers of inflammatory cytokines, including interleukin six and interleukin 10, TNFα, and apoptotic markers while increasing the anti-apoptotic protein Bcl2 (Al-Yahya, 2016). Other studies found that Ajwa provides cardioprotection in myocardial infarction due to the abundance of phenolic and flavonoid compounds. Ajwa constituent helps build up the reserves and organize circulating progenitor cells from bone marrow and circulation (Alhaider, 2017). These all previously performed animal experimental studies confirm the trial result that Ajwa has a hypolipidemic effect due to nutritional constituents and can play a significant role in protecting against various cardiovascular diseases.
5 Limitation
The sample for the analysis was not significant and was single-center-based; for validation and confirmation of the study results, large-scale and multicenter clinical trials are required. Confounders existed in the study, including diet variation and activity level of the participants.
6 Conclusion
Consumption of Ajwa date with a regular diet in daily life is well tolerated and improves the lipid profile particularly HDL that protects against ischemic heart diseases. Further studies, including trials with diet control, monitoring of activity, and individuals with cardiovascular diseases and type 2 diabetes mellitus, should be conducted to validate the trial findings.
Acknowledgments
Thankful to the “Researchers Supporting Project Number (RSP-2021/47), King Saud University, Riyadh, Saudi Arabia”.The authors greatly acknowledge the mentorship, continuous support, and guidance of Prof. Anwar-ul Hassan Gilani. We are very thankful to Mr. Ghulam Haider and MDL/ML lab staff for technical support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Comparison of Antioxidant Activity and Total Phenol Contents of some Date Seed Varieties from Iran. Iranian Journal of pharmaceutical research: IJPR. 2010;9(2):141-146.
- [Google Scholar]
- Date fruit (Phoenix dactylifera L.): An underutilized food-seeking industrial valorization. NFS Journal. 2017;6:1-10.
- [Google Scholar]
- Antibacterial Properties and Effects of Fruit Chilling and Extract Storage on Antioxidant Activity, Total Phenolic and Anthocyanin Content of Four Date Palm (Phoenix dactylifera) Cultivars. Molecules. 2016;21(4):419.
- [Google Scholar]
- Review Ajwa date (Phoenix dactylifera)- an emerging plant in pharmacological research. Pak J Pharm Sci. 2014;27(3):607-616.
- [Google Scholar]
- A review of the chemistry and pharmacology of the date fruits (Phoenix dactylifera L.) Food Research International. 2011;44(7):1812-1822.
- [Google Scholar]
- In Vivo Evaluation of Anti Diabetic, Hypolipidemic, Antioxidative Activities of Saudi Date Seed Extract on Streptozotocin-Induced Diabetic Rats. J Clin Diagn Res. 2016;10(3):Ff06-12.
- [Google Scholar]
- Therapeutic effects of date fruits (Phoenix dactylifera) in the prevention of diseases via modulation of anti-inflammatory, antioxidant and anti-tumor activity. International Journal of Clinical and Experimental Medicine. 2014;7(3):483-491.
- [Google Scholar]
- Date (Phoenix dactylifera) Polyphenolics and Other Bioactive Compounds: A Traditional Islamic Remedy's Potential in Prevention of Cell Damage, Cancer Therapeutics and Beyond. Int J Mol Sci. 2015;16(12):30075-30090.
- [Google Scholar]
- A. El Arem E.B. Saafi F. Ghrairi A. Thouri M. Zekri A. Ayed A. Zakhama L. Achour Aqueous date fruit extract protects against lipid peroxidation and improves antioxidant status in the liver of rats subchronically exposed to trichloroacetic acid.
- Oxidative damage and alterations in antioxidant enzyme activities in the kidneys of rat exposed to trichloroacetic acid: protective role of date palm fruit. J Physiol Biochem. 2014;70(2):297-309.
- [Google Scholar]
- Use of mixture design to construct a consortium of date palm (Phoenix dactylifera L.) fruit extract and potentially probiotic Bacillus strain to confer protection against vibriosis in Artemia culture. J Sci Food Agric. 2013;93(15):3850-3855.
- [Google Scholar]
- Evaluation of antioxidant and neuroprotective effect of date palm (Phoenix dactylifera L.) against bilateral common carotid artery occlusion in rats. Indian J Exp Biol. 2011;49(8):627-633.
- [Google Scholar]
- Anti-cancer effects of Ajwa dates (Phoenix dactylifera L.) in diethylnitrosamine induced hepatocellular carcinoma in Wistar rats. BMC Complement Altern Med. 2017;17(1)
- [Google Scholar]
- Comparison of Antioxidant Activity, Anthocyanins, Carotenoids, and Phenolics of Three Native Fresh and Sun-Dried Date (Phoenix dactylifera L.) Varieties Grown in Oman. Journal of Agricultural and Food Chemistry. 2005;53(19):7592-7599.
- [Google Scholar]
- Effect of processing conditions on phenolic compounds and antioxidant properties of date syrup. Industrial Crops and Products. 2013;44:634-642.
- [Google Scholar]
- Chapter 28 – Polyphenols and Inflammation. In: Watson R.R., Preedy V.R., eds. Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases. San Diego: Academic Press; 2013. p. :379-392.
- [Google Scholar]
- Chemical characterization and the anti-inflammatory, anti-angiogenic and antibacterial properties of date fruit (Phoenix dactylifera L.) J Ethnopharmacol. 2016;194:457-468.
- [Google Scholar]
- Date fruit consumption at term: Effect on length of gestation, labour and delivery. Journal of Obstetrics and Gynaecology. 2017;37(5):595-600.
- [Google Scholar]
- South Asians and cardiovascular risk: what clinicians should know. Circulation. 2006;113(25):e924
- [Google Scholar]
- The Effect of Late Pregnancy Consumption of Date Fruit on Cervical Ripening in Nulliparous Women. Journal of Midwifery and Reproductive Health. 2014;2(3):150-156.
- [Google Scholar]
- The effect of late pregnancy consumption of date fruit on labor and delivery. J Obstet Gynaecol. 2011;31(1):29-31.
- [Google Scholar]
- Muhammad Kaleem, Antioxidant activity and phenolic contents of Ajwa date and their effect on lipo-protein profile. Functional Foods in Health and Disease. 2017;2017:396-410.
- [Google Scholar]
- Effect of flax seeds and date palm leaves extracts on serum concentrations of glucose and lipids in alloxan diabetic rats. Pak J Biol Sci. 2010;13(23):1141-1145.
- [Google Scholar]
- EFFECT OF AJWA DATE SEED ON LIPID PROFILE OF DIET INDUCED HYPERLIPIDEMIC RABBITS. Khyber Medical University Journal. 2017;9(3):135-139.
- [Google Scholar]
- Antioxidant and antihyperlipidemic effects of Ajwa date (Phoenix dactylifera L.) extracts in rats fed a cholesterol-rich diet. J Food Biochem. 2019;43(8):e12933
- [Google Scholar]
- 'Ajwa' dates (Phoenix dactylifera L.) extract ameliorates isoproterenol-induced cardiomyopathy through downregulation of oxidative, inflammatory, and apoptotic molecules in a rodent model. Phytomedicine. 2016;23(11):1240-1248.
- [Google Scholar]
- Date Palm (Phoenix dactylifera) Fruits as a Potential Cardioprotective Agent: The Role of Circulating Progenitor Cells. Front Pharmacol. 2017;8:592.
- [Google Scholar]







