Translate this page into:
Responses of Enterococcus faecalis resistance and cytolysin up-regulation to nutrients in constructed mesocosms
⁎Corresponding author. yudaojin@yeah.net (Daojin Yu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Spreading of multidrug-resistant bacteria has become a growing and significant threat to environmental and public health. Our understanding of environmental variables such as nutrients which contribute to the dissemination of antibiotic resistance and pathogenesis is still limited. In this study, we operated outdoor mesocosm experiments to evaluate how nitrate and phosphate ions with different concentrations influence on an adaptation of Enterococcus faecalis to aquatic environments. E. faecalis were isolated from the mesocosms at 8 sampling events for 96 days to evaluate biofilm production, quorum-sensing-related genes expression, and sensitivity to oxytetracycline, erythromycin, ciprofloxacin, ampicillin, vancomycin, and chloramphenicol. Quantitative real-time PCR was used to compare mRNA levels of E. faecalis quorum-sensing-related genes. E. faecalis isolates exhibited resistance to oxytetracycline, ampicillin, and ciprofloxacin, respectively. We observed that the biofilm production of E. faecalis isolates on day 60 and 96 was significantly increased (p < 0.01). The expressions of quorum-sensing-related genes were significantly up-regulated (∼tenfold) at the transcriptional level in nutrient-enriched treatments. Our findings indicate that nitrate and phosphate ions facilitate resistance to commonly used antibiotics, increase biofilm production and intra-species communication, which could be a major reviving strategy of E. faecalis in the aquatic environments.
Keywords
Antibiotic resistance
Aquatic environment
Enterococcus faecalis
Nitrate and Phosphate ions
Nutrient pollution
1 Introduction
Enterococcus faecalis, a low-GC ratio (37.5%), aerotolerant Gram-positive bacterium that ubiquitously presents in the gut of hosts, ranging from humans to insects, has emerged as a leading causes of nosocomial infections on a global scale (Guzman Prieto et al., 2016). Over the past three decades, E. faecalis is extensively dispersed in a different environmental habitats, including sediments, soil, aquatic vegetation, and heterothermic environments (temperature ranging from 10 °C to 45 °C) (Byappanahalli et al., 2012). The spreading of E. faecalis in aquatic ecosystems is due to industrial inputs, agricultural runoff, and domestic wastewaters (Cho et al., 2020). Similarly, antibiotics and antibiotic resistance genes (ARGs) are also released into the surface waters and marine sediments, which contribute a major risk to humans health (Amarasiri et al., 2020).
The proliferation of antibiotic-resistant bacteria/genes in the marine environments is a growing public health threat worldwide (Ayandiran and Dahunsi, 2017; Nasri et al., 2020; Sanjay et al., 2020). Enterococcal ARGs have been reported in different aquatic environments (Naeem-Ullah et al., 2020) and the dissemination of these genes in Enterococcus are due to horizontal gene transfer mechanisms in marine sediments (Rashid et al., 2020). The pathogenesis of E. faecalis is associated with numerous virulence factors. Virulence factors are extracellular molecules released by microbial pathogens that allow overcoming host defense mechanisms and causing disease in a host (Islam et al., 2020; Noman et al., 2021; Noman et al., 2019). In E. faecalis, most of these factors are controlled by quorum-sensing system and biofilm formation. In quorum-sensing; bacteria communicate with each other through sensing molecules called autoinducers, which are regulating bacterial gene expression in response to changes in cell-population density (Hmelo, 2017). In E. faecalis, two autoinducer molecules, including, gelatinase biosynthesis-activating pheromone (GBAP) and the small subunit of cytolysin (CylLS) are environmentally regulated by the fsr operon and cyl regulatory signaling, respectively (Weaver et al., 2019). The mechanisms whereby quorum-sensing contributes to antibiotic resistance and biofilm development in E. faecalis are not completely understood. However, quorum-sensing-related genes have been identified that play a role in biofilm development (Li et al., 2019). In addition, Dale and coworkers reported that fsr-mediated quorum-sensing involved in the biofilm-associated antibiotic resistance (Dale et al., 2015).
In the fsr system, an extracellular accumulation of GBAP triggers FsrC (transmembrane protein) and FsrA (response regulator), both together work as two-component signal transduction (TCST) system. The TCST is phosphorylated upon the binding of GBAP to FsrC (Littlewood et al., 2020). The phosphorylated FsrA then binds to the fsr promoter region that controls the regulation of downstream virulence factors genes i.e. gelE and sprE that encode gelatinase and serine protease, respectively (Dundar et al., 2015).
Cytolysin is a two-subunit toxin of E. faecalis that is regulated by a cyl operon (Van Tyne et al., 2013). The cyl operon contains two promoters: PL promoter for the structural genes (cylLL, cylLS, cylM, cylB, cylA, and cylI) and PR promoter for the two regulatory genes (cylR1 and cylR2). The two-subunit toxin, CylLL and CylLS form a multimeric complex that leads to pore formation in the target (eukaryotic and prokaryotic) cells membranes. CylM post-translationally modify the two-subunit toxin (CylLL and CylLS), which then further processed and transported by the CylB transmembrane protein. In the extracellular milieu, CylA protease further activated the subunits of cytolysin. CylR1 and CylR2 regulate cyl operon in response to extracellular CylLS. Last but not least, CylI confer self-immunity against the bactericidal activity of the cytolysin (Van Tyne et al., 2013).
Aquatic sediments are significant reservoirs of enterococci. Sediments provide sufficient nutrients so that E. faecalis can withstand for three to four months in the aquatic sediments. Similarly, mesocosms are useful tools to study antibiotic resistance and virulence factors in E. faecalis (Ali et al., 2016). The main advantages of mesocosms are to simulate realistic environmental conditions, replicates of different treatments, and extended periods of experimentation. Recently, attention has focused on mesocosms environments to understand the mechanisms of antibiotic resistance in different environmental conditions. It has been reported that the mesocosm approaches appropriately represent the interactions between E. faecalis, nutrients and soil within a complex sediment ecosystem (Byappanahalli et al., 2012).
Previously, we found that supplementation of cornmeal and nutrients such as nitrogen (N) and phosphorus (P) to the freshwater mesocosms lead to the antibiotic resistance in E. faecalis (Ali et al., 2016; Chen et al., 2018; Yu et al., 2012). Therefore, the current studies investigate the hypothesis that N and P influences antibiotic resistance, biofilm development, and quorum-sensing regulation in E. faecalis. In the present study, we established outdoor experimental mesocosms with different concentrations of N and P. Our study seeks to explore the effects of nutrients on survival, antibiotic resistance, biofilm development and quorum-sensing in E. faecalis.
2 Materials and methods
2.1 Experimental design of model ecosystem
Mesocosms used in this study were located at the Fujian Agriculture and Forestry University (Fuzhou, China, latitude: 26°05′24.9″ N, longitude: 119°13′53.2″ E). The average precipitation during the study period was ranges from 74 to 288 mm. These mesocosms were identical to those described previously (Ali et al., 2016). Briefly, the mesocosms consisted of twelve sterile plastic pails (w × d × h = 20 × 20 × 20 cm) in approximately 27 m3 polyethylene enclosure established in May 2010. Prior to filling with five liters of tap water to each pail, uncontaminated soil from the Fuzhou National Forest Park was placed symmetrically to a depth of 5 cm at the bottom of each pail. The average annual temperature of the water was 24 ± 4.45 °C during the entire experiment. The pH, dissolved oxygen, Secchi Depth (SD) and primary conductivity in mesocosms were inspected regularly in situ, using Water Checker Field Monitor (Horiba Instruments) to ensure that the environmental parameters of all the mesocosms were equivalent at the start of the study. During heavy rain, the pails were temporarily covered with lids. To avoid the evaporation effect, tap water was sprayed daily to nearly re-establish the original water levels.
The mesocosms consisted of four treatments (T1; control without N and P supplementation), T2 (low-dose of N and P), T3 (mid-dose of N and P), T4 (high-dose of N and P) and were arranged in parallel triplicates: (i) T1: unenriched control treatment (ii) T2: P was supplemented with KH2PO4 at a final concentration of 0.15 mg/L P and N (1.5 mg/L) supplemented with NH4NO3 (iii) T3: the concentration of P (0.45 mg/L) supplemented with KH2PO4 and N (4.5 mg/L) supplemented with NH4NO3 (iv) T4 was enriched by 1.25 mg/L P and 12.5 mg/L N. These nutrient-enrichment concentrations were selected to modulate the bacterioplankton community structure under different spatial aquatic environments and mesocosm experiments (Ali et al., 2016). Furthermore, these nutrients were chosen since E. faecalis is likely adapted to the aquatic environments containing certain nutrients including N and P. All treatments were inoculated with E. faecalis, resulting in a final concentration of ≈4 × 104 CFU/mL on day 0. Before starting the experiment, trophic assessment standards were established and evaluated by conducting a pre-experiment, to make sure that the different treatment groups have different trophic levels.
2.2 Sample collection
The experiments reported here were conducted from August to November 2015. The nutrients (N and P) were added to the treatments (T2, T3, and T4) at a day 0. Ten samples from each treatment pail were collected on different sampling points to avoid overlapping. These samples (100 µL) were taken from the top of the mesocosms sediment in sterile Eppendorf tubes on day 0, 1, 7, 14, 28, 40, 60 and 96. At the same days, water samples (20 mL) from the pails were also collected in glass jars for the determination of Total Nitrogen (TN), Total Phosphorus (TP), Chemical Oxygen Demand (CODMn), pH, and Conductivity (Cond) as described previously (Hou et al., 2013).
2.3 Isolation and identification of enterococci
Ten sediment samples each at 8 sampling events of the mesocosms were taken. Each sample was inoculated in Mueller-Hinton (MH) broth at 25 °C for 24 h with gentle shaking. Then, the samples were spread on Slanetz-Bartley agar medium (CM0377, Oxoid Ltd, Hampshire, UK) at 37 °C for 48 h. Deep red colored colonies were selected and further spread on bile-aesculin agar plates (CM0888, Oxoid Ltd, Hampshire, UK) at 37 °C for 18–24 h and small, brown colonies were selected for subsequent analyses. A total of n = 382 isolates were obtained from day 0 to day 96. DNA of the E. faecalis isolates was extracted using MiniBEST Bacteria Genomic DNA Extraction Kit (Takara, Dalian, China) according to the to the manufacturer's instructions. E. faecalis 16S rRNA gene was identified using primers 72-F (5′-CCG AGT GCT TGC ACT CAA TTG G-3′) and 210-R (5′-CTC TTA TGC CAT GCG GCA TAA AC-3′). E. faecalis ATCC 29,212 was used as a reference strain. For each sampling of the selected days, only one strain was identified and preceded for quantitative real-time PCR (qPCR) analysis.
2.4 Enterococcus faecalis sensitivity testing
Antibiotic resistances of all isolated E. faecalis strains (3 8 2) were grown on MH broth overnight. Broth microdilution method was used according to the (Wikler et al., 2007) breakpoints (Table 1) to determine the resistance of the following antibiotics: ampicillin (AMP), oxytetracycline (OXY), ciprofloxacin (CIP), vancomycin (VAN), chloramphenicol (CHL), and erythromycin (ERY). These antibiotics were selected primarily because they are most frequently found on surface water and sediment in China due to anthropogenic activities. T1 = without nutrient-enrichment, T2 = low-dose of N and P, T3 = mid-dose of N and P, T4 = high-dose of N and P, nd = not determined.
Antimicrobial agent
MIC breakpoint (μg/mL)
No. of resistant isolates (%)
T1
T2
T3
T4
Oxytetracycline
≥16
nd
43 (11.26%)
65 (17.02%)
80 (20.94%)
Erythromycin
≥8
6 (1.57%)
4 (1.05%)
7 (1.83%)
9 (2.36%)
Ciprofloxacin
≥4
nd
nd
17 (4.45%)
4 (1.05%)
Ampicillin
≥16
nd
12 (3.14%)
29 (7.59%)
42 (10.99%)
Vancomycin
≥32
nd
nd
nd
4 (1.05%)
Chloramphenicol
≥32
nd
3 (0.78%)
2 (0.52%)
nd
2.5 Biofilm assay
Biofilm assay of the E. faecalis isolates was performed as described previously (O’Toole, 2010) with minor modifications. Briefly, overnight cultures of E. faecalis isolates were diluted 100-fold in MH broth. 150 μL of diluted cell suspensions were added to 96-well polystyrene plates and incubated at 37 °C for 24 h. After incubation, media from the wells were gently removed, washed with phosphate-buffered saline, left to dry and then stained with 150 μL of 1% crystal violet for 10 min at room temperature. The wells were then washed twice with de-ionized water and left to dry. To destain the biofilm, 150 μL of (33%) glacial acetic acid was added to each well. The optical densities of the wells were measured at 630 nm (OD630) in triplicate by using a microplate reader (Tecan, Austria).
2.6 Gelatinase assay
For the qualitative detection of gelatinase activity, cultures of E. faecalis were transferred into gelatin broth (Guangdong Huankai Microbial Sci. & Tech. Co. Ltd. China) having 12% gelatin in 0.8% nutrient broth. The tubes were incubated at 37 °C for 24–48 h in a shaking incubator (190 rpm) and then held at 4 °C for 1 h. The solidified tubes were phenotypically considered gelatinase negative. We used E. faecalis ATCC 29,212 strain because it possesses gelE gene and cyl operon, however, this strain does not contain fsr operon.
2.7 Physiochemical analyses
Standard stock solution (250 mM NaOH, 55 mM K2S2O8, and 242 mM H3BO3) was prepared and stored at room temperature. The standard calibration curves of TN and TP were constructed as previously described (Lei Yanzhi, 2007). Water samples were collected to measure TN, TP using a persulfate oxidation method (Valderrama, 1981). For the simultaneous analysis of TN and TP, 10 mL of a water sample from each pail was added to 10 mL standard stock solution and autoclaved at 124 °C for 30 min and further processed as described previously (Valderrama, 1981). The absorbance of the samples were analyzed for TN at 220 nm and 275 nm and for TP at 700 nm using Beckman DU-800 spectrophotometer (Fullerton, USA) and Milli-Q water was used in the reference cuvette. CODMn was measured by acid titration method (Ma et al., 2016).
2.8 Quantitative real-time PCR (qPCR)
Total RNA was extracted from 28 sediment samples (5 g) (4 treatments × 7 different time point) using TRIzol method (Shanghai Biological Engineering Co. LTD) as previously described (Ran et al., 2013). The purity of mRNA was determined using Nano-200 Nucleic Acid Analyzer at 260 and 280 nm: A260/A280 optical density ratios range from 1.8 to 2.0 was accepted for further analysis. cDNA was synthesized from 2 µg of total RNA using Prime Script RT Reagent Kit with gDNA Eraser. In qPCR, using SYBR Premix Ex Taq II (Takara, Japan) for the subsequent genes expression of cytolysin large subunit gene (cylLL), cytolysin small subunit (cylLS), cytolysin synthetase (cylM), ATP-binding transporter (cylB), component A (cylA), cytolysin regulatory genes (cylR1 and cylR2), cytolysin immunity (cylI), and gelatinase (gelE) as described previously (Ran et al., 2013). The results of these genes were based upon three independent experiments and the relative expression values were internally normalized against reference gene (23S rRNA) expression. Primers used for PCR and qPCR, along with the corresponding size of the amplified fragments are listed in Table S1.
2.9 Statistical analysis
E. faecalis biofilm development was determined by one-way ANOVA and Tukey's multiple pair-wise comparisons among different treatments, and quorum-sensing-related genes expression analyses were performed using Student’s t-test. These analyses were analyzed in MS Excel and Minitab v.18 (State College, PA) at a 5% significance level. A canonical discriminant analysis was conducted on the environmental variables in R version 3.4..
3 Results
3.1 Mesocosms appearance and survival of E. faecalis
After enrichment of the nutrients, no changes were seen in the physiological appearance of the mesocosms across treatments. The nutrient contents have impacted by several factors, such as the type of organic matter, sediment type, water SD rate, evaporation, and redox conditions. However, the average SD was decreased from the beginning (day 0) to end date (day 96) of the experiment. It was observed that TN concentrations of the different treatments generally decreased over time. The decrease in the relative humidity and longer-term (14 weeks) of the experiment offset the evaporation effect (Table S2). Consequently, the concentration of TN in T3 (2.78 ± 0.35 mg/L N) and T4 (6.64 ± 1.16 mg/L N) treatments were significantly decreased on day 96. In addition, TP concentration also significantly decreased (p < 0.05) on day 96 in the enriched treatments (Table S2). However, control, T2, and T3 was significantly similar on day 1. In the aquatic environments, TN and TP both are the key factors to measure Trophic Level Index (TLI), which indicate a gradual decreased in mesocosm TLI. In addition, the approximate ratio of N:P (14:1) in T3 and T4 treatments was closed to redfield value (16:1), however, this ratio was either high or low in different mesocosms on different days. The multivariate linear model showed that the canonical scores of two dimensions account for 99.2% in the environmental variables (Fig. 1). The first dimension (horizontal) was significantly correlated with conductivity, pH, CODMn, TN, and TP while the second dimension (vertical) was significantly correlated with SD and temperature. T4 treatment was more separated from control treatment compared to the T2 and T3 treatments.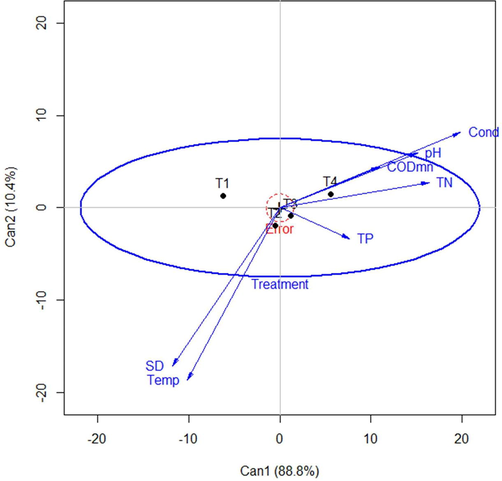
Multivariate linear model (canonical heplot), visualizing the two canonical dimensions of the environmental variables, with alpha = 0.05. T1; control (without nutrient-enrichment), T2; (low-dose of N and P), T3; (mid-dose of N and P), and T4; (addition of nitrogen and phosphorus with high concentration) are four treatments. The lengths of environmental variables indicate their relative contribution to discrimination among the group means. The close angles between variables show high correlations between them. From right to left, the first canonical dimension (88.8%) essentially corresponds to the treatments. The variables such as conductivity (Cond), pH, chemical oxygen demand (CODMn), and Total Nitrogen (TN) are largely associated with this dimension (Can1), indicates that they mostly explain by T4. Whereas, Secchi Depth (SD), and Temperature (Temp) is point towards the second canonical dimension (Can2) and negatively correlated with the other four variables.
Electric conductivity of the mesocosms on day 1 in nutrient amended treatments were 10.43 ± 0.75, 11.67 ± 0.96, and 12.27 ± 0.45 mS/cm in T2, T3 and T4, respectively, significantly different from control 7.43 ± 0.76. The conductivity of mesocosms was gradually increased from day 1 to day 96 in all treatments (Table S2).
The average pH of all the treatments was slightly decreased, however, no significant difference was found among the treatments or days. The lowest pH was 6.8 ± 0.10 on day 14 in control mesocosm and highest was 8.43 ± 0.05 on day 96 in T2 treatment (Table S2). The CODMn ranged from 1.56 ± 0.17 to 11.28 ± 8.61 mg/L in all treatments. The overall CODMn of T4 was significantly high (p < 0.05) than T2, T3 and control treatments. The number of E. faecalis isolates declined over time in all treatments (Fig. S1). On day 0, the average isolation rates among all four treatments were 100% (10 isolates). However, on day 96, the number of isolates decreased to 5%, 5%, 0% and 15% in T1, T2, T3 and T4, respectively (Fig. S1).
3.2 Effect of nitrogen and phosphorus on Enterococcus faecalis antibiotic resistance
The prevalence of antibiotic (AMP, OXY, CIP, and ERY) resistant E. faecalis strains was detected in four different treatments (Table 1). In a total of 382 isolates, ERY (MIC, 8–256 μg/mL), was the most common of all the treatments. In the nutrient-enriched treatments, 188 (49.2%), 83 (21.7%), and 21 (5.5%) E. faecalis isolates exhibited resistance to OXY, AMP, and CIP, respectively (Table 1). Resistance to VAN and CHL was the least prevalent in the mesocosm experiment. Overall, statistical analysis revealed a significant increase of antibiotic resistance in E. faecalis after exposure to the water environment.
3.3 Biofilm development
To determine whether nutrients and different isolation time point (day 0 to day 96) effect E. faecalis attachment to surfaces, we evaluated biofilm development with the microtiter dish assay. The biofilm development of E. faecalis isolates on day 60 and day 96 were significantly increased (F7,24 = 62.26, p < 0.01; Fig. 2). Notably, on day 0 no significant difference (F3,8 = 2.86, p > 0.05) was found among treatments, however, biofilm development was increased significantly in different treatments over time (Fig. 2; Tukey's test (p < 0.05).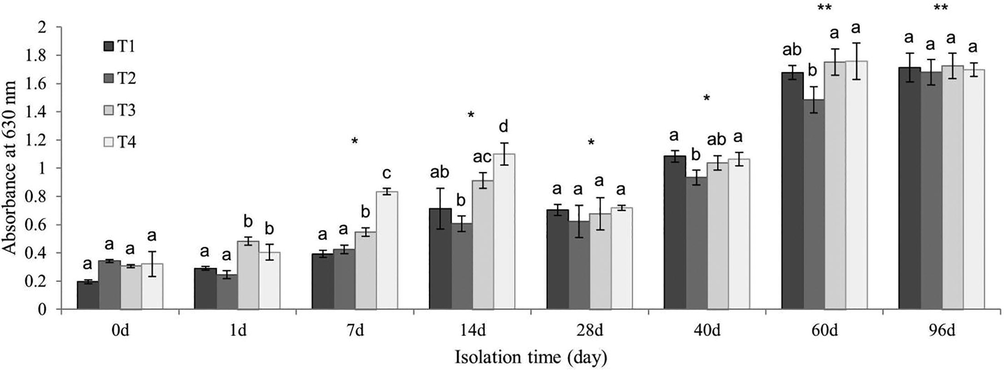
E. faecalis biofilm formation with different time points. E. faecalis biofilm formation shows in T1 (without nutrient-enrichment), T2 (low-dose of N and P), T3 (addition of nitrogen and phosphorus with mid concentration) and T4 (addition of nitrogen and phosphorus with high concentration) treatments. Different letters indicate a significant difference between treatments on the same day (One-way ANOVA and Tukey's test). Error bars represent standard deviation (n = 3). Asterisks indicate statistically significant difference of isolates on different days (* p < 0.05; ** p < 0.01).
3.4 Analysis of Quorum-sensing gene expression
The expressions of E. faecalis cyl operon genes in response to different concentrations of nutrients in mesocosms were examined by qPCR. The relative expression of quorum-sensing-related genes was made between experimental mesocosms and with respect to those obtained from day 0 (calibrator), regarding strain-dependent expression. The level of cylLL expression for E. faecalis isolates was determined for each of the different treatments relative to control for seven different time points (Fig. 3a). A significant increase in cylLL expression was observed on day 7 in T4 treatment (18.93 ± 4.31) compared to control (5.43 ± 1.36), T2 (5.90 ± 0.46), and T3 (6.43 ± 0.83) treatments. Although, cylLL abundance was significantly similar in all treatments on day 14 and day 28. On day 40, down-regulation was observed in T2 treatment (10.07 ± 1.46) compared to control (15.17 ± 4.30). Fig. 3b illustrates the relative abundance (mRNA relative level in fold difference) of cylLS at different sampling points of the four different treatments. On day 0, the values (3.5 ± 0.3), (2.53 ± 0.40), (1.83 ± 0.21), and (1.47 ± 0.25) of E. faecalis cylLS were obtained for control, T2, T3, and T4 treatments, respectively. The cylLS abundances on day 1 were slightly increased in control (3.87 ± 0.11), T2 (3.63 ± 0.21), T3 (5.77 ± 0.15), and T4 (4.57 ± 0.15) treatments. A significant level of up-regulation (14.7 ± 0.95) was observed in T4 treatment on day 7 compared to control (4.13 ± 0.21). Similar trends were observed for the abundance of cylLS at day 28, day 40, and day 60. Relative levels of cylR1 expression in the initial isolation (day 0) were less than twofold in all treatments (Fig. 3c). However, the expression of cylR1 in T3 treatment was approximately tenfold (10.73 ± 1.80) increased on day 1. The gradual up-regulation of was observed in the four treatments on day 7. The expression of cylR1 was significantly different (p < 0.05) in T2 (15.83 ± 4.37), T3 (15.8 ± 0.5), and T4 (20.16 ± 6.36) as compared to control (8.5 ± 4.43) on day 7 and 14 (Fig. 3c). In contrast, cylR gene expression was stimulated much more in control than other treatments on day 28 and day 40. The transcription of cylR2 gene was also showed similar trends like cylR1 on first two isolation time points, and the patterns for the day 7 and day 14 were parallel those observed for cylLS and cylR1 (Fig. 3b; Fig. 3c). The nutrient supplement treatments were significantly higher than control on day 7 and 14. The highest abundance of cylR2 transcription was observed in T4 treatment on day 14 (18.47 ± 2.53), while the lowest expression was observed in T2 (0.83 ± 0.15) treatment on day 0 (Fig. 3d). The level of cylM expression for E. faecalis isolates was determined for each of the different treatments relative to MH broth for seven different time points (Fig. 3e). A significant increase in cylM expression was observed on day 1 in T3 treatment (15.9 ± 1.18) compared to control (3.27 ± 1.55), T2 (2.23 ± 0.32), and T4 (3.13 ± 0.15) treatments. Although overall cylM abundance was significantly higher in T4 treatment on day 7, day 14 and day 28. On day 40, down-regulation was observed in T4 treatment (8.73 ± 0.85) compared to T2 (16.6 ± 0.92). The level of cylB gene was also showed similar trends like cylM on first two isolation time points. However, the patterns for the day 7 in control and T2 were down-regulated (Fig. 3f). On the other hand, the abundances of the cylB gene were up-regulated on other time points. On day 14 the expression of cylB in the T3 and T4 treatments were significantly different from control, nevertheless, by day 28, 40, and 60 a student t-test of this gene abundance in all treatments indicated that both N and P addition were not significantly different from control mesocosm. On the other hand, neither gelE expression by qPCR nor gelatinase positive phenotype by gelatinase assay was detected throughout the experiment (Fig. S 2).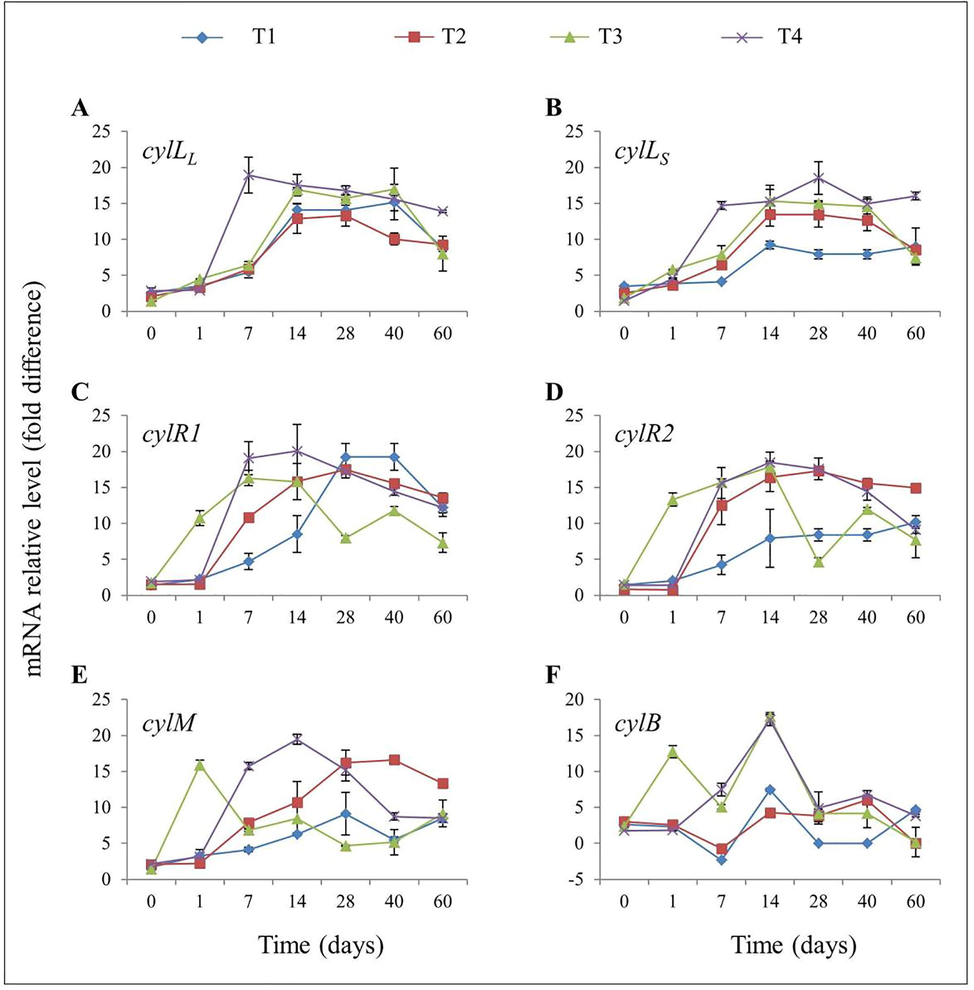
Expression of E. faecalis cyl operon genes isolated at different time points, relative to expression levels in Mueller-Hinton broth. (a) Abundances of the cylLL gene (b) cylLS gene (c) cylR1 gene (d) cylR2 gene (e) cylM gene, and (f) cylB gene, during the time course of the mesocosm experiment. Error bars represent the standard deviation (n = 3).
4 Discussion
4.1 Correlation of nitrogen and phosphorus concentrations on Enterococcus faecalis survival
The persistence and survival of E. faecalis in aquatic environment have been largely influenced by environmental conditions. Hartke et al. (1998) evaluated the survival rate of E. faecalis in tap water over a period of 85 days in an oligotrophic model ecosystem. In addition, our data indicated that the survivals of E. faecalis strain in the mesocosm experiments were gradually decreased from day 0 to day 96 in all treatments (Fig. S1). The natural habitat of E. faecalis is a gastrointestinal tract of humans and animals, however, in aquatic (secondary) environments, E. faecalis adapting the survival strategy against environmental conditions including complex nutrients (Lebreton et al., 2014). On the contrary, mesocosm experiments retain oligotrophic conditions which may affected the survival of E. faecalis (Irankhah et al., 2016). The isolation rates in nutrient-enriched treatment were similar to, or higher than control (Fig. S1), endorses the hypothesis that oligotrophic conditions could somehow influence the survival of E. faecalis in a water environment. Nonetheless, other factors such as salinity, sunlight, and temperature may influence the survival of E. faecalis in environmental settings (Byappanahalli et al., 2012).
4.2 Correlation of nutrients with antibiotic resistance
High levels of N and P in the water environments can cause eutrophication. Eutrophication is highly increased the spreading of antibiotic-resistant bacteria in the aquatic environment. Thus, we hypothesized that N and P play a role in emergence of antibiotic resistance in the water. Consistent with this hypothesis, the supplementations of N and P to mesocosms were induced resistance in E. faecalis over time (Table 1). E. faecalis in the nutrient-enriched treatments were shown resistance to OXY, CHL, AMP, and CIP, indicating that this bacterium evolves resistance with increased nutrients availability. It is apparent that nutrients would affect E. faecalis, as this bacterium had developed resistance in enriched treatments compared to control (Table 1). This finding is consistent with the previous observation in which showing that E. faecalis develop resistance to antibiotics that inhibit protein synthesis (Ali et al., 2016). However, ERY resistant bacteria were found in all treatments including control. Besides N and P, it is likely that other environmental factors may influence antibiotic resistance in aquatic environments. However, additional research is needed to explore environmental factors related to wastewater and sediment which contribute to the development of resistant bacteria.
4.3 Nutrients and biofilm formation
In the aquatic environment, like other Gram-positive bacteria, E. faecalis is able to develop biofilms and increase their acquired and intrinsic resistance to antibiotics. However, the factors which are implicated in the biofilm development remain poorly understood. The nutrients such as nitrates and phosphates are crucial for bacterial growth and often found in oligotrophic environments. To some extent, these nutrients are considered as limiting factors of bacterial growth in drinking water (Fish et al., 2016). In this study, biofilm-forming E. faecalis on day 0 (under nutrient-limited conditions) were significantly similar (Fig. 2). This is in accordance with observations by (Villanueva et al., 2011), concerning the biofilm development of bacteria in a nutrient-limited mesocosm experiment. Nutrient addition was showed a gradual but significant biofilm production in E. faecalis (Fig. 2). It is likely that increased total biofilm formation was favored with increased nutrients availability in the aquatic environment (Miao et al., 2021). Furthermore, in the presence of N and P, bacteria can stimulate their growth rate and reproduction to take advantage of nutrients (Adams et al., 2015). Similarly, bacterial attachment and biofilm accretion to microplate wells were tended to increase over time. A plausible reason for this phenomenon could be the optimal adaptability of E. faecalis in the oligotrophic microcosm environments (Hartke et al., 1998). Based on our findings, it appears that N and P availability in the water might be associated with the biofilm development. In addition to nutrients, other factors, such as genetic and environmental (pH, oxygen, and temperature) may influence the biofilm development (Fish et al., 2016). In the future, it will be of major interest to explore those factors and their mechanisms which are potentially developed bacterial biofilm in the water.
4.4 Influence of nutrients on Quorum-sensing in Enterococcus faecalis
In the aquatic environment, quorum-sensing mechanisms in bacteria usually depend on growth physiology and environmental conditions. Bacteria also regulate the expression of autoinducers in response to N and P within sequencing batch reactors (Liu et al., 2006). To gain insight into how E. faecalis quorum-sensing response to the nutrients, qPCR was used to determine the abundance of structural and regulatory genes of cyl operon for two months on seven different occasions (Fig. 3). In this study, we noted a gradual increase in the expression of cylLS within nutrient-enriched treatments compared to the unenriched control over time (Fig. 3b). The cylLS encodes CylLS protein, a small subunit of cytolysin toxin that is implicated in the autoinduction and control of cyl operon (Van Tyne et al., 2013). The expression of cylLS in T4 treatment at day 7 was nearly tenfold higher compared to control, suggesting that water containing nitrate and phosphate ions may play a pivotal role in E. faecalis quorum-sensing. In contrast, quorum-sensing regulating genes were induced under P-limited condition in water; N and P with ratio of 5:0.05, respectively (Liu et al., 2006). CylLS interacts with CylR1 (membrane protein) and further leads to the dissociation of CylR2 from the promoter region that triggers high-level activation of cyl operon (Ali et al., 2017). Since E. faecalis are capable of expressing a cyl operon, we expected to see that there would be a correlation between structural and regulatory genes of cyl operon in the nutrients enriched and control mesocosms. However, relative abundance of mRNA in the control mesocosms was either lower or uniform. This may indicate that the expression of quorum-sensing-related mRNA degradation or regulation might be controlled by environmental and nutritional signals. In response to environmental signals, mRNA decay or regulations lead to express a co-transcribed genes at distinct levels (Mohanty and Kushner, 2016). Furthermore, nutrient alterations in a variety of bacteria can lead to altering quorum-sensing regulation expression. Such studies are needed to explore the quorum-sensing regulation mechanism at high E. faecalis density in the nutritional environment.
E. faecalis is also used fsr quorum-sensing system that regulates social activity, virulence and biofilm development. The role of fsr quorum-sensing in the aquatic environment is thus far undocumented. A complete fsr operon is required for the expression of gelatinase phenotype in E. faecalis (Dundar et al., 2015). The partial deletions or complete absence of fsr operon in E. faecalis are eliminating the gelatinase ability to be expressed in standard assays (Dundar et al., 2015). Furthermore, it has been suggested that a partial deletion of fsr operon is more likely due to recombination and horizontal transfer (Galloway-Peña et al., 2011). Thus, we postulated that as developing of antibiotic resistance, E. faecalis might also be restored gelatinase phenotype in the aquatic environment. However, we did not detect gelatinase positive phenotypes which appear consistent with the previous results (Galloway-Peña et al., 2011). This suggests that complete fsr operon in E. faecalis is essential for the expression of gelE gene in the aquatic environment.
5 Conclusion
Different level of N and P fertilizers from wastewater to the aquatic environment can affect various features related to fecal indicator bacteria. This model ecosystem study provides evidence that E. faecalis strongly influence by nutrients such as N and P. We found that E. faecalis can withstand approximately three months in tap water mesocosms. The isolation rate of E. faecalis in control mesocosm was reached to 50% on day 7, however, this bacterium was likely to be isolated over a period of 96 days from all treatments including control. This work also demonstrated that nutrients not only induced antibiotic resistance but also increased biofilm-forming capability in E. faecalis over time. In addition, the transcriptional profiles of quorum-sensing-related genes were also comparatively high in the nutrient-enriched mesocosms. Taken together, these findings may yield insight into how E. faecalis switch over from commensal to pathogenic within aquatic environments. To address their fate in different environmental conditions, future work will focus on the pathogenic response mechanisms of E. faecalis to environmental settings.
Acknowledgements
This work was mainly financed by the National Natural Science Foundation of China (grant number 31272606), Post Expert of Swine Industry of Fujian Province, China and Research Center for Advanced Materials Science (RCAMS) at King Khalid University Abha, Saudi Arabia through project number RCAMS/KKU/002-21.
Author Contributions
Liaqat Ali, and Daojin Yu conceived and designed the study; Liaqat Ali, Muhammad Mustafa, Waqar Islam, Ulfat Ara, Zheng Run Xiao, Muhammad Ajmal analyzed the data and wrote the paper. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Isolating the effects of storm events on arctic aquatic bacteria: Temperature, nutrients, and community composition as controls on bacterial productivity. Front. Microbiol.. 2015;6:250.
- [CrossRef] [Google Scholar]
- Molecular mechanism of quorum-sensing in Enterococcus faecalis: Its role in virulence and therapeutic approaches. Int. J. Mol. Sci.. 2017;18:960.
- [CrossRef] [Google Scholar]
- Nutrient-induced antibiotic resistance in Enterococcus faecalis in the eutrophic environment. J. Global Antimicrob. Resist.. 2016;7:78-83.
- [CrossRef] [Google Scholar]
- Understanding human health risks caused by antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARG) in water environments: Current knowledge and questions to be answered. Crit. Rev. Environ. Sci. Technol.. 2020;50(19):2016-2059.
- [CrossRef] [Google Scholar]
- Microbial evaluation and occurrence of antidrug multi-resistant organisms among the indigenous Clarias species in River Oluwa, Nigeria. J. King Saud Univers. – Sci.. 2017;29(1):96-105.
- [CrossRef] [Google Scholar]
- Enterococci in the Environment. Microbiol. Mol. Biol. Rev.. 2012;76(4):685-706.
- [CrossRef] [Google Scholar]
- Biofilm formation plays a role in the formation of multidrug-resistant Escherichia coli toward nutrients in microcosm experiments. Front. Microbiol.. 2018;9:367.
- [CrossRef] [Google Scholar]
- The prevalence and antimicrobial resistance phenotypes of Salmonella, Escherichia coli and Enterococcus sp. in surface water. Lett. Appl. Microbiol.. 2020;71(1):3-25.
- [CrossRef] [Google Scholar]
- Multiple roles for Enterococcus faecalis glycosyltransferases in biofilm-associated antibiotic resistance, cell envelope integrity, and conjugative transfer. Antimicrob. Agents Chemother.. 2015;59(7):4094-4105.
- [CrossRef] [Google Scholar]
- The fsr quorum-sensing system and cognate gelatinase orchestrate the expression and processing of proprotein EF_1097 into the mature antimicrobial peptide enterocin O16. J. Bacteriol.. 2015;197(13):2112-2121.
- [CrossRef] [Google Scholar]
- Characterising and understanding the impact of microbial biofilms and the extracellular polymeric substance (EPS) matrix in drinking water distribution systems. Environ. Sci. Water Res. Technol.. 2016;2(4):614-630.
- [CrossRef] [Google Scholar]
- Diversity of the fsr-gelE region of the Enterococcus faecalis genome but conservation in strains with partial deletions of the fsr operon. Appl. Environ. Microbiol.. 2011;77(2):442-451.
- [CrossRef] [Google Scholar]
- Global emergence and dissemination of enterococci as nosocomial pathogens: Attack of the clones? Front. Microbiol.. 2016;7:788.
- [CrossRef] [Google Scholar]
- Survival of Enterococcus faecalis in an oligotrophic microcosm: changes in morphology, development of general stress resistance, and analysis of protein synthesis. Appl. Environ. Microbiol.. 1998;64(11):4238-4245.
- [Google Scholar]
- Quorum Sensing in Marine Microbial Environments. Ann. Rev. Mar. Sci.. 2017;9(1):257-281.
- [CrossRef] [Google Scholar]
- Effects of environmental factors on nutrients release at sediment-water interface and assessment of trophic status for a typical shallow lake, northwest china. Scient. World J.. 2013;2013:1-16.
- [CrossRef] [Google Scholar]
- Ex situ study of Enterococcus faecalis survival in the recreational waters of the southern coast of the Caspian Sea. Iran. J. Microbiol.. 2016;8:101-107.
- [Google Scholar]
- Plant-insect vector-virus interactions under environmental change. Sci. Total Environ.. 2020;701:135044.
- [CrossRef] [Google Scholar]
- Enterococcus Diversity, Origins in Nature, and Gut Colonization. In: Gilmore M.S., Clewell D.B., Ike Y., Shankar N., eds. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection. Boston: Massachusetts Eye and Ear Infirmary; 2014. p. :3-44.
- [Google Scholar]
- Experiment of water environmental chemistry, first ed.. Chinese Agriculture Press; 2007.
- Regulation of Enterococcus faecalis Biofilm Formation and Quorum Sensing Related Virulence Factors with Ultra-low Dose Reactive Species Produced by Plasma Activated Water. Plasma Chem. Plasma Process.. 2019;39(1):35-49.
- [CrossRef] [Google Scholar]
- The gelatinase biosynthesis-activating pheromone binds and stabilises the FsrB membrane protein in Enterococcus faecalis quorum sensing. FEBS Lett.. 2020;594(3):553-563.
- [CrossRef] [Google Scholar]
- Effect of phosphorus limitation on microbial floc structure and gene expression in activated sludge. Water Sci. Technol.. 2006;54:247-255.
- [CrossRef] [Google Scholar]
- Accurate determination of low-level chemical oxygen demand using a multistep chemical oxidation digestion process for treating drinking water samples. Anal. Methods. 2016;8(18):3839-3846.
- [CrossRef] [Google Scholar]
- Effects of biofilm colonization on the sinking of microplastics in three freshwater environments. J. Hazard. Mater.. 2021;413:125370.
- [CrossRef] [Google Scholar]
- Regulation of mRNA Decay in Bacteria. Annu. Rev. Microbiol.. 2016;70(1):25-44.
- [CrossRef] [Google Scholar]
- Toxicity of four different insecticides against Trilocha varians (Bombycidae: Lepidoptera) J. King Saud Univers. – Sci.. 2020;32(3):1853-1855.
- [CrossRef] [Google Scholar]
- Chronic ecotoxicity of ciprofloxacin exposure on taxonomic diversity of a meiobenthic nematode community in microcosm experiments. J. King Saud Univers. – Sci.. 2020;32(2):1470-1475.
- [CrossRef] [Google Scholar]
- Insects–plants-pathogens: Toxicity, dependence and defense dynamics. Toxicon. 2021;197:87-98.
- [CrossRef] [Google Scholar]
- Zinc finger protein transcription factors: Integrated line of action for plant antimicrobial activity. Microb. Pathog.. 2019;132:141-149.
- [CrossRef] [Google Scholar]
- Survival of Enterococcus faecalis during alkaline stress: Changes in morphology, ultrastructure, physiochemical properties of the cell wall and specific gene transcripts. Arch. Oral Biol.. 2013;58(11):1667-1676.
- [CrossRef] [Google Scholar]
- Benchmark taxonomic classification of chicken gut bacteria based on 16S rRNA gene profiling in correlation with various feeding strategies. J. King Saud Univers. – Sci.. 2020;32(1):1034-1041.
- [CrossRef] [Google Scholar]
- Isolation and identification of chromium reducing bacteria from tannery effluent. J. King Saud Univers. – Sci.. 2020;32(1):265-271.
- [CrossRef] [Google Scholar]
- The simultaneous analysis of total nitrogen and total phosphorus in natural waters. Mar. Chem.. 1981;10(2):109-122.
- [CrossRef] [Google Scholar]
- Structure, function, and biology of the Enterococcus faecalis cytolysin. Toxins. 2013;5:895-911.
- [CrossRef] [Google Scholar]
- Biofilm formation at warming temperature: acceleration of microbial colonization and microbial interactive effects. Biofouling. 2011;27(1):59-71.
- [CrossRef] [Google Scholar]
- Performance Standards for Antimicrobial Susceptibility Testing; Seventeenth Informational Supplement. Clin. Laborat. Standars Institute - NCCLS. 2007;27:1-182.
- [Google Scholar]
- Cornmeal-induced resistance to ciprofloxacin and erythromycin in enterococci. Chemosphere. 2012;89(1):70-75.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2021.101680.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







