Translate this page into:
Residual toxicity and sublethal effects of fenvalerate on the development and physiology of Spodoptera exigua reared on different hosts
⁎Corresponding author. junaidali206@gmail.com (Junaid Ali Siddiqui)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Beet armyworm Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) is one of the most devastating pests of different key crops. The present study deals with the toxic effect of fenvalerate insecticide on the development, reproduction and the detoxification enzyme’s activity of S. exigua feeding on tomato, cabbage, and artificial diet. The developmental period of the second instar to adult emergence was prolonged (16.74 days) reared on tomato followed by cabbage (15.97 days) and artificial diet (15.62 days). The fecundity of S. exigua was lowest on the tomato plant (279 eggs), followed by an artificial diet (347 eggs), while the highest number of eggs was observed on cabbage after the control diet (421eggs). Moreover, the lowest survival rate of the second to the third instar, fourth to the fifth instar, and hatchability were recorded in insects reared on tomato (70.89%) compared to that reared on cabbage (77.08%) and artificial diet (79.66%). S. exigua reared on tomato had the lowest intrinsic rate of increase (r: 0.133) and the highest mean generation time (T: 31.36). Furthermore, detoxifying enzyme activity in S. exigua was much lower on tomato than on cabbage and diet. Moreover, the fenvalerate toxicity was synergized by either piperonyl butoxide (PBO) or S,S,S tributylphosphorotrithioate (DEF) due to the likely participation of monooxygenases or esterase; this suggests a general metabolic resistance. This research provides a solid base for devising an applicable and successful strategy for the controlling the resistance development in beet armyworms.
Keywords
Beet armyworm
Fenvalerate
Sublethal effects
Resistance mechanism
Detoxification enzymes activity
1 Introduction
Spodoptera exigua (Hubner) is a polyphagous insect belonging to the Lepidoptera family (Noctuidae) that is a destructive pest of many valuable crops around the world including cotton cereals, pulses, vegetables, and fruits (Abbas et al., 2012; Abhilash and Singh, 2009). Feeding on 30 different plant species makes this pest a polyphagous insect (Dingha et al., 2004; Rizwan-ul-Haq et al., 2009). The use of synthetic insecticides generally manages S. exigua, especially organochlorines, organophosphates, carbamates and pyrethroids. Chemical pesticides can have sublethal effects on exposed arthropods in addition to lethal ones (Desneux et al., 2007; Ullah et al., 2019). On the other hand, overuse of these chemicals has resulted in high resistance to newer and traditional insecticides (Ahmad and Arif, 2010; Hafeez et al., 2019; Ishtiaq et al., 2012). Often, failure of a pest management tactic for S. exigua is due to insecticides resistance in several field crops (Ahmad et al., 2018). The overproduction of detoxifying enzymes and oxidative metabolism of S. exigua is responsible for variation in resistance in different field populations (Tian et al., 2014).
Pyrethroids include synthetic compounds with a structure based on natural pyrethroid insecticides (Colombo et al., 2011). Synthetic pyrethroids have been extensively used in agriculture, forestry, and the public health sector to manage different pests (Colombo et al., 2011; Liu et al., 2010). Fenvalerate is a highly potent pyrethroid insecticide that kills a wide range of insects (Ma et al., 2009). In general, insect resistance is based on (1) increased detoxification and (2) alteration of target enzyme/receptor sites, making them immune to pesticide exposure (Zhu et al., 2016). Three major groups of enzymes contribute metabolic resistance to pesticides in insects: cytochrome P450, esterase, and glutathione S-transferases (GST) (Joußen et al., 2012; Khan et al., 2021b, 2021a, 2020; Zinjarde et al., 2017). Monooxygenases boost oxidative metabolism, which is one of the key mechanisms behind resistance against pyrethroid. GST is an enzyme that facilitates glutathione-pesticide conjugation, resulting in organophosphate resistance (Zinjarde et al., 2017). It is necessary to investigate the diverse impact of host plants on insecticide susceptibility and pesticide detoxification metabolic alterations.
The present study aims to determine susceptibility and sublethal effects of fenvalerate on the growth and development of beet armyworm larvae feeding on different food regimes to understand more about the mechanism of fenvalerate resistance and the involvement of metabolic enzymes were evaluated by using synergists. The findings will demonstrate fenvalerate resistance characteristics for S. exigua, which could better understand resistance against this pesticide and create effective management techniques.
2 Materials and methods
2.1 Toxicity bioassays
Toxicity of fenvalerate was determined using the leaf dipping method for host plants, as Shao et al. (2013) reported with slight modification and the diet incorporation method described by Moulton et al. (2002). Fresh leaf discs of tomato and cabbage (6.5 cm Ø) were picked from the greenhouse plants described above. For five to six needed concentrations, the technical grade fenvalerate was combined in acetone and serially diluted in distilled water containing 0.1% Triton X-100. Three fresh leaf discs were joined together and submerged in pesticide solutions for ten seconds each. Only acetone containing 0.1% Triton X-100 solution was used on the control discs. For around 30 min, all of the dipped leaf discs were left to dry at room temperature. Individual discs were placed in disposable Petri dishes (9.0 cm Ø). Each plate contained 15 s-instar larvae, and three replicates were established and kept under the same conditions as stated earlier. After 72 h of fenvalerate exposure, mortality was determined. When larvae could not move when brushed with a brush, they were pronounced dead.
2.2 Selection for fenvalerate susceptibility
The selection experiments for susceptibility insecticides to S. exigua on different food regimes were carried out in the laboratory condition. Following the toxicity test results, 400–700 newly molted S. exigua second-instar larvae were divided into three groups and treated with a dose that causes 50–70% mortality. The larvae that survived 72 h of exposure were fed tomato, cabbage, and an artificial diet until adulthood. The toxicity of fenvalerate to S. exigua on the selected host plants and diet was assayed for three generations for resistance selection. Each experiment was repeated three times.
2.3 Life table construction
From the fourth generation onwards, approximately 90 freshly molted second-instar S. exigua larvae from each cohort were chosen and treated with LC40 concentration of fenvalerate and reared to maturity on tomato, cabbage and artificial, separately in the small transparent plastic Petri plates (9.0 cm). Each one was raised independently in small translucent plastic containers on cabbage, tomato leaves and 5–7 g of artificial diet. Before pupation, larvae were tested every day for molts and survival from the second to fifth instars. Individuals who made it to pupation were taken out, sorted by sex, and weighed to compare strains. On the third day of pupation, the male and female were identified. Each larva contributed one replicate for this experiment (Chi and Yang, 2003). We evaluated survival, developmental period and pupal bodyweights. Male and female freshly emerging adults were assigned into groups individually. We formed 18 families, which were then separated into three equal groups. Every group consisted of six families and acted as a single strain replication. Plastic boxes with 8 × 11 cm dimensions, were used and a liner nappy was vertically hung for oviposition. The adult longevity and fecundity (eggs/female) were measured. To avoid any impacts of spoilage throughout the trial, the diet was changed once every two days.
2.4 Enzyme activity assays
Four to six midguts of third instar larvae from each treatment were obtained to assess the total protein contents of the midgut. Collected samples were weighed before homogenization. The total protein content of supernatants from the insect homogenates was determined using bovine albumin serum (BSA) as standard, as described by Bradford (1976).
The samples were all pulverized with ice-cold 0.05 M sodium phosphate buffer at room temperature (pH 7.3). The homogenized samples were centrifuged at 12,000 rpm for 10 min at 4 °C (rotation per minute). The supernatant was pipetted out and inserted into new tubes and centrifuged for 15 min at 4 °C at 12,000 rpm. For detoxification enzymes (P450, esterase and GST), activity was determined by the commercially available kits purchased from Nanjing Jiancheng Bioengineering Research Institute. The method followed was as per the instructions provided by the manufacturer.
2.5 Statistical analysis
Probit analysis was used to examine concentration-mortality data (Finney, 1982). The LC50 values, standard errors, slopes, and the 95 % fiducial limit were calculated using POLO software. Using age-stage two-sex life table theory, developmental period of different stages, adult survival, adult lifespan, and fecundity were statistically evaluated (Chi, 1988; Chi and Liu, 1985; Chi et al., 2020). The means and standard errors (SEs) of different variables were assessed by using 100,000 bootstrap replicates. The paired bootstrap test was used to analyze all treatments; TWOSEX-MSChart was used to perform both the bootstrap and paired bootstrap tests, and graphical work was done via Sigmaplot 12.5 for all population life table parameters. The statistical software SPSS was used to examine all of the data, including enzyme activity (version 19.0, SPSS Inc., Chicago, IL, USA). ANOVA was used to evaluate the statistically significant mean values of the treatments, and the Student Newman-Keuls test (P < 0.05) was used to determine the major variations among the treatments.
3 Results
3.1 Toxicity of fenvalerate
Fenvalerate was used to test beet armyworm, S. exigua larvae (7 days old), raised on four different diets and the LC50 values are shown in Table S1. From the first to the third generation on a diet, cabbage-fed larvae had the highest LC50 values, followed by tomato-fed larvae. The insecticides toxicity against larvae grown on various diets was assessed.
3.2 Pre-adult developmental time of S. exigua when fed on different food regimes
Pre-adult development time comparisons of S. exigua larvae after feeding on different food regimes are given in Table 1. There was a considerable difference between the developmental period of second instar larval of S. exigua on tomato leaves exposed to an LC40 concentration of fenvalerate compared with the control and diet. While the prolonged mean larval duration of the second instar was also observed on tomato leaves compared to artificial diet and cabbage in Table 1. Furthermore, the mean larval duration of the third and fourth instar was extended on tomato and cabbage leaves compared to the control (Table 1). A similar mean larval duration of the third instar was found among cabbage and tomato and the mean larval duration of the fourth instar between tomato and diet compared with the control. According to the paired bootstrap test at the 5% significance level, means followed by the same letters in the same rows are not significantly different. For each treatment, 50 insects were employed.
Parameters
CK
Diet
Cabbage
Tomato
Egg period (d)
3.12 ± 0.05 a
3.00 ± 0.00 a
3.06 ± 0.03 a
3.06 ± 0.03 a
2nd Instar (d)
3.04 ± 0.05 d
3.18 ± 0.08 bd
3.09 ± 0.07 cbd
3.42 ± 0.08 a
3rd Instar (d)
2.98 ± 0.02 c
2.98 ± 0.02 c
3.21 ± 0.08 b
3.31 ± 0.08 ab
4th Instar (d)
2.95 ± 0.03 bc
3.0 8 ± 0.05 dc
3.2 ± 0.07 a
3.03 ± 0.03 bd
5th Instar (d)
3.00 ± 0.38 cd
3.21 ± 0.07 bc
3.16 ± 0.07 ac
3.56 ± 0.23 ab
larval (d)
15.12 ± 0.1 d
15.62 ± 0.13 c
15.97 ± 0.16 bc
16.74 ± 0.3 a
Pupal (d)
7.49 ± 0.09 d
8.77 ± 0.11 b
8.54 ± 0.1 bc
9.29 ± 0.11 a
Pupal wet (mg)
123.26 ± 1.14 a
115.95 ± 0.499b
114.06 ± 0.64 b
107.24 ± 1.00 c
Furthermore, results indicate that the mean larval duration of the fifth instar was extended when S. exigua larvae were fed on tomato leaves. The mean pupal period was significantly increased on diet, cabbage and tomato as compared with the control. The mean of total larval duration was significantly prolonged after feeding on the diet, cabbage and tomato. In the meantime, the mean pupal weight continued to trend in the same manner (Table 1).
3.3 Adult longevity, oviposition period, fecundity, and MGT of S. exigua when fed on different food regimes
The insects feed on host plants and diet exposed to the LC40 concentration of fenvalerate adult longevity, APOPs, TPOPs, Oviposition period, fecundity, and MGT (mean generation time) of S. exigua listed in (Table 2). These results show that among the treatment, larvae fed on tomato, cabbage, and diet treated with the LC40 concentration of fenvalerate recorded the shorter a comparison of average adult longevity to a the control group. Similarly, the mean longevity of females was significantly shorter when the second instar larvae fed on tomato, cabbage and diet treated with the LC40 concentration of fenvalerate. When S. exigua larvae were fed tomato and diet, there was a substantial increase in APOP compared to the control diet, but there was no substantial difference between diet and cabbage consumption. Furthermore, TPOP levels were significantly higher in the diet, cabbage, and tomato groups but not in the diet and cabbage groups (Table 2). Total oviposition days of females were significantly decreased among the treatments as compared with the control. Also, results show that female fecundity was significantly lowered in tomato, diet and cabbage compared with the control, and the lowest eggs were produced by a female when of S. exigua larvae fed on tomato leaves exposed to LC40 compared with diet and cabbage. The mean growth time of S. exigua was significantly extended in tomato and diet (Table 2). According to the paired bootstrap test at the 5% significance level, means followed by the same letters in the same rows are not substantially different. For each treatment, 50 insects were employed.
Parameters
CK
Diet
Cabbage
Tomato
Adult longevity (d)
29.02 ± 1.32 a
25.18 ± 171 bd
23.94 ± 1.75 cd
21.86 ± 1.87 d
Female longevity (d)
12.13 ± 0.57 a
10.17 ± 0.76 b
10.17 ± 0.8 b
8.58 ± 1.11 c
Male longevity (d)
9.54 ± 0.34 a
9.56 ± 0.59 a
8.56 ± 1.04 b
7.75 ± 1.25 b
APOP (d)
1.2 ± 0.13 d
1.47 ± 0.14 b
1.27 ± 0.14 cd
1.6 ± 0.22 ab
TPOP (d)
23.87 ± 0.24 d
25.55 ± 0.31 c
25.82 ± 0.35 bc
27.3 ± 0.37 a
Ovi-day
7.27 ± 0.27 a
5.45 ± 0.28 bcd
5.36 ± 0.28 cd
5.3 ± 0.47 d
Fecundity (eggs/female)
532.47 ± 7.13 a
347 ± 35.4 b
421.33 ± 32.49 ac
279.17 ± 42.8 d
MGTa
33.07 ± 0.38 c
34.27 ± 0.47 ab
33.89 ± 0.71 bc
34.42 ± 0.79 ab
3.4 Survival and hatchability of immature stage and adults of S. exigua
Percent survival and percent hatchability of S. exigua fed on cabbage, tomato, and an artificial diet treated with the LC40 concentration of fenvalerate compared to the control are given in Table 3. The average survival rate of all treatments from the second to the third instar, diet, cabbage, and tomato was significantly lower than the control, and significant differences were noted between the diet and tomato and diet and cabbage, respectively (Table 3). The percent survival and percent hatchability of S. exigua fed on cabbage, tomato, and an artificial diet treated with the LC40 concentration of fenvalerate compared to the control. Similarly, the mean pupal survival rate in a diet and tomato was significantly lower than the control. The emergence rate of healthy adults in tomato and diet were lower compared with the control, a similar trend was observed among the treatments at the same time, while the results show mean hatchability percentage of S. exigua fed on tomato leaves exposed to LC40 concentration of fenvalerate was significantly lower (Table 3). All means ± S.E. are based on three replicates within rows, means followed by the same letter did not differ significantly.
Parameters
CK
Diet
Cabbage
Tomato
2nd −3rd instar survival (%)
92.33 ± 1.65 a
80.43 ± 3.01 c
71.86 ± 1.35 b
70.89 ± 2.289 b
4th – 5th instar survival (%)
91.22 ± 1.12 a
87.32 ± 1.67 a
78.747 ± 1.75 b
77.08 ± 0.56 b
Pupal survival (%)
95.79 ± 1.198 a
88.62 ± 1.91 b
89.56 ± 1.44 ab
88.03 ± 1.56 b
Emergence rate of healthy Adult (%)
94.96 ± 1.56 a
82.17 ± 2.98 b
90.32 ± 2.29 ab
80.53 ± 2.31 b
Hatchability (%)
92.47 ± 0.96 a
85.08 ± 0.56 b
84.71 ± 0.90 b
79.66 ± 1.08c
3.5 Fitness traits comparison of S. exigua after feeding on different food regimes
Compared to the control, feeding of S. exigua on tomato resulted in a considerable decrease in the intrinsic rate of population expansion (r) (Table 4). These results also showed that the net reproductive rate (R0) of S. exigua fed on tomato and cabbage was significantly lower compared with the control, while no significant difference was observed among the treatments (Table 4). In addition, compared to the control group, the mean generation time (T) of S. exigua fed on tomato cabbage and diet dropped significantly. The finite rate of increase (λ) was drastically lowered in all treatments compared to the control; simultaneously, no significant difference was observed among the treatments (Table 4). Means followed by the same letters in the same rows are not significantly different based on the paired bootstrap test at the 5% significance level. 50 insects were used for each treatment
Parameters
CK
Diet
Cabbage
Tomato
Intrinsic rate of increase (r) day−1
0.181 ± 0.009 a
0.149 ± 0.01 bcd
0.145 ± 0.099 c
0.133 ± 0.01 cd
Net reproductive rate (R0)
159.7 ± 34.58 a
83.422 ± 22.45 abc
77.121 ± 20.79 c
67.02 ± 19.51 bc
Mean generation time (T)
27.88 ± 0.26 c
29.38 ± 0.32 b
29.77 ± 0.32 b
31.36 ± 0.38 a
Finite rate of increase (λ)(day−1)
1.198 ± 0.01 a
1.161 ± 0.012 b
1.155 ± 0.011 bc
1.141 ± 0.011 bcd
3.6 The survival rate, fecundity, reproduction value and life expectancy of the S. exigua
The age-stage survival rate (Sxj) for male and female adults of S. exigua was considerably influenced in all treatments (Fig. 1). The curves showed that both sexes reared on tomato emerged after 23 d (females) and 24 d (males). However, female and male of S. exigua emergence occurred after 20 d and 19 d in the control, respectively, while a similar trend was observed between the diet and cabbage (Fig. 1). These results showed that survivorship significantly decreased selected in lx of S. exigua feeding on the diet, tomato, and cabbage compared with the control, as well as the graph for fxj, mx, and lxmx are represented in Fig. 2. These results indicate that fecundity level of S. exigua female when the second instar fed on host plants and diet exposed with the LC40 concentration of fenvalerate were lower than the control. The fertility levels in treated populations were lower than the control populations, as shown by graphs for fxj, mx, and lxmx (Fig. 2). Correspondingly, results indicate that the age-stage reproductive values Vxj of S. exigua fed on the diet, cabbage, and tomato treated with the LC40 concentration of fenvalerate decreased markedly compared with the control (Fig. 3). In the treatment fed on an artificial diet, the life expectancy of freshly deposited eggs (36.0 d), cabbage (36.0 d), which were similar to those of eggs from the control (36.0 d), but the peak exj of freshly laid eggs can be seen in the tomato (39.0 d) (Fig. 4).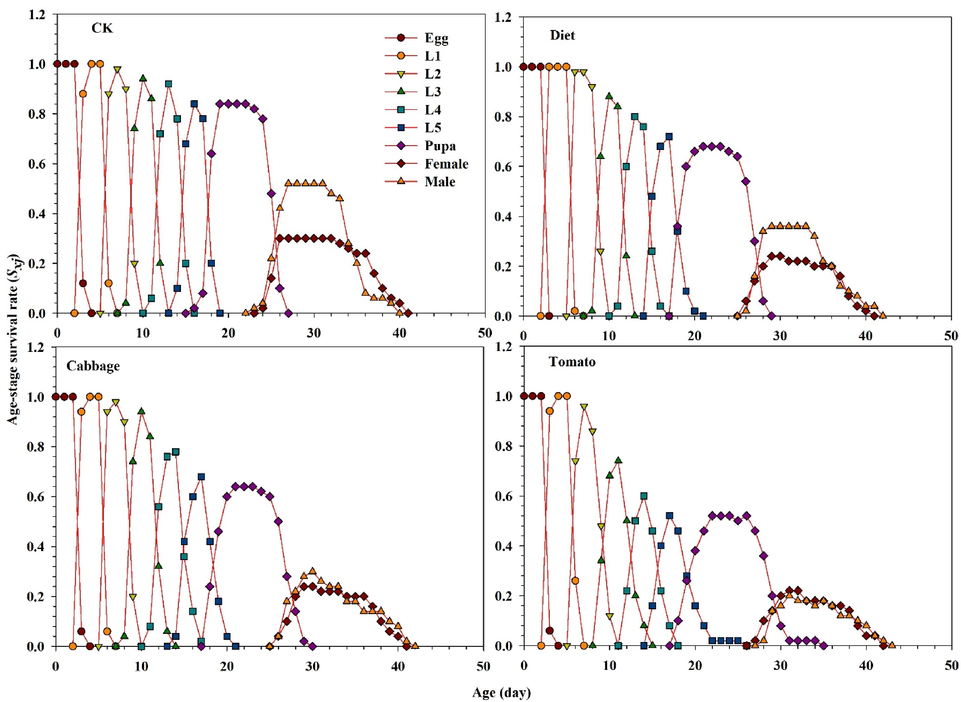
Graphs showing Age-stage specific survival rate (sxj) for S. exigua larvae fed on different food regimes (CK), (Diet), (Cabbage) and (Tomato) exposed with fenvalerate.
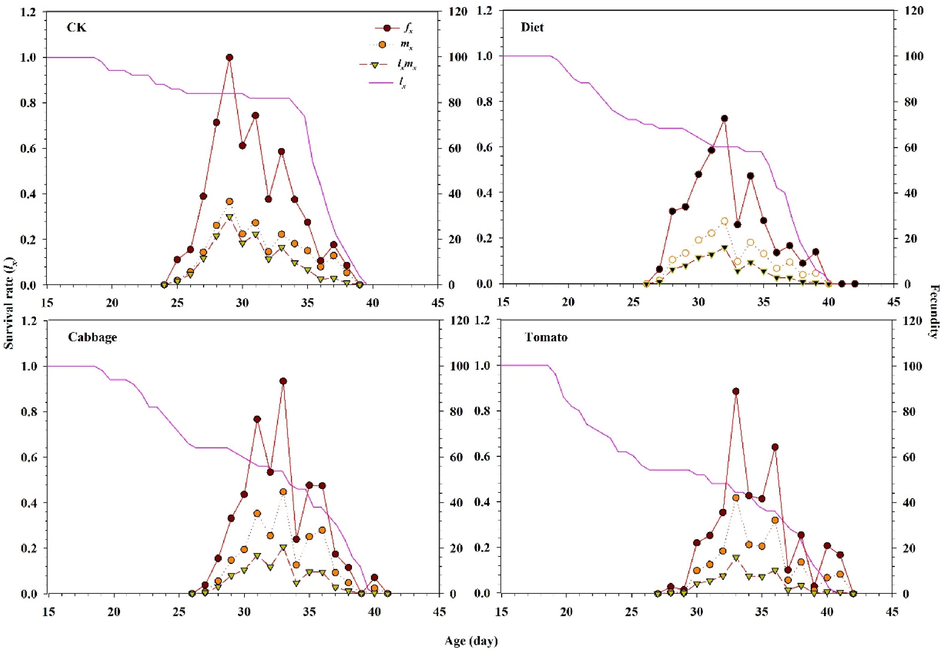
Graphs showing the Age-specific survival rate, female age-specific fecundity (fx), age-specific fecundity (mx), and age specific maternity (lxmx) for S. exigua larvae fed on different food regimes (CK), (Diet), (Cabbage) and (Tomato) exposed with fenvalerate.
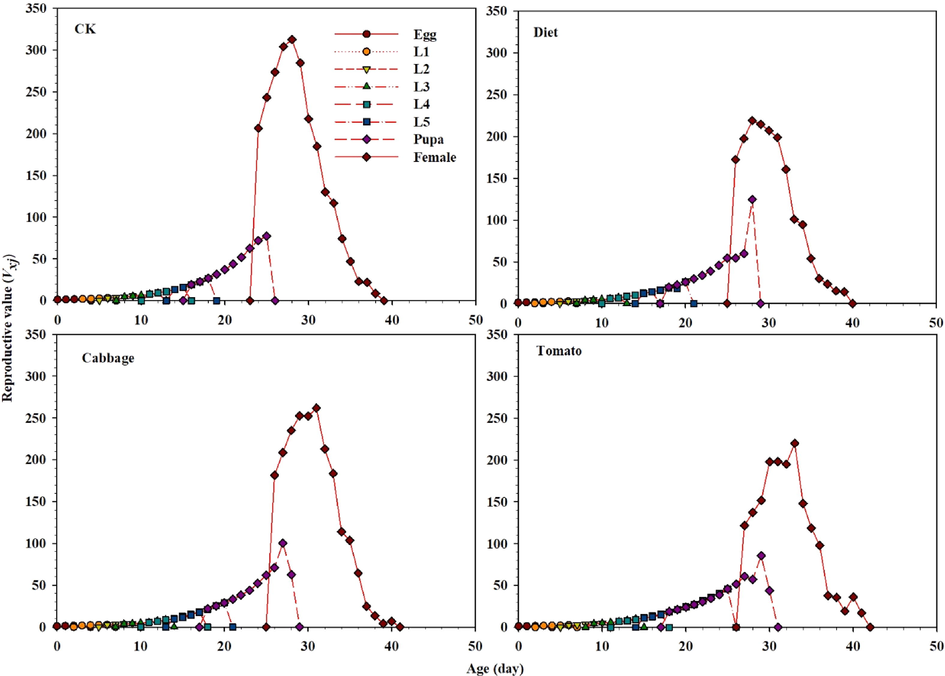
Graphs showing Age-stage specific reproductive values (Vxj) for S. exigua larvae fed on different food regimes (CK), (Diet), (Cabbage) and (Tomato) exposed with fenvalerate.
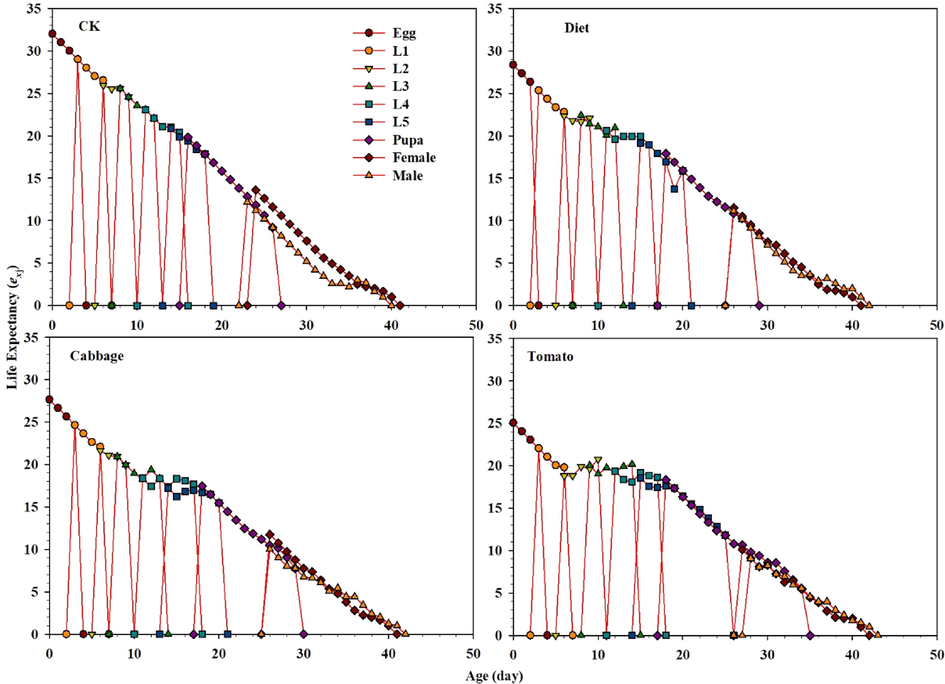
Graphs showing Life expectancy (exj) values for S. exigua larvae fed on different food regimes (CK), (Diet), (Cabbage) and (Tomato) exposed with fenvalerate.
3.7 The activity of detoxification enzymes of S. exigua
The esterase (ESTs), cytochrome P450 monooxygenases (CYPs), and glutathione S-transferases (GST) of S. exigua fed on host plants and standard artificial diet subjected to fenvalerate pesticide were examined. On cabbage, the relative activities of P450, EST were significantly higher, while on tomato, they were significantly lower. Moreover, when compared to the control, a diet showed higher relative GST activity (Table 5). All means ± S.E. are based on three replicates within rows, means followed by the same letter did not differ significantly.
Treatments
Esterase (nmol/min/mg of protein) (±SE)
Relative activity
GST (nmol/min/mg of protein) (±SE)
Relative activity
Cytochrome P450 mono-oxygenase (nmol/min/mg of protein) (±SE)
Relative activity
CK
92.153 ± 2.283 c
1
40.52 ± 1.417 b
1
0.52 ± 0.0173 d
1
Diet
105.73 ± 1.546 b
1.26
47.52 ± 1.779 a
1.17
1.58 ± 0.0231 b
3.23
Cabbage
131.50 ± 3.125 a
1.42
44.26 ± 1.186 ab
1.06
2.68 ± 0.0231 a
5.15
Tomato
102.50 ± 2.572 b
1.11
44.24 ± 1.071 ab
1.09
1.68 ± 0.0279 b
1.31
3.8 PBO and DEF synergism for fenvalerate
The synergism of PBO and DEF to fenvalerate in S. exigua on different food regimes are presented in Table S2. The results indicated that PBO and DEF had significant synergistic effects with fenvalerate in cabbage, diet and tomato. The synergistic effect of PBO in S. exigua fed with cabbage was significantly higher than that of the tomato and diet. While no effect on the S. exigua fed tomato leaves and artificial diet. This implied that P450 monooxygenase plays a significant role in fenvalerate resistance in S. exigua. In addition, esterase might also play a certain role in the fenvalerate resistance development in S. exigua fed on cabbage and diet.
4 Discussion
In this study, fitness traits and three significant detoxification enzyme activities in the larval midgut of S. exigua in response to feeding on different food regimes exposed to a low lethal concentration of fenvalerate insecticides were measured. The current findings suggest that their host plants heavily impact insect susceptibility to pesticides. Polyphagous pests such as S. exigua are exposed to a wide range of secondary plant chemicals under varied dietary regimes, resulting in differential detoxifying enzyme activity. As a result, susceptibility levels may vary with different host plants. Plant allelochemicals stimulated the activation of insect detoxification enzymes, with induction levels modulated by plant nutrients, allelochemical structures and distributions, insect species and developmental stages, and temperature (Yang et al., 2005).
Similarly, in our study, the effects of the selected host plant species and diet exposed with a low lethal concentration of fenvalerate insecticides on the development, fecundity, and the activity of three detoxification enzymes of S. exigua were significant either. Thus, S. exigua had different fitness on these host plant species and diet compared with the control. The present study supports a faster developmental rate of larvae, longer pupal duration, and higher pupal weight on Chinese cabbage than the tomato reported by Greenberg et al. (2002) and Tripathi (2001). Compared to pigweed, Chinese cabbage is a favorite meal for beet armyworms. Our results indicate that the host plants that S. exigua larvae were fed substantially impacted their pesticide responsiveness. Cabbage-fed S. exigua larvae were more resistant to fenvalerate than tomato-fed larvae; similar results were reported by Hannig et al., 2009 and Wei et al., 2010, when Helicoverpa armigera and Plutella xylostella fed on tomato, cabbage and other host plants exposed with fenvalerate at third instar larval stage.
The current observation suggests that, in the presence of continual selection pressure from pesticides feeding on two separate host plants and standard artificial diets for three generations, S. exigua fed on tomato plants exposed to the LC40 of fenvalerate had a significantly decreased survival rate, lower pupal weight, the more extended period of male and female, extended the time duration of larval instar, increased the total developmental time from egg to adults as well as compared to the cabbage, diet, and the control diet (without fenvalerate). This is because insecticide-exposed insects must devote more energy to detoxification rather than growth (Hannig et al., 2009), and fenvalerate may prevent insects from feeding on tomato, leading to insufficient nutrients for normal insect growth induce greater susceptibly against fenvalerate. Greenberg et al. (2002) and Perveen and Miyata (2000) reported that the larvae were feeding on asparagus, lettuce and Chinese cabbage, having a shorter pupal stadium than on the other host plant species.
Feeding on diverse host plants exposed to fenvalerate severely affected the life table parameters of S. exigua, reflecting the negative impacts on population growth attributes. When fed on host plants and diet exposed to fenvalerate, Sxj, fxj, and mx substantially lowered compared to the control, and Vxj statistically influenced in the tomato plant. Furthermore, exj, a measure of new individuals’ contribution to population expansion, drops dramatically in stage larval instars in host plants and diet. These results, therefore, show that after feeding on different host plants, the S. exigua population may become more susceptible to insecticides.
In the present study, significantly higher relative activities of cytochrome P450 monooxygenase (P450), esterase (EST) on cabbage and lowest on tomato were observed. Furthermore, the higher relative activity of GST was observed on a diet compared to the control, while no significant difference was found among the treatments. Our results coincide with Shen and Dowd (1992) and Chen et al. (2017). The results indicate that feeding on these host plants might cause a change in sensitivity to insecticides in S. exigua by affecting the activity of esterase and P450 monooxygenase. The changes of enzymatic activity in beet armyworm have been well documented when fed on different host plants (Chintkuntla, 2015; Zhang et al., 2011). Because the metabolism of induced detoxifying enzymes varies with the types of pesticides used, insect susceptibility varies.
The metabolic activity of various detoxifying enzymes plays a major role in developing resistance in insect species (Kang et al., 2006). The synergistic effect of PBO and DEF with fenvalerate was tested for chosen and unselected populations of S. exigua to discover the mechanistic pathway involved in resistance development. The toxicity of fenvalerate selection fed on cabbage and diet was significantly affected by PBO and DEF. However, this is not the case with tomatoes or the general population. The activity of cytochrome P450 monooxygenases and esterase appears to play a role in establishing fenvalerate resistance because PBO and DEF synergized the toxicity of the fenvalerate-selected population.
5 Conclusion
In conclusion, our study found that S. exigua larvae fed different diet regimes had distinct reproductive characteristics and cytochrome P450 monooxygenase and esterase activities, and that exposed larvae exhibited different pesticide susceptibility. The current work has provided some pertinent data on the life attributes and enzyme activity of S. exigua larvae fed on various food regimes, which will aid in the understanding of pesticide resistance mechanisms and develop improved management tactics. However, before building a more rational pest the control plan for a certain crop system, we must first establish the relationship between insect, plant, and insecticide. Binding and interaction with different kinds of pesticide and insecticide removal capabilities may make host plants interesting systems bioremediation of contaminated pesticide environments.
Acknowledgements
This work was funded by Researchers Supporting Project number (RSP-2021/165), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Fitness cost, cross resistance and realized heritability of resistance to imidacloprid in Spodoptera litura (Lepidoptera: Noctuidae) Pestic. Biochem. Physiol.. 2012;103(3):181-188.
- [CrossRef] [Google Scholar]
- Pesticide use and application: An Indian scenario. J. Hazard. Mater.. 2009;165(1-3):1-12.
- [CrossRef] [Google Scholar]
- Resistance of beet armyworm Spodoptera exigua (Lepidoptera: Noctuidae) to endosulfan, organophosphorus and pyrethroid insecticides in Pakistan. Crop Prot.. 2010;29:1428-1433.
- [CrossRef] [Google Scholar]
- Resistance to new insecticides and their synergism in Spodoptera exigua (Lepidoptera: Noctuidae) from Pakistan. Crop Prot.. 2018;107:79-86.
- [CrossRef] [Google Scholar]
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72(1-2):248-254.
- [CrossRef] [Google Scholar]
- Elevated carboxylesterase activity contributes to the lambda-cyhalothrin insensitivity in quercetin fed Helicoverpa armigera (Hübner) PLoS One. 2017;12(8):e0183111.
- [CrossRef] [Google Scholar]
- Life-Table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol.. 1988;17:26-34.
- [CrossRef] [Google Scholar]
- Chi, H., Liu, H., 1985. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin.
- Two-sex life table and predation rate of Propylaea japonica Thunberg (Coleoptera: Coccinellidae) fed on Myzus persicae (Sulzer) (Homoptera: Aphididae) Environ. Entomol.. 2003;32(2):327-333.
- [CrossRef] [Google Scholar]
- Photo-Fenton degradation of the insecticide fenvalerate in aqueous medium using a recirculation flow-through UV photoreactor. J. Hazard. Mater.. 2011;198:370-375.
- [CrossRef] [Google Scholar]
- Finney, D.J., 1982. Probit Analysis. Cambridge Univ. Press. doi: 10.1038/161417a0.
- The Sublethal Effects of Pesticides on Beneficial Arthropods. Annu. Rev. Entomol. 2007;52:81-106.
- [CrossRef] [Google Scholar]
- Effects of Bacillus thuringiensis Cry1C toxin on the metabolic rate of Cry1C resistant and susceptible Spodoptera exigua (Lepidoptera: Noctuidae) Physiol. Entomol.. 2004;29(5):409-418.
- [CrossRef] [Google Scholar]
- Beet armyworm (Lepidoptera: Noctuidae) host plant preferences for oviposition. Environ. Entomol.. 2002;31(1):142-148.
- [CrossRef] [Google Scholar]
- Gossypol-induced fitness gain and increased resistance to deltamethrin in beet armyworm, Spodoptera exigua (Hübner) Pest Manag. Sci.. 2019;75(3):683-693.
- [CrossRef] [Google Scholar]
- Feeding cessation effects of chlorantraniliprole, a new anthranilic diamide insecticide, in comparison with several insecticides in distinct chemical classes and mode-of-action groups. Pest Manag. Sci.. 2009;65(9):969-974.
- [CrossRef] [Google Scholar]
- Monitoring of resistance in Spodoptera exigua (Lepidoptera: Noctuidae) from four districts of the Southern Punjab, Pakistan to four conventional and six new chemistry insecticides. Crop Prot 2012
- [CrossRef] [Google Scholar]
- Resistance of Australian Helicoverpa armigera to fenvalerate is due to the chimeric P450 enzyme CYP337B3. Proc. Natl. Acad. Sci.. 2012;109(38):15206-15211.
- [CrossRef] [Google Scholar]
- Synergism of enzyme inhibitors and mechanisms of insecticide resistance in Bemisia tabaci (Gennadius) (Hom., Aleyrodidae) J. Appl. Entomol.. 2006;130(6-7):377-385.
- [CrossRef] [Google Scholar]
- Phototoxicity of ultraviolet-A against the whitefly Bemisia tabaci and its compatibility with an entomopathogenic fungus and whitefly Parasitoid. Oxid. Med. Cell. Longev.. 2021;2021:1-13.
- [Google Scholar]
- Insecticide resistance and detoxification enzymes activity in Nilaparvata lugens Stål against neonicotinoids. J. Agric. Sci.. 2020;12:24.
- [CrossRef] [Google Scholar]
- Emamectin benzoate induced enzymatic and transcriptional alternation in detoxification mechanism of predatory beetle Paederus fuscipes (Coleoptera : Staphylinidae) at the sublethal concentration. Ecotoxicology. 2021;1–15
- [CrossRef] [Google Scholar]
- Photodegradation mechanism of deltamethrin and fenvalerate. J. Environ. Sci.. 2010;22(7):1123-1128.
- [CrossRef] [Google Scholar]
- Enantioselectivity in aquatic toxicity of synthetic pyrethroid insecticide fenvalerate. Ecotoxicol. Environ. Saf.. 2009;72(7):1913-1918.
- [CrossRef] [Google Scholar]
- Pro-active management of beet armyworm (Lepidoptera: Noctuidae) resistance to tebufenozide and methoxyfenozide: Baseline monitoring, risk assessment, and isolation of resistance. J. Econ. Entomol.. 2002;95(2):414-424.
- [Google Scholar]
- Effects of sublethal dose of chlorfluazuron on ovarian development and oogenesis in the common cutworm Spodoptera litura (Lepidoptera: Noctuidae) Ann. Entomol. Soc. Am.. 2000;93:1131-1137.
- [CrossRef] [Google Scholar]
- Rizwan-ul-Haq M, Hu QB, Hu MY, Lin QS, Z.W., 2009. Biological impact of harmaline, ricinine and their combined effects with Bacillus thuringiensis on Spodoptera exigua (Lepidoptera: Noctuidae. J. Pest Sci. (2004). 82, 3327–33.
- Detoxifying enzymes and insect symbionts. J. Chem. Educ.. 1992;69(10):796.
- [CrossRef] [Google Scholar]
- Biochemical mechanisms for metaflumizone resistance in beet armyworm, Spodoptera exigua. Pestic. Biochem. Physiol. 2014
- [CrossRef] [Google Scholar]
- Effect of single generation feeding of different host plants on susceptibility to different insecticides and certain enzymes of Helicoverpa armigera (Hubner) Ann. Plant Prot. Sci.. 2001;9:205-208.
- [Google Scholar]
- Imidacloprid-induced hormesis effects on demographic traits of the melon aphid. Aphis gossypii. Entomol. General. 2019;39:325-337.
- [CrossRef] [Google Scholar]
- Sub-lethal effects of fenvalerate on the development, fecundity, and juvenile hormone ehafeezerase activity of diamondback moth, Plutella xylostella (L.) Agric. Sci. China. 2010;9:1612-1622.
- [CrossRef] [Google Scholar]
- Relative contribution of detoxifying enzymes to pyrethroid resistance in a resistant strain of Helicoverpa armigera. J. Appl. Entomol.. 2005;129:521-525.
- [CrossRef] [Google Scholar]
- Guideline for insecticide resistance monitoring of Plutella xylostella (L.) on cruciferous Vegetables. Beijing: China Agric. Press; 2013.
- Effect of host plants on development, fecundity and enzyme activity of Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) Agric. Sci. China. 2011;10(8):1232-1240.
- [CrossRef] [Google Scholar]
- Insecticide resistance and management strategies in urban ecosystems. Insects. 2016;7(1):2.
- [CrossRef] [Google Scholar]
- Role of induced glutathione-S-transferase from Helicoverpa armigera (Lepidoptera: Noctuidae) HaGST-8 in detoxification of pesticides. Ecotoxicol. Environ. Saf.. 2017;147:612-621.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2021.101593.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







