Translate this page into:
Rearing water quality and zootechnical parameters of Litopenaeus vannamei in rapid Biofloc® and conventional intensive culture system
⁎Corresponding authors. okomodavictor@yahoo.com (V.T. Okomoda), norazman@umt.edu.my (N.A. Kasan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Rapid Biofloc® Technology (R-BFT) is a bio-augmentation protocol designed for biofloc. In this study, five production cycles were conducted to investigate the effect of R-BFT on water quality, and the performance of Litopenaeus vannamei reared on an industrial scale in comparison with a control group (C-SOP). Bacillus infantis was used to inoculate the R-BFT group at the start of each cycle, while the C-SOP was conditioned fortnightly with commercial probiotics following the standard operating procedure for intensive shrimp culture. The C/N balance was maintained at 15:1 in both treatments using molasses one hour after feeding. Zero-wastewater discharge was maintained throughout each cycle for the R-BFT group, while 20% water exchange was done in the C-SOP weekly. The result showed similarity in the mean values of temperature, pH, and salinity. However, phosphate concentration and biofloc volume were higher in the R-BFT compared to the control, while the reverse trend was observed for dissolved oxygen, nitrite, and ammonia. Although the final weight and weight gained were similar in the treatments, the quantity of feed fed per shrimp and feed conversion ratio were substantially reduced for the R-BFT (14.82 g and 1.52 respectively) compared to the C-SOP group (18.33 g and 2.49 respectively). Survival was significantly higher in the R-BFT (91%) compared to the C-SOP (66%), while the mean yield was 57% higher in the former compared to the latter. Thus, R-BFT substantially improved water quality and the zootechnical performance of L. vannamei under intensive culture.
Keywords
Growth performance
EHP
Biofloc volume
1 Introduction
Aquaculture is prided as the fastest-growing food production sector in the world with the increase predicated on the need to feed an ever-growing population and the perceived health benefit of the consumption of fish and shellfishes (Oladimeji et al., 2020a; Oladimeji et al., 2020b). However, as aquaculture intensifies, so is the problem of environmental pollution and economic losses. This is because an improvement in aquaculture productivity is dependent on the inputs of higher quality fish feed, which in turn increases waste production (Liu et al., 2019). About 70–80% of the dietary protein is wasted during feeding as it dissolves in the water and can accumulate to toxic levels (Avnimelech, 1999). The deterioration in water quality can cause a disease outbreak, which is a huge financial loss to the fish farmer (Samocha et al., 2004). Therefore, to prevent these losses, frequent water exchange is often done in the conventional aquaculture system which results in the eutrophication/degradation of the environment and increases the water budget for the aquaculture venture (Ataguba and Okomoda, 2012; Salin and Arome Ataguba, 2018).
Biofloc technology has been proven to solve many of these aquaculture problems. The underlining principle involves the generation of an appropriate mixotrophic microbial growth in the culture system to assimilate the deleterious nitrogenous waste while also serving as an additional food source to the fish (Kasan et al., 2019). Consequently, the quality of wastewater is improved, and feed conversion efficiency increased (Crab et al., 2009; Hari et al., 2004). This has been demonstrated in many studies on shrimp culture and has been observed to improve immune defences as well as biosecurity of these shrimps (Zhao et al., 2012; Liu et al., 2018; Das and Mandal, 2021). However, enhancing the growth of the appropriate microorganisms for the biofloc system requires changing the dominant microorganism community from autotrophic to mixotrophic (Browdy et al., 2001). This is done by manipulating the carbon–nitrogen ratio (C/N ratio) for the establishment of the heterotrophic microbiota community (Crab et al., 2012).
The time taken for the establishment of beneficial microorganisms varies under different conditions (Ferreira et al., 2017). However, a prolonged period could be detrimental especially when a disease outbreak occurs before the development of biofloc in a system. Therefore, cutting down the time of flocculation through bio-inoculation with a floc-forming bacterium is advantageous for aquaculture practices. Although the idea that biofloc can be accelerated through the addition of certain bacteria has not been convincingly demonstrated in pond systems (Liu et al., 2018; Liu et al., 2019), however, our earlier study has shown the effectiveness of Bacillus infantis over Nitratireductor aquimarinus as a floc-forming bacterium under laboratory conditions (Harun et al., 2019). Alongside the reduction of flocculation time observed, bio-inoculation with B. infantis increased the growth performance of white leg shrimp Litopenaeus vannamei and improved the water quality during the rearing period (Kasan et al., 2019; Harun et al., 2019). This technology was named Rapid biofloc® (Patent file - PI 2017703679) because of the reduced flocculation time characterized by its use (Yee et al., 2021). In this study, we tested the commercialization potential of this technology on the rearing water quality and the performance of white leg shrimp L. vannamei on an industrial scale. This was compared to the conventional method used for intensive shrimp production in Malaysia. The outcome of this study can help to reduce biofloc flocculation time, improve water quality and shrimp yield as well as to prevent economic losses during a disease outbreak in commercial shrimp culture.
2 Materials and methods
The study was conducted at the Hannan Corporation located in Kuala Gula, Perak, Malaysia (4°92′93″ N, 100°49′32″ E). It is one of the commercial shrimp farms in Malaysia producing about 3000 tons of L. vannamei annually (Kasan et al., 2019). This industrial-scale experiment was conducted in two pairs of ponds (1500 m2 each, 1 m depth) lined with high-density polyethylene. This was to accommodate the treatment with Rapid biofloc® technology (R-BFT), and the control group which utilized the standard operational procedure (C-SOP) normally employed for the intensive culture of L. vannamei on the farm (i.e. Experimental design is 2 treatments × 2 replicates). In each pond, water depth was set at 0.70 m and 400,000 post-larvae shrimp of similar breeding history (PL20; mean weight = 0.7 ± 0.03 g) were stocked (stocking density = 260 ± 3.55 shrimps/m3) at the start of every experimental cycle. Since the shrimp were obtained from the Hannan Corporation farms, they were not acclimatized before stocking. The broodstocks used for production at the Hannan Corporation farms were maintained in the indoor hatchery throughout the production cycle. Also, the post-larvae were maintained indoors for twenty days before stocking in the outdoor tanks.
Pre-treatment of the seawater from the source and disinfection of the treatment ponds were done before and after every trial respectively. In brief, the incoming seawater was stored in holding/reservoir tanks, where it was disinfected with calcium hypochlorite (30 mg/L). De-chlorination of the seawater happens naturally by the action of the ultraviolet rays of the sun during the water residency of two weeks in the reservoir.
After stocking the shrimps, one litter of the Rapid biofloc® obtained from the bioflocculation-bacteria collection at the Institute of Tropical Aquaculture (AKUATROP), Universiti Malaysia Terengganu (stored at room temperature) was used to inoculate the designated ponds (i.e. R-BFT group inoculated at 1 × 109 CFU mL−1 of B. infantis). The R-BFT operated a zero-wastewater exchange protocol; hence, the pond water was only emptied at the end of every cycle, replaced with fresh seawater, and re-inoculated with B. infantis (at 1 L per 1000 m2 of water) following the protocol of the Rapid biofloc® Technology. The C-SOP group, on the other hand, was inoculated fortnightly with 1 kg of dry PondPlus® probiotics (heterotrophic bacteria composed of Bacillus; Bacterial count: 1 × 109 CFU/gram) (Zhou et al., 2017). The pond water was also refreshed periodically (i.e. 20% water exchange every week). At the end of every cycle, the water was emptied and replaced with fresh seawater before re-inoculation with the commercial probiotic (at 1 kg per 1000 m2 of water). Since the probiotic was in powdered form, it was first dissolved in water (1 kg dissolved in 1L of water) and aerated in a bowl for 30 min before pouring into the designated pond for the C-SOP.
The ponds for the R-BFT and C-SOP were installed with three paddle wheels (1hp capacity) each strategically positioned to maximize flow and aeration efficiency in each pond. The paddle wheels were operated non-stop throughout each cycle. The shrimp were fed to satiation four times a day with Gold Coin commercial diets (45% CP; 10% Lipid and 14% Ash) from crumble (i.e. 0.5–1.1 mm) to pellets (i.e. Ø1.8 × 2.0–3.3 mm) depending on the size of shrimps. Each experimental cycle in this study lasted for 110 days (i.e. February – May 2017; June – September 2017; October 2017 – January 2018; February – May 2018; June – September 2018 respectively for the five cycles reported in this study). The addition of commercial molasses (24% carbon w/w) to maintain the carbon nitrogen−1 (C-N) ratio of 15:1 was done one hour after feeding (Crab et al., 2012). The choice of C-N ratio of 15:1 was based on our previous laboratory optimized of the same and the routine SOP used for shrimp rearing in the farm where the study was conducted. The result reported in this study represents the five cycles of production done within the two years of this study.
Water quality parameters such as temperature, dissolved oxygen, pH, and salinity of the ponds were recorded in-situ on the farm every ten days using the YSI professional plus multi-parameter water quality meter (Model 13M10065, Made in the USA). Water samples were also collected for the determination of ammonium and phosphate in the laboratory (APHA, 2005). Biofloc volume was measured on-site using the Imhoff cone after allowing sample water to settle for an hour (Kasan et al., 2019; Harun et al., 2019).
Shrimp weight was recorded every ten days (i.e., by collecting about 1000 subsamples of the shrimps using sampling nets) till they were completely harvested on the 110th day. The growth indices were computed using the relations below:
-
Mean Weight Gained (mg) = W2 - W1
-
Growth rate (mg/day) =
Where W1 = initial weight (mg)
W2 = final weight (mg)
t2 - t1 = duration between W2 and W1 (days)
Specific growth rate (%/day) =
Feed conversion ratio (FCR) =
Survival rate (%) =
The yield of the shrimp was also determined as the total biomass at harvest in each pond. Periodically (4 times in every production cycle), shrimp and water samples were taken and screened for the presence of common shrimp pathogens in Malaysia using polymerase chain reaction (PCR). White spot syndrome virus (WSSV) was screened according to Tan et al. (2001). Early mortality disease (EMD) aetiological agent was screened according to Sirikharin et al. (2015) with modification, whereas Enterocytozoon hepatopenaei (EHP) screening followed Tangprasittipap et al. (2013) method. During the disease outbreak, the shrimps in both treatments were not subjected to any curative management practice to demonstrate the efficacy of the Rapid biofloc® and PondPlus® used against the disease.
Summary statistics of the water quality, growth parameters, survival, and yield were obtained using Minitab 14 for Windows. The normality and homogeneity of variance were then tested before a Two-way Analysis of Variance was performed for the water quality parameters. However, the means of growth, survival, and yield were subjected to Student T-test (p ≤ 0.05). Analyzed data were presented in bar charts, line graphs, and tables.
3 Results and discussion
Water parameters such as temperature, pH, and salinity showed some levels of fluctuation during this study (Figs. 1–3 respectively). Despite these fluctuations, they were still similar in both treatments (p ≥ 0.05) and fall within the favourable ranges earlier reported to support the growth of shrimp (Kasan et al., 2019; Harun et al., 2019). Dissolved oxygen was however lower in the R-BFT compared to the C-SOP (p < 0.001) (Fig. 4). In line with this observation, the study by Kamilya et al. (2017) reported a reduction in DO levels of the biofloc system compared to the control group. Ray et al. (2009) had earlier hypothesized that higher oxygen consumption for nitrification and nitrogen immobilization by heterotrophic microbial communities were responsible for reduced oxygen content observed in the biofloc system. This probably explained the low DO observed in the R-BFT compared to the C-SOP.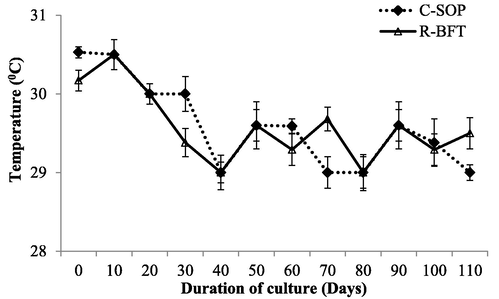
Water temperature in biofloc and control ponds of Litopenaeus vannamei. Line graphs with error bars are means ± standard errors. Line graphs did not differ significantly (p ≥ 0.05), (C-SOP = Control standard operational procedure; R-BFT = Rapid biofloc®).
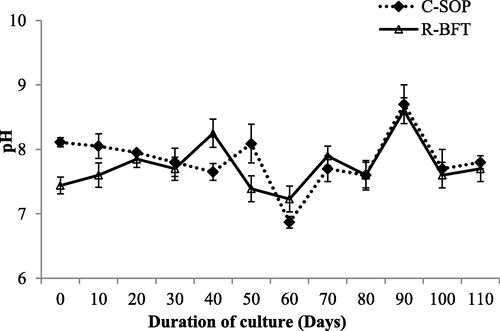
Water pH in biofloc and control ponds of Litopenaeus vannamei. Line graphs with error bars are means ± standard errors. Line graphs did not differ significantly (p ≥ 0.05), (C-SOP = Control standard operational procedure; R-BFT = Rapid biofloc®).
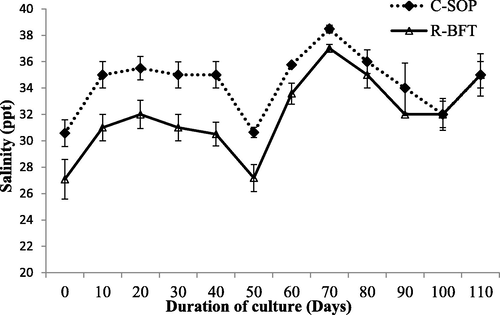
Water salinity in biofloc and control ponds of Litopenaeus vannamei. Line graphs with error bars are means ± standard errors. Line graphs did not differ significantly (p ≥ 0.05), (C-SOP = Control standard operational procedure; R-BFT = Rapid biofloc®).
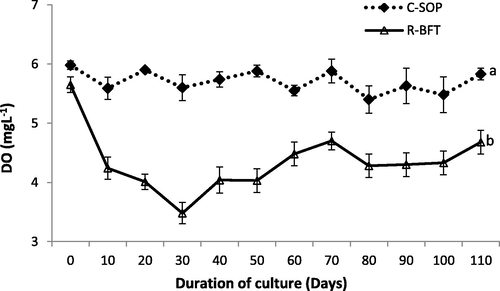
Dissolved oxygen in biofloc and control ponds of Litopenaeus vannamei. Line graphs with error bars are means ± standard errors. Line graphs with different lower case letters differed significantly (p < 0.05), (C-SOP = Control standard operational procedure; R-BFT = Rapid biofloc®).
The generally moderate to low levels of ammonia in the C-SOP might be due to the use of probiotics in addition to periodic water exchange done. Probiotics are known to improve water quality in aquaculture ponds because the bacteria in it also participate in the absorption of organic nutrients (Moriarty, 1997). At the end of this current study, however, the ammonia concentration was significantly reduced in the R-BFT than the C-SOP group (p < 0.05) (Fig. 5) which was in line with the finding of Avnimelech (1999). Efficient assimilation and oxidation of nitrogenous waste to useful metabolites by the action of heterotrophic bacteria have long been implicated for the low level of Total Ammonia Nitrogen (TAN), and NO3– N in a biofloc system (Xu et al., 2016; Mandal and Das, 2018). Hence, the ammonia levels in this study might be linked to the biofloc content (with different heterotrophic bacteria) of both treatments as a steady increase in biofloc volume accompanied the gradual reduction of ammonia observed over time. Therefore, the higher biofloc volume in the R-BFT probably resulted in a higher nitrification process compared to the C-SOP group, consequently, a reduced ammonia level was observed in the former than in the latter.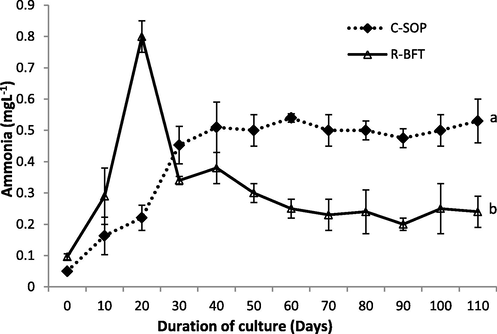
Ammonia concentration in biofloc and control ponds of Litopenaeus vannamei. Line graphs with error bars are means ± standard errors. Line graphs with different lower case letters differed significantly (p < 0.05), (C-SOP = Control standard operational procedure; R-BFT = Rapid biofloc®).
Likewise, Phosphate concentrations have been earlier attributed to the abundance of microbial floc deposited in the bottom of the tank which releases phosphorus through the decomposition action of heterotrophic bacteria (Xu et al., 2016). Hence, the low phosphate observed in the C-SOP group (p < 0.05) (Fig. 6) might also be connected to the low level of biofloc developed or/and the periodic water exchange done in this control group. In general, the changes and dynamism in the duo inorganic nitrogen reported in this study are indicative of the level of immobilization and/or nitrification by nitrifying microbes in both treatments (Burford et al., 2004).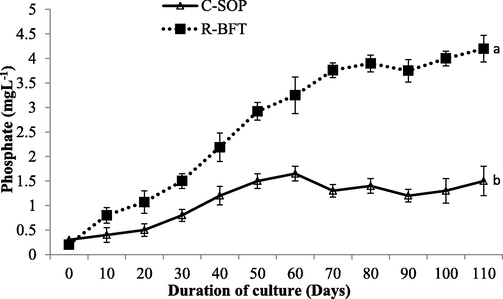
Phosphate concentration in biofloc and control ponds of Litopenaeus vannamei. Line graphs with error bars are means ± standard errors. Line graphs with different lower case letters differed significantly (p < 0.05), (C-SOP = Control standard operational procedure; R-BFT = Rapid biofloc®).
The development of biofloc in the control was not surprising as PondPlus® commercial probiotic used to inoculate the C-SOP group fortnightly contained heterotrophic bacteria composed mainly of Bacillus (Fig. 7). However, the establishment of biofloc was only observed after 30 days despite fortnight inoculation with the probiotic. Many studies have shown that not all bacteria species can accelerate biofloc formation in pond systems (Liu et al., 2018; Liu et al., 2019; Harun et al., 2019). While the exact microbiota composition of the PondPlus® commercial probiotic was unknown, this study may have demonstrated the efficacy of B. infantis in the Rapid biofloc® over the microbiota in PondPlus® as regards accelerating biofloc formation. Also, the biofloc volume was significantly lower in the C-SOP compared to the R-BFT (p < 0.05). Avnimelech, (1999) and Hari et al. (2004) had earlier opined that the reduction in nitrogenous waste through the addition of carbon sources significantly increased microbial flocs. This was in line with the finding of this study as an inverse trend was observed between the nitrogenous compounds and the biofloc volume of both treatments. The inadequate practice of biofloc development characterized by periodic water exchange may explain the low level of biofloc volume in the C-SOP.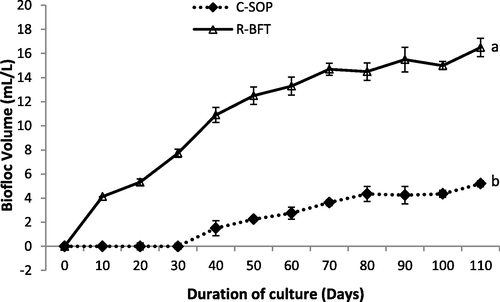
Biofloc volume in biofloc and control ponds of Litopenaeus vannamei. Line graphs with error bars are means ± standard errors. Line graphs with different lower case letters differed significantly (p < 0.05), (C-SOP = Control standard operational procedure; R-BFT = Rapid biofloc®).
An earlier study by Zhao et al. (2012) had reported overall better growth performance in shrimp cultured with biofloc system compared to control. However, in our study, final weight, weight gained, growth rate, and specific growth rate was similar in both treatments (Table 1). Interestingly, a lesser feed requirement was used in the R-BFT group to get a similar weight of shrimps in the C-SOP group as dictated by the value of feed fed per shrimp and feed conversion ratio (FCR) (p < 0.05). Microbial flocs have earlier been demonstrated to be an effective feed source for aquaculture species such as Nile tilapia Oreochromis niloticus, white leg shrimps L. vannamei (Xu and Pan, 2012), and Common carp Cyprinus carpio (Dinda et al., 2018). Hence, the microbial flocs could have augmented the nutrition of shrimps reared in the R-BFT group, hence increasing feed utilization. This consequently reduced significantly the amount of feed needed to gain 1 kg of flesh as denoted by the FCR in the R-BFT group (1.52) compared to the C-SOP (2.49). Mean in the same row with different superscript differ significantly (p < 0.05) (C-SOP = Control standard operational procedure; R-BFT = Rapid biofloc®).
C-SOP
R-BFT
P-Value
Initial weight (g)
0.70 ± 0.10
0.73 ± 0.09
0.23
Final weight (g)
9.09 ± 1.37
10.63 ± 0.87
0.11
Weight gain (g)
8.39 ± 1.38
9.91 ± 0.87
0.15
Growth rate (gday−1)
0.076 ± 0.013
0.09 ± 0.008
0.09
SGR
2.31 ± 0.18
2.45 ± 0.12
0.35
Feed fed (g)
18.33 ± 0.73a
14.82 ± 0.92b
0.01
FCR
2.49 ± 0.50a
1.52 ± 0.09b
0.001
Likewise, survival is another zootechnical parameter that reflects the profitability or losses in an aquaculture venture (Sgnaulin et al., 2018). We observed significantly higher survival in the R-BFT group compared to the C-SOP group (p < 0.05) (Fig. 8). The better performance in the R-BFT group despite the occurrence of EHP in the first month of the fourth cycle of production (i.e. between PL20 and PL 40) demonstrated the ability of Rapid biofloc® to reduce the susceptibility of the shrimps to disease infections. Use of biofloc technology especially those characterized by Bacillus sp. has been shown to exhibit antagonistic tendencies with pathogenic Vibrio thereby reducing outbreak and enhancing survival (Avnimelech et al., 2012; Haslun et al., 2012; Mandal and Das, 2018; Das and Mandal, 2021). The early biofloc formation in the R-BFT group may have resulted in a swift action against the pathogen compared to those cultured in the C-SOP. Consequently, the EHP lasted till the second month in the C-SOP but eliminated in the first month in the R-BFT group, hence, resulting in improved survival in the latter than the former. However, the water quality of both treatments was not significantly affected during the outbreak.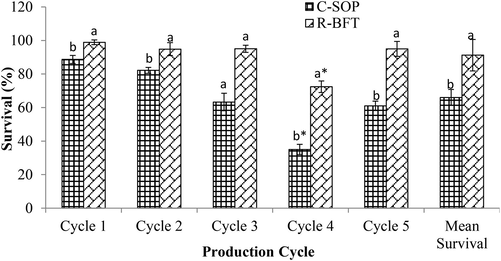
Survival of Litopenaeus vannamei in biofloc and control ponds under intensive culture system. Bars with different lower case letters under the same parameter (n = 5 cycles) differed significantly (p < 0.05) (C-SOP = Control standard operational procedure; R-BFT = Rapid biofloc®). Note: (*) represent cycle of production with disease outbreak.
With the better survival observed in the R-BFT under normal and diseased conditions, the mean yield was 57% higher than the C-SOP group (p < 0.05) (Fig. 9). Extensive culture of Marsupenaeus japonicus in grow-out ponds has been reported to give a low yield ranging between 0.030 and 0.075 kg m−3 (Mu et al., 2008). Under intensive culture, however, yield could range between 0.55 and 1.0 kg m−3 depending on the number of days of culture and the initial stocking density used (Lin et al., 2001; Zhou et al., 2008). The study by Zhao et al. (2012) however, demonstrated that biofloc technology potentially resulted in a 41.3% increase in the yield of M. japonicus compared with conventional intensive culture. Although yield values recorded for M. japonicus in the referenced studies are lower compared to the present study for L. vannamei, their finding was still in line with the fact that biofloc increases final yield compared to conventional systems. The findings of this industrial-scale study also affirm laboratory and field trials previously done on L. vannamei using the Rapid Biofloc® technology (Kasan et al., 2019; Harun et al., 2019).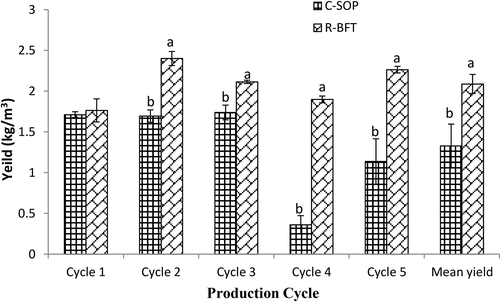
Yield of Litopenaeus vannamei in biofloc and control ponds under intensive culture system. Bars with different lower case letters under the same parameter (n = 5 cycles) differed significantly (p < 0.05) (C-SOP = Control standard operational procedure; R-BFT = Rapid biofloc®).
4 Conclusion
This study has demonstrated the commercial applicability of the R-BFT for better performance of the L. vannamei shrimp. In addition to the reduced feed requirement of the shrimp for optimum growth, the study also showed that R-BFT improves survival during the outbreak of EHP. It was therefore concluded that the Rapid biofloc® Technology was commercially viable and advantageous for use in the intensive culture of L. vannamei shrimp. Future studies might focus on the suitability of the technology for use in culturing other shrimp species and fin fishes. This could include the evaluation of various carbon sources, C-N ratio and biofloc volumes on the growth and physiological parameters of the aquaculture species after the inoculation of the rearing water with Rapid biofloc® technology.
Acknowledgments
This work was funded by the Ministry of Higher Education (MOHE), Malaysia under Higher Institution Centre of Excellence (HICoE), Institute of Tropical Aquaculture and Fisheries (AKUATROP) program [Vot. No. 63933, JPT.S(BPKI) 2000/016/018/ 015 Jld.3 (23) and Vot. No. 56050, UMT/PPPI/2-2/5 Jld.2 (24)]. We will also like to give special gratitude to those participated in the data collection for this research project.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Standard Methods of Examination of Water and Wastewater (21st ed.). Washington, D.C.: APHA; 2005.
- Aquaculture and the environment. In: 26th Annual Conference of the Fisheries Society of Nigeria (FISON), 28 Nov – 2 Dec 2011. Nigeria: Minna; 2012. p. :39-47.
- [Google Scholar]
- Carbon nitrogen ratio as a control element in aquaculture systems. Aquaculture. 1999;176:227-235.
- [Google Scholar]
- Biofloc Technology – A Practical Guide Book (second ed.). Baton Rouge, Louisiana, EUA: The World Aquaculture Society; 2012. p. :272.
- Browdy, C.L., Bratvold, D., Stokes, A.D., McIntosh, R.P., 2001. Perspectives on the application of closed shrimp culture systems. In: Browdy, C.L., Jory, D.E. (Eds.), The New Wave, Proceedings of the Special Session on Sustainable Shrimp Culture, Aquaculture 2001. The World Aquaculture Society, Baton Rouge, USA, pp. 20–34.
- The contribution of flocculated material to shrimp (Litopenaeus vannamei) nutrition in a high-intensity, zero-exchange system. Aquaculture. 2004;232(1-4):525-537.
- [Google Scholar]
- Biofloc technology in aquaculture: beneficial effects and future challenges. Aquaculture. 2012;356-357:351-356.
- [Google Scholar]
- Bio-flocs technology application in over-wintering of tilapia. Aquac. Eng.. 2009;40(3):105-112.
- [Google Scholar]
- Environmental amelioration in biofloc based rearing system of white leg shrimp (Litopenaeus vannamei) in West Bengal, India. Aquatic Living Resour.. 2021;34:17.
- [Google Scholar]
- Neem (Azadirachta indica A. Juss)-supplemented biofloc medium as alternative feed in common carp (Cyprinus carpio var. communis Linnaeus) culture. J. Appl. Aquac.. 2018;32(4):361-379.
- [Google Scholar]
- Bioremediation and biocontrol of commercial probiotic in marine shrimp culture with biofloc. Lat. Am. J. Aquat. Res.. 2017;45(1):167-176.
- [Google Scholar]
- Effects of carbohydrate addition on production in extensive shrimp culture systems. Aquaculture. 2004;241(1-4):179-194.
- [Google Scholar]
- Effect of different aeration units, nitrogen types and inoculum on biofloc formation for improvement of Pacific White leg shrimp production. Egypt. J. Aquat. Res.. 2019;45(3):287-292.
- [Google Scholar]
- Characterization of bioflocs in a no water exchange super-intensive system for the production of food size pacific white shrimp Litopenaeus vannamei. Int. J. Aquaculture. 2012;2:29-38.
- [Google Scholar]
- Biofloc technology application in indoor culture of Labeo rohita (Hamilton, 1822) fingerlings: The effects on inorganic nitrogen control, growth and immunity. Chemosphere. 2017;182:8-14.
- [Google Scholar]
- Production of Pacific white leg shrimp, Litopenaeus vannamei through implementation of rapid biofloc technology. IOP Conf. Series: Earth Environ. Sci.. 2019;370(1):012005
- [Google Scholar]
- Studies on high density culture of Penaeus japonicus in shrimp hatchery in autumn in north China. J. Oceanogr. Taiwan Strait. 2001;20:510-514.
- [Google Scholar]
- Inorganic nitrogen control, growth, and immunophysiological response of Litopenaeus vannamei (Boone, 1931) in a biofloc system and in clear water with or without commercial probiotic. Aquac. Int.. 2018;26(4):981-999.
- [Google Scholar]
- Biofloc formation improves water quality and fish yield in a freshwater pond aquaculture system. Aquaculture. 2019;506:256-269.
- [Google Scholar]
- Comparative efficacy of neem (Azadirachta indica) and non-neem supplemented biofloc media in controlling the harmful luminescent bacteria in natural pond culture of Litopenaeus vannaemei. Aquaculture. 2018;492:157-163.
- [Google Scholar]
- The role of microorganisms in aquaculture ponds. Aquaculture. 1997;151(1-4):333-349.
- [Google Scholar]
- New techniques in effective aquaculture of Marsupenaeus japonica. Sci. Fish Farm.. 2008;11:38-39.
- [Google Scholar]
- Aquaponics production of Catfish and Pumpkin: comparison with conventional production systems. Food Sci. Nutr.. 2020;8(5):2307-2315.
- [Google Scholar]
- Effects of different hydroponics growth media on water quality and plant yield in a Catfish-pumpkin Aquaponics system. J. King Saud Univ. Sci.. 2020;32:60-66.
- [Google Scholar]
- Ray, A.J., Shuler, A.J., Leffler, J.W., Browdy, C.L., 2009. Microbial ecology and management of biofloc systems. In: Browdy, C.L., Jory, D.E. (Eds.), The Rising Tide. Proceedings of the Special Session on Sustainable Shrimp Farming. World Aquaculture Society, Baton Rouge, LA, USA, pp. 231–242.
- Aquaculture and the environment: Towards sustainability. In: Hai F.I., Visvanathan C., Boopathy R., eds. Sustainable Aquaculture. Cham: Springer International Publishing; 2018. p. :1-62.
- [Google Scholar]
- Production of the Pacific white shrimp, Litopenaeus vannamei, in high-density greenhouse-enclosed raceways using low salinity groundwater. J. Appl. Aquac.. 2004;15(3-4):1-19.
- [Google Scholar]
- Biofloc technology (BFT): an alternative aquaculture system for Piracanjuba Brycon orbignyanus? Aquaculture. 2018;485:119-123.
- [Google Scholar]
- Characterization and PCR detection of binary, Pir-like toxins from Vibrio parahaemolyticus isolates that cause acute hepatopancreatic necrosis disease (AHPND) in shrimp. PLoS ONE. 2015;10(5):e0126987.
- [Google Scholar]
- Quantitative analysis of an experimental white spot syndrome virus (WSSV) infection in Penaeus monodon Fabricius using competitive polymerase chain reaction. J. Fish Dis.. 2001;24(6):315-323.
- [Google Scholar]
- The microsporidian Enterocytozoon hepatopenaei is not the cause of white feces syndrome in white leg shrimp Penaeus (Litopenaeus) vannamei. BMC Vet. Res.. 2013;9:139.
- [Google Scholar]
- Effects of C/N ratio on bioflocs development, water quality, and performance of Litopenaeus vannamei juveniles in a bioflocs- based, high-density, zero-exchange, outdoor tank system. Aquaculture. 2016;453:169-175.
- [Google Scholar]
- Effects of bioflocs on growth performance, digestive enzyme activity and body composition of juvenile Litopenaeus vannamei in zero-water exchange tanks manipulating C/N ratio in feed. Aquaculture. 2012;356-357:147-152.
- [Google Scholar]
- Marine microalgae co-cultured with floc-forming bacterium: insight on growth and lipid productivity. Peer J. 2021;9:e11217
- [Google Scholar]
- The application of bioflocs technology in high-intensive, zero exchange farming systems of Marsupenaeus japonicus. Aquaculture. 2012;354–355:97-106.
- [Google Scholar]
- Zhou, C.G., Chen, J.Y., Huang, X.K., 2008. Preliminary study of high productive aquaculture of Marsupenaeus japonica. Shandong Fish., 25, 27–27.
- Effect of three commercial microbial products on bacterial community in a freshwater fish polyculture system. Aquac. Res.. 2017;48(8):4449-4460.
- [Google Scholar]







