Translate this page into:
Quantifying anticancer drug toxicity on white blood cell count in cancer patients: A mathematical and computational approach
⁎Corresponding author. adeel@ntu.edu.pk (Muhammad Adeel)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
White blood cells (WBCs) are immune cells that fight infections and cancers. However, many cancers and their treatment with chemotherapy negatively affect the WBCs count. For oncologists and physicians, it is important to know how much the WBCs count is affected to optimize drug administration. The data of breast cancer patients were included in this study because the largest number of cancer patients were suffering from breast cancer in the under-study hospital. This study presents a computational and mathematical approach to measure the effect on the WBCs count of breast cancer patients treated with chemotherapy with two anticancer drug combinations: docetaxel with cyclophosphamide (TC) and doxorubicin with cyclophosphamide (AC).
Methods
Between July 2016 and October 2021, to breast cancer patients, 4 cycles of chemotherapy (one cycle of 21 days) were administered intravenously using TC (75 mg/m2 + 600 mg/m2). Similarly, varying in cases, intravenous AC (60 mg/m2 + 600 mg/m2) was administered in each of the 4 cycles. The WBCs counts of the subjects affected by both TC and AC combinations were observed for comparison. An equation was derived to calculate the effect of TC and AC on WBCs count (eWBCc). The eWBCc of 171 patients who received TC and 154 patients who received AC were calculated by implantation of the derived equation using Python 3.8.
Results
Comparing the results of 171 patients, it is observed that, TC affected WBC count of a patient at lowest level by 12.29%, at maximum level by 69.64%, and on average by 34.13%. Whereas, comparing the results of 154 patients, AC affected WBC count of a patient at lowest level by 19%, at maximum level by 65.03%, and on average, by 44.36%. A paired t-test was used to analyze the statistical differences between the eWBCc of both TC and AC cohorts. This test indicates a statistically significant difference between the eWBCc by TC and AC, as evidenced by the P-value of 0.000, which is less than the chosen significance level (alpha) of 0.05.
Conclusion
We conclude that TC remains less toxic than AC in affecting white blood cells count of the studied patients.
Keywords
Breast cancer
White blood cells count
Chemotherapy
Equation for effect on WBC
Impact of anticancer on WBC
1 Introduction
Cancer is a threatening disease that is treated by highly toxic anticancer drugs that leave many side effects (Adeel et al., 2019). The deficiency in white blood cells (WBCs) count is seen in different types of cancer and during chemotherapy using different anticancer drugs (Wu et al., 2019). WBCs also known as leukocytes play a major role in infection immunity. WBCs are of five major types including eosinophils, basophils, monocytes, lymphocytes (T cells, B cells, and Natural Killer cells), and neutrophils. WBCs make up 1 % of human blood. MedlinePlus, US National Library of Medicine (developed by National Institute of Health, US), reported that a cancer patient can acquire a low WBCs count from the tumor or from its chemotherapy (Gersten, 2021). Cancer in the bone marrow can cause fewer WBCs production. The WBCs count can also be decreased when cancer is treated with chemotherapy. The chemotherapy can negatively affect bone marrow hematopoiesis which causes the reduction of WBCs production.
Breast cancer in women has become a global cause for concern owing to its high occurrence all over the world (Kashyap et al., 2022). The most common cancers are breast cancer (in women) in the USA and lung cancer in China whereas lung cancer is the leading cause around the world (Xia et al., 2022). It was reported that through 23 years (between 1998 and 2020), 104,753 women with breast cancer were admitted in three major hospital of Punjab province of Pakistan. The immune system of patients with breast cancer is affected by a deficiency in WBCs. Immune status is a useful marker for predicting the risk of primary, recurrent, or metastatic (secondary) breast cancer (Standish et al., 2008).
Anticancer drugs can be categorized into different types (Fig. 1, see Supplementary Material). Depending on the stage, grade, and immune system of the patient, different drugs are selected for chemotherapy in breast cancer patients. Chemotherapy for breast cancer comprises eight cycles (Saarto et al., 1997). It was observed from the data of a government hospital that, in most breast cancer cases, patients were treated with two combinations. These combinations include docetaxel, cyclophosphamide (TC), and doxorubicin with cyclophosphamide (AC). Breast cancer and its treatment both affect WBCs (Park et al., 2019). These cells move through patients’ tissues and bloodstream to respond to illness or injury by beating any strange microbes, such as viruses and bacteria that arrive in their body. Once a patient’s WBCs reach the location of infection in the body, they attack the aggressor by supplying antibodies to the microbial tissue and clearing it.
Chemotherapy usually lowers the patient's WBCs count. Some cancer types that affect the bone marrow and blood can also decrease the WBCs count. These include lymphomas and multiple myeloma. A higher-than-normal range of monocytes or lymphocytes can signify the possibility of other types of malignancies. Some malignancies and their therapies may lead to neutropenia. Neutropenia is developed because of low counts of neutrophils which raises the possibility of microbial infections. To prevent a patient from developing neutropenia, clinicians lower the dose of chemotherapy, including diarrhea, sore throat, rash, chills, drainage from the ear, pain or burning with urination, fever, last longer cough, drainage from the central venous catheter site, stiff neck, any areas of warmth, redness, or other pain, which are sings of infections (Hassan et al., 2010). Thus, to improve the production of body neutrophils, clinicians usually prescribe medicine, such as WBC growth factors, in addition to antibiotics (Meckler and Lindemulder, 2009).
For oncologists and physicians, it is important to know how much WBCs count is affected to optimize drug administration (Ono et al., 2022) and WBCs count is also associated with cancer mortality (Jee et al., 2005). Thus, this study presents a mathematical and computational method to determine the effect on WBCs count of breast cancer patients treated with chemotherapy for four cycles with TC followed by four cycles with AC. This study may help clinicians to assess 1) the administration of drug dosage, 2) the best time for chemotherapy, 3) the time for medication to cure infections, 4) to select or reject the type of understudied drugs, 5) the mathematical observation of WBCs count, 6) the drug’s overall trend in affecting the WBCs count, 7) the formula for calculating the effect on WBCs count, and 8) the comparison of TC and AC in affecting WBCs count.
2 Material and methods
The study was divided into two parts. In the first part, an equation was derived to calculate the effect on WBCs count in the understudied patients. The second part presents a comparative analysis of the data retrieved by the implementation of the derived equation using the WBCs count during the four cycles of chemotherapy for each cohort (TC and AC).
2.1 Study design
In this study, women with breast cancer treated chemotherapy in a governmental hospital were selected to observe the effect of TC and AC on their WBCs counts during four cycles of chemotherapy with both combinations. This work was permitted (approval number 675/2016) by the ethical review committee, in Faisalabad, Pakistan. All patients provided written informed consent for the use of their white WBCs. Data from 668 breast cancer patients treated with chemotherapy were collected. After applying the exclusion and inclusion criteria (see the next subsection), we examined 459 cases in our records. Out of the 459, 134 subjects were excluded from the study because they were being treated with other drugs including tamoxifen, paclitaxel, and epirubicin. Thus, 171 of the remaining 325 patients were treated or were being treated for four cycles of chemotherapy with TC, and 154 women with breast cancer received or were being treated for the next four cycles of chemotherapy with AC.
2.2 Inclusion and exclusion criteria
The records of this study had data from different patients, because other studies were conducted along with this work. Thus, only patients who met the inclusion criteria (Table 1) were included in this study. (ECOG, Eastern Cooperative Oncology Group).
Inclusion Criteria
Exclusion Criteria
Gender
female
Gender
male
Age
above 26 and below 61 years
Age
below 26 and above 61 years
Stage
I-III
Stage
IV
Grade
1 and 2
Grade
3
Histology
invasive ductal carcinoma
Histology
Other than invasive ductal carcinoma
Diabetes
non-diabetic
Diabetes
diabetic
Renal function tests
normal
Renal function tests
abnormal
ECOG
0–1
ECOG
two and above
Ejection fraction in echocardiography
5 %–70 %
Ejection fraction in echocardiography
less than 50 %
Data from the participants’ charts and their follow-up reports were saved for this study in different sets of weights, ages, and geographical areas. Fig. 1 shows the comparison of age, weight, and area between the AC and TC cohorts.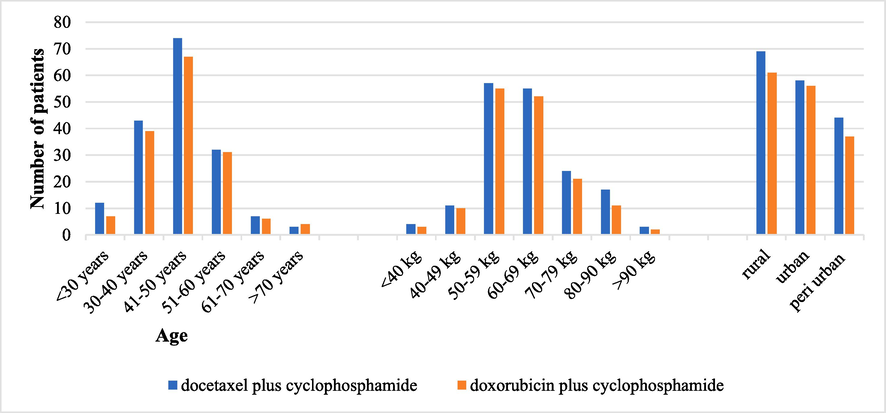
TC and AC cohorts’ classification by age, weight, and geographical area (TC, docetaxel with cyclophosphamide; AC, doxorubicin with cyclophosphamide).
2.3 Treatment
Intravenous TC (75 mg/m2 + 600 mg/m2) was administered in each of the 4 cycles of chemotherapy (one cycle was of 21 days).Varying in cases, intravenous AC (60 mg/m2 + 600 mg/m2) was administered in each of the 4 cycles. The WBCs counts of the subjects affected by both TC and AC combinations were observed for comparison.
2.4 Equation for the effect of anticancer drugs on patient’s WBCs count
The percentage change in the new value in accordance with the old value was calculated mathematically using the following formula:
Based on the above formula, Eq. (2) is derived which calculates the effect of TC and AC on WBCs count (eWBCc) of patients:
3 Results
3.1 Effect of TC and AC on patient’s WBCs count
Python 3.8 was used to implement Eq. (2) providing a WBCs count of 154 patients during four cycles of chemotherapy with AC and a WBCs count of 171 patients during four cycles of chemotherapy with TC. Tables 2 and 3 show eWBCc values for AC and TC patients, respectively. (eWBCc, effect of AC on WBCs count). (eWBCc, effect of TC on WBCs count).
#
eWBCcby AC
#
eWBCc by AC
#
eWBCc by AC
#
eWBCc by AC
#
eWBCc by AC
#
eWBCc by AC
#
eWBCc by AC
1
59.50
23
62.00
45
44.00
67
46.00
89
39.80
111
33.00
133
48.33
2
63.03
24
56.00
46
44.00
68
37.00
90
37.00
112
32.00
134
42.00
3
48.33
25
51.00
47
42.00
69
49.00
91
36.00
113
34.00
135
39.33
4
44.00
26
50.00
48
55.00
70
48.00
92
38.00
114
37.00
136
49.20
5
61.00
27
44.00
49
58.50
71
63.00
93
31.50
115
46.00
137
39.80
6
47.20
28
43.00
50
62.03
72
57.00
94
41.00
116
35.00
138
37.00
7
37.80
29
35.00
51
47.33
73
48.00
95
62.00
117
40.00
139
30.43
8
35.00
30
44.00
52
39.00
74
33.00
96
56.00
118
46.00
140
36.00
9
42.00
31
35.00
53
38.33
75
37.00
97
60.50
119
61.00
141
31.50
10
43.00
32
39.00
54
46.20
76
33.00
98
64.03
120
55.00
142
36.00
11
38.00
33
46.00
55
36.80
77
36.00
99
49.33
121
46.00
143
62.00
12
43.00
34
61.00
56
34.00
78
45.00
100
61.00
122
51.00
144
56.00
13
61.00
35
60.50
57
32.00
79
36.00
101
40.33
123
54.00
145
59.50
14
39.00
36
64.03
58
33.00
80
37.00
102
48.20
124
31.00
146
63.03
15
37.00
37
49.33
59
34.00
81
47.00
103
38.80
125
34.00
147
48.33
16
35.00
38
43.00
60
33.00
82
62.00
104
36.00
126
43.00
148
34.00
17
33.00
39
40.33
61
34.00
83
61.50
105
39.00
127
34.00
149
39.33
18
36.00
40
48.20
62
35.00
84
65.03
106
35.00
128
59.00
150
47.20
19
45.00
41
38.80
63
41.00
85
50.33
107
30.50
129
45.00
151
37.80
20
36.00
42
36.00
64
46.00
86
34.00
108
34.00
130
60.00
152
35.00
21
41.00
43
41.00
65
53.00
87
41.33
109
35.00
131
59.50
153
39.00
22
47.00
44
39.00
66
35.00
88
49.20
110
19.00
132
63.03
154
54.00
#
eWBCcby TC
#
eWBCc by TC
#
eWBCc by TC
#
eWBCc by TC
#
eWBCc by TC
#
eWBCc by TC
#
eWBCc by TC
1
14.29
26
18.00
51
49.91
76
28.73
101
14.00
126
43.24
151
68.00
2
21.19
27
26.00
52
43.24
77
18.64
102
14.29
127
66.64
152
45.00
3
35.85
28
20.00
53
66.64
78
28.14
103
21.19
128
65.00
153
51.00
4
25.57
29
35.00
54
65.00
79
43.27
104
37.85
129
25.00
154
45.00
5
27.40
30
23.00
55
25.00
80
47.36
105
27.57
130
33.00
155
47.00
6
41.92
31
40.00
56
33.00
81
45.85
106
29.40
131
19.00
156
43.00
7
24.44
32
26.00
57
19.00
82
51.91
107
43.92
132
14.00
157
30.00
8
34.65
33
34.00
58
14.00
83
45.24
108
26.44
133
12.29
158
20.00
9
21.64
34
20.00
59
50.91
84
68.64
109
34.65
134
19.19
159
28.00
10
27.73
35
15.00
60
44.24
85
65.00
110
21.64
135
33.85
160
22.00
11
17.64
36
15.29
61
67.64
86
42.00
111
27.73
136
23.57
161
37.00
12
27.14
37
22.19
62
66.00
87
48.00
112
17.64
137
25.40
162
25.00
13
42.27
38
36.85
63
26.00
88
42.00
113
27.14
138
39.92
163
42.00
14
46.36
39
26.57
64
34.00
89
44.00
114
40.27
139
22.44
164
28.00
15
44.85
40
28.40
65
20.00
90
40.00
115
44.36
140
32.65
165
36.00
16
50.91
41
42.92
66
15.00
91
27.00
116
42.85
141
19.64
166
22.00
17
44.24
42
25.44
67
15.29
92
17.00
117
48.91
142
25.73
167
17.00
18
67.64
43
33.65
68
22.19
93
25.00
118
42.24
143
15.64
168
17.29
19
66.00
44
20.64
69
36.85
94
19.00
119
65.64
144
25.14
169
24.19
20
43.00
45
26.73
70
26.57
95
34.00
120
64.00
145
40.27
170
22.00
21
49.00
46
16.64
71
28.40
96
22.00
121
24.00
146
44.36
171
36.00
22
43.00
47
26.14
72
42.92
97
39.00
122
32.00
147
42.85
23
45.00
48
41.27
73
25.44
98
25.00
123
18.00
148
48.91
24
41.00
49
45.36
74
35.65
99
33.00
124
13.00
149
42.24
25
28.00
50
43.85
75
22.64
100
19.00
125
49.91
150
69.64
From Table 2, it is observed that comparing the results of 154 patients obtained from the implementation of Eq. (2), AC affects WBC count of patient number 110 at the lowest level is 19 %. However, at the maximum level, in these patients, the WBC count of patient number 84 was affected 65.03 % by AC. However, on average, AC remains toxic, affecting these patients WBC count of 44.36 %. Interviewing patient no. 110, it was found that the food and environment provided to this patient collectively played a vital role in controlling his WBC count. The same factors affected the WBC count of patient no. 84 at the maximum level among these patients. It has also been reported that WBC count is controlled by these factors (Mahdi and Kadhim, 2022). The WBC count of most of the patients in this study affected by AC remained in the range of 30 % to 40 %.
From Table 3, it is observed that comparing the results of 171 patients obtained from the implementation of Eq. (2), TC affects WBC count of the patient number 133 at the lowest level of 12.29 %. Whereas, At maximum level, in these patients, the WBC count of patient number 150 was affected 69.64 % by TC. However, on average TC remains toxic in affecting these patients WBC count by 34.13 %. Upon interviewing patient no. 133, it was found that the food and environment provided to this patient collectively played a vital role in controlling his WBC count. The same factors affected the WBC count of patient no. 150 at the maximum level among these patients. The WBC count of most of the patients in this study affected by AC remained in the range of 20 % to 30 %. The data listed in Tables 2 and 3 can be graphically observed in Fig. 2, which shows a dramatic difference between the effects of TC and AC on the patient’s WBCs count. Fig. 2 shows that TC and AC both cause a low white blood cell count (neutropenia). However, the duration and magnitude of neutropenia varies between these two chemotherapies. AC is generally more likely to cause severe neutropenia than TC. The base WBC count normally takes place 7 to 10 days after TC administration and 5 to 7 days after AC administration. The retrieval of WBC count after administration of TC is faster than after the administration of AC. Patients treated using TC are required to have their WBC count monitored more closely than those receiving AC, as the start of a low white blood cell count is more rapid and the salvage is slower.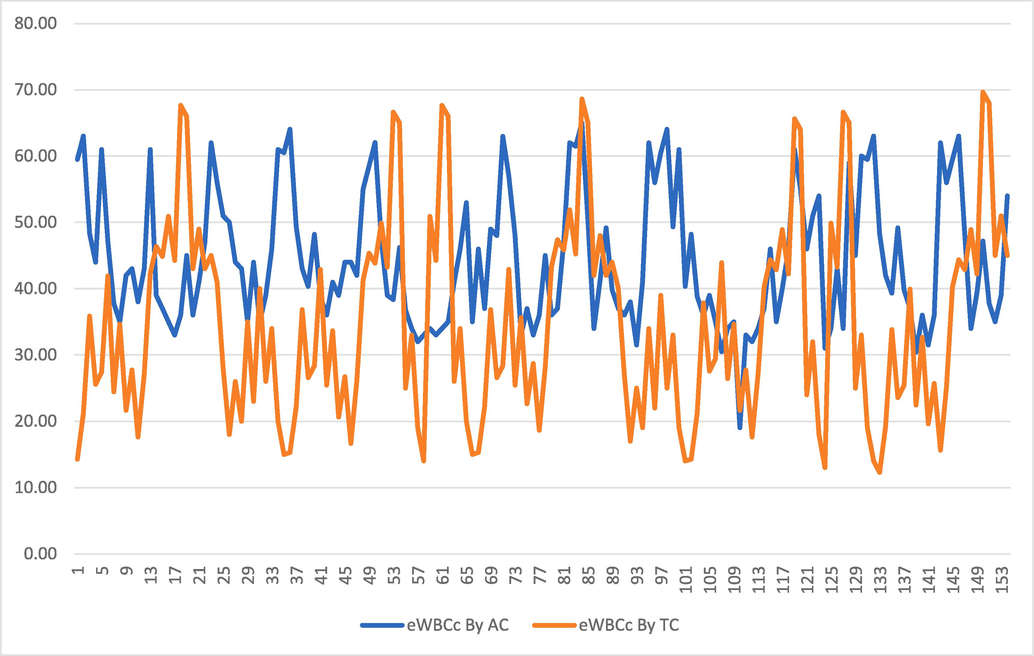
Difference between effects of TC and AC on patient’s WBCs counts (TC, docetaxel with cyclophosphamide; AC, doxorubicin with cyclophosphamide; WBC, White Blood Cells; eWBCc, effect of TC/AC on WBCs count).
3.2 Comparative WBC between AC and TC
A paired t-test was applied to analyze the statistical difference between the overall effect of TC and AC on patient WBCs count. Table 4 presents the results of the analysis. From Table 4, it is found that the p-value is 0.000, which is less than the chosen significance level of 0.05. Therefore, we reject the null hypothesis and conclude that there is a statistically significant difference between the eWBCc by TC and AC. 95 % CI for mean difference: (6.93, 12.56). T-Test of mean difference = 0 (vs ≠ 0): T-Value = 6.84P-Value = 0.000. TC, docetaxel with cyclophosphamide; AC, doxorubicin with cyclophosphamide; WBC, White Blood Cells.
Paired T-Test and CI: AC, TC
N
Mean
StDev
SE Mean
AC
154
44.36
10.02
0.81
TC
154
34.62
14.60
1.18
Difference
154
9.74
17.68
1.42
4 Discussion
In this study, the drug dosage was optimized by assessing the eWBCc of patients. For example, the case of patient 1 was affected 14.29 % by TC and 59.50 % by AC (Tables 2 and Table 3). Based on these values, physicians can determine the dosage and duration of chemotherapy. Because TC affects 59.50 % that means patient’s immune system has been disturbed, so cannot receive chemotherapy. When a patient recovers a WBCs count in the normal range, it can be the best time for chemotherapy. Similarly, if a patient’s eWBCc is adversely affected, it may lead to the development of any infection. This may assist doctors in deciding on the time to provide medication to cure any infection. Based on eWBCc of the patient, the doctor may also reject drugs and select a suitable drug for the patient. For example, in the case of patient 150, eWBCc was 69.64 % that means doctor may change the drug for the remaining chemotherapy. Some studies have provided assistance with the administration of drug dosage. Several studies have discussed the optimal time for chemotherapy. Three papers discussed the time taken for medication to cure the infections. Four articles reported drug rejection or selection. The mathematical observation of WBCs count has been presented in a few studies. Table 5 presents a comparison of assistance observed in other studies. TC, docetaxel with cyclophosphamide; AC, doxorubicin with cyclophosphamide; WBC, White Blood Cells.
Assistance for Observing
Wu
et al.
(Wu et al., 2019)
Park
et al.
(Park et al., 2019)
Shankar
et al.
(Shankar et al., 2006)
Ono
et al.
(Ono et al., 2022)
Jee
et al.
(Jee et al., 2005)
Zhao
et al.
(Zhao et al., 2022)
Hao
et al.
(Hao et al., 2018)
This study
the administration of drugs dosage
✓
×
✓
×
✓
×
✓
✓
the best time for chemotherapy
×
×
×
✓
×
×
×
✓
the time for medication to cure infections
×
✓
✓
×
✓
✓
×
✓
to select or reject type of understudy drugs
✓
×
×
✓
✓
×
✓
✓
the mathematical observation of WBCs count
×
×
×
×
×
✓
✓
✓
the drugs overall trend in affecting the WBCs count
×
×
×
×
×
×
×
✓
the formula for calculating effect on WBCs count
×
×
×
×
×
×
×
✓
the comparison of TC and AC in affecting WBCs count
×
×
×
×
×
×
×
✓
The literature makes many important contributions by observing the white blood cell count of patients with cancer. A recent study have explored the medical importance of the perioperative counts of white blood cells count, along with other bio markers (Wang et al., 2021). Lien et al. found higher increase of WBCs with Chinese Herbal Medicine with chemo than with chemotherapy only in randomized controlled trials (Lien et al., 2021). The results of a study determined intracellular reactive oxygen species decline without impairing the chemotherapeutical activity of doxorubicin in K562 cells or inducing WBCs death (Dos Santos et al., 2018). Changes in the WBCs, platelet, neutrophil, and hemoglobin indices on fist day before chemo of small cell lung cancer patients and 5th, 8th, 11th, 14th, 21st, and on 28th days after chemo were compared by a study among two different groups of medicines (Zhao et al., 2021). Hao et al. shown the predictive value of WBCs after chemotherapy of small cell lung cancer (Hao et al., 2018). A high lymphocyte-to-white blood cell ratio was observed by a study to predict the effectiveness of chemotherapy with the oxaliplatin and capecitabine regimen to gastric cancer patients (Tang et al., 2018). Yuksel et al. investigated WBCs counts and neutrophil to lymphocyte ratio in the identification of localized testicular cancer (Yuksel et al., 2016). A study investigated the analytical impact of elevated WBCs count of cervical cancer recurrence at the time of the diagnosis (Mabuchi et al., 2012). Shen et al. found that doxorubicin directly destroy neutrophils in a very short time and thus may not be used to make as a model chemo nanodrug to prepare an innovative cell chemotherapeutic drug (Shen et al., 2021). A study determined that the baseline, cycle-1 day-1 median absolute neutrophil count was lower in Black breast cancer patients versus Non-Black with same results at cycle-3 day-1 (Schreier et al., 2022). Hoshino et al. investigated the association between neutrophil-to-lymphocyte ratio change after neoadjuvant chemotherapy and histologic response and oncologic outcomes in patients of esophageal malignancy (Hoshino et al., 2022). Neutrophil-to-lymphocyte ratio was described to be related with diagnosis of patients of urothelial cancer who were receiving organized chemo or immunotherapy (Kobayashi et al., 2022). Though, even patients with high pre-chemo neutrophil-to-lymphocyte ratio attained favorable overall and progression-free survivals if they had the neutrophil-to-lymphocyte ratio decreased by chemo, while those with high pre-chemo neutrophil-to-lymphocyte ratio produced uncomplimentary overall and progression-free survivals if they had the neutrophil-to-lymphocyte ratio continued high after chemo, signifying that chemo may had disparity effect on the efficiency of following pembrolizumab medication in patients of urothelial cancer (Kobayashi et al., 2022). Méndez et al. evaluated the probiotic fermented milk management on the side effects of chemo drug, capecitabine and on its anticancer effects and they found that capecitabine organization reduced the number of WBCs compared to both the probiotic fermented milk and milk groups, without accomplishment of the control group standards (Méndez Utz et al., 2021). A study reviewed that many trials indicated that β-glucan concomitant administration with chemotherapy or with radiotherapy decreased immune decline due to such medications and enhanced the recovery of WBCs counts (Steimbach et al., 2021). Orhan et al. studied the data of perianal-complications and examined the association among the perianal lesion, WBCs to neutrophil count, and the kind of treatment in understudy patients with hematologic cancers in the neutropenic period. They derived a statistical significance consideration in favor of acute-myeloid-leukemia was found between those patient findings and anal-abscess-development and they also found that there was a converse relationship between the number of WBCs at hospitalization with an anal pathologic operation. They observed that patients with high WBCs counts underwent fewer operations due to anal pathology (Orhan et al., 2022).
5 Conclusions
The results of this study concluded that TC remains less toxic than AC in affecting white blood cells count of understudied patients. It was also concluded that both drugs disturbed the immune system of the patients, resulting in a slow recovery of their health. However, this study did not compare the levels of eosinophils, basophils, monocytes, and lymphocytes affected by anticancer drugs. In the future, we would like to formulate a calculation for the effect of chemotherapy on platelets of patients with cancer.
6 Research ethics approval
All methods adopted in this study were based on the standards of the ethical review committee of Faisalabad, Pakistan, who approved this study (approval number 675/2016). All patients provided written informed consent for using their data.
Data availability
The data used for this study can be provided on request to corresponding author.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project number (RSP 2023R75), King Saudi University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Comparative study of adjuvant chemotherapeutic efficacy of docetaxel plus cyclophosphamide and doxorubicin plus cyclophosphamide in female breast cancer. Cancer Manag. Res.. 2019;11:727.
- [Google Scholar]
- Guazuma ulmifolia Lam. decreases oxidative stress in blood cells and prevents doxorubicin-induced cardiotoxicity. Oxidative Med. Cellular Longevity 2018
- [Google Scholar]
- Gersten, T. 2021, 4/28/2021. Low white blood cell count and cancer. Retrieved 6/26/2022, 2022, from https://medlineplus.gov/ency/patientinstructions/000675.htm.
- Prognostic value of white blood cells detected for the first time after adjuvant chemotherapy in primary operable non-small cell lung cancer. Technol. Cancer Res. Treatment. 2018;17:1533033818802813
- [Google Scholar]
- Fever/clinical signs and association with neutropenia in solid cancer patients: bacterial infection as the main cause. Asian Pac. J. Cancer Prev.. 2010;11(11):1273-1277.
- [Google Scholar]
- Neutrophil-to-lymphocyte ratio change predicts histological response to and oncological outcome of neoadjuvant chemotherapy for esophageal squamous cell carcinoma. Esophagus 2022:1-10.
- [Google Scholar]
- White blood cell count and risk for all-cause, cardiovascular, and cancer mortality in a cohort of Koreans. Am. J. Epidemiol.. 2005;162(11):1062-1069.
- [Google Scholar]
- Global increase in breast cancer incidence: risk factors and preventive measures. BioMed Res. Int. 2022
- [Google Scholar]
- Pre-pembrolizumab neutrophil-to-lymphocyte ratio (NLR) predicts the efficacy of second-line pembrolizumab treatment in urothelial cancer regardless of the pre-chemo NLR. Cancer Immunol. Immunother.. 2022;71(2):461-471.
- [Google Scholar]
- Chinese Herbal Medicine, Guilu Erxian Glue, as alternative medicine for adverse side effects of chemotherapy in doxorubicin-treated cell and mouse models. Evidence-Based Complement. Alternat. Med. 2021
- [Google Scholar]
- Elevated white blood cell count at the time of recurrence diagnosis is an indicator of short survival in patients with recurrent cervical cancer. Int. J. Gynecologic Cancer. 2012;22(9)
- [Google Scholar]
- Mahdi, M.S., Kadhim, N.Q., 2022. The role of alpha klotho protein and FGF-23 in serum of breast cancer patients. In: AIP Conference Proceedings, AIP Publishing LLC.
- Fever and neutropenia in pediatric patients with cancer. Emergency Med. Clin.. 2009;27(3):525-544.
- [Google Scholar]
- Milk fermented by Lactobacillus casei CRL431 administered as an immune adjuvant in models of breast cancer and metastasis under chemotherapy. Appl. Microbiol. Biotechnol.. 2021;105(1):327-340.
- [Google Scholar]
- Impact of white blood cell count on clinical outcomes in patients treated with aspirin-free ticagrelor monotherapy after percutaneous coronary intervention: insights from the GLOBAL LEADERS Trial. Eur. Heart J.-Cardiovasc. Pharmacother.. 2022;8(1):39-47.
- [Google Scholar]
- The role of white blood cell count in perianal pathologies: a retrospective analysis of hematologic malignancies. Mediterranean J. Hematol. Infect. Dis.. 2022;14(1):e2022051
- [Google Scholar]
- Association of white blood cell count with breast cancer burden varies according to menopausal status, body mass index, and hormone receptor status: a case-control study. Sci. Rep.. 2019;9(1):1-10.
- [Google Scholar]
- Haematological toxicity: a marker of adjuvant chemotherapy efficacy in stage II and III breast cancer. Br. J. Cancer. 1997;75(2):301-305.
- [Google Scholar]
- Racial disparities in neutrophil counts among patients with metastatic breast cancer during treatment with CDK4/6 inhibitors. Breast Cancer Res. Treat. 2022:1-15.
- [Google Scholar]
- Association between circulating white blood cell count and cancer mortality: a population-based cohort study. Arch. Intern. Med.. 2006;166(2):188-194.
- [Google Scholar]
- Neutrophil-mediated clinical nanodrug for treatment of residual tumor after focused ultrasound ablation. J. Nanobiotechnol.. 2021;19(1):1-16.
- [Google Scholar]
- Fungal beta-glucans as adjuvants for treating cancer patients–A systematic review of clinical trials. Clin. Nutr.. 2021;40(5):3104-3113.
- [Google Scholar]
- Platelet-to-lymphocyte ratio and lymphocyte-to-white blood cell ratio predict the efficacy of neoadjuvant chemotherapy and the prognosis of locally advanced gastric cancer patients treated with the oxaliplatin and capecitabine regimen. Onco. Targets Ther.. 2018;11:7061.
- [Google Scholar]
- Perioperative Circulating Tumor Cells (CTCs), MCTCs, and CTC-white blood cells detected by a size-based platform predict prognosis in renal cell carcinoma. Dis. Markers 2021
- [Google Scholar]
- Values of applying white blood cell counts in the prognostic evaluation of resectable colorectal cancer. Mol. Med. Rep.. 2019;19(3):2330-2340.
- [Google Scholar]
- Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin. Med. J. (Engl.). 2022;135(05):584-590.
- [Google Scholar]
- White blood cell counts and neutrophil to lymphocyte ratio in the diagnosis of testicular cancer: a simple secondary serum tumor marker. Int. Braz. J. Urol.. 2016;42:53-59.
- [Google Scholar]
- To explore the effects of acupuncture and medical treatment at different times on the gastrointestinal reaction and white blood cell count of patients with lung cancer chemotherapy. Appl. Bionics Biomech. 2022
- [Google Scholar]
- Randomized efficacy trial of conventional, TCM herb, and TEAS on bone marrow suppression in patients with small cell lung cancer after initial chemotherapy. Evidence-Based Complement. Alternat. Med. 2021
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.103024.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







