Translate this page into:
Purification, characterization and molecular docking studies of analogous alpha amylase inhibitor compounds
⁎Corresponding author. drrattandeep@gmail.com (Rattandeep Singh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
A large number of protein inhibitors are found in higher plants, cereals and legumes. These inhibitors are helpful in the prevention as well as medical treatment of metabolic syndromes such as type 2 diabetes. Basically, the diabetes mellitus is a chronic disorder that occurs either due to inadequate insulin secretions or when body fails to utilize the produced insulin. The alpha amylase inhibitors are termed as starch blockers. They catalyze the hydrolysis of α-(1,4)-D-glycosidic linkages of starch and other glucose polymer. They play a significant role in inhibition of the activity of salivary and pancreatic amylase in vitro and in vivo.
Method
The present study was based on the purification, characterization and molecular docking studies of the alpha amylase inhibitor isolated from the kidney bean sample. The seed sample was collected from G.B. Pant Nagar University. The crude extract was prepared, the in-vitro studies and heat stability were determined. Following it, purification was carried out using ammonium sulphate precipitation and size exclusion chromatography was done. Later, characterization and molecular docking studies of the purified sample was done after obtaining GC–MS results.
Result
The in-vitro analysis was done and noted that the inhibitory activity was 96.5 ± 0.84 % post size exclusion chromatography. The molecular weight was about 54 kDa. The molecular docking studies revealed that there were interactions between the ligand molecules (the constituents that were selected from the GC–MS chromatogram peaks based on the height and area) and the human pancreatic alpha amylase (1HNY). The hydrogen bonding as affinity binding capacity was also been observed in each of the ligand–protein interaction.
Conclusion
From the tests, it has been elucidated that the alpha amylase inhibitor isolated from the sample has a great potential to serve as an anti-diabetic drug. However, in order to check the potential effects and to explore the optimization and development of the anti-diabetic drug the in-vivo studies can also be done further.
Keywords
Alpha amylase inhibitor
Diabetes
GC–MS analysis and molecular docking
1 Introduction
The diabetes mellitus is a chronic disorder that give birth to the number of ailments that ultimately affects the body. Basically, DM is caused either due to inadequate insulin secretions or when body fails to utilize the produced insulin. It initiates with the signs and symptoms that include polyuria (a condition causing increased urination), glycosuria (presence of reducing sugars in the urine), excessive hunger and fatigue (Bhosale et al., 2011). There are various different subclassifications of the diabetes such a diabetes mellitus type I and type II, gestational and neonatal diabetes and maturity-onset diabetes of the young (MODY). Above all, the type II diabetes mellitus is more prevalent in all the nations as compared to the other types. As per world health organisation (WHO), the type II diabetes contributes about 85 % in developed countries (Brayer et al., 2000). For the treatment of diabetes various therapeutic drugs and diets are prescribed to reduce the post prandial hyperglycaemia. The drugs belongs to the classes of thiazolidinediones (TZDs), sulfonylureas, DPP-4 inhibitors and SGLT2 inhibitors. Despite the advantage of reducing blood glucose levels they have adverse effects on the body. Thus, an alternative approach to tackle with this problem is by using of plant-based diets and drugs (Demir et al., 2019). As per World health Organization, it has been reported that in case of diabetic patients the consumption of more leguminous food is beneficial due to low glycemic index. Additionally, they also contain various bioactive compounds and alpha amylase inhibitor that aids in inhibiting the activity of alpha amylase. Thus, reducing the blood glucose level.
Basically, the alpha amylase inhibitor has been used for generation of drugs that are curative in case of diabetes such as voglibose, miglitol (Dilip et al., 2006). It works on the principle of the breaking down of large complex carbohydrates starch into smaller starch molecules. Primarily, these inhibitors cleave the α (1 → 4) glycosidic linkage present in starch by the process of the endohydrolysis. In the present study, in-vitro evaluation, purification and characterization of the alpha amylase inhibitor that has been extracted from the sample was done. (See Figs. 2 and 3.
2 Material and methodology
2.1 Sample collection and analysis
Phaseolus vulgaris seed sample was collected from G.B Pant Nagar University of Agricultural and Technology, Pantnagar, Uttarakhand. The collected sample was stored in airtight container.
2.2 Methodology
2.2.1 Preparation of crude extract
The crude extract was prepared using 100 mg of seed sample in extraction buffer (10 mM Tris −HCl, 500 mM NaCl, 1 % 2-Mercaptoethanol, 0.1 % Triton- X-100, 2 mM PMSF). The seeds were grinded into fine powder and about 0.1gm of sample was weighed and dissolved in 100 ml of extraction buffer. The contents were homogenized using pestle and motorand transferred into falcon tubes, followed by centrifugation at 10,000 rpm at 4℃ for 15 mins. The supernatant was collected and utilized for further experimentations (Iguti et al., 1991).
2.2.2 In-vitro assessment of alpha amylase inhibitory activity
The analysis of alpha amylase inhibitory activity was done by using soluble starch solution (0.4 ml, 1 % w/v) prepared in 80 mM phosphate buffer (pH 6.9), porcine pancreatic alpha amylase (0.2 ml, 0.05 % w/v) in 20 mM acetate buffer (pH- 4.5) was added and then incubated for 15mins at 37 °C. Then, the reaction was stopped by addition of 0.8 ml dinitrosalicyclic acid reagent. The contents were heated in boiling water for about 5 mins and then cooled immediately. The optical density was measured at 540 nm. Alpha-amylase inhibitory activity was determined according to equation shown below:
Where, Mo and Mi are amount of maltose (mg/ml) produced in absence and presence of inhibitor respectively, under the same conditions (Ishimoto et al., 1989).
2.2.3 Purification of α-amylase inhibitor
The crude protein extract was taken and subjected to ammonium sulphate precipitation (30–90 % saturation) at 4 °C. The precipitate was then dissolved in 10 mM Tris-HCl and was dialyzed against buffer in batches. Then, the size exclusion chromatography was performed using Sephadex G-50 column (Singh et al., 2021).
2.2.4 Characterization of the purified alpha amylase inhibitor
Heat stability of alpha-amylase inhibitor − For determination of optimum temperature for activity of the enzyme, the assay was carried out by taking 0.5 ml of the enzyme extract. The reaction mixture was incubated for 10 to 15 min at 40 °C to 100 °C, followed by the addition of 1.0 ml of dinitrosalicylic acid reagent (DNS reagent). The optical density was measured at 540 nm. (Qian et al., 1994).
2.2.5 Molecular weight determination
The purified sample was characterized to determine molecular weight of purified alpha amylase inhibitor. SDS-PAGE was done as per the following protocol (Marshall et al., 1975). Initially, gels were prepared using acrylamide and bisacrylamide following it 1.875 M Tris- HCl (pH 8.8) separating gel and 0.6 M Tris- HCl (pH 6.8) were used. Then, the gels were cast. The sample was mixed with sample buffer (1 M Tris HCl (pH = 6.8), SDS, beta-mercaptoethanol, glycerol and 1 %) before loading. The sample was loaded along with ladder. The gel was run at 120–140 V for about 1.5––2 h in electrophoretic buffer containing. Then, the gels were stained for 45–50 mins in a solution containing Coomassie brilliant blue following it, the gel was kept in destaining solution (glacial acetic acid and methanol) for overnight (Moreno et al., 1990).
2.2.6 GC–MS analysis of analogous alpha amylase inhibitor compounds
In this case, the 25gm of seed sample was homogenised properly in 150 ml of methanol and it was kept for 5 days. Then, the solution was filtered using Whatman filter paper. After filtration, the sample was centrifuged at 15000 rpm for 15mins. The supernatant was collected and stored in cooling conditions (Mosca et al., 2008). Then, the GC–MS analysis was done in order to find out the chemical constituents that are present in the purified extract. The SH-Rxi-5Sil MS column (30 m, 0.25mmID, 0.25um df) was used for the analysis. During the experimentation the helium gas was used as a carrier gas and 1 ml/min of the flow rate was maintained. The temperature of 280 °C was set for the injection transfer and for the ion source whereas the ionization energy was 70 electron volts. At last, the data was collected (Mubarak et al., 2005). (See Table 1).
Peak#
Name
R.Time
Area
Area%
Height
Height%
1
Hexadecanoic acid, methyl ester
29.987
3,282,061
3.92
1,487,003
5.46
2
9,12-Octadecadienoic acid (Z, Z)-, methyl ester
32.681
3,576,152
4.27
1,614,437
5.93
3
9,12,15-Octadecatrienoic acid, methyl ester
32.776
8,357,197
9.97
3,363,820
12.35
4
Stigmasterol
47.562
10,510,315
12.54
2,813,135
10.33
5
Gamma-Sitosterol
48.467
18,968,903
22.64
4,450,414
16.34
2.2.7 Molecular docking
The molecular modeling software, Autodock-vina was used to study molecular docking. PDB or proteins 1HNY alpha-amylase was selected and extracted from the protein data bank. The structure of this molecule was downloaded in 3D conformation in the sdf format that was converted into pdb using pymol software. Then, the pdbqt file was prepared for both ligand as well as the protein molecule using autodock. The molecular docking was performed using autodock vina tool and the results were analyzed in discovery studio to see the ligand and protein interactions (Huang et al., 2010).
3 Results and Discussion
3.1 Purification and alpha amylase inhibitory activity
The Chromatography fractions were collected at a constant flow rate of 100 µl/min. The in-vitro enzymatic inhibitory activity was carried out and it has been found that the crude extract of the sample Phaseolus vulgaris shows 60.3 ± 0.49 % inhibition, whereas after ammonium sulphate precipitation it shows the maximum inhibitory activity of 86.8 ± 0.97 % and protein content was 10.5 %. After purification via sephadex G-50 column the inhibitory activity was 96.5 ± 0.84 % while protein content reduced to 7.96 %. It has been noted that the inhibitory activity was increasing after each step of the purification. Thus, the specific activity was also rising as it was 4.0 in case of crude extract but rise to 12.12 after purification.
3.2 Heat stability
In this case, the sample was incubated at different temperatures ranging from 40℃- 100℃ for 10––15 mins. After incubation, the optical density of sample was measured at 540 nm. It was observed that the sample has shown the good enzymatic activity at temperature ranging from 40℃- 70℃ but above this temperature range the alpha amylase inhibitor starts degrading and the enzymatic activity started declining. (See Fig. 1)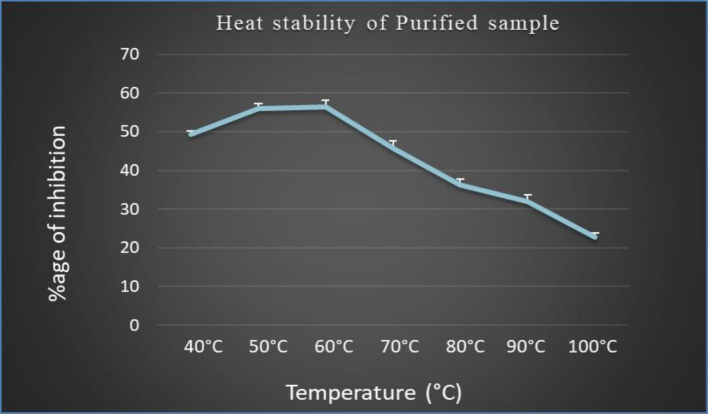
The graph represents the effect of temperature on the in-vitro alpha amylase inhibitory activity of the purified sample.
3.3 Characterization of purified alpha amylase inhibitor
3.3.1 Molecular weight determination
The purified fraction after size exclusion chromatography was loaded in SDS-PAGE gel in order to determine the molecular weight. Both Native-Page as well as SDS-PAGE characterization has been done. The Fig. 2, represents the Native- Page of the purified sample (Pant Anupama) a single band has been observed corresponding to the molecular weight of 54KDa. While, the Fig. 3 shows the bands as two subunits of 22KDa and 28KDa this might be due to the denaturation of the pure amylase inhibitor into two subunits.
Native-Page of the purified sample was carried out, the image shows the protein ladder and the bands of the sample at 54KDa.
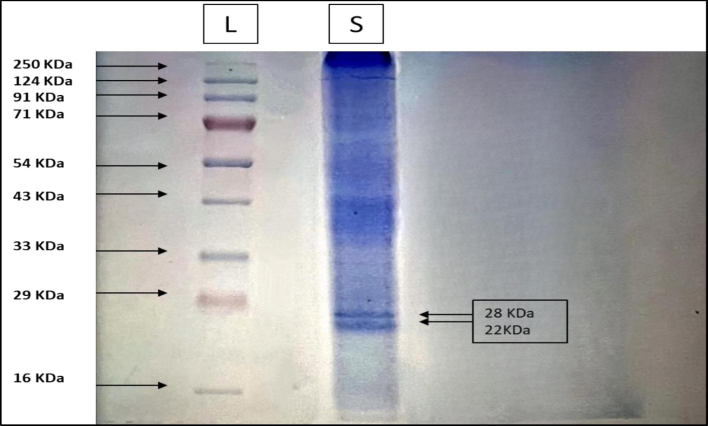
SDS-Page of the purified sample was carried out, the image shows the protein ladder and the bands of the sample showing two subunits of 28 KDa and 22 KDa.
3.3.2 GC–MS analysis and molecular docking
The purified methanol extract of the Phaseolus vulgaris has shown twenty-five peaks from time ranging between twenty-nine to forty-nine minutes. The identities of these compounds in terms of the retention time, area and height percentage have been studied and it has been observed that five compounds have covered maximum area and height in terms of the percentage (Fig. 4). They are namely, hexadecanoic acid-methyl ester which has 3.92 % and 5.46 % of area and height respectively. After this, the 9,12, Octadecanoic acid (Z, Z)-methyl ester has covered 4.27 % of area and 5.93 % of height. The maximum peak height and area is covered by gamma sitosterol i.e., 22.64 % of area and 16.34 % of height. Whereas, the second highest in number was stigmasterol with area and height percentage of 12.54 and 10.33. In case of the 9,12,15, Octadecanoic acid, methyl ester the third highest percentage of the area and height has been covered i.e. area 9.97 % and height 12.35 %.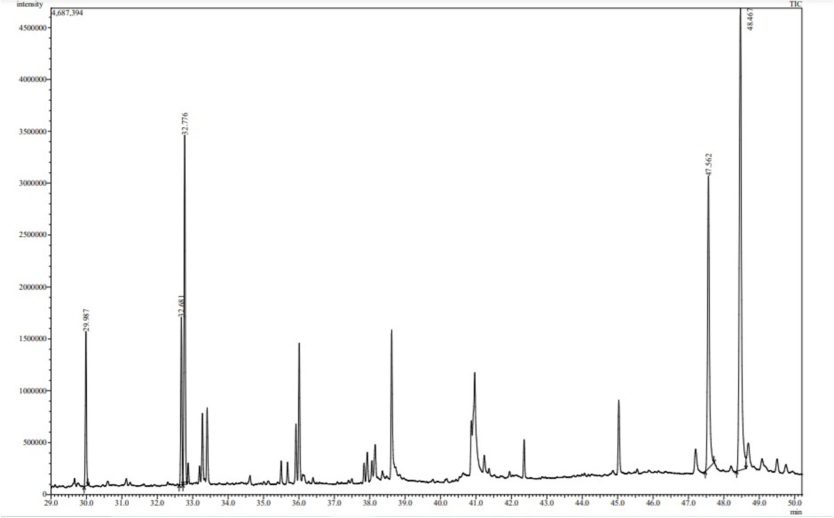
Chromatogram of methanolic extract of Phaseolus vulgaris along with the table below describing each peak name, area and height.
In addition to the study height as well as area percentages of the peaks it has also been studied that these 5 constituents have some specific characteristics in terms of the pharmacological properties. According to already present reports (Mukherjee et al., 2006), the octadecanoic acid exhibits anti-diabetic properties same as alpha-glucosidase inhibitors. Whereas, as per the research done by (Renganatha et al., 2021) the in-silico study has revealed that the hexadecenoic acid possess the properties of the potential alpha amylase inhibitor. It has been elucidated by Adewole et al. (2022) that the stigmasterol has a potential mechanism in order to act against alpha amylase and alpha glucosidase enzymes, thus it can be a great therapeutic component that can be utilized in lowering the blood glucose level. Therefore, these components can be widely used as a curative for the treatment of the diabetes mellitus. Furthermore, the molecular docking studies has been done that reveals the interactions between the ligand molecules (the constituents that were selected from the GC–MS chromatogram peaks based on the height and area) and the human pancreatic alpha amylase (1HNY). The hydrogen bonding has been observed in each of the ligand–protein interaction. Also, the affinity binding has also been calculated. The details are as follows. The Fig. 5 represents the molecular docking between the ligand Hexadecanoic acid, methyl ester and the protein namely 1HNY. It has been observed that the there were total nine conformations of the binding between the ligand and the protein but of the nine the one that has shown maximum hydrogen bonding was mode 2 which shows 2 hydrogen bonds with affinity of −6.5 kcal/mol. In case of the docking between the ligand 9,12, Octadecanoic acid (Z, Z)-methyl ester and 1HNY (Fig. 6) out of the nine conformations of the binding between the ligand and the protein the one that has shown maximum hydrogen bonding was mode 2 which shows 3 hydrogen bonds with affinity of −7.4 kcal/mol. Likewise, in case of the 9,12,15, Octadecanoic acid, methyl ester and 1HNY binding (Fig. 7) the total hydrogen bonds noticed was 1 with affinity value −6.0 kcal/mol. The total 2 hydrogen bonds with affinity −8.7 kcal/mol and −9.5 kcal/mol was observed in both Stigmasterol (Fig. 8) and gamma sterol (Fig. 9) respectively. In each figure, the hydrogen bonding is represented as pink-white-green color which depicts the donor and the acceptor sites between the binding molecules.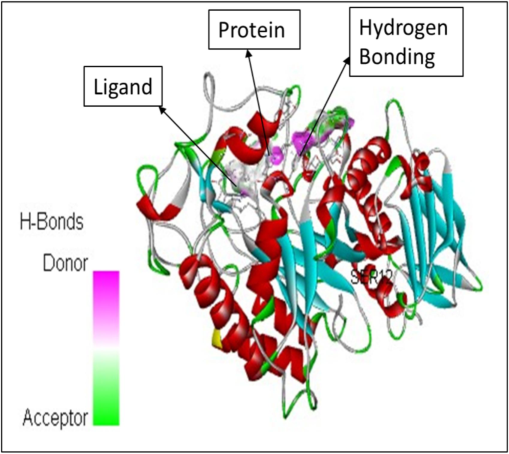
The molecular docking between the ligand Hexadecanoic acid, methyl ester and the protein namely 1HNY.
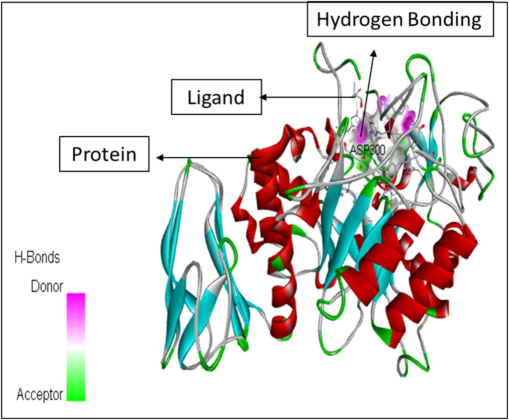
The figure represents the molecular docking between the ligand 9,12,Octadecanoic acid (Z,Z)-methyl ester and 1HNY.
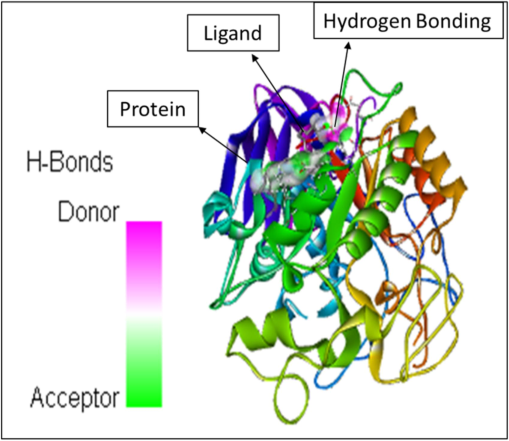
The molecular docking between 9,12,15, Octadecanoic acid, methyl ester and 1HNY.
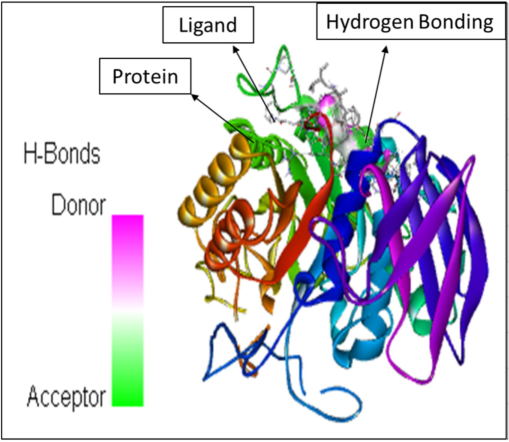
The molecular docking between the ligand stigmasterol and the protein 1HNY.
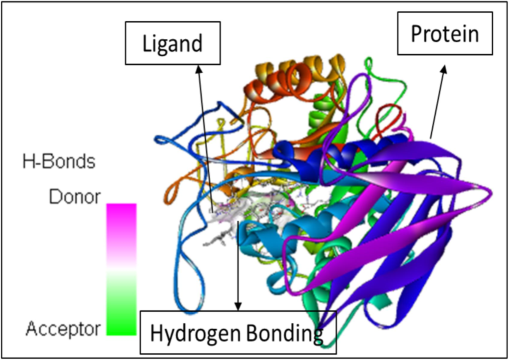
The molecular docking between the ligand gamma-sterol and the protein 1HNY.
4 Discussion
The alpha amylase hydrolyzes the starch molecule to yield low molecular weight dextrin’s and sugars. Therefore, causing in rise in blood glucose level. The inhibition action of α-amylase is carried out by α-amylase inhibitors. They catalyse the hydrolysis of α-(1,4)-D-glycosidic linkages of starch and other glucose polymer. Furthermore, they affect the process of the carbohydrate absorption in the small intestine by inhibiting the activity of the carbohydrate hydrolysing enzymes (Remah et al., 2019). Thus, they are very helpful in management of the postprandial hyperglycaemia (PPHG) in diabetic patients. They also play a significant role in inhibition of the activity of salivary and pancreatic amylase in vitro and in vivo (Narkhede et al., 2011).
Currently, the research is focused on the fabrication of anti-diabetic drug that has a great potential in lowering blood glucose level with least or no side effects. In order to attain the aim, in this study the alpha amylase inhibitor and its analogues were isolated and purified from the sample of the kidney beans (Phaseolus vulgaris). The sample was collected and stored in air tight container. Then, the in-vitro analysis of the sample was done against porcine pancreatic amylase and around 60.3 ± 0.49 % inhibitory activity of crude extract was observed. It has been previously reported by Marshall and Lauda (1975) that the percentage of inhibition tends to increase after purification as compare to crude extract. A similar trend was noticed in this study that after purification via ammonium sulphate and sephadex G-50 column the percentage of inhibition reached to 96.5 ± 0.84 %. The results were compared with already published data by (Tamil et al., 2010) that the Phyllanthus amarus has shown 75.32 % alpha amylase inhibitory activity that is quite less than the inhibition percentage obtained in current study.
Similar study has been reported by (Imad et al., 2008) the in-vitro analysis of the Jordanian flora samples the study reveals that out of 35 species only 4 were able to show inhibitory activity but even their percentage of inhibition was low in comparison to one reported in the present study. The characterization and molecular docking has been done in addition to this the components revealed after the GC–MS analysis possess great hydrogen bonding.
The characterization of the purified extract was done and it was found that a single band of 54KDa was observed in case of Native- Page while two subunits of 28 and 22 KDa were noticed in case of SDS-Page that might be due to denaturation of the pure alpha amylase inhibitor into two subunits. The obtained results were compared with the already published data that shows the molecular weight of the alpha amylase inhibitor was 56KDa (Singh et al., 2021). Further, the GC–MS analysis has been carried out and it has been observed that the components like hexadecenoic acid and octadecanoic acid have previously been discovered to exhibit a variety of pharmacological properties (Remah et al., 2019). The hexadecenoic acid act as an alpha amylase inhibitor whereas octadecanoic acid exhibits properties same as of alpha-glucosidase inhibitor. Also, the docking studies reveals that there is presence of hydrogen bonding between the protein and the ligand molecules. This shows that the inhibitor molecules attack at the binding site of the alpha amylase in order to slow down its process of starch hydrolysis (Ghosh et al., 2014). Therefore, it can be concluded that the alpha amylase inhibitor isolated from the sample has a putative potential of being as alternative herbal medicine against human pancreatic amylase to prevent the spiking of postprandial blood glucose levels in the body.
5 Conclusion
To conclude with, this study provided the insight knowledge about the treatment of the post prandial hyperglycemia with the purified alpha amylase inhibitor isolated from the kidney beans. The methodology implemented for this study has proven to be very effective against the in-vitro alpha amylase inhibitory activity as well as in addressing the future challenges for the treatment of the diabetes mellitus. The results obtained from the study shows that there is interaction of protein and ligand molecules through hydrogen bonding which culminate that the purified extract can serve as a great potential for the treatment of the diabetes mellitus. The future research is focused on the effect of the purified extract on the in-vivo effect on the rat model. This study would give a great implication on the development of the novel anti-diabetic drug that would serve a great medication against post prandial hyperglycemia.
6 Compliance with ethical standards
There is no ethical issue concerning this article.
CRediT authorship contribution statement
Lovepreet Kaur: Project administration, Data curation, Conceptualization. Rattandeep Singh: Writing – review & editing. Ashish Suttee: Software, Resources. Mohammad Raish: Visualization, Validation, Methodology.
Funding
The work was supported by School of Bioengineering and Biosciences, Lovely Professional University, Phagwara, Punjab. The authors are grateful to the Researchers Supporting Project Number (RSPD2024R957) at King Saud University, Riyadh, Saudi Arabia.
Acknowledgement
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSPD2024R957), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- GC-MS compound Identification in Phaseolus vulgaris- A Low-Cost Cataract Prevention Food. Food Sci. Technol.. 2022;10(3):112-119.
- [Google Scholar]
- Gamma radiation-induced mutation in black grain (Vigna mungo (L.) Hepper) Asian J. Plant Sci. Res.. 2011;1:96-100.
- [Google Scholar]
- Subsite mapping of the human pancreatic alpha-amylase active site through structural, kinetic and mutagenesis techniques. Biochemistry.. 2000;39(18):4778-4791.
- [Google Scholar]
- Toxicity Studies of Blockal, a Dietary Supplement Containing Phase 2 Starch Neutralizer (Phase 2), a Standardized Extract of the Common White Kidney Bean (Phaseolus vulgaris) Int. J. Toxicol.. 2006;25(5):361-371.
- [Google Scholar]
- Demir, Y., Durmaz, L., Taslimi, P., 2019. Antidiabetic, G. İ. Properties of Dietary Phenolic Compounds:Inhibition Effects on Alpha-Amylase, Aldose Reductase, and Alpha- Glycosidase. Biotechnol. Appl. Biochem, 66(5), 781–786.
- Molecular Docking and Inhibition Studies of α-Amylase Activity by Labdane Diterpenes from Alpinia Nigra Seeds. Med. Chem. Res.. 2014;23(11):4836-4852.
- [Google Scholar]
- Screening of Jordanian Flora for α-Amylase Inhibitory Activity. Pharm. Biol.. 2008;46(10–11):746-750.
- [Google Scholar]
- Advances and challenges in protein ligand docking. Intl J Mol Sci.. 2010;11:3016-3034.
- [Google Scholar]
- Occurrence and purification of alpha-amylase isoinhibitor in bean (Phaseolus vulgaris L.) varieties. J. Agric. Food Chem.. 1991;39:2131-2136.
- [Google Scholar]
- Growth inhibitory effects of an α-amylase inhibitor from the kidney bean, Phaseolus vulgaris (L.) on three species of bruchids (Coleoptera: Bruchidae) Appl. Ent. Zool.. 1989;24:281-284.
- [Google Scholar]
- Purification and properties of Phaseolamin, an inhibitor of α-amylase, from the kidney bean, Phaseolus vulgaris. J. Biol. Chem.. 1975;250:8030.
- [Google Scholar]
- Characterization of alpha-amylase inhibitor, a lectin- like protein in the seeds of Phaseolus vulgaris. Plant Physiol. 1990;92:703-709.
- [Google Scholar]
- Determination of Alpha-Amylase Inhibitor Activity of Phaseolamin from Kidney Bean (Phaseolus Vulgaris) in Dietary Supplements by HPAEC-PAD. Anal. Chim. Acta.. 2008;617:192-195.
- [Google Scholar]
- Nutritional composition and antinutritional factors of mung bean seeds (Phaseolus aureus) as affected by some home traditional processes. Food Chem.. 2005;89:489-495.
- [Google Scholar]
- Leads from Indian medicinal plants with hypoglycemic potentials. J Ethnopharmacol.. 2006;106(1):1-28.
- [Google Scholar]
- In vitro anti-diabetic activity of Caesalpina digyna (R.) methanol root extract. Asian Journal of Plant Science and Research.. 2011;1(2):101-106.
- [Google Scholar]
- The active center of mammalian a- amylase. Structure of the complex of a pancreatic aamylase with a carbohydrate inhibitor refined to 2.2-A˚ resolution. Biochemistry. 1994;24:6284-6294.
- [Google Scholar]
- Renganathan S, Manokaran S, Vasanthakumar P, Singaravelu U, Kim PS, Kutzner A, Heese K, 2021. Phytochemical Profiling in Conjunction with In Vitro and In Silico Studies to Identify Human α-Amylase Inhibitors in Leucaena leucocephala (Lam.) De Wit for the Treatment of Diabetes Mellitus. ACS Omega., Jul 15;6(29):19045-19057.
- Singh, Ravinder & Dobriyal, Anoop & Singh, Rattan & De Los Ríos-Escalante, Patricio, 2021. Evaluation of Inhibitory Activity, Purification and X-Ray Crystallography of Alpha—Amylase Inhibitor from Phaseolus Vulgaris Cultivars of Uttarakhand. 10.21203/rs.3.rs-154559/v1.
- Remah Sobhy, Mohamed Eid, Fuchao Zhan, Hongshan Liang, Bin Li a.,2019. Toward understanding the in vitro anti-amylolytic effects of three structurally different phytosterols in an aqueous medium using multispectral and molecular docking studies, Journal of Molecular lipids, 283, 225-234.
- In vitro study on α-amylase inhibitory activity of an Indian medicinal plant, Phyllanthus Amarus. Indian J Pharmacol.. 2010 Oct;42(5):280-282. PMID: 21206618; PMCID: PMC2959209
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103521.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







