Translate this page into:
Purification and characterization of pectinase from gut-associated Klebsiella oxytoca af-G4 of dwarf honey bee, Apis florea
⁎Corresponding author. sneharaniah@gmail.com (A.H. Sneharani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Pectinases are enzymes that hydrolyze pectin or pectic compounds. Microorganisms in the gut of bees are involved in the breakdown of these macromolecules. In this study, we aimed to optimize the physicochemical parameters for enhancing enzyme pectinase production by bacteria Klebsiella oxytoca af-G4 isolated from Apis florea gut; in addition, purification and characterization of purified enzyme were carried out.
Methods
Optimization of growth conditions for pectinase production using different synthetic and agro-industrial wastes by Klebsiella oxytoca af-G4 were performed. Purification and characterization of the pectinase were carried out. Application of the pectinase in juice extraction was evaluated.
Results
Pectinase activity was maximum at 30 °C at pH 5.0, in media supplemented with lactose (1 % w/v) and ammonium sulfate (1 % w/v) as carbon and nitrogen sources, respectively, with a growth period of 72 h. The pectinase production with agro-industrial wastes showed higher production with pomegranate peel (1 % w/v) as substrate at a growth condition of 40 °C, pH 6.0, and a growth period of 92 h. The enzyme was purified to homogeneity (4.8-fold) using DEAE cellulose ion-exchange chromatography. The purified enzyme showed the molecular weight of ∼ 60 kDa on SDS-PAGE with an activity of 23.26 U/ml and specific activity of 14.36 U/mg. The pectinolytic activity was confirmed on the zymogram. The purified enzyme was more active at an optimum pH of 6.0, and stable at a pH range between 5 and 8. The enzyme was stable at temperatures ranging from 30 °C to 80 °C, with 60 °C being the optimum. The enzyme showed Km and Vmax values of 6.04 mg/ml and 4.92 U/ml, respectively. The enzyme showed maximum activity with the addition of metal ion Mn2+ at pH 6 and 60 °C. The enzyme enhanced the extraction of apple juice and clarification significantly.
Conclusion
The enzyme characteristics and kinetics as well as the higher production with agro-waste support its pectin-based industrial application. The enzyme properties prove that it is a good candidate for the hydrolysis of pectin and juice clarification for industrial applications.
Keywords
Pectinase
Polysaccharides
Chromatography
Agro-industrial wastes
Juice clarification
Gut microbiome
1 Introduction
Pectinases are a class of enzymes with a wide range of uses, including plant fibre processing, treating pectin effluent, pulping of paper, extraction and clarification of fruit juice. Given the growing demand for this enzyme, it is essential in the industrial sector to characterize organisms that can secrete the enzyme on a large scale along with the capacity to stay stable at a wide temperature and pH range.
The honey bee natural diet mainly consists of plant nectar along with pollen. The formation of distinct layers of the pollen wall is aided by pectin, a polysaccharide. Pollen digestion in the honey bee gut is achieved through bacteria-producing pectin digestive enzymes (Klungness and Peng, 1984). Engel et al. (2012) identified the genes that code for pectin-degrading enzymes in honey bee gut bacteria. Pectin has also been found to be poisonous to honey bees (Barker, 1977), and the breakdown of pectin by gut microbes may help the bees avoid intoxication. On the other hand, Polygalacturonase (PG), a type of pectinase assists in hydrolysis of α-1,4 glycosidic bonds, converting them to galacturonic acid units. Polygalacturonases are useful in the food industry because they reduce viscosity, enhance fruit juice yield, and aid in exploring fibre crystalline structure (Jayani et al., 2010).
The microbiota in the gastrointestinal tract is thought to play a significant role in decomposing plant detritus by giving a battery of polysaccharide hydrolysing enzymes to the bacterial host (carbohydrases). Bacterial extracellular pectinase was found to be significant compared to plants, animals, and other microbial pectinases. Comparatively, 10–25 % of global enzyme production is of microbial pectinases and their market is growing by the day (Jayani et al., 2005).
Enzymatic breakdown of these polymers has recently gained a lot of interest because it is a more promising alternative to mechanical and chemical approaches. The synthesis of polysaccharide-degrading enzymes is generally performed with fungal and bacterial strains since these microorganisms secrete a diverse range of enzymes responsible for cell wall degradation (Juturu and Wu, 2014). As a result, it is obvious that the growing demand for progressive biotechnological strategies employing microorganisms and enzymes such as cellulases and pectinases to replace old physical and chemical procedures (Bajpai, 1999).
However, developing economically cost-effective enzymes for polysaccharides breakdown is challenging. Many microorganisms, including bacteria and fungi, produce enzymes like cellulases and pectinases, which break down cellulose and pectin, respectively. As a result, isolating and characterizing the bacteria that produce such industrially significant enzymes is important, as they have unique advantages over other microorganisms. Furthermore, the production of enzymes under various physicochemical conditions will be helpful for future industrial claim.
There are only limited number of reports on bacterial pectinases production (Jayani et al., 2005). In this study, we aimed to optimize the physicochemical parameters for enhancing enzyme pectinase production by bacteria K. oxytoca af-G4 isolated from A. florea gut; in addition, purification and characterization of the enzyme were carried out.
2 Materials and Methods
2.1 Chemicals
The solvents and chemicals utilized in the present study were of high analytical grade procured either from Sigma Aldrich, USA, or HIMEDIA, Mumbai, India. The bacteria K. oxytoca af-G4 (Accession no.: MN512297) was isolated from the honey bee, Apis florea, and identified at NCMR-NCCS, Pune, India (Ganeshprasad et al., 2022).
2.1.1 Pectinase production media
The production of pectinase in basic mineral salt medium was determined using liquid state or submerged fermentation process (0.6 % Na2HPO4, 0.3 % KH2PO4, 0.2 % NH4Cl, 0.5 % NaCl, 0.01 % MgSO4·7H2O, 1.5 % agar) supplemented with 1 % pectin.
2.2 Quantitative determination of pectinase enzyme activity
Pectinase activity was determined by measuring the release of reducing sugars from pectin according to the method described by Soares et al., (1999) with slight modification, using 3,5-dinitrosalicylic acid (DNS) reagent.
2.2.1 Pectinase activity in selected cultures
The pure culture of K. oxytoca af-G4 were grown overnight in nutrient broth and the cell-free supernatant (CFS) was collected by centrifugation at 10,000 rpm at 4 °C for 10 min. The CFS was used as crude enzyme source. The crude enzyme (200 µl) was mixed with 1 % pectin (800 µl) solution in 0.2 M citrate phosphate buffer of pH 6.0 and were incubated at 37 °C for 15 min. The reaction was terminated by adding 1 ml DNS and then boiled for 5 min. The absorbance was determined using a spectrophotometer (Model: UV-1900i; Shimadzu, Japan) at 550 nm. The activity of pectinase was calculated by released galactose in comparison with the galactose standard graph. One unit of pectinase activity is defined as the amount of pectinase necessary to catalyse the generation of reducing sugar equal to 1 μmol of galactose per minute under assay conditions (Hussain et al., 2019).
2.3 Optimization of pectinase production
2.3.1 Carbon and nitrogen sources
Various carbon sources (1 % w/v) such as sucrose, maltose, mannose, lactose, and starch; nitrogen sources (1 % w/v) such as peptone, tryptone, yeast extract, sodium nitrite and ammonium sulfate were supplemented in the enzyme production medium to investigate their influence on enzyme production.
2.3.2 Incubation temperature and media pH
The production media for pectinase was prepared with different pH (acidic, neutral and basic pH) using respective buffers along with carbon (lactose) and nitrogen (ammonium sulfate) sources. Similarly, the effect of temperature on pectinase production was carried out by incubating the culture at various temperatures from 30 °C to 50 °C.
2.3.3 Incubation period
Nutrient broth (pH 5) supplemented with filter-sterilized lactose (1 %) and ammonium sulfate (1 %) was inoculated with K. oxytoca af-G4 culture (1 %) and incubated at 30 °C. At regular intervals of time (24, 48 and 72 h), an aliquot was drawn and checked for bacterial growth and pectinase activity.
2.4 Effect of different agricultural wastes in pectinase production
2.4.1 Production of pectinase using agro-industrial waste
Various agro-industrial wastes (1 % w/v) were studied to analyze the effect on the pectinase production medium. The agro-industrial wastes used were peels of pomegranate, orange, chikku and lemon. All of the selected agro-industrial wastes were dried and finely ground in an electric grinder. The powder was then sieved to obtain uniformly sized substrate particles.
2.4.2 Pectinase production
The nutrient broth was adjusted to pH 5 using 1 M HCl and supplemented with selected agro-waste powder at a concentration of 1 % separately. After sterilization, the broth was inoculated (1 %) with freshly grown culture (K. oxytoca af-G4) and then incubated for 72 h at 30 °C. After incubation, CFS was obtained by centrifugation (10,000 rpm, 10 min) and analyzed for pectinase activity as described earlier.
2.5 Optimization of growth conditions for pectinase using agro-industrial waste
2.5.1 Concentrations of pomegranate peel as substrate
The effect of various concentrations of substrate (pomegranate peel) was checked. The nutrient broth was supplemented with carbon source (pomegranate peel) at a final concentration of 0.5, 1.0, 1.5, and 2.0 % and studied for microbial growth, enzyme production and analyzed for pectinase activity as described earlier.
2.5.2 Temperature and pH
The pectinase production was measured in media with different pH. Similarly, the effect of temperature on pectinase production was carried out at various temperatures ranging from 30 °C to 50 °C.
2.5.3 Incubation time
Nutrient broth adjusted to pH 6 was supplemented with 1 % pomegranate peel and inoculated with bacterial culture (1 %) and was incubated at 45 °C. At regular intervals of time (24, 48, 72, 96, 120 h), an aliquot was drawn and measured for bacterial growth and pectinase activity.
2.6 Purification of pectinase from K. oxytoca af-G4
2.6.1 Ammonium sulfate precipitation
The bacterial culture was grown in 1L of optimized synthetic media under specified conditions (synthetic; lactose, ammonium sulfate, pH 5, 30 °C, 72 h) and then centrifuged at 10, 000 rpm for 15 min at 4 °C to collect the CFS. The protein content in the CFS was precipitated at 75 % saturation. The overall methodology was carried out at 4 °C, under aseptic conditions. The precipitate was recovered by centrifugation at 10,000 rpm for 15 min. The precipitate collected was dissolved in 5 ml of sodium phosphate buffer (0.2 M; pH 7) and dialyzed using 10 kDa cut-off dialysis membrane against sodium phosphate buffer for 24 h with 3–4 changes of buffer. The dialyzed samples were checked for total protein content and pectinase activity as described earlier.
2.6.2 Anion exchange chromatography
The dialyzed sample (50 mg protein) was injected onto a DEAE cellulose column (18 X 3 cm) that was already pre-equilibrated with 0.2 M sodium phosphate buffer (pH 7). The column was washed to remove unbound proteins using 0.2 M sodium phosphate buffer, pH 7, containing no salt. Protein fractions were eluted in a linear gradient of 0.0–1 M NaCl in the same buffer at 1 ml per min flow rate. The crude, ammonium sulfate precipitate and column fraction were measured for total protein content and pectinase activity as described earlier and were subjected to SDS-PAGE (Laemmli, 1970). The gel was treated with Coomassie brilliant blue (CBB) R-250 after electrophoresis, and then destained until distinct bands were seen. The gel picture was documented using GELDOC system (BIO-RAD, India) and the column fraction was also checked for activity by zymogram.
2.6.3 Zymogram
Zymogram for pectinase activity was carried out under native conditions. The resolving gel (10 %) was prepared according to Laemmli (1970) by copolymerization with pectin. Briefly, PAGE was prepared by copolymerizing with 0.2 % pectin by replacing it with an equal volume of distilled water. The sample was loaded onto the gel and performed electrophoresis under non-denaturing and non-reducing conditions.
After electrophoresis, the gel was washed with buffer (2.5 % triton X-100) for 1 h to restore the enzyme activity and later washed 3–4 times to remove triton X-100, followed by incubating the gel in reaction buffer (0.3 M Tris-HCl; 0.2 M NaCl; 0.1 M CaCl2) for 6–8 h. After incubation, the gel was stained with iodine solution for 15 min and then washed repeatedly with water until a clear zone was observed.
2.7 Characterization of pectinase
2.7.1 Temperature and pH
The optimum temperature was detected by incubating the enzyme (4.65 U/ 200 µl) at different temperatures (30, 40, 50, 60, 70, 80 and 90 °C) and analyzing the activity. Similarly, the optimum pH for the enzyme activity was determined by incubating the enzyme at various pH buffers (4, 5, 6, 7, 8 and 9) at 30 °C for 30 min and the activity was recorded as described earlier. Various buffers used in the study include 0.1 M Citrate phosphate buffer: pH 4–7; 0.1 M sodium phosphate buffer: pH 8–9.
2.7.2 Thermostability and pH stability
To assess the thermal stability, the enzyme (4.65 U/ 200 µl) was treated at different temperatures (30–90 °C) for 30 min. The reaction carried out at 30 °C was considered as control. The effect of pH on stability was assessed by mixing 1:1 ratio of enzyme solution in respective buffer (pH 4–9). The mixture without substrate was incubated for 30 min at 30 °C. The reaction mixture with enzyme and pH 6 buffer (1:1) was considered as control.
2.7.3 Metal ions
The impact of metal ions on enzyme activity was analyzed by direct incorporation of metal ions in the reaction mixture to a final concentration of 5 mM. The metal ions examined include CaCl2, NH4Cl, MnCl2, KCl and NaCl.
2.7.4 Km and Vmax value
Km and Vmax of the enzyme were calculated by evaluating the enzyme activity at different substrate (Pectin) concentrations, i.e. 2–8 mg/ml. The activity was plotted with the reciprocal of the enzyme activity (1/V) on Y-axis and the reciprocal of the substrate concentration (1/[S]) on X-axis to define the Km and Vmax values according to the Lineweaver Burk plot.
2.7.5 UV absorption spectra
The absorption spectra of the purified pectinase enzyme (23.26 U) and substrate (1.0 %, w/v) were recorded between 190 and 450 nm using a spectrophotometer (Model: UV-1900i; Shimadzu, Japan).
2.8 Application in juice extraction
For extraction of fruit juice, an apple was washed, sliced into small pieces, and weighed. To obtain juice, the apple pieces (3.2 g) were mashed in a mortar and pestle in the presence of enzyme (11.63 U). After washing the mortar and pestle with 1.5 ml distilled water, the apple paste was placed into a 15 ml centrifuge tube. After 1 h of incubation at 50 °C, the mixture was centrifuged for 20 min at 5000 rpm. The amount of clear supernatant was quantified and kept in a separate tube. The percentage of recovered juice as well as the clarity of the recovered apple juice were measured (Shrestha et al., 2021). The clarity of the apple juice was assessed by measuring absorbance at 660 nm with a UV visible spectrophotometer. The juice recovery percentage was calculated by using an equation,
2.9 Statistical analysis
All the experiments were performed in triplicates, and the results were statistically analyzed using one-way ANOVA, and mean separation was accomplished by Duncan’s multiple range test. All analyses were performed using GraphPad Prism 9.0 software (San Diego, CA).
3 Results and discussion
Earlier studies primarily describe the gut microbial diversity in the honey bees (Kwong and Moran, 2016), however, there are limited data on polysaccharide degrading bacteria, especially no reports from the gut of A. florea available. The present isolate, Klebsiella oxytoca af-G4 was observed to produce carbohydrases, in addition to pectinase that enabled the degradation of starch and other polysaccharides (Ganeshprasad et al., 2021).
3.1 Pectinase production by Klebsiella oxytoca af-G4
3.1.1 Optimization of pectinase production using synthetic sources
Various carbon and nitrogen sources were used to optimize the growth condition of the pectinase production medium. The most efficient sources of the various carbon and nitrogen sources examined for pectinase production were lactose and ammonium sulphate (Fig. 1a, b). Further, the pectinase production was checked at different pH and temperature levels by keeping the optimized supplementation of carbon and nitrogen sources to determine the optimum pH and temperature (Fig. 1c, d). The maximum pectinase activity was measured at 30 °C and pH 5 with the activity of 5.25 U/ml and 6.11 U/ml; specific activity of 2.93 U/mg and 3.10 U/mg. The pectinase production medium incubated for the period of 72 h was best with 3.23 U/mg pectinase specific activity.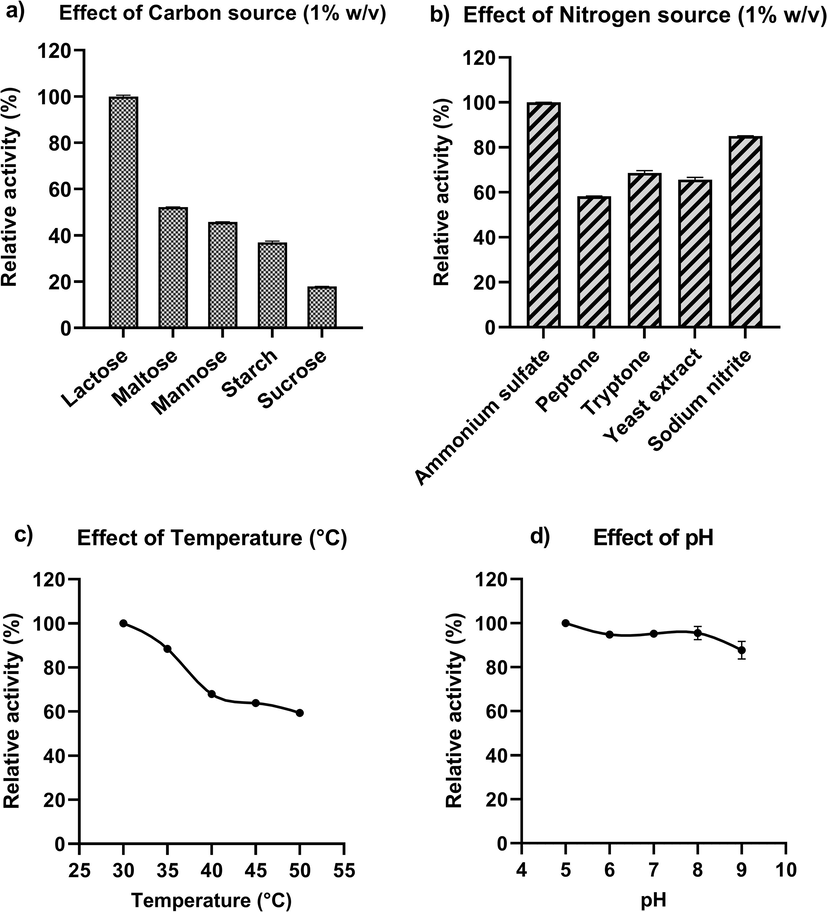
Optimization of the growth conditions for maximum pectinolytic activity from Klebsiella oxytoca af-G4 by using synthetic sources. a) Influence of various carbon sources on pectinase production; b) Influence of various nitrogen sources on pectinase production; c) Influence of temperature; and d) Influence of pH on pectinase production.
Prakash et al., 2014 reported lactose and glucose as the best carbon sources in pectinase production and Jayani et al., 2010 observed the maximum production of pectinase with citrus pectin by Bacillus sphaericus. Literature support maximal pectinase production in the presence of pure pectin followed by wheat bran by thermophilic Aspergillus fumigatus (Phutela et al., 2005). In general, enhanced pectinase production is studied in relation to the different sugars and complex agro-wastes at 1 % (w/v). In addition, nitrogen source also has a key influence on pectinase production and nitrogen limitation significantly reduced the pectinase production.
In the present study, cultivation of K. oxytoca af-G4 in a liquid medium containing 1 % lactose and 1 % ammonium sulfate as carbon and nitrogen sources resulted in maximum enzyme production after 72 h of incubation and the production time observed is much less than reported earlier. Oyeleke et al., (2012) have reported higher pectinase production by Aspergillus niger after 96 h. In another study, the pectinase was best produced after 120 h of incubation by Aspergillus flavus (Gewali et al., 2007), prolonged incubation had reduced the enzyme production, which could be due to the formation of waste products with insufficient nutrient sources, limiting microbial growth. In a similar study, after 72 h of incubation, the maximal pectinase production from Bacillus subtilis ADI1 and Chryseobacterium indologenes was detected (Nawawi et al., 2017; Roy et al., 2018), which well agreed with our findings. Bacillus subtilis isolated from soil has been reported to produce the enzyme after the incubation period of 48 h (Datta Tripathi et al., 2014). The enzyme production is dependent on both microbial sources and medium composition.
3.1.2 Optimization of growth conditions for pectinase production using agro-industrial wastes.
Various agro-industrial wastes were used to optimize the growth condition of the pectinase production medium. Among them, pomegranate peel was the best source with the highest pectinase specific activity of 2.22 U/mg (Fig. 2a). Again the growth was measured at different concentrations of pomegranate substrate. The pectinase activity was found to be 2.30 units/mg at 1 % of substrate (Fig. 2b). Further, the enzyme production was measured at different pH and temperature levels by keeping the optimized concentration of the substrate. The maximum pectinase production was detected at 40 °C and pH 6 with the specific activity of 2.19 U/mg and 2.72 U/mg (Fig. 2c & d). The pectinase production medium incubated for the period of 96 h was best with 3.47 U/mg pectinase specific activity.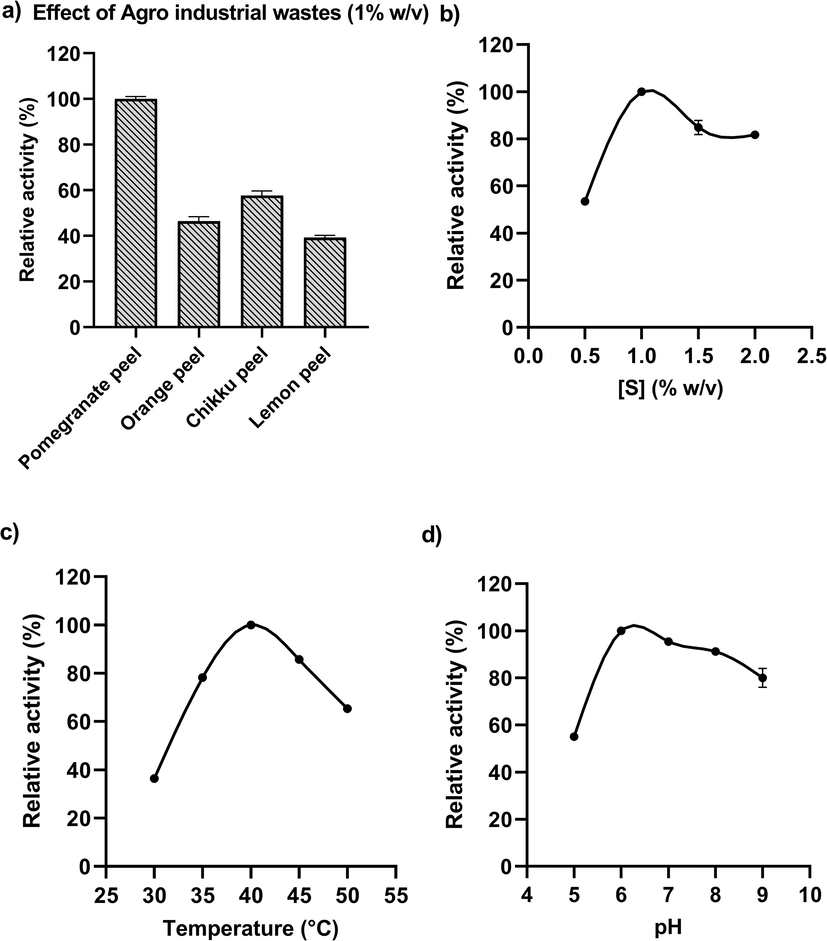
Optimization of the growth conditions for maximum pectinolytic activity from Klebsiella oxytoca af-G4 by using agro industrial wastes. a) Effect of various agro-industrial wastes on pectinase production; b) Effect of different concentrations of substrate (pomegranate); c) Effect of temperature; and d) Effect of pH on pectinase production medium.
3.2 Purification and molecular weight determination
The total protein content in the cell-free supernatant or crude sample was 1.98 mg/ml showing the specific activity of 2.96 U/mg. The crude enzyme sample was subjected to ammonium sulfate precipitation at a saturation level of 75 %. The precipitated protein was dialyzed using a 10 kDa cut-off dialysis membrane. The total protein content after dialysis was 5.27 mg/ml with 1.5 fold of enzyme purification. As the next purification step, dialyzed protein (50 mg) was passed through DEAE cellulose column. The bound protein finally eluted was 4.8 folds purified with a specific activity of 14.36 U/mg (Table 1). The protein purity was ascertained by SDS–PAGE under non-reducing conditions and the molecular weight of the desired protein was identified by comparing it with the reference medium-range protein marker (M.W. 97.4 to 14.3 kDa; Fig. 3a). The molecular weight of the pectinase was found to be ∼ 60 kDa. To visualize the pectinolytic activity, zymogram was carried out by copolymerized pectin as substrate in native PAGE. Pectinase hydrolyses the pectin copolymerized in the gel and a clear zone is observed against a dark background (Fig. 3b) after staining with iodine, confirming the presence of pectinase.
Sample
Total protein (mg)
Total activity (U) a
Specific Activity (U/mg)
Fold Purified
% Yield
Crude extract (1000 ml)
1980
5890
2.96
1.0
100
Dialysate (25 ml)
131.75
570.375
4.33
1.5
9.7
Ion exchange fraction (8 ml)
12.96
186.08
14.36
4.8
3.2
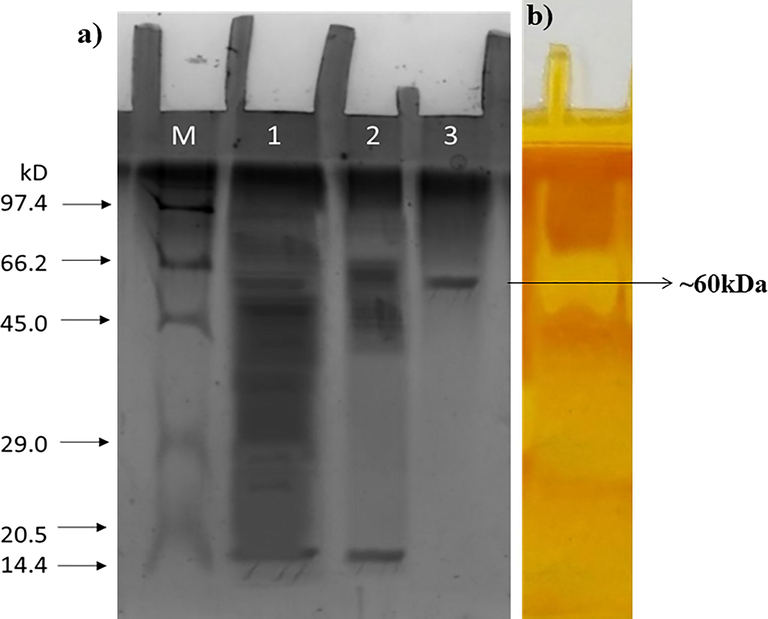
(a) SDS-PAGE analysis of lane (M) protein marker (M.W. 97.4 to 14.3 kDa; (1) crude; (2) ammonium sulfate precipitate and dialyzed; (3) ion-exchange fraction. (b) Zymogram of fraction after iodine staining.
The protein band corresponding to ∼ 60 kDa indicated that it is a novel enzyme from K. oxytoca af-G4 and it was comparable to pectinase from Mucor sp. and Fusarium oxysporium f. sp. melonis (58 kDa) (Martlnez et al., 1991). However, which is much higher than that of pectinase from Penicillium frequentans (20 kDa) (Borin et al., 1996) and Bacillus stearothermophilus (24 kDa) (Karbassi and Vaughn, 1980) reported in the literature.
3.3 Characterization of pectinase enzyme
The enzyme purified by anion exchange chromatography displayed optimum activity at a temperature of 60 °C and pH 6 (Fig. 4 a, c). The enzyme was stable in a pH range of 5 to 8, with being optimal pH of 6 (Fig. 4 b). According to data obtained, more acidic or more alkaline pH led to a marked decline in the stability of the enzyme. The enzyme was found stable within the temperature range of 30 °C to 80 °C and beyond 80 °C was unstable (Fig. 4 d). The pectinase from thermophilic fungus Rhizomucor pusillus showed an optimum temperature of 60 °C (Trindade et al., 2016). Thermostable enzymes show high rigidity due to complex folding and hence require a high temperature for activity (>40 °C) that promote structural flexibility for catabolism of substrate. In general, bacterial pectinases are mesophilic, with optimum activity in the temperature ranging between 50 and 60 °C. Comparatively, pectinase from Klebsiella oxytoca af-G4 in our study had a similar optimum temperature for enzyme activity, in addition, it also exhibited stable activity over a wide range of temperature (>95 % relative activity from 30 °C to 70 °C).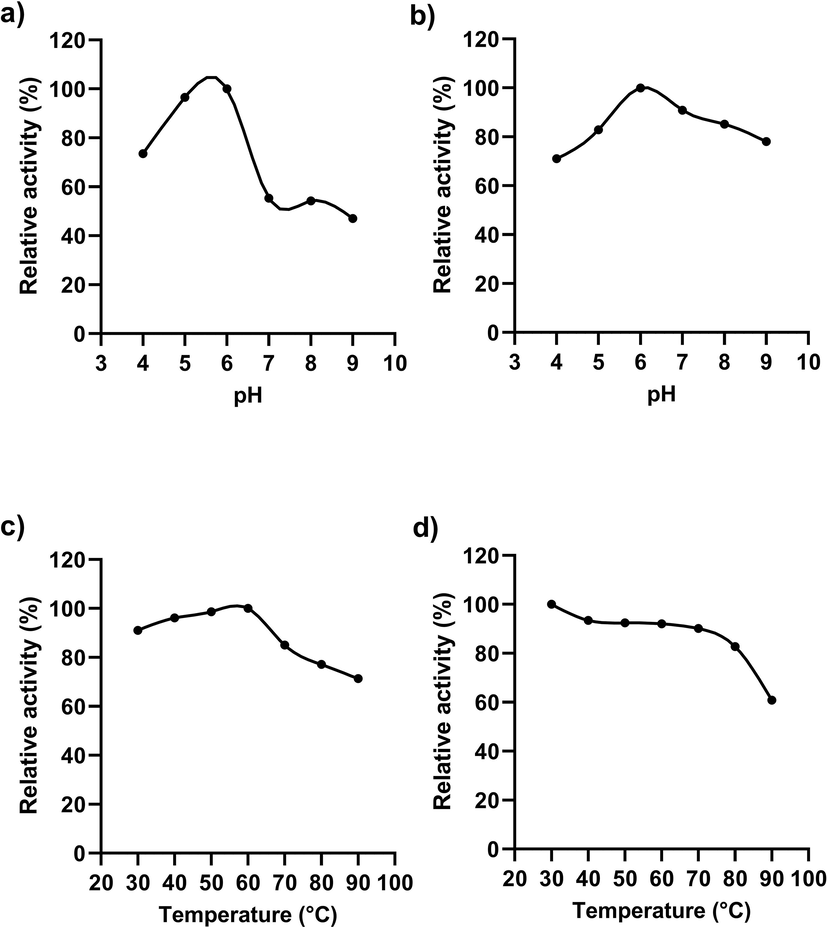
Activity of enzyme at different pH (a) and temperature (c) pH stability (b), thermostability (d).
In addition to temperature, pH also plays a significant role in enzyme stability and activity. The data from the present study shows that the pectinase is thermostable and acidic. These properties increase the applicability of enzymes in various industries employing high temperature and acidic pH in the production process. The relative enzyme activity in the present study showed that the pectinase was also active even in alkaline pH retaining its activity of >60 % at pH 9. The previous studies support the presence of alkaline pectinase in bacteria especially Bacillus species. The pectinase characterized by Bacillus stearothermophilus, Bacillus halondurans M29, Paenibacillus xylanolyticusm were found to be more active at high temperature and pH (Paudel et al., 2015). The cysteine residue present in the pectinase of Bacillus licheniformis is known to contribute to the thermostability of the enzyme (Singh et al., 2012). The formation of disulfide bond and the strong hydrophobic effect of cysteine residue contribute to thermostability. The possible presence of such residue in the pectinase isolated from K. oxytoca af-G4 may be the reason for thermostability. Temperature and pH stability are the two vital criteria of a biocatalyst for their application in several industries.
The activity of the enzyme was checked in the presence of various monovalent and divalent ions. The enzyme activity declined by 15–20 % in the presence of metal ions such as Ca2+, [NH4]+, K+ and Na+ whereas in the presence of Mn2+ supported an additional 60 % increase in the enzyme activity compared to control (Fig. 5). The presence of divalent metal ion, Mn2+ increased the enzyme activity by ∼ 1.6 fold. The addition of Mn2+ enhanced the alpha-glucosidase, acid phosphatase and phytase activities of Lactobacillus reuteri (Hayek et al., 2013); alkaline protease of Bacillus aquimaris VITP4 (Shivanand and Jayaraman, 2011). On the contrary, studies on pectinase from Penicillium chrysogenum, Bacillus sp. KSMp576 and mango peel revealed that Ca+2 ions greatly accelerated the pectinase activity. In the present isolate, pectinase activity was enhanced by more than 60 % in the presence of Mn+2. Whereas, the pectinase activity from Mucor circinelloides ITCC 6025 was inhibited by 1 mM Mn+2 ions. This implies that the metal ion requirements for pectinase activity differ based on their sources (Thakur et al., 2010).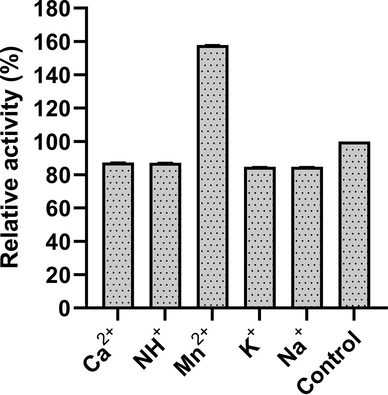
Impact of different metal ions on pectinase activity at a final concentration of 5 mM.
The values Km and Vmax of pectinase were found to be 6.04 mg/ml and 4.92 µmol/min/mg, respectively, with the substrate concentration ranging from 2 to 8 mg/ml (Fig. 6). The activity of an enzyme with a high Km will vary as the substrate concentration varies, hence the rate of product production will be determined by the availability of substrate. Pectinase from Bacillus sp. KSMP 443 (Kobayashi et al., 2014) and Penicillium frequentans (Barense et al., 2001) were found to have comparable Vmax values.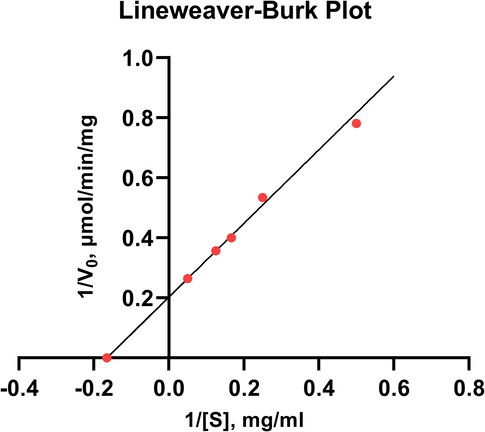
Lineweaver-Burk plot for the purified pectinase. GraphPad Prism 9.0 (San Diego, CA) was used to analyze the kinetic data.
3.4 Ultraviolet–visible spectral studies
The spectrophotometric studies of the purified enzyme are shown in Fig. 7. 23.26 U of enzyme showed maximum absorption at 271 nm. In the presence of pectin substrate (1 %, w/v), the absorption maxima were shifted to 276 nm. A redshift in peak maxima of ∼ 5 nm was observed. Peak maxima at wavelength ∼ 271 nm of enzyme alone are attributed to the ultraviolet absorption of residual or side groups from aromatic amino acids such as tryptophan, tyrosine and phenylalanine on protein chains (Sneharani, 2016; Sneharani et al., 2019). The redshift in peaks suggests binding of substrate to enzyme leading to exposure of these aromatic amino acids onto the outer surface of the enzymatic structure or to an aqueous environment (Sneharani et al., 2011).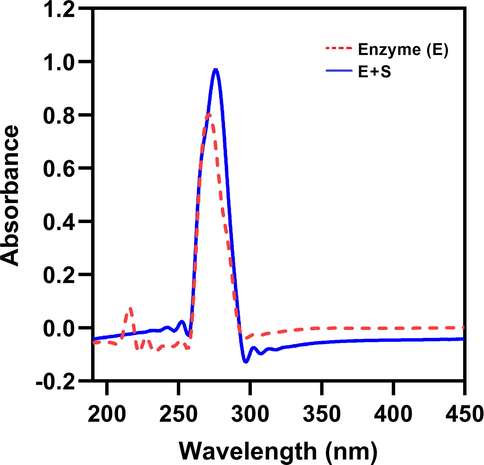
UV absorption spectra of purified enzyme. The UV–visible spectra of pectinase enzyme (red) and enzyme-substrate (pectin) complex (blue) were taken from 190 to 450 nm.
3.5 Application of pectinase in juice extraction
For extraction of juice, the enzyme was added to mashed apples, incubated, then centrifuged. The volume of juice extracted was evaluated and compared to a control, as shown in Table 2 (no enzyme extract).
Sample
Juice Extraction (ml)
Juice Recovery %
Clarity (Transmittance %)
Control
0.35 ± 0.05b
20.6 ± 0.01b
31.3 ± 1.1b
Enzyme
2.53 ± 0.03a
37.6 ± 0.1a
55.64 ± 0.64a
In addition, the purified enzyme was employed to investigate its application in the extraction of apple juice. The amount of extracted apple juice was calculated and used to determine the enzyme's ability in juice extraction. The enzyme enhanced the extraction of apple juice, as shown in Table 2. The juice was extracted in greater volume than the control without enzyme extract, and the extraction was statistically significant (p < 0.05).
Pectinase aids in apple juice extraction by breaking down the pectin found in apples. In extracting and clarifying juice, the synergistic activity of certain hydrolytic enzymes including pectinase, xylanase, cellulase and amylase is a key factor (Sharma et al., 2016). Enzyme treatment degrades residual pectin in the fruit, resulting in decreased water-holding ability by pectin, hence releasing water into the system thereby increasing juice yield (Shrestha et al., 2021). Enzymatic extraction of fruit juice is a novel, eco-friendly and efficient technology (Sharma et al., 2016). The present study suggests that gut-associated isolates from honey bees can significantly extract juice from apples. The pectinase secreted by these isolates could be beneficial in industries other than fruit juice, and it needs further exploration. In a similar line, the gut-associated Klebsiella sp. of the Pila globosa, an apple snail produced multiple polysaccharide degrading enzymes (Imran et al., 2016). In contrast, Klebsiella sp. observed in the gastrointestinal tract of giant snails has been reported to degrade only carboxy methylcellulose (Pawar et al., 2015). The relationship of autochthonous Klebsiella sp. with wild honey bees needs more attention and further investigation studies.
So, this bacterial pectinase might be a promising alternative to fungal pectinase and may be claimed for degumming and retting of fibre crops, paper industries and wastewater treatment from fruit juice industries. In addition, neutral to slightly alkaline pH enzyme is used to prepare vegetable purees and other food products (Soares et al., 1999). In the present investigation, pectinase from K. oxytoca af-G4 has been effectively purified and characterized and this newly isolated bacteria can produce extracellular pectinase during Submerged or Liquid state fermentation, in which different pectinases are produced. This investigation suggests the application of the microbes in fruit juice manufacturing and allied industries for cost-effective process compared to fungal pectinase. The bacteria K. oxytoca af-G4 showed multi-enzyme function, suggesting that they are potentially feasible candidate for a wide range of industrial applications and require further study. However, the enzyme characteristics can be enhanced further by efficient immobilization on a suitable matrix. Further improvement of enzyme properties by immobilizing onto a suitable matrix benefits the industries in terms of strength, availability, inertness, low cost, reusability, and temperature stability.
4 Conclusion
In conclusion, a catalytically efficient enzyme for pectin hydrolysis was purified from K. oxytoca af-G4. The present work shows that it is feasible to use agro-industrial wastes for the production of pectinase by K. oxytoca af-G4. From the industrial point of view, a shorter fermentation cycle is advantageous. Hence, this isolate have potential biotechnological as well as industrial applications. The pectinase from Klebsiella oxytoca af-G4 has superior properties including high temperature tolerance and being active in acidic/alkaline conditions. The functionality of excellent adaptation to a wide range of temperature and pH, and ease of purification by a single purification step, enhance its possible acceptance. The enzymes derived from the bacteria play a potential role in juice extraction and could be used as a substitute for the commercial pectinase production. However, additional investigation is necessary to analyse the optimization of juice extraction processes in order to increase juice yield.
Acknowledgements
KAK, HAG and BMA extend their appreciation to the Deanship of Scientific Research at King Khalid University, Saudi Arabia, for funding this work through Large Groups Project under grant number RGP.2/28/43.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Application of enzymes in the pulp and paper industry. Biotechnol. Progr.. 1999;15:147-157.
- [CrossRef] [Google Scholar]
- Partial purification and characterization of exopolygalacturonase II and III of Penicillium frequentans. Brazilian J. Microbiol.. 2001;32:327-330.
- [CrossRef] [Google Scholar]
- Some carbohydrates found in pollen and pollen substitutes are toxic to honey bees. J. Nutr.. 1977;107:1859-1862.
- [Google Scholar]
- Puri®cation and biochemical characterization of an extracellular endopolygalactu- ronase from Penicillium frequentans. J. Agric. Food Chem.. 1996;44:1616-1620.
- [Google Scholar]
- Pectinase production and purification from Bacillus subtilis isolated from soil. Pelagia Res. Libr. Adv. Appl. Sci. Res.. 2014;5:103-105.
- [Google Scholar]
- Functional diversity within the simple gut microbiota of the honey bee. Proc. Natl. Acad. Sci. U. S. A.. 2012;109:11002-11007.
- [CrossRef] [Google Scholar]
- Polysaccharide hydrolyzing enzyme activity of bacteria, native to Apis florea gut. Biomedicine. 2021;41:768-775.
- [CrossRef] [Google Scholar]
- Gut bacterial flora of open nested honeybee, Apis florea. Front. Ecol. Evol. 2022:126.
- [CrossRef] [Google Scholar]
- Studies on Polygalacturonase from Aspergllus Flavus. Sci. World. 2007;5:19-22.
- [CrossRef] [Google Scholar]
- Hayek, S.A., Shahbazi, A., Worku, M., Ibrahim, S.A., 2013. Enzymatic activity of Lactobacillus reuteri grown in a sweet potato based medium with the addition of metal ions. https://doi.org/10.1186/2193-1801-2-465.
- Hussain, K., Wajid, A., Babar, M.E., Anwar, Z., Farooqi, S., Siddiqa, A., Noreen, S., Iqbal, J., 2019. Production and optimization of pectinase from pomelo by Aspergillius niger through solid state fermentation annals of life sciences production and optimization of pectinase from pomelo by Aspergillius niger through solid state fermentation department of B.
- The gut-associated Klebsiella sp. Of the apple snail produces multiple polysaccharide degrading enzymes. Curr. Sci.. 2016;110:2170-2172.
- [CrossRef] [Google Scholar]
- Microbial pectinolytic enzymes: A review. Process Biochem.. 2005;40:2931-2944.
- [CrossRef] [Google Scholar]
- Screening of bacterial strains for polygalacturonase activity: Its production by bacillus sphaericus (MTCC 7542) Enzyme Res.. 2010;2010
- [CrossRef] [Google Scholar]
- Microbial cellulases: Engineering, production and applications. Renew. Sustain. Energy Rev.. 2014;33:188-203.
- [CrossRef] [Google Scholar]
- Purification and properties of polygalacturonic acid trans-eliminase from Bacillus stearothermophilus. Can. J. Microbiol.. 1980;26:377-384.
- [CrossRef] [Google Scholar]
- A histochemical study of pollen digestion in the alimentary canal of honeybees (Apis mellifera L.) J. Insect Physiol.. 1984;30:511-521.
- [CrossRef] [Google Scholar]
- Purification and properties of a galacturonic acid-releasing exopolygalacturonase from a strain of Bacillus. OUP. 2014;65:842-847.
- [CrossRef] [Google Scholar]
- Gut microbial communities of social bees. Nat. Rev. Microbiol. Nature Publishing Group 2016
- [CrossRef] [Google Scholar]
- Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-685.
- [CrossRef] [Google Scholar]
- Pectic activities from Fusarium oxysporum f. sp. melonis: Purification and characterization of an exopolygalacturonase. FEMS Microbiol. Lett.. 1991;81
- [CrossRef] [Google Scholar]
- Extracellular xylanopectinolytic enzymes by Bacillus subtilis ADI1 from EFB’s compost. Int. Sch. Res. Notes. 2017;2017:1-7.
- [CrossRef] [Google Scholar]
- Cellulase and pectinase production potentials of <em>Aspergillus Niger</em> Isolated from Corn Cob. Bayero. J. Pure Appl. Sci.. 2012;5:78-83.
- [CrossRef] [Google Scholar]
- Characterization of pectin depolymerising exo polygalacturonase by Bacillus sp. HD2 isolated from the gut of Apis mellifera L. Microbiol. Discov.. 2015;3:2.
- [CrossRef] [Google Scholar]
- Enrichment and identification of cellulolytic bacteria from the gastrointestinal tract of Giant African snail, Achatina fulica. Appl. Biochem. Biotechnol.. 2015;175:1971-1980.
- [CrossRef] [Google Scholar]
- Pectinase and polygalacturonase production by a thermophilic Aspergillus fumigatus isolated from decomposting orange peels. Brazilian J. Microbiol.. 2005;36:63-69.
- [CrossRef] [Google Scholar]
- Prakash, S., Karthik, R., M, T.V., Sridhar, B., Bharath, P.G., 2014. of Recent Scientific optimization and production of pectinase from Bacillus subtilis (MTCC 441) by using orange peel as a substrate Int. J. Recent Sci. Res. 5, 1177–1179.
- Extracellular pectinase from a novel bacterium Chryseobacterium indologenes strain SD and its application in fruit juice clarification. Enzyme Res.. 2018;2018
- [CrossRef] [Google Scholar]
- Enzymatic extraction and clarification of juice from various fruits – A review. Crit. Rev. Food Sci. Nutr.. 2016;2
- [Google Scholar]
- Isolation and characterization of a metal ion-dependent alkaline protease from a halotolerant Bacillus aquimaris VITP4. Indian J. Biochem. Biophys.. 2011;48:95-100.
- [Google Scholar]
- Screening and molecular identification of novel pectinolytic bacteria from forest soil. Fermentation. 2021;7:1-11.
- [CrossRef] [Google Scholar]
- Cloning, expression and characterization of a metagenome derived thermoactive/thermostable pectinase. Mol. Biol. Rep.. 2012;39:8353-8361.
- [CrossRef] [Google Scholar]
- Curcumin as a tool to assess the surface hydrophobicity of proteins. Spectrosc. Lett.. 2016;49:568-572.
- [CrossRef] [Google Scholar]
- Inhibition of lipoxygenase-1 by tetrahydrocurcumin. Eur. Food Res. Technol.. 2011;4:561-568.
- [CrossRef] [Google Scholar]
- Effect of phytochemicals on optical absorption spectra during biogenic synthesis of self-assembled silver nanoparticles and studies relevant to food applications. Spectrosc. Lett.. 2019;52:413-422.
- [CrossRef] [Google Scholar]
- Screening of bacterial strains for pectinolytic activity: characterization of the polygalacturonase produced by Bacillus sp. Rev. Microbiol.. 1999;30:299-303.
- [CrossRef] [Google Scholar]
- Production, purification, and characterization of polygalacturonase from mucor circinelloides ITCC 6025. Enzyme Res.. 2010;2010
- [CrossRef] [Google Scholar]
- Trindade, L.V., Desagiacomo, C., Polizeli, M.D.L.T.D.M., Damasio, A.R.D.L., Lima, A.M.F., Gomes, E., Bonilla-Rodriguez, G.O., 2016. Biochemical characterization, thermal stability, and partial sequence of a novel exo-polygalacturonase from the thermophilic fungus rhizomucor pusillus a13.36 obtained by submerged cultivation. Biomed Res. Int. 2016. https://doi.org/10.1155/2016/8653583.
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102301.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







