Translate this page into:
Protective effects of Viscum album L. leaf extract on chlorpyrifos-induced hepatotoxicity in Wistar rats
⁎Corresponding author at: Department of Biology and Ecology, Faculty of Science, University of Kragujevac, Radoja Domanovića 12, P.O. Box 60, 34000 Kragujevac, Serbia. milos.matic@pmf.kg.ac.rs (Miloš M. Matić)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Objectives
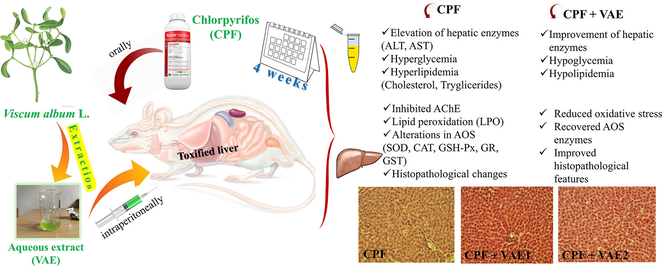
The goal of this study was to examine the antioxidant activity of Viscum album L. (VA, European mistletoe) extract (VAE) and to investigate whether VAE could provide protection against chlorpyrifos-induced hepatotoxicity.
Methods
Male Wistar albino rats were divided into five groups and treated two times per week during four weeks: I group without treatment served as control; II, IV and V group were treated with chlorpyrifos (CPF, 35 mg/kg b.w) via gavage; III and IV group received a higher dose of VAE (350 mg/kg b.w); and V group received a lower dose of VAE (175 mg/kg b.w) intraperitoneally. The extract was obtained by ultrasound-assisted extraction. HPLC-DAD analysis was performed to investigate the chemical composition of the extract, and antioxidant assays to determine its antioxidant activities. Biochemical parameters, antioxidant enzyme activities in liver tissue, and histopathology were also determined.
Results
The VAE caused significant decrease in glucose, TC, TG, albumins, AST, and ALT compared to CPF-treated rats. The cotreatment with VAE also significantly recovered the antioxidative system parameters (SOD, CAT, GSH-Px, GR, and GST) and alleviated some histopathological changes caused by CPF.
Conclusions
Our data revealed that the simultaneous VAE administration modulated the negative changes induced by CPF. This study suggests that VAE could be useful in providing protection against hepatotoxicity and oxidative stress due to its antioxidative properties.
Keywords
Chlorpyrifos
Viscum album L.
Hepatotoxicity
Oxidative stress
- TPC
-
total phenolic content
- TFC
-
total flavonoid content
- CT
-
condensed tannins
- GA
-
gallotannins
- TAC
-
total antioxidant capacity
- DPPH
-
2,2-di-phenyl-1-picrylhydrazyl
- ABTS
-
2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- ILP50
-
lipid peroxidation activity
- OH50
-
hydroxyl radical scavenging activity
- IC50
-
half maximal inhibitory concentration
- LD50
-
medial lethal dose
- BHT
-
butylated hydroxytoluene
- ND
-
not detected
- BD
-
ballooning degeneration
- CN
-
confluent necrosis
- C
-
congestion
- FN
-
focal necrosis
- HD
-
hydropic degeneration
- KCH
-
Kupffer cell hyperplasia
- I
-
infiltration of lymphocytes
Abbreviations
1 Introduction
The extensive use of synthetic chemicals causes numerous environmental issues (Al-Enizi et al., 2020; Naughton and Terry, 2018; Ubaidullah et al., 2020). Chlorpyrifos (CPF) [O,O-diethyl-o-(3,5,6-trichloro-2-pyridyl) phosphorothionate] is a frequently utilized organophosphate (OP) insecticide against flies, mosquitoes and household pests (Uzun and Kalender, 2013). Exposure to OPs, including CPF, can lead to irreversible inhibition of the enzyme acetylcholinesterase (AChE), which results in overstimulation of the postsynaptic cells (Mohamed et al., 2018). While the inhibition of AChE is a crucial event in OP-toxicity, there is evidence of other mechanisms involved (Naughton and Terry, 2018). One of the molecular mechanisms in CPF intoxication is the overproduction of free radicals, which in turn may induce various organs toxicities (Tanvir et al., 2015). The liver is the primary organ involved in xenobiotic metabolism and first major organ exposed to various toxins ingested owing to its portal blood supply. The liver is rich in mitochondria (which have a significant role in energy regulation as a source of free radicals), and therefore mitochondrial impairment could cause oxidative stress leading to liver damage (Taha et al., 2021). Albasher and collegues (2019) reported CPF leads to liver injury in rats mediated via oxidative stress, inflammation, and apoptosis. Recent study by Taha et al. (2021) documented the implication of free radicals in CPF toxicity evidenced in hepato-mitochondrial dysfunction in male rats.
To prevent cell damage and reduce adverse effects from reactive radical structures, components of the antioxidant defense system (AOS) function as free radical scavengers (Uzun and Kalender, 2013). However, in the case of OP-poisoning, the effectiveness of AOS in maintaining of the cell redox balance is not efficient enough (Milošević et al., 2018). Viscum album L. (European mistletoe, VA) is a semi-parasitic shrub that grows on a variety of host trees and has been used for centuries in the traditional medicine of Europe. VA contains a variety of phytochemicals such as polysaccharides, phenylpropanoids, lignans, lectins, viscotoxins, alkaloids and flavonoids (Szurpnicka et al., 2020). The antioxidative capacity of mistletoes depends on the host tree and the harvesting time (Szurpnicka et al., 2020). Stefanucci et al. (2020) investigated the biological activities of mistletoe leaves, fruits, and seeds extracts, and among them the leaves extract exerted the best antioxidative properties.
Although VA is used as an effective treatment of different diseases, the antioxidative potential of VA extracts (VAEs) remains elusive. Therefore, the purpose of this study is to investigate if VAE can protect against CPF-induced hepatotoxicity by focusing on oxidative stress, blood biochemistry, and histological alterations in liver tissue. Accordingly, we monitored changes in the activity of AOS enzymes (superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), glutathione reductase (GR) and glutathione-S-transferase (GST)), lipid peroxidation levels, and AChE activity. Besides, HPLC analysis and antioxidant activities of VAE (total antioxidant capacity, lipid peroxidation assay, hydroxyl radical scavenging activity, DPPH radical scavenging activity, and ABTS radical scavenging assay) were performed.
2 Material and methods
2.1 Chemicals
The chlorpyrifos insecticide (Radar 300EW, CAS No. 64742-95-6) was obtained from Galenika Phytopharmacy a.d. (Belgrade, Serbia). The standards for HPLC analysis were purchased from Sigma Chemicals Co. (St. Louis, MO, USA) and Alfa Aesar (Karlsruhe, Germany).
2.2 Plant material and preparation of the extract
The Viscum album L. was collected from a pear tree on Zlatibor mountain (Serbia) in September 2016, and plant leaves were used for the extraction. The leaves were dried naturally in the draft and dark during one month, grounded in the blender and kept in paper bags. The leaves (75.0 g) were crushed and homogenized with a cylinder crusher and put into Soxhlet apparatus. The extraction process lasted eight hours, with the 96% ethanol used as solvent (600 ml). The ultrasound-assisted extraction was performed in an ultrasonic water bath. A sample (10 g) was placed in a volumetric flask, and after the solvent was added (250 ml of double-distilled water) the mixture was sonificated for 30 min at the frequency of 40 kHz and the ultrasound power of 90% (216 W).
2.3 Chemical composition of the extract
The total phenolics (TPC) and flavonoids (TFC) contents were established according to Mašković et al. (2015). The results were expressed as mg of gallic acid equivalents (mg GAE) and mg of rutin equivalents (mg RU) per g of dry extract. Condensed tannins (CT) and gallotannins (GA) were determined using the potassium iodate assay (Mašković et al., 2015). Both results were expressed as mg GAE/g.
2.4 HPLC analysis
The quantitative analyses of individual phenolic compounds in VAE were conducted using the reversed phase HPLC analysis. During the process, the following equipment was used: an HPLC Agilent-1200 series with UV–vis DAD detector for multi wavelength detection (Suppl).
2.5 Determination of antioxidant activity
The antioxidant activity of the extract was determined using: total antioxidant capacity, lipid peroxidation assay, hydroxyl radical scavenging activity, DPPH radical scavenging activity, and ABTS radical scavenging assay (Mašković et al., 2015). The total antioxidant capacity (TAC) was expressed as micrograms of ascorbic acid per gram of dry extract (μg AA/g). The results for lipid peroxidation assay were expressed as ILP50 in μg/ml, for hydroxyl radical scavenging activity as OH50 in μg/ml, for DPPH and ABTS as IC50 in μg/ml. All tests were performed in triplicates. Gallic acid, ascorbic acid, butylated hydroxytoluene (BHT) and α-tocopherol were used as reference standards.
2.6 Animals and treatment
Thirty male Wistar albino rats (2 months old, weighing 220 ± 50 g) were used throughout the experiments. The animals were kept under standard laboratory conditions, and were given rodent laboratory pellet. All animal procedures were in accordance with the EU Directive (2010/63/EU) and were approved by the University Ethics Committee for Animal Experimentation.
After acclimation, the animals were weighed and randomly divided into five equal groups of six animals per each and treated as follows:
– Control group (without treatment);
– Group was treated with CPF (35 mg/kg b.w);
– Group was treated with higher dose of extract,VAE1 (350 mg/kg);
– Group was given CPF (35 mg/kg b.w) and higher dose of extract, VAE1 350 mg/kg), and
-
V
– Group was given CPF (35 mg/kg b.w) and lower dose of extract, VAE2 (175 mg/kg).
The chemicals were dissolved in distilled water and administered two times per week in the morning for four weeks. The acute oral LD50 of CPF for rats is 229 mg/kg b.w. (World Health Organization, 2009). Therefore, we used the dose of 35 mg/kg two times per week (1/7.6 LD50). The treatment twice weekly was chosen based on the study of Elsharkawy et al. (2013). We used two doses of VAE (350 mg/kg/twice weekly and 175 mg/kg/twice weekly) (Adaramoye et al., 2012).
Forty-eight hours after the last treatment, animals were anesthetized with diethyl ether, sacrificed by decapitation, and blood and liver samples were collected for further analyses. The liver tissue was excised, rinsed in ice-cold saline and one part was stored at −80 °C. The second part was fixed in 4% formalin and processed for histopathological examination.
2.7 Biochemical assays of serum
The blood samples in non-anticoagulant tubes were centrifuged at 1000g for 10 min and serum was used. Albumins, glucose, total cholesterol (TC), triglycerides (TG), and activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured with a Cobas Integra 400+ (Roche Diagnostics, Switzerland).
2.8 Tissue preparation
The liver tissue was minced and homogenized with an Ultra-Turrax homogenizer (Janke & Kunkel, IKA-Werk, Staufen, Germany) at 0–4 °C (10% w/v) using 0.25 M sucrose, 1 mM EDTA and 0.05 M Tris-HCl solution pH 7.4. The homogenates were centrifuged (15,000g for 30 min at 4 °C) and the supernatant was used for total protein determination, antioxidant enzyme activity assays, and estimation of AChE.
2.9 Lipid peroxidation assay and enzymes activity assays
Lipid peroxides (LPO) were determined by measuring the malondialdehyde (MDA) concentration (Rehncrona et al., 1980). The protein content was determined by the biuret method (Lowry et al., 1951). SOD activity was determined by using pyrogallol as a substrate (Marklund and Marklund, 1974). CAT activity was measured by the Beutler method (Beutler, 1982). The activity of GSH-Px was assayed according to Tamura et al. (1982) using t-butyl hydroperoxide as a substrate. GR activity was assayed according to Glatzle et al. (1974). To determine the GST activity, 1-chloro-2,4-dinitrobenzene was used as a substrate (Habig et al., 1974). The activity of AChE was determined by the Ellman method (Ellman et al., 1961). All measurements were performed in triplicate and were read using UV/visible spectrophotometer (Jenway 6105, Bibby Scientific Limited, Staffordshire, UK).
2.10 Histopathological examinations
The liver sections were dissected, washed in normal saline, and fixed in 4% formalin for 24 h. The dehydrated pieces of liver tissues were embedded in paraffin wax, then cut into 4–6 µm thick sections and stained with hematoxylin and eosin (H&E). The sections were examined microscopically for histopathological changes. Photographs of each slide were taken at 100× magnification.
2.11 Statistical analyses
All data were evaluated using IBM-SPSS 23 software for Windows (SPSS Inc., Chicago, IL, USA), and presented as a mean ± standard error (S.E.M). The statistical significance was determined using one-way ANOVA, followed by Tukey's/Dunnett's T3 post-hoc tests. The IC50 values were determined by nonlinear regression analysis.
3 Results
3.1 Phytochemicals results/Identification of phenolic compounds in Viscum album L. extract
The data given in Table 1 showed the levels of different compounds (TPC, TFC, CT, and GA). The results of the HPLC-DAD analysis (Table 2, Fig. 1) showed that phenolic compounds in VAE were represented with five flavonoids: rutin, quercetin, kaempferol, apigenin and apigenin glycoside. In comparison to the standards (Table 3), VAE exerted better DPPH scavenging activity than BHT and greater inhibition of lipid peroxidation compared to gallic and ascorbic acids. The scavenging activity against hydroxyl radicals was the highest in VAE compared to all used standards. The ABTS assay showed that only gallic acid exerted better scavenging ability when compared to VAE.
Extract
Content
TPC (mg GA/g)
94.38 ± 0.62
TFC (mg RU/g)
65.24 ± 0.23
CT (mg GAE/g)
44.65 ± 0.09
GA (mg GAE/g)
29.64 ± 0.38
TAC (µg AA/g)
128.65 ± 0.92
Compound
Content (µg/g)
p-Hydroxybenzoic acid
ND
Caffeic acid
0.090
Vanillic acid
ND
Chlorogenic acid
0.060
p-Coumaric acid
0.125
Ferulic acid
0.076
Sinapic acid
ND
Rutin
4.467
Apigenin-glycoside
0.792
Rosmarinic acid
ND
Quercetin
2.145
Luteolin
ND
Naringenin
ND
Kaempferol
2.229
Apigenin
1.874
∑
11.858
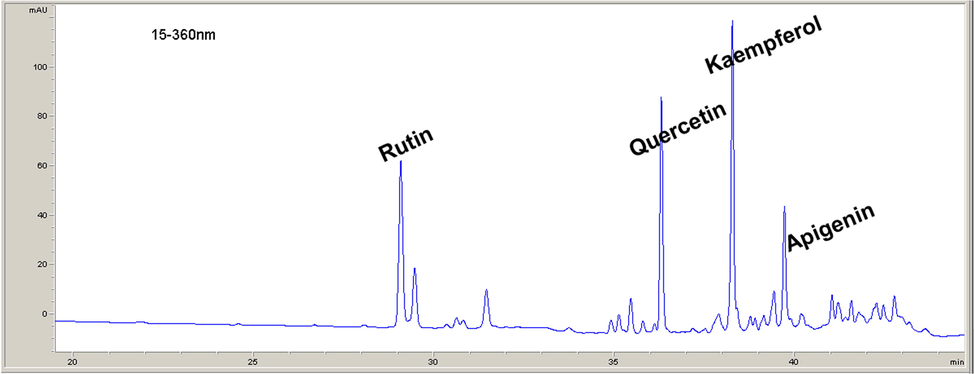
Polyphenolic compounds identified in VAE.
Sample
IC50(µg/mL)
DPPH scavenging activity
Inhibitory activity against lipid peroxidation
Hydroxyl radical scavenging activity
ABTS radical scavenging assay
Viscum album L.
7.28 ± 0.83
18.11 ± 0.82
22.02 ± 0.83
5.09 ± 0.23
Gallic acid
3.79 ± 0.69
255.43 ± 11.68
59.14 ± 1.10
1.96 ± 0.41
Ascorbic acid
6.05 ± 0.34
>1000
160.55 ± 2.31
10.98 ± 0.95
BHT
15.61 ± 1.26
1.00 ± 0.23
33.92 ± 0.79
7.23 ± 0.87
α-Tocopherol
–
0.48 ± 0.05
–
–
3.2 Body and liver weight changes and serum biochemical parameters
The treatments did not significantly differ in body weight change and liver/b.w. ratio between groups (Table 4). Data are expressed as mean ± SEM, (n = 6 animals).
Parameters
Experimental groups
Control
CPF
VAE1
CPF + VAE1
CPF + VAE2
Body weight change (%)
10.06 ± 1.01
5.49 ± 0.64
19.68 ± 2.37
8.98 ± 1.54
18.37 ± 1.54
Liver/b.w. ratio × 1000
24.09 ± 0.24
32.54 ± 0.37
28.97 ± 0.93
28.74 ± 0.1
30.03 ± 1.40
Glucose (mmol/L)
7.05 ± 0.46
10.00 ± 0.73*
7.66 ± 0.44#
6.43 ± 0.15#
6.93 ± 0.31#
TC (mmol/L)
1.90 ± 0.03
2.24 ± 0.15*
1.78 ± 0.09
1.32 ± 0.10*#
1.28 ± 0.10*#
TG (mmol/L)
0.35 ± 0.01
0.50 ± 0.04*
0.38 ± 0.04
0.32 ± 0.07#
0.45 ± 0.03
Albumins (g/L)
46.23 ± 1.39
43.54 ± 0.76
43.15 ± 0.41
20.50 ± 1.46*#
24.03 ± 1.50*#
ALT (U/L)
30.50 ± 1.76
44.25 ± 1.65*
42.75 ± 2.17
30.00 ± 4.14#
37.00 ± 4.71
AST (U/L)
112.00 ± 3.49
167.60 ± 13.3*
111.60 ± 7.78#
109.00 ± 6.62#
116.00 ± 16.4
The exposure of rats to CPF showed a significant increase in glucose, TC and TG (p < 0.05), and an insignificant decrease in albumins compared to the control group. The liver function markers ALT and AST were significantly elevated (p < 0.05) when the CPF group was compared to the control. The cotreatments with VAE reduced glucose and TC levels in comparison to the treated CPF rats. Interestingly, the TC values in cotreatments were lower than those in the control group. The reduction of TG was noted in both cotreatments, but only in the group with higher dose of VAE it was significant. The albumins were significantly lower in cotreatment groups when compared to the CPF-treated and control animals. The VAE showed decrement of ALT and AST in dose-dependent manner. The control and VAE groups did not differ significantly in all the mentioned parameters (Table 4).
3.3 Estimation of AChE activity in liver tissue
In comparison to the control animals, the AChE was significantly reduced (p < 0.05) in CPF-treated group (Fig. 2). The cotreatments with VAE did not significantly change the AChE values compared to CPF-treated group. In the group treated with VAE1, the alteration in the AChE activity was not significant compared to the control.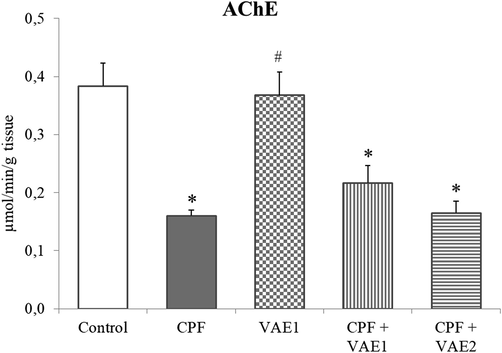
Effects of the VAE on AChE activity in liver of CPF-treated rats. Data are expressed as mean ± SEM, (n = 6 animals). *p < 0.05 compared to control animals; #p < 0.05 compared to CPF exposed animals.
3.4 Antioxidant enzyme activities and LPO levels
The CPF caused a significant increase in SOD and GST activities, while CAT, GSH-Px and GR were significantly decreased (p < 0.05) in comparison to the control group (Fig. 3a–e). The activities of all enzymes were attenuated by VAE coadministration, and their amelioration was dose-dependent. Among the CPF-administered rats, a significant elevation (p < 0.05) was noted in LPO, when compared to control rats (Fig. 3f). The VAE caused an insignificant decrease in LPO levels when compared to the CPF. No significant difference regarding these parameters was observed between the VAE and control groups.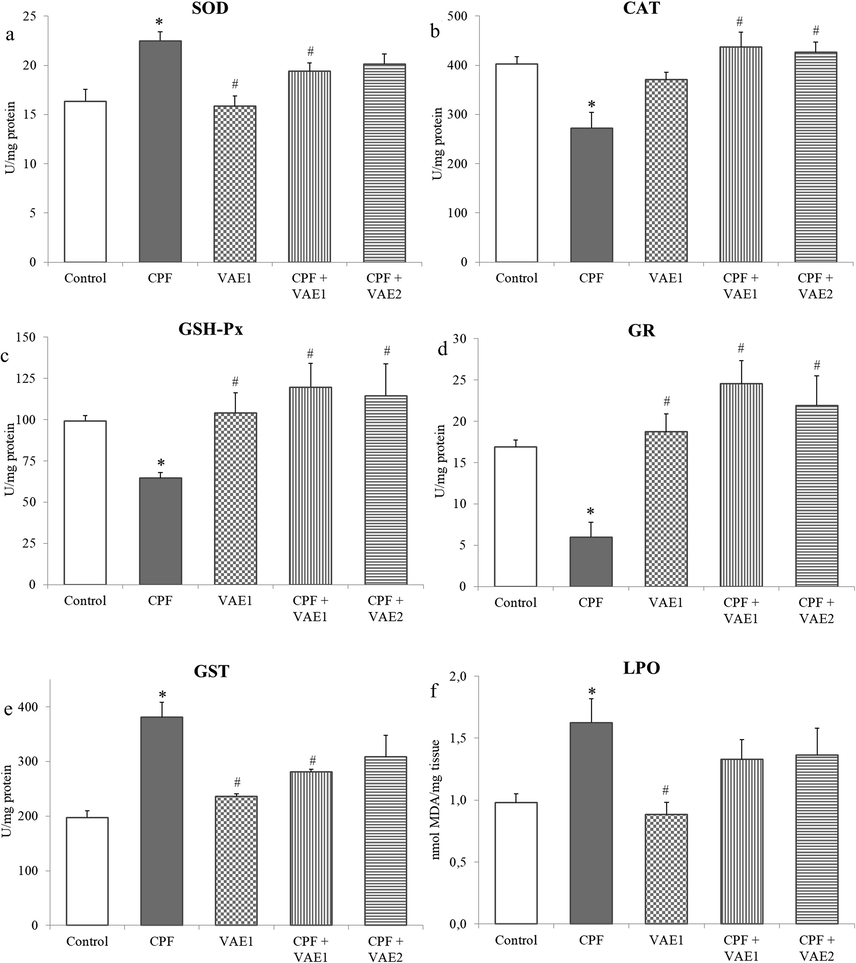
Effects of the VAE on the activity of antioxidant enzymes (SOD, CAT, GSH-Px, GR and GST) and the concentration of LPO in liver of CPF-treated rats. Data are expressed as mean ± SEM, (n = 6 animals). *p < 0.05 compared to control animals; #p < 0.05 compared to CPF exposed animals.
3.5 Histopathological examination
The histopathological examination of liver sections revealed the ability of VAE to prevent liver injury in two doses (Fig. 4). The control group displayed a normal arrangement of hepatocytes with weak congestion and infiltration in some samples. Within the group exposed to CPF, abnormal cellular morphology manifested with ballooning degeneration, weak focal and confluent necrosis, congestion, Kupffer cell hyperplasia and moderate infiltration of lymphocytes were noted. However, supplementation with both high and low doses of VAE showed an improvement when compared to the CPF group. Both doses displayed moderate congestion, slight or moderate hydropic degeneration and mild infiltration of lymphocytes, and slight focal necrosis, while confluent necrosis and ballooning degeneration were not observed. In the VAE1 group weak congestion and cell swelling was noted.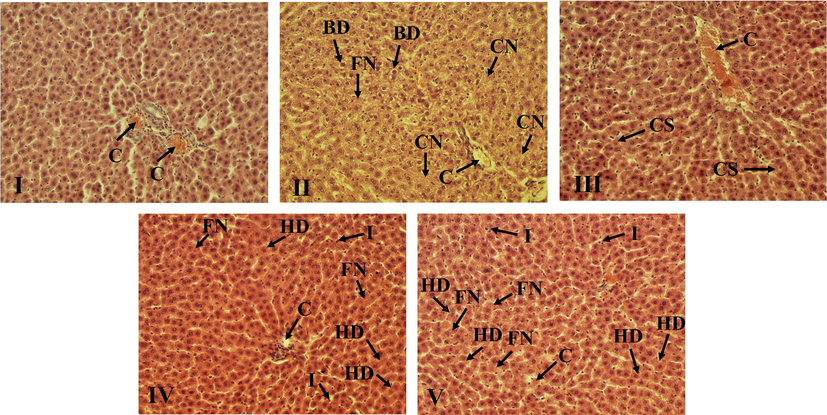
The VAE limits the CPF-induced histopathological changes in liver. Representative photomicrographs of sections from liver tissue (100× magnification). (I) – Normal rats without treatment, (II) – rats treated with CPF (35 mg/kg b.w), (III) – VAE1 (350 mg/kg b.w), (IV) – CPF (35 mg/kg b.w) and VAE1 (350 mg/kg b.w), and (V) – CPF (35 mg/kg b.w) and VAE2 (175 mg/kg b.w).
4 Discussion
The mistletoes proved to be effective in the treatment of various diseases according to their antidiabetic, anticancer, immunomodulatory, hepatoprotective and cardiac properties (Adaramoye et al., 2012; Szurpnicka et al., 2020). However, there is still a lack of data regarding whether VAEs can reduce the adverse effects of OPs. Thus, the present study was designed to examine if aqueous VAE from a pear tree exerts protective effects against hepatic injuries caused by sub-chronic exposure to CPF.
After hepatic metabolism of CPF, the active metabolite CPF oxon is generated supported by cytochrome P450 2B6 enzyme (Albasher et al., 2019). Oxon forms possess the AChE inhibiting potency that is about two to three orders of magnitude higher than among the parent compounds (Flaskos, 2012). In our study, the AChE activity was decreased in the livers of the CPF-intoxicated rats. The inhibition of AChE could be the consequence of lipid peroxidation (Mohamed et al., 2018). Since coadministration of CPF and VAE has not exerted significant changes of AChE activity compared to CPF group we can conclude that VAE does not affect cholinesterase activity in the liver.
The observed increase in ALT and AST activities in serum of CPF-treated rats could be due to hepatic dysfunction and disturbance in the biosynthesis of these enzymes, and the alteration in the membrane permeability of hepatocytes (Tanvir et al., 2015), which was confirmed by previous studies (Mohamed et al., 2018; Uzun and Kalender, 2013). The supplementation with VAE alleviated hepatic serum markers in CPF-intoxicated rats, and demonstrates that VAE exerts protective potential against liver damages by maintaining membrane integrity and reducing leakage of these enzymes. Abdel-Salam et al. (2010) also observed these preventive effects of VAE on serum transaminases in rat hepatotoxicity induced by CCl4. The phenolics presented in VAE, such as chlorogenic acid and quercetin can prevent the leakage of these enzymes in liver injury (Ali et al., 2017; El-Shafey et al., 2015). Thus, it can be concluded that these compounds can stabilize the membrane of hepatocytes.
The treatment with CPF has disturbed the metabolism of carbohydrates, proteins and lipids (Table 4). Hyperglycemia is a characteristic output in OPs exposure, as reported in previous study (Milošević et al., 2018). There are a few possible underlying mechanisms of hyperglycemia induced by OP. One of them involves the activation of the glycogenolysis pathway (Acker and Nogueira, 2012). The OPs increase glycogenolysis via insulin inhibition and stimulation of glucagon activity. Furthermore, elevated glucose levels could increase the peroxide levels in pancreatic islets. As a result, this hyperglycemic state decreases insulin secretion (Ambali et al., 2011). The increase in levels of TC and TG in the serum may be related to the increased permeability of hepatocyte membranes caused by pesticides (Uzun and Kalender, 2013). These marked elevations in glucose, TC and TG are significantly restored within the group co-administered with VAE, which thus demonstrates its hypoglycemic and hypolipidemic properties. These results are in accordance with the findings of Adaramoye et al. (2012), who showed that VA methanolic extract improved carbohydrate metabolism and hyperlipidemia in streptozotocin-induced diabetic rats. The reduced levels of glucose after VAE supplementation may be attributed to several processes, such as suppressed hepatic gluconeogenesis, stimulated glycolysis, and insulin release (Ohiri et al., 2003). Although the exact mechanism of VAE action in decreasing glucose and lipid levels is not known, it can be assumed that these effects are due to constituents presented in VAE. Additionally, previous findings demonstrated the beneficial effects of rutin and quercetin against liver injuries, manifested by attenuated TC and TG levels (El-Shafey et al., 2015; Pan et al., 2014). The hydroxycinnamic acid derivatives also identified in VAE, proved to be effective in lowering glucose and improving lipid metabolism in metabolic disorders and liver injuries, helping alleviate oxidative stress (Chen et al., 2019; Shen et al., 2019).
In the presence of some stressors, the balance between antioxidative potential and cellular pro-oxidants was disrupted in favor of pro-oxidants. This results in the oxidative stress phenomenon, manifested through the overproduction of reactive oxygen species. This further causes damage to macromolecules such as lipids, nucleic acids, and proteins. Lipid peroxidation is the oxidation of the lipid layer in cellular membranes, which leads to impaired membrane structure and function, reduced fluidity, and inactivation of membrane-bound enzymes (Tanvir et al., 2015). The CPF is a lipophilic molecule and can easily cross the cell membrane, causing damage inside the cell (Tanvir et al., 2015). The levels of MDA, the main indicator of lipid peroxidation, are significantly increased in treatment with CPF, whereas VAE exerted no significant restoration of MDA levels (Fig. 3f). The disruption of redox homeostasis in CPF intoxicated rats can be also noticed from alterations in enzymatic components of AOS (Fig. 3a–e). The SOD and CAT are the two basic antioxidative enzymes, which neutralize free radicals that protect macromolecules from oxidative damage (El-Demerdash and Nasr, 2014; Uzun and Kalender, 2013). In our study, the CPF significantly increased SOD, but decreased CAT (Fig. 3a,b). This indicates that CPF led to the overproduction of free radicals, which increased the activity of SOD in order to confront the oxidative stress. Perturbations in the SOD and CAT showed that their antioxidative activities were insufficient to compensate reactive species (Yonar, 2018). The depletion of GSH-Px and GR could be caused by the direct effect of OP on the enzyme and due to excessive production of free radicals (El-Demerdash and Nasr, 2014). The increase in GST activity in CPF treated rats (Fig. 3e) can be explained by the fact that OPs consume GSH in the detoxification process, and that these compounds are supposed to induce the GST activity as a protection mechanism against lipid peroxidation (Yonar, 2018). The VAE supplementation ameliorates AOS enzymes activities (SOD, CAT, GSH-Px, GR and GST). Medical plants are rich in flavonoids known for their antioxidative properties, which stem from their chemical composition and ability to donate electrons or hydrogen molecules (Alanazi et al., 2020). Flavonoids have shown to act as free radical scavengers and prevent oxidative damage, related to their ability to stabilize membranes (Uzun and Kalender, 2013). The phytochemical screenings of VAE showed that flavonoids in the extract are represented with rutin, kaempferol, quercetin and apigenin (Table 2). The previous study showed that rutin and quercetin had more potent effects in restoring LPO level (Pan et al., 2014; Uzun and Kalender, 2013). The protective antioxidant activity of kaempferol is also reported in redox imbalance and decreased AOS enzyme activities in propacetamol-induced oxidative stress in mice liver (Tsai et al., 2018). Besides, hydroxycinnamic acid derivatives (caffeic acid, chlorogenic acid, p-coumaric acid, and ferulic acid) that are present in VAE can act as chain-breaking antioxidants and quench free radicals (Pisoschi and Pop, 2015). The histopathological changes in the CPF group, including focal and confluent necrosis and ballooning degeneration, clearly indicate liver damage and confirm perturbations of parameters measured in the serum and liver (Fig. 4). These changes could be a consequence of induced oxidative stress (Uzun and Kalender, 2013). The cotreatment with VAE caused less histopathological changes than in CPF group, indicating its protective effects.
5 Conclusion
Our findings showed that VAE ameliorates the toxic effects of CPF by restoring the values of biochemical parameters, preventing histopathological changes, and reducing oxidative stress parameters in a dose-dependent manner. These protective effects of VAE could be attributed to its antioxidative constituents. Further investigations are needed to examine the precise mechanisms of VAE action at the cellular and molecular level, and to test different doses and extracts from various host trees, harvested in different times of the year, in order to find the most efficient extract composition and dose against different toxicants.
Acknowledgements
This study was supported by the Serbian Ministry of Education, Science and Technological Development (Agreement No. 451-03-68/2022-14/200122).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effect of Viscum album on acute hepatic damage caused by carbon tetrachloride in rats. Turk. J. Med. Sci.. 2010;40:421-426.
- [CrossRef] [Google Scholar]
- Chlorpyrifos acute exposure induces hyperglycemia and hyperlipidemia in rats. Chem.. 2012;89:602-608.
- [CrossRef] [Google Scholar]
- Methanolic extract of African mistletoe (Viscum album) improves carbohydrate metabolism and hyperlipidemia in streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Med.. 2012;5:427-433.
- [CrossRef] [Google Scholar]
- Protective role of Loranthus regularis against liver dysfunction, inflammation, and oxidative stress in Streptozotocin diabetic rat model. Evid.-Based Complement. Altern. Med.. 2020;2020:1-8.
- [Google Scholar]
- Ameliorative effect of Beta vulgaris root extract on chlorpyrifos-induced oxidative stress, inflammation and liver injury in rats. Biomolecules. 2019;9:261.
- [CrossRef] [Google Scholar]
- Utilization of waste polyethylene terephthalate bottles to develop metal-organic frameworks for energy applications: A clean and feasible approach. J. Clean. Prod.. 2020;248:119251
- [CrossRef] [Google Scholar]
- Protective effect of chlorogenic acid against methotrexate induced oxidative stress, inflammation and apoptosis in rat liver: An experimental approach. Chem. Biol. Interact.. 2017;272:80-91.
- [Google Scholar]
- Hyperglycemia induced by subchronic co-administration of chlorpyrifos and lead in Wistar rats: Role of pancreatic lipoperoxidation and alleviating effect of vitamin C. Biol. Med.. 2011;3:6-14.
- [Google Scholar]
- Beutler, E., 1982. Catalase, in: Beutler, E. (Ed.), Red Cell Metabolism, a Manual of Biochemical Methods. Grune and Stratton, New York, pp. 105–106.
- Hepatoprotective effect of chlorogenic acid against chronic liver injury in inflammatory rats. J. Funct. Foods. 2019;62:e103540
- [CrossRef] [Google Scholar]
- Antioxidant effect of selenium on lipid peroxidation, hyperlipidemia and biochemical parameters in rats exposed to diazinon. J. Trace Elem. Med. Biol.. 2014;28:89-93.
- [CrossRef] [Google Scholar]
- Determination of acetylcholinesterase activity. Biochem. Pharmacol.. 1961;7:88-95.
- [CrossRef] [Google Scholar]
- Quercetin protects against acetaminophen-induced hepatorenal toxicity by reducing reactive oxygen and nitrogen species. Pathophysiology. 2015;22:49-55.
- [CrossRef] [Google Scholar]
- Sub-chronic exposure to chlorpyrifos induces hematological, metabolic disorders and oxidative stress in rat: Attenuation by glutathione. Environ. Toxicol. Pharmacol.. 2013;35:218-227.
- [CrossRef] [Google Scholar]
- The developmental neurotoxicity of organophosphorus insecticides: A direct role for the oxon metabolites. Toxicol. Lett.. 2012;209:86-93.
- [CrossRef] [Google Scholar]
- Glutathione reductase test with whole blood, a convenient procedure for the assessment of the riboflavin status in humans. Exp.. 1974;30:665-667.
- [CrossRef] [Google Scholar]
- Protein measurement with the Folin phenol reagent. J. Biol. Chem.. 1951;193(1):265-275.
- [Google Scholar]
- Involvement of superoxide anion radical in the autoxidation of pyrogallol and a constituent assay for superoxide dismutase. Eur. J. Biochem.. 1974;47:469-479.
- [CrossRef] [Google Scholar]
- Onosma aucheriana: A source of biologically active molecules for novel food ingredients and pharmaceuticals. J. Funct. Foods. 2015;19:479-486.
- [CrossRef] [Google Scholar]
- Role of selenium and vitamin C in mitigating oxidative stress induced by fenitrothion in rat liver. Biomed. Pharmacother.. 2018;106:232-238.
- [CrossRef] [Google Scholar]
- Alpha lipoic acid protects against chlorpyrifos-induced toxicity in Wistar rats via modulating the apoptotic pathway. Environ. Toxicol. Pharmacol.. 2018;59:17-23.
- [CrossRef] [Google Scholar]
- Neurotoxicity in acute and repeated organophosphate exposure. Toxicology. 2018;408:101-112.
- [CrossRef] [Google Scholar]
- Hypoglycemic properties of Viscum album (Mistletoe) in alloxan-induced diabetic animals. Pharm. Biol.. 2003;41:184-187.
- [CrossRef] [Google Scholar]
- Protective effects of rutin on liver injury induced by biliary obstruction in rats. Free Radic. Biol. Med.. 2014;73:106-116.
- [CrossRef] [Google Scholar]
- The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem.. 2015;97:55-74.
- [CrossRef] [Google Scholar]
- Peroxidative changes in brain cortical fatty acids and phospholipids, as characterized during Fe2+ - and ascorbic acid-stimulated lipid peroxidation in vitro. J. Neurochem.. 1980;34:1630-1638.
- [CrossRef] [Google Scholar]
- Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed. Pharmacother.. 2019;111:579-587.
- [CrossRef] [Google Scholar]
- Viscum album L. homogenizer-assisted and ultrasound-assisted extracts as potential sources of bioactive compounds. J. Food Biochem.. 2020;44:13377.
- [CrossRef] [Google Scholar]
- Biological activity of mistletoe: in vitro and in vivo studies and mechanisms of action. Arch. Pharm. Res.. 2020;43:593-629.
- [CrossRef] [Google Scholar]
- Mitochondrial dysfunction and oxidative stress in liver of male albino rats after exposing to sub-chronic intoxication of chlorpyrifos, cypermethrin, and imidacloprid. Pestic. Biochem. Physiol.. 2021;178:104938
- [CrossRef] [Google Scholar]
- Some characteristics of hydrogen and alkylhydro-peroxides metabolizing systems in cardiac tissue. J. Biochem.. 1982;92:1019-1031.
- [CrossRef] [Google Scholar]
- Honey has a protective effect against chlorpyrifos-induced toxicity on lipid peroxidation, diagnostic markers and hepatic histoarchitecture. Eur. J. Integr. Med.. 2015;7:525-533.
- [CrossRef] [Google Scholar]
- Kaempferol protects against propacetamol-induced acute liver injury through CYP2E1 inactivation, UGT1A1 activation, and attenuation of oxidative stress, inflammation and apoptosis in mice. Toxicol. Let.. 2018;290:97-109.
- [CrossRef] [Google Scholar]
- Waste PET plastic derived ZnO@NMC nanocomposite via MOF-5 construction for hydrogen and oxygen evolution reactions. J. King Saud Univ. – Sci.. 2020;32:2397-2405.
- [CrossRef] [Google Scholar]
- Chlorpyrifos induced hepatotoxic and hematologic changes in rats: The role of quercetin and catechin. Food Chem. Toxicol.. 2013;55:549-556.
- [Google Scholar]
- World Health Organization, WHO specifications and evaluations for public health pesticides, 2009. Chlorpyrifos O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate, http://www.who.int/whopes/quality/Chlorpyrifos_WHO_specs_eval_Mar_2009.pdf (accessed 23 February 2017).
- Chlorpyrifos-induced biochemical changes in Cyprinus carpio: Ameliorative effect of curcumin. Ecotoxicol. Environ. Saf.. 2018;151:49-54.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.101957.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







