Translate this page into:
Protective effects of aucubin against nonylphenol-induced liver toxicity by improving biochemical, inflammatory and histopathological indices
⁎Corresponding authors. umar.ijaz@uaf.edu.pk (Muhammad Umar Ijaz), smarazi@ksu.edu.sa (Suhail Razak)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Nonylphenol (NP) is a ubiquitous environmental toxicant that is known to cause hepatotoxicity via generation of reactive oxygen species (ROS). Aucubin (AUC), an iridoid glucoside, is a potential phytochemical possessing antioxidant and anti-inflammatory features. The current study was intended to ascertain the hepatoprotective role of AUC against NP-generated liver toxicity in rats. 48 male rats were distributed into four groups. viz. Control, NP-intoxicated group (50 mg/kg), NP + AUC-treated group (50 mg/kg + 40 mg/kg) and AUC-treated group (40 mg/kg). All the doses were administered orally. Our findings indicated that the NP intoxication upregulated the alanine aminotransferase (ALT), alkaline phosphatase (ALP) and aspartate aminotransferase (AST) level. Moreover, it brought down the activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GSR) and glutathione-S-transferase (GST), as well as the glutathione (GSH) content. Conversely, the levels of malondialdehyde (MDA) and ROS were escalated. Besides, the levels of nuclear factor-kappa B (NF-κB), tumor necrosis factor-alpha (TNF-α), interleukin-1beta (IL-1β) and IL-6 as well as the cyclooxygenase-2 (COX-2) activity were elevated due to the NP administration. In addition to it, histopathological assessment displayed the prominent morphological alterations in hepatic tissues of rats. Nevertheless, treating the rats with AUC significantly (p < 0.05) abated all the NP-instigated liver damages in rats. Therefore, it was evinced that AUC could be used as a hepatoprotective agent against the NP-prompted liver toxicity owing to its antioxidant and anti-inflammatory properties.
Keywords
Aucubin
Nonylphenol
Protective effect
Liver damage
Hepatotoxicity
Antioxidant
1 Introduction
Nonylphenol (NP) is a synthetic xenoestrogen and breakdown product of alkylphenol polyethoxylates, which possesses endocrine-disruptive properties. However, it is proved to be more reactive, toxic and persistent as compared to its precursor compound (Bai et al., 2017). NP is a non-ionic surfactant that is used in the formulation of various detergents, plastic objects, paints, herbicides, resins, cosmetics, emulsifiers, solubilizers and various other daily-use items (Amaro et al., 2014). Humans are exposed to the NP via direct absorption, inhalation as well as ingestion of NP-contaminated water and foods such as, vegetables, fish, milk, fruits and cereals (Li et al., 2010). The concentration of NP in various food items ranges from 0.1 to 19.4 μg/kg and its approximate intake in humans is 7.5 μg/day. Owing to its lipophilic potential and long half-life, NP and its metabolites are accumulated in body that can be detected in milk, blood, urine and body tissues of humans (Acir and Guenther, 2018).
NP is reported to generate multiple toxicities in body such as immunotoxicity, neurotoxicity, embryotoxicity (Soares et al., 2008) as well as male reproductive toxicity. NP mainly generates toxicity via elevation of ROS that leads to the imbalance of oxidants and antioxidants and increase lipid peroxidation (Ijaz et al., 2021). The imbalance of antioxidant-oxidants damages the macromolecules such as, DNA, proteins and lipids, which ultimately produces oxidative damage in various organs including liver. It is well-established that liver is center of metabolic events as well as site for detoxification and storehouse of xenobiotics. Besides, NP has shown the tendency to concentrate in liver cells (Uguz et al., 2003). It was evinced that the NP is a xenoestrogen having capability to mimic the activities of natural estradiol and therefore, it interacts with the estrogen receptors on hepatocytes and affect the liver (Lu and Gan, 2014). Mohamed et al. (2019) stated that NP exposure causes hepatotoxicity via increasing lipid peroxidation and level of inflammatory markers as well as by inducing hyperbilirubinemia. Moreover, NP is evidently metabolized in liver and therefore, it results in the impairment of liver parenchymal cells and steatosis, thereby disrupting the overall structure and function of hepatic system (Kazemi et al., 2016).
Scientists are striving to seek safer and effective hepatoprotective phytochemicals possessing antioxidant properties to counter liver damages (Singh et al., 2016). Aucubin (AUC), an iridoid glucoside, is a phytochemical possessing numerous remedial effects. It is extracted from the various Chinese pharmacological plants such as hardy rubber tree and Chinese plantain as well as Japanese aucuba (Ma et al., 2019). Moreover, AUC is reported to exhibit anti-oxidant, anti-fibrotic, anti-inflammatory, anti-carcinogenic and neuroprotective features (Zeng et al., 2020). Therefore, aforementioned pharmacological properties of AUC impelled us to use it as a therapeutic agent to cure the NP-prompted liver toxicity in rats via assessing liver serum enzymes, oxidative stress markers, inflammatory markers and histoarchitectural indices.
2 Material and methods
2.1 Animals
A total of 48 male albino rats of 8 weeks of age (180–230 g) were held in Animal facility of University of Agriculture, Faisalabad for trial. Standard conditions (24 ± 2 °C; 12 h light/dark cycle) were sustained. Moreover, open access to standard food and water was given to rats. Rats were kept in laboratory for a week to acclimatize them to laboratory conditions. Besides, all the animal handling and research was performed according to the rules of Ethical Committee of University of Agriculture, Faisalabad.
2.2 Experimental design
Rats were distributed into 4 groups and NP and AUC was given for 30 days. Group 1 was control. Group 2 was intoxicated with 50 mg/kg of NP dissolved in DMSO orally. Group 3 was co-treated with the NP (50 mg/kg) as well as 40 mg/kg of AUC by oral gavage. Group 4 was only treated with the 40 mg/kg of AUC orally. The dose of NP (50 mg/kg) was chosen according to our previous investigation (Woo et al., 2007). 40 mg/kg of AUC was selected as per the investigation of Yang et al. (2017). After the experiment, rats were anesthetized by CO2 inhalation and then decapitated. Cardiac blood was collected in syringes. Serum was separated from blood and stored at − 80 °C for the evaluation of hepatic function enzymes. Liver was detached and cut into equal halves. Half of it was kept in zipper bags and stored at −20 °C. Homogenization was performed with the chilled phosphate buffer saline (25 mM; pH: 7.4) at 11,000g for 20 min using sonicator homogenizer. The homogenate was later on used for the determination of inflammatory markers and antioxidative enzymes. Other half of liver was fixed with the 10% formalin for histoarchitectural observation.
2.3 Hepatic function enzymes
Levels of ALT, AST and ALP in samples were evaluated using ELISA kits (Abcam, MA, USA) and the standard technique given along with the kits was followed.
2.4 Biochemical assays
The SOD activity was evaluated using a technique presented by Kakkar et al. (1984). CAT activity was determined by the protocol of Aebi (1974). GSH was analyzed by protocol of Jollow et al. (1974). GPx activity was determined according to a process explained by Rotruck et al. (1973). GSR activity was analyzed by a protocol given by Carlberg and Mannervik (1975). GST was determined by the protocol of Habig et al. (1974). MDA level was estimated to measure the lipid peroxidation in accordance with a method stated by Ohkawa et al. (1979). ROS level was estimated by methodology of Hayashi et al. (2007).
2.5 Inflammatory markers analysis
Level of NF-kΒ (CSB-E13148r), TNF-α (CSB-E07379r), IL-1β (CSB-E08055r) and IL-6 (CSB-E04640r) in addition to activity of COX-2 (CSB-E13399r) were determined by ELISA kits (Cusabio Technology Llc, Houston, TX, USA) and the instructor’s guide was followed.
2.6 Histopathological examination
For the histoarchitectural observation, liver tissues were fixed in formalin (10%), dehydrated with ascending grades of alcohol and then embedded in the wax blocks. After that, thin sections of approximately 5 μm were made via a microtome. Staining was done by the hematoxylin and eosin. Finally, photomicrographs were taken by observing the slides under the microscope (Leica DM4000B; Leica Microsystems GmbH).
2.7 Statistical analysis
Data was depicted as Mean ± SEM. Data was investigated by the one-way ANOVA. Finally, Tukey test was applied and GraphPad prism 5 software was used. Significance level was kept as p < 0.05.
3 Results
3.1 Role of NP and AUC on hepatic serum enzymes
Assessment of varying levels of liver serum enzymes indicate the condition of hepatic system. NP exposure led to a significant upsurge in ALT (P < 0.001), AST (P < 0.01) and ALP (P < 0.001) level in NP-administered rats compared with control (Fig. 1). Conversely, co-treatment of AUC and NP substantially reduced the level of ALT (P < 0.01), AST (P < 0.001) and ALP (P < 0.001) in comparison to NP-gavage rats. Additionally, the levels of ALT (P < 0.001), AST (P < 0.01) and ALP (P < 0.001) were noticed to be significantly reduced in AUC (only) treated group compared with the NP-intoxicated group. However, no significant difference was observed between AUC group and control.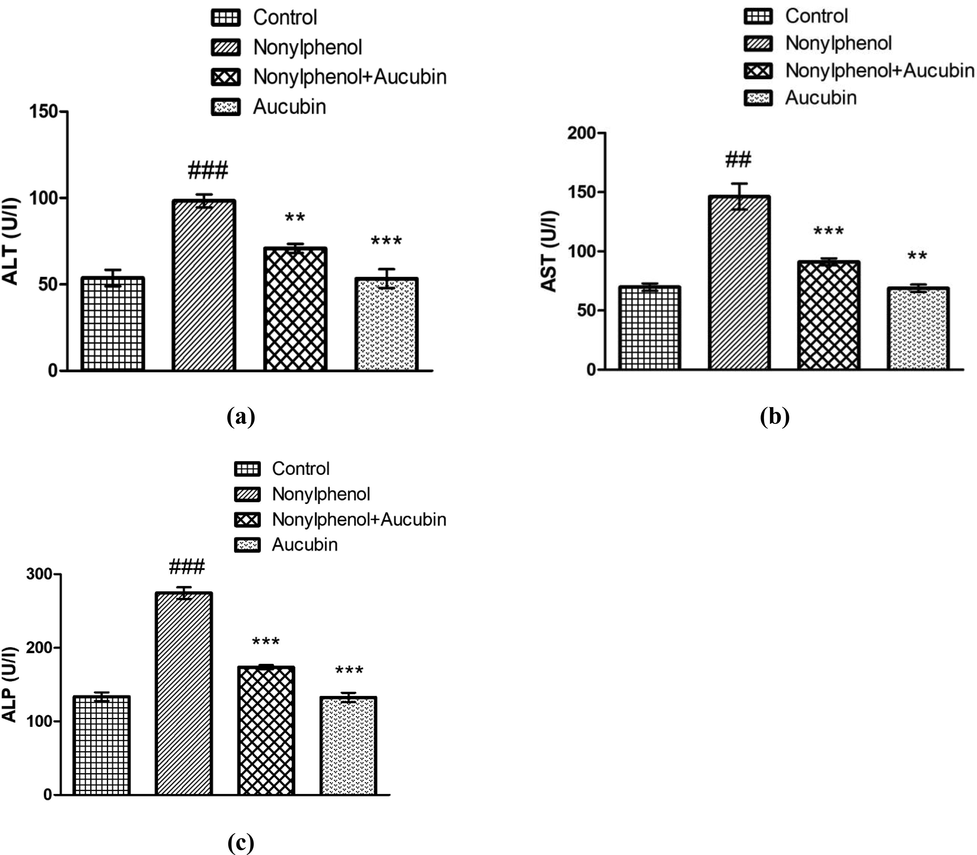
Role of NP and AUC on liver function markers. (a) ALT, (b) AST, and (c) ALP. Bars depict the mean ± SEM values (n = 12 rats/group). Significant differences shown as #P < 0.05, ##P < 0.01, ###P < 0.001 compared to control; *P < 0.05, **P < 0.01, ***P < 0.001 compared to NP-intoxicated group.
3.2 Role of NP and AUC on antioxidant profile
The antioxidant enzymes hold the capacity to counter free radicals. Whereas, the reduction in the antioxidative enzymatic activities implicates oxidative damage. The NP intoxication caused significant reduction in activity of SOD (P < 0.001), CAT (P < 0.01), GPx (P < 0.001), GSR (P < 0.001) and GST (P < 0.01), along with GSH level (P < 0.001) in NP group compared to control (Fig. 2). However, treating rats with NP + AUC remarkably escalated the activities of SOD (P < 0.001), CAT (P < 0.05), GPx (P < 0.001), GSR (P < 0.001) and GST (P < 0.01), as well as level of GSH (P < 0.001) in NP + AUC-treated rats compared to NP group. Furthermore, the activities of SOD (P < 0.001), CAT (P < 0.01), GPx (P < 0.001), GSR (P < 0.001) and GST (P < 0.01), as well as GSH level (P < 0.001) were seen to be significantly high in AUC (only) treated group in comparison to NP-administered group. Besides, no significant difference was noted among AUC group and control.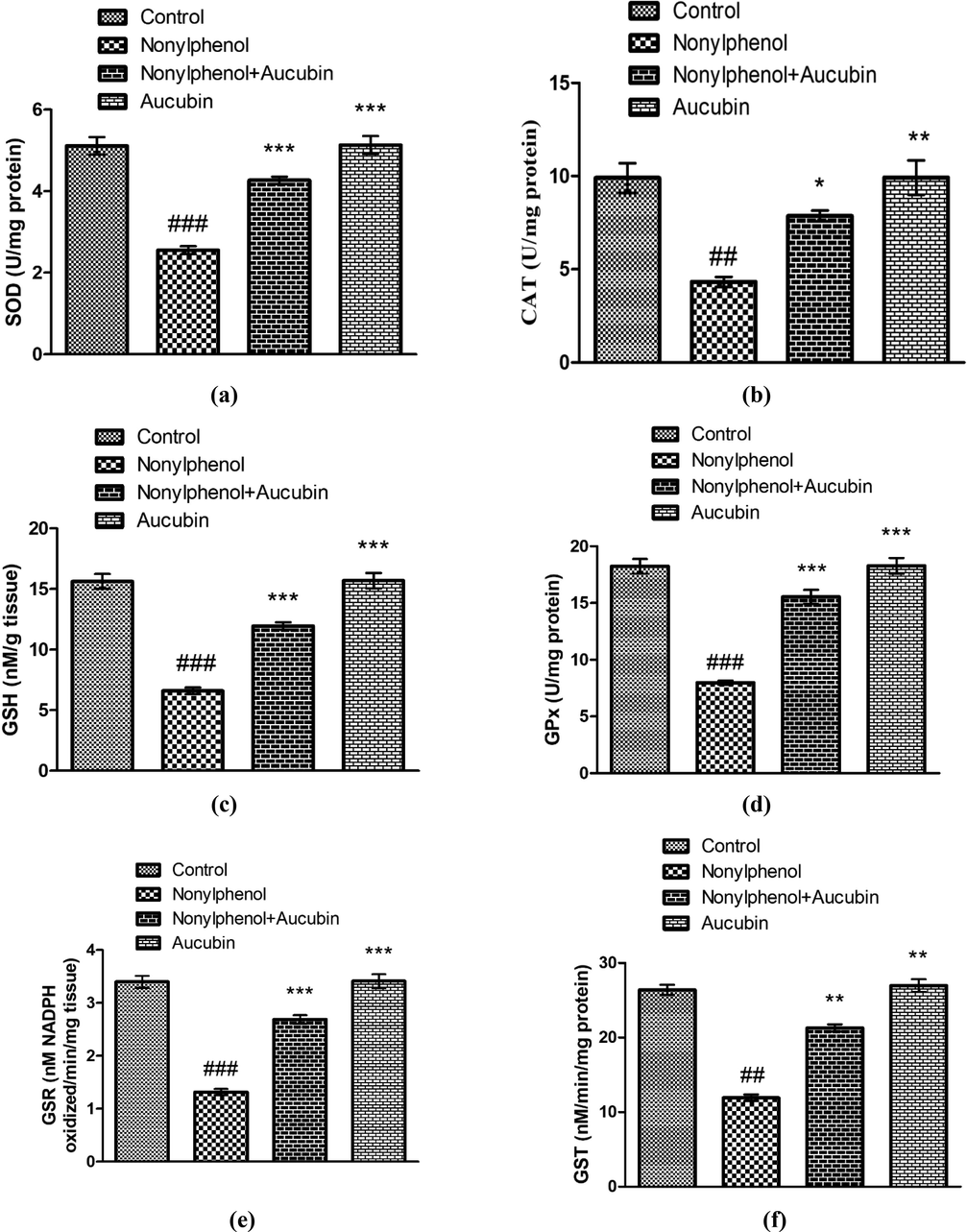
Role of NP and AUC on the antioxidant profile of hepatic tissues. (a) SOD, (b) CAT, (c) GSH, (d) GPx, (e) GSR, and (f) GST. Bars depict the mean ± SEM values (n = 12 rats/group). Significant differences shown as #P < 0.05, ##P < 0.01, ###P < 0.001 compared to control; *P < 0.05, **P < 0.01, ***P < 0.001 compared to NP-intoxicated group.
3.3 Role of NP and AUC on oxidative stress markers
The MDA is considered as the indicator of lipid peroxidation. NP administration brought a pronounced escalation in level of MDA (P < 0.001) and ROS (P < 0.05) in NP group compared to control (Fig. 3). However, AUC and NP co-treatment significantly reduced the level of MDA (P < 0.001) and ROS (P < 0.01) in NP + AUC-administered rats compared with NP group. Moreover, the levels of MDA (P < 0.001) and ROS (P < 0.01) were noted to be significantly low in AUC (only) treated group in comparison to NP-administered group. Whereas, no significant difference was observed among AUC group and control.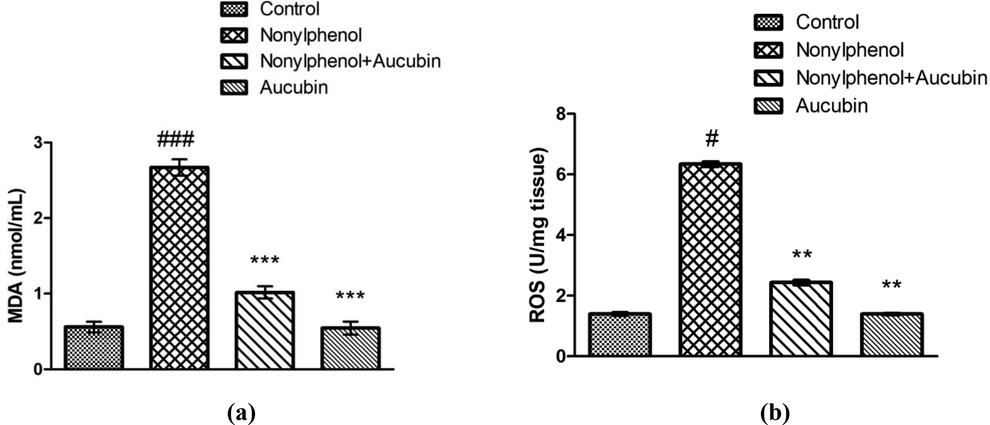
Role of NP and AUC on the oxidative stress markers of hepatic tissues. (a) MDA and (b) ROS. Bars depict the mean ± SEM values (n = 12 rats/group). Significant differences shown as #P < 0.05, ##P < 0.01, ###P < 0.001 compared to control; *P < 0.05, **P < 0.01, ***P < 0.001 compared to NP-intoxicated group.
3.4 Role of NP and AUC on inflammatory markers
NP provision significantly resulted in a visible increase in level of NF-kΒ (P < 0.001), TNF-α (P < 0.05), IL-1β (P < 0.001) and IL-6 (P < 0.01) in addition to increased COX-2 activity (P < 0.001) in liver of NP group compared with control (Fig. 4). Whereas, AUC treatment significantly suppressed the level of NF-kΒ (P < 0.001), TNF-α (P < 0.01), IL-1β (P < 0.001) and IL-6 (P < 0.01) as well as COX-2 activity (P < 0.01) in co-treated (NP + AUC) group in contrast to NP group. Additionally, the levels of NF-kΒ (P < 0.001), TNF-α (P < 0.01), IL-1β (P < 0.001) and IL-6 (P < 0.01) apart from COX-2 activity (P < 0.001) were observed to be significantly lower in AUC (only) treated group compared to NP-administered group. Besides, no significant difference was noticed among inflammatory marker levels of AUC (only) treated group and control.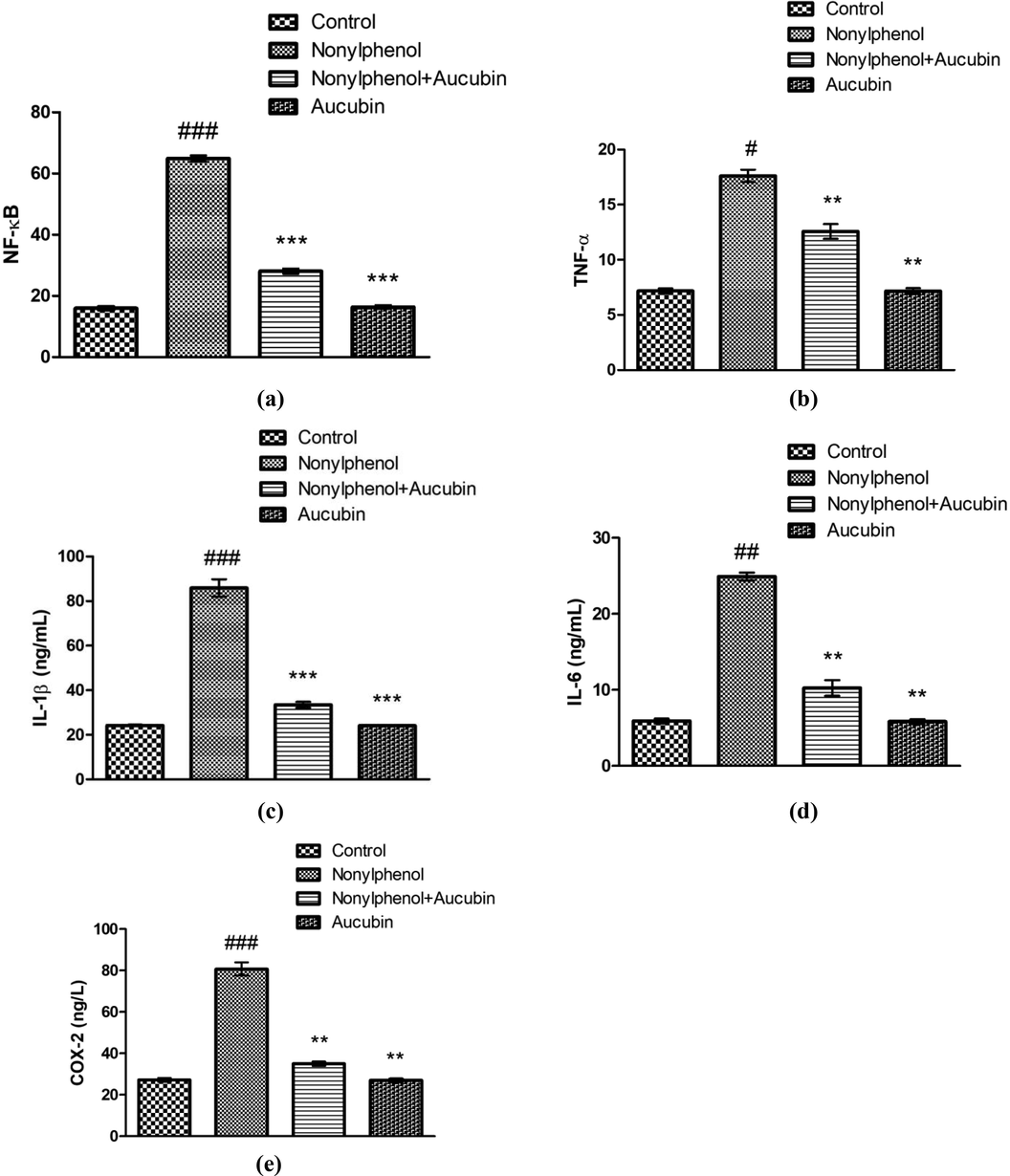
Role of NP and AUC on the inflammatory markers in hepatic tissues. (a) NF-kΒ, (b) TNF-α, (c) IL-1β, (d) IL-6, and (e) COX-2. Bars depict the mean ± SEM values (n = 12 rats/group). Significant differences shown as #P < 0.05, ##P < 0.01, ###P < 0.001 compared to control; *P < 0.05, **P < 0.01, ***P < 0.001 compared to NP-intoxicated group.
3.5 Role of NP and AUC on histopathological parameters
NP exposure significantly induced the histoarchitectural changes in hepatic tissues. NP exposure led to the dilated sinusoids and disrupted central venules as well as adverse alterations in Kupffer cells and nuclei of hepatocytes in comparison to tissue samples of control (Fig. 5). Whereas, AUC supplementation remarkably mitigated all structural alterations in liver morphology in NP + AUC-treated group compared to NP-intoxicated rats. Additionally, the histopathological state of hepatic tissues in AUC group was remarkably better in the AUC (only) treated group compared with NP-intoxicated group. Moreover, no significant difference was noticed among rats of AUC group and control.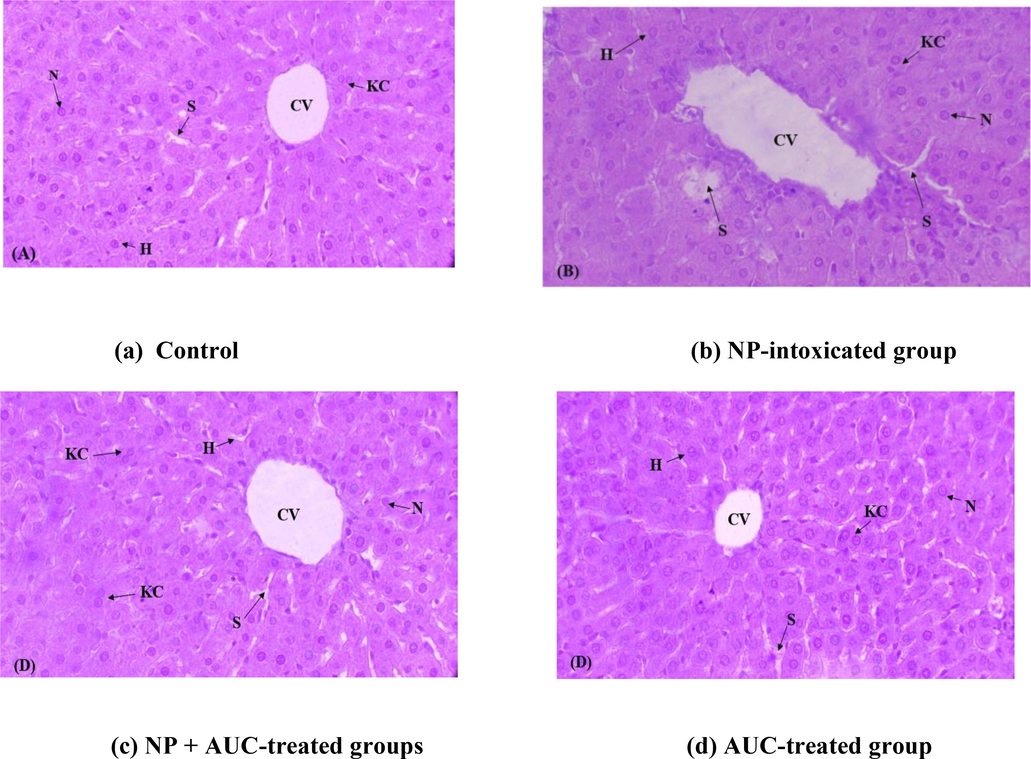
Role of NP and AUC on morphology of liver tissues (400x). (a) Control group exhibiting normal histology, (b) NP group displaying necrosis all over the hepatic tissues, (c) NP + AUC group revealing prominent improvements in morphology of hepatic tissues, and (d) AUC group showing noticeable structural similarities with the control group. NP, nonylphenol; AUC, aucubin; S, sinusoids; CV, central vein; KC, kupffer cell; N, nucleus; H, hepatocytes.
4 Discussion
In the current study, NP administration exhibited a remarkable rise in levels of ALT, AST and ALP, which indicated significant hepatic damage. A most prominent approach to analyze role of toxicants on liver functionality is via detection of aforementioned hepatic function enzymes in the serum (Ulasoglu et al., 2019). Rupture in membrane of hepatocytes results in loss of membranous integrity, which leads to the release of hepatic markers from cytosol into the blood, thereby indicating the hepatic dysfunction (Nagai et al., 2016). Previous findings have revealed that excess generation of ROS damage the membrane integrity of hepatocytes, which brought an uprise in level of the hepatic biomarkers (Pratibha et al., 2006). Therefore, it was deduced that the NP exposure brought an escalation in level of ROS and the lipid peroxidation, which could impair the membrane integrity of liver cells, subsequently leading to upregulation in levels of these markers. However, AUC supplementation lowered the activities of the hepatic enzymes in serum. It was assumed that AUC has tendency to exhibit palliative effect on membranous structure of hepatocytes by bringing down the lipid peroxidation and ROS level, thereby abrogating the release of hepatic enzymes in the blood.
Findings of our research demonstrated a decline in activity of antioxidant enzymes in hepatocytes due to the administration of NP. Liver cells convert the lipophilic xenobiotics to the hydrophilic state and facilitate their ejection from the body by the assistance of activity of antioxidant enzymes (Das and Roychoudhury, 2014). However, the NP-prompted decline in enzymatic activities inhibited the elimination of NP from the body, thereby causing cascade of damages in hepatic tissues. The SOD enzyme potentially cleaves the O−2 into H2O2 and O2, which also prevents the generation of toxic OH• ions (Kheradmand et al., 2010). CAT plays essential role in the breakdown of H2O2 into H2O and O2. However, reduction in CAT activity leads to the accumulation and transformation of H2O2 to ·OH, a highly toxic ion (Vicente-Sánchez et al., 2008). GSH, a cofactor of GPx, shields hepatocytes against the damages by reducing levels of H2O2 and other peroxides as well as plays a crucial role in xenobiotic detoxification (Fenoglio et al., 2005). GST assists in prevention of oxidative damages from the free radicals and is involved in removal of toxic substances (Hayes et al., 2005). It is well-established that the cell membranes are enriched in poly-unsaturated fatty acids (PUFAs). The NP-generated ROS react with the lipid content of the PUFAs, and cause lipid peroxidation as evinced by the elevated level of MDA. Conversely, treating rats with the AUC led to a prominent escalation in activities of antioxidant enzymes and GSH, while level of MDA and ROS was reduced. The antioxidant property of AUC owes to its ROS scavenging feature as well as its protective effect on antioxidant defense system in the body. It is indicated that the polyphenols have tendency to inhibit the oxidative stress, due to the presence of high number of hydroxyl groups in their structure (Dion et al., 2015). AUC is a polyphenolic agent, having several hydroxyl groups in its structure, which plausibly contributed to its antioxidant effects. Therefore, it was presumed that the possible reason behind the antioxidant nature of AUC are the multiple hydroxyl groups in its structure.
To ascertain the effect of NP and AUC on inflammatory index in liver, inflammatory marker profile was assessed. NP administration in rats resulted in substantial elevation in level of inflammatory markers. Oxidative stress leads to activation of NF-κB, which results in the liberation and shifting of activated NF-κB in the nucleus, thereby transcribing various inflammatory markers (TNF-α, IL-1β and IL-6) in addition to the inflammatory enzyme (COX-2) (Wang et al., 2018). NF-κB has tendency to control the numerous stages of inflammation simultaneously. Lee et al. (2004) reported that the COX-2 holds the tendency to elicit inflammatory responses and damages tissues following the NF-κB activation. Our study revealed that the NP caused inflammation in hepatocytes of rats due to elevation in levels of inflammatory markers. However, supplementation of AUC remarkably brought down their levels validating that it could play the role of potent anti-inflammatory agent. It was assumed that the NP led to the uprise in level of inflammatory biomarkers possibly by activating NF-κB signaling pathway, whereas the AUC treatment reduced the level of these markers by suppression of NF-κB activation.
Histomorphological assessment of liver tissues exhibited various adverse effects of NP on hepatic tissues such as, dilated sinusoids and disrupted central venules as well as adverse alterations in kupffer cells and nuclei of hepatocytes. NP prompted these structural damages owing to the lipid peroxidation and generation of free radicals. These free radicals have capability to disrupt the structure of macromolecules and cause oxidative damages, which subsequently affect the structure and normal functioning of liver (Zeashan et al., 2008). It was previously reported that NP exposure results in a prominent decline in antioxidative enzymes’ activities, which resulted in the induction of histoarchitectural alterations in the testicular tissues of rats (Ijaz et al., 2021). The current study evidenced that that the morphological damages were observed in hepatic tissues due the decrease in activities of antioxidant enzymes. However, administration of AUC profoundly abrogated the NP-instigated histopathological damages in the liver of rats. Therefore, it was inferred that AUC could confer histoprotective effect on the morphology of liver tissues due to its antioxidant and anti-inflammatory property.
5 Conclusion
Taken together, NP administration caused hepatotoxicity by elevating levels of ALT, AST and ALP as well as by decreasing the antioxidative enzymes activity and GSH content. Moreover, levels of MDA and ROS, along with the levels of inflammatory biomarkers were scaled up. In addition to it, histoarchitectural alterations were observed in the liver tissues due to NP intoxication. Nonetheless, AUC could efficaciously rectify the damages in hepatic system of rats suggesting that the AUC could be used as a potential hepatoprotective agent against NP-prompted liver toxicity owing to its antioxidant and anti-inflammatory nature.
6 Declarations
Ethics approval: The experimental protocol (Zoo#01213) was approved by the ethical board of the University of Agriculture, Faisalabad, Pakistan.
Consent to participate: Not applicable.
Consent for publication: Not applicable.
Availability of data and materials: All the data is contained in the manuscript.
Funding
The Authors extend their appreciation to the Researcher supporting project number (RSP2022R502), King Saud University, Riyadh Saudi Arabia for funding this project.
Author contribution
AR, AT, NE, and MU designed the study, conceived the study, and analyzed the results. SM and MUI conceived an initial part of the study, performed the experiment, histology and helped in compiling the results. SR, AA, AT, HA, ZH, and TA helped in writing the results. MU, SR, AT, MU, NE, TA, HA, ZH, NWA, and AA made a substantial contribution in the interpretation of data and revising the manuscript for intellectual content. All authors read and approved the final manuscript.
Acknowledgement
The Authors extend their appreciation to the Researcher supporting project number (RSP2022R502), King Saud University, Riyadh Saudi Arabia for funding this project.
Competing interests
The authors declare that they have no competing interests.
References
- Endocrine-disrupting metabolites of alkylphenol ethoxylates–a critical review of analytical methods, environmental occurrences, toxicity, and regulation. Sci. Total Environ.. 2018;635:1530-1546.
- [CrossRef] [Google Scholar]
- Catalase estimation. In: Bergmeyer H.U., ed. Methods of Enzymatic Analysis. Weinheim, New York: Verlag chemic; 1974. p. :673-684.
- [Google Scholar]
- Endocrine disruptor agent nonyl phenol exerts an estrogen-like transcriptional activity on estrogen receptor positive breast cancer cells. Curr. Med. Chem.. 2014;21:630-640.
- [Google Scholar]
- Nonylphenol biodegradation characterizations and bacterial composition analysis of an effective consortium NP-M2. Environ. Pollut.. 2017;220:95-104.
- [CrossRef] [Google Scholar]
- Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem.. 1975;250:5475-5480.
- [CrossRef] [Google Scholar]
- Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci.. 2014;2:53.
- [CrossRef] [Google Scholar]
- Dion, C., Haug, C., Guan, H., Ripoll, C., Spiteller, P., Coussaert, A., Lamottke, K., 2015. Evaluation of the anti-inflammatory and antioxidative potential of four fern species from China intended for use as food supplements. Nat. Prod. Commun., 10, 1934578X1501000416. https://doi.org/10.1177/1934578X1501000416.
- Effects of environmental pollution on the liver parenchymal cells and Kupffer-melanomacrophagic cells of the frog Rana esculenta. Ecotoxicol. Environ. Saf.. 2005;60:259-268.
- [CrossRef] [Google Scholar]
- Glutathione-S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem.. 1974;249(22):7130-7139.
- [Google Scholar]
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res. Genet. Toxicol Environ. Mutagen.. 2007;631:55-61.
- [CrossRef] [Google Scholar]
- Glutathione transferases. Annu. Rev. Pharmacol. Toxicol.. 2005;45:51-88.
- [CrossRef] [Google Scholar]
- Nobiletin ameliorates nonylphenol-induced testicular damage by improving biochemical, steroidogenic, hormonal, spermatogenic, apoptotic and histological profile. Hum. Exp. Toxicol.. 2021;40:403-416.
- [CrossRef] [Google Scholar]
- Bromobenzeneinduced liver necrosis. Protective role of glutathione and evidence for 3,4- bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11(3):151-169.
- [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys.. 1984;21:130-132.
- [Google Scholar]
- Nonylphenol induces liver toxicity and oxidative stress in rat. Biochem. Biophys Res. Commun.. 2016;479:17-21.
- [CrossRef] [Google Scholar]
- Ghrelin promotes antioxidant enzyme activity and reduces lipid peroxidation in the rat ovary. Regul. Pept.. 2010;162:84-89.
- [CrossRef] [Google Scholar]
- Spinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur. J. Neurosci.. 2004;19(12):3375-3381.
- [Google Scholar]
- Simultaneous determination and assessment of 4-nonylphenol, bisphenol A and triclosan in tap water, bottled water and baby bottles. Environ. Int.. 2010;36:557-562.
- [CrossRef] [Google Scholar]
- Analysis, toxicity, occurrence and biodegradation of nonylphenol isomers: a review. Environ. Int.. 2014;73:334-345.
- [CrossRef] [Google Scholar]
- Aucubin, a natural iridoid glucoside, attenuates oxidative stress-induced testis injury by inhibiting JNK and CHOP activation via Nrf2 up-regulation. Phytomedicine. 2019;64:153057
- [CrossRef] [Google Scholar]
- Palliative effects of zinc sulfate against the immunosuppressive, hepato-and nephrotoxic impacts of nonylphenol in Nile tilapia (Oreochromis niloticus) Aquac. 2019;504:227-238.
- [CrossRef] [Google Scholar]
- Protective effects of taurine on doxorubicin-induced acute hepatotoxicity through suppression of oxidative stress and apoptotic responses. Anti-cancer Drugs. 2016;27:17-23.
- [CrossRef] [Google Scholar]
- Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [Google Scholar]
- Enzymatic studies of cisplatin induced oxidative stress in hepatic tissue of rats. Eur. J. Pharmacol.. 2006;532:290-293.
- [CrossRef] [Google Scholar]
- Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588-590.
- [Google Scholar]
- Hepatoprotective effect of trans-chalcone on experimentally induced hepatic injury in rats: inhibition of hepatic inflammation and fibrosis. Can. J. Physiol. Pharmacol.. 2016;94:879-887.
- [CrossRef] [Google Scholar]
- Nonylphenol in the environment: a critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ. Int.. 2008;34:1033-1049.
- [CrossRef] [Google Scholar]
- The bioaccumulation of nonyphenol and its adverse effect on the liver of rainbow trout (Onchorynchus mykiss) Environ. Res.. 2003;92:262-270.
- [CrossRef] [Google Scholar]
- Characterization of patients with biopsy-proven non-alcoholic fatty liver disease and normal aminotransferase levels. J. Gastrointestin. Liver Dis.. 2019;28:427-431.
- [CrossRef] [Google Scholar]
- Effect of the flavonoid quercetin on cadmium-induced hepatotoxicity. Food Chem. Toxicol.. 2008;46:2279-2287.
- [CrossRef] [Google Scholar]
- Alleviation of cadmium-induced oxidative stress by trehalose via inhibiting the Nrf2-Keap1 signaling pathway in primary rat proximal tubular cells. J. Biochem. Mol. Toxicol.. 2018;32(1)
- [CrossRef] [Google Scholar]
- A repeated 28-day oral dose toxicity study of nonylphenol in rats, based on the ‘Enhanced OECD Test Guideline 407’for screening of endocrine-disrupting chemicals. Arch. Toxicol.. 2007;81(2):77-88.
- [Google Scholar]
- Gastroprotective effect of aucubin against ethanol-induced gastric mucosal injury in mice. Life Sci.. 2017;189:44-51.
- [Google Scholar]
- Hepatoprotective activity of Amaranthus spinosus in experimental animals. Food Chem. Toxicol.. 2008;46:3417-3421.
- [CrossRef] [Google Scholar]
- A review of the pharmacology and toxicology of aucubin. Fitoterapia. 2020;140:104443
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102033.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







