Translate this page into:
Protective effect of Gymnema sylvestre leaf extract against uranium toxicity in human peripheral blood mononuclear cells
⁎Corresponding authors. dean.cenr@srmist.edu.in (Kantha Deivi Arunachalam), sasikala@ucsiuniversity.edu.my (Sasikala Chinnappan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Uranium is a toxic heavy metal, naturally present in soil and water, that causes several health effects due to free radical generation and damage to DNA. Gynmenma sylvestre is a pharmacologically important medicinal plant that has been used in the treatment of diabetes and several other ailments. Accordingly, in this analysis, we intended to examine the protective effect of G. sylvestre leaf extract (GSE) against uranium toxicity.
Methods
Aqueous leaf extract of Gymnema sylvestre was prepared and its qualitative and quantitative phytochemical analysis was carried out. The antioxidant potential of the extract was analysed. Human lymphocytes isolated from freshly collected blood samples were treated with uranyl nitrate and GSE independently and in combination. The toxic effects of uranium and the protective effect of the leaf extract have been analyzed by MTT assay, micronucleus and γH2AX DNA damage response detection.
Results
Phytochemical analysis revealed the presence of major and important phytochemical groups such as alkaloids, flavonoids, saponins, tannins, etc. 15 μg/mL of GSE significantly reduced toxicity in the 3 different uranium concentrations (0.25, 0.50 & 0.75 mM). Cell viability percentage increased from 90.44 to 94%, 80.40 to 86% and 72.03 to 77.40% in the GSE and uranium co-exposure groups. GSE treatment led to statistically significant reduction in the average number of micronuclei observed per 1000 binucleate cells in all three groups with values 30.66, 40 and 48.33 micronuclei respectively. GSE was successful in reducing the γH2AX percentages in the treated cell groups with statistically significant protection with values 22.11 ± 1.56, 48.43 ± 0.83 and 51.14 ± 1.78 respectively.
Conclusion
This study is the first to report the in vitro toxic effects of uranium in human peripheral blood mononuclear cells as well as the protective effect of Gymnema sylvestre against uranium induced damages. GSE could alleviate DNA damage in cells treated with uranium in a dose-dependent way. Together these findings emphasize that GSE could be a promising protective agent against uranium-induced damages and should be further evaluated for the identification of active principle(s).
Keywords
Protective effect
Gymnema sylvestre extract
DNA damage
γH2AX
Micronucleus assay
Toxicity
Lymphocytes
- MTT
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MN
-
Micronucleus Assay
- GSE
-
Gynmenma sylvestre leaf extract
- FBS
-
Foetal bovine serum
- PHA
-
phytohemagglutinin
- DPPH
-
1,1–diphenyl–2–picrylhydrazyl
- ANOVA
-
Analysis of variation
Abbreviations
1 Introduction
Uranium is an abundant heavy metal present in the earth's crust. However human activities such as uranium mining, over-pumping of groundwater from archaic rock reservoirs, use of uranium in ammunition, nuclear reactor, and nuclear processing facilities, have led to elevated exposure of uranium to humans (Council, 1999). Occupational exposure and communities living in uranium mining regions are often exposed to long-term implications of uranium toxicity (Redvers et al., 2021). Uranium toxicity generates free radicals in the cellular systems which affects biomolecules and causes genetic damage (Gerber et al., 2018). The major toxicological concern is biochemical rather than radiochemical. Uranium has known to have direct genotoxic effects leading to DNA adduct, DNA damage due to chromosome breakage and nongenotoxic effects such as generation of reactive oxygen species which cause indirect genomic damage (Dashner-Titus et al., 2020; Stearns et al., 2005). This in turn leads to hypermutability, genomic instability, loss of growth; greatly increasing the risk of cancer (Council, 2012). Increased genotoxic and micronuclei damage in uranium miners' blood lymphocytes have been observed in short as well as long-term impact studies in cytogenetic analysis (Kryscio et al., 2009; Zölzer et al., 2012).
Health effects of uranium on humans have come from epidemiological studies done on accidental exposures and studies carried on occupationally exposed groups (Kelly‐Reif et al., 2020). Cytogenetic analysis of lymphocytes in such studies has indicated genomic damages but has provided inconsistent results. Epidemiological studies are not controlled experiments and therefore difficult to establish causal relationship (Checkoway et al., 2019). Therefore, there is a need to understand molecular mechanism and toxic effects of uranium to human cells and tissues. The limited work done to assess uranium effects invitro has yielded positive results but calls for further research in this area. Even though renal tissues have been long considered the primary target of uranium effects, many studies have shown deleterious effects on other organ systems including testes, spleen, thyroid, lymph nodes, and nervous system and cells (Homma-Takeda et al., 2019). While the standard procedure for estimating uranium, exposure is by urine testing, it is considered that 60–70% of uranium is excreted out (Keith et al., 2013).
Therefore, an estimate of uranium toxicity in blood cells would provide a better estimate of the systemic burden and indicate exposure to uranium. No such invitro studies on human lymphocytes had been done so far. Although there have been few epidemiological researches for estimating the toxicity of uranyl nitrate, very less has been explored in terms of protection. Several reports point towards the antioxidant capacity and drug detoxification potential of plant extracts, without themselves causing any side effects (Flieger et al., 2021). Ayurvedic medicine in India has tapped into this potential of phytochemicals to treat and alleviate symptoms of several diseases. Gymnema sylvestre R. Br., also known as “Meshasringi” in India, has been used to treat diabetes mellitus for centuries (Khan et al., 2019). Bioactive components of Gymnema sylvestre extract have been demonstrated to help with eye diseases, asthma, snakebite, piles, chronic cough, dyspesis, haemorrhoids, cardiopathy, colic discomfort and wound healing (Patel et al., 2012; Subramanian et al., 2020). GSE has been shown to have anti-diabetic, anti-inflammatory, anti-ulcer, anti-cancer and wound healing properties (Aleisa et al., 2014; Arunachalam et al., 2014; B. Aggarwal et al., 2011; Khan et al., 2019; Subramanian et al., 2021). Recent studies also indicated radioprotective effects of GSE in the alleviation of gamma radiation-induced toxicity (Sinha et al., 2018a). Several studies in the past have demonstrated the detoxifying capability of Gymnema sylvestre, however its protective action against uranium induced toxicity has never been reported so far.
The present study aims to explore the protective capability of the aqueous extract of Gymnema sylvestre against cellular damage caused by uranium in human lymphocytes in vitro. In this study, we carried out preliminary investigations to determine the NOAEL value of the GSE on human lymphocytes in vitro and to examine the potential of GSE in alleviating the uranium-induced toxic effects by micronucleus assay and Gamma-H2AX measurement.
2 Materials & methods
2.1 Chemicals
RPMI 1640, foetal bovine serum (FBS), L-glutamine, phytohemagglutinin (PHA), Monoclonal antibody anti-phospho- Histone H2A.X, FITC Conjugate were procured from Sigma Aldrich, India. Uranyl nitrate hexahydrate (UO2(NO3)2·6H2O, analytical grade was bought from Merck, India. 1,1-diphenyl-2-picrylhydrazyl (DPPH), MTT, Ficoll HiSep, and other chemicals were purchased from Himedia, India.
2.2 Preparation of the aqueous leaf extract
Leaves of the G. sylvestre plant were obtained from the “Herbal Garden at Tamil University, Thanjavur, Tamil Nadu”, and the herbarium was prepared for authentication (CENR/PTC/2018/05). The plant samples were validated by botanist, Prof. P. Jayaraman, “Plant Anatomy Research Centre, Chennai”. The leaves were washed with tap water twice, followed by rinsing in deionized water thrice. The leaves were shade dried at room temperature and powdered. To avoid the toxicity induced by the solvents, aqueous extract of leaf sample was prepared. Powdered leaf material was extracted thrice in distilled water at an orbital shaker for 72 h. Followed by this, the Whatman No.1 filter paper was used to filter the extract, lyophilized, and stored at −80 °C until further use (Aiyegoro and Okoh, 2010). The percentage yield (w/w %) was calculated from the product that was obtained after lyophilization using the given formula (Dowlath et al., 2020).
2.3 Preliminary phytochemical investigations
The qualitative phytochemical analysis for the aqueous extract of G. sylvestre was carried out by previously established methods. The extract was analyzed for the presence of phenolics (alkaline test, lead acetate reagent test), flavonoids (alkaline reagent test), alkaloids (Mayer’s, Wagner’s, Dragendorff’s test), triterpenes (Liberman-Burchard test), phenolics (lead acetate, alkaline reagent test), tannins (gelatine test), glycosides (Kellar Killani’s test), triterpenes (Tschugajen test) and saponins (foam test). The presence and absence of these phytochemicals in the extract were determined (Kumar et al., 2013).
2.4 Quantitative phytochemical analysis
2.4.1 Determination of total phenolic content
The Folin Ciocalteu method was performed to determine the phenolic contents present in the prepared extract (Hatami et al., 2014). In this assay, the extract was mixed for 2 h in dark conditions with FC reagent at 37 °C and the absorption was measured using a UV–Vis spectrophotometer at 765 nm (Lab India, India). Gallic acid of 0.1 mg/mL concentration was used as the standard and values were represented in mg Gallic acid equivalent per gram of dry extract.
2.4.2 Determination of total flavonoids
The aluminum chloride colorimetric technique was used to identify the total flavonoids in the plant extract (Fattahi et al., 2014). The extract has been dissolved in deionized water and mixed with 5% sodium nitrite and incubated for 6 min. Further, following the addition of aluminum chloride (10%), the tube was incubated for 5 min. 1 M sodium hydroxide was added and the tubes were incubated at room temperature for 5 min. Spectrophotometrically, absorbance was recorded at 510 nm (UV–VIS spectrophotometer, Lab India). The total flavonoids were calculated in mg catechin equivalent (CE)/g of plant extract.
2.5 Total antioxidant capacity
This capacity can be determined by dissolving the extract in water in equal volume and to it, a reagent solution containing 4 M ammonium molybdate, 28 mM sodium phosphate, and 0.5 M sulfuric acid, was added (Jan et al., 2013). The solution obtained was mixed thoroughly and incubated for 5 min. The absorption was read spectrophotometrically at 695 nm (at UV–Vis spectrophotometer, Lab India, India). The reaction mixture has been incubated and absorbance determined at 695 nm with a reagent blank. The standard used was Gallic acid and the results were calculated as ascorbic acid equivalent.
2.6 DPPH radical scavenging assay
This assay was performed to estimate the free radical scavenging activity of GSE (Rahman et al., 2014). In this method, GSE was mixed with 0.1 mM DPPH solution. The mixture was thoroughly mixed and incubated for 30 min at 25 °C. Ascorbic acid (1 mM) was considered the standard. The absorbance was read spectrophotometrically at 517 nm (UV–VIS spectrophotometer, Lab India, India). The DPPH radical scavenging ability value is represented as ascorbic acid equivalents per grams of the plant extract.
2.7 Cytoprotective ability
2.7.1 Lymphocytes isolation
A total of five healthy male volunteers were enrolled in this study who belonged to an age group of 20 to 30 years with a mean age of 28 ± 1.6 years and were non-alcoholic and non-smoking without recent exposure to mutagens or drug therapy. After receiving written consent from the volunteers, 5 mL of blood sample was collected by the venipuncture method. The study was approved by the “Ethics Committee, SRM Medical College Hospital and Research Centre” (IEC:1400A). The blood taken in heparin vacutainers was thoroughly mixed with the same volume of PBS (“Phosphate Buffered Saline”). The diluted blood sample was then gently stacked and centrifuged to produce the buffy layer using Ficoll HiSep. The buffy layer of peripheral blood mononuclear cells was washed three times with PBS. The cells (1x106 cells/mL) were then poured in a medium RPMI 1640, mixed with 10% FBS, also stimulated with 2% PHA and cultured at 37 °C with an atmosphere of 5% CO2. All experiments were performed invitro on isolated human peripheral blood mononuclear cells obtained from the study participants’ blood samples.
2.7.2 Treatment and MTT assay
Lymphocytes were analyzed for toxicity induced by GSE in the concentration range of 1–50 μg/mL. As no adverse effect level (NOAEL) was found, the maximum dosage of extract which is not substantially different from the control response was determined (Tualeka et al., 2019). Combination treatment of NOAEL dose of G. sylvestre with three uranyl nitrate concentrations; 0.25, 0.50 & 0.75 mM were thereafter performed with filter sterilization. The cells were cultured for 96 h after respective treatments and then the cytotoxic and cytoprotective ability was evaluated using the standard MTT assay. Briefly, after the treatment, the cells were incubated with an MTT reagent (5 mg/mL) at 37 °C for 4 h. The absorbance of the formazan crystals formed was analyzed by dissolving in dimethyl sulfoxide and reading at 570 nm and 720 nm with an ELISA reader (Multiscan EX, Thermo Scientific, United States). The percentage of cell viability was measured by the following equation (Meerloo et al., 2011):
2.7.3 Micronucleus (MN) assay
MN assay was done by addition of cytochalasin B, 44 h after initiation of culture (Akyıl et al., 2015). The cells were then centrifuged and treated with hypotonic solution (0.125 M KCl). The pellet was then harvested by resuspending in KCl, mixing, and repeating the steps three times. Carnoy's fixative was poured into the tube and thereafter slides were prepared. The slides were stained by Giemsa and inspected with a full filter at 800X1000 magnification with Nikon Eclipse 80i light microscope. These slides have been coded for scoring, and 1000 binucleated (BN) cells were evaluated for the presence of one or more micronuclei for every exposure.
2.7.4 Gamma-H2AX measurement
Gamma-H2AX (γH2AX) detection was carried out according to the manufacturer's protocol using flow cytometry and FITC tagged antibody (Muslimovic et al., 2008). Cells were incubated for a 96 h study period, after which the cells were fixed by adding paraformaldehyde (4%). The samples were washed once with PBS and 0.5 percent Triton X-100 was applied to permeabilize the cell wall. Thereafter, the cells were centrifuged, the supernatant removed and monoclonal antibody anti-phospho- Histone H2A.X, FITC Conjugate was added to the cells. The cells were then resuspended in PBS after incubation at 37 °C for 2 h and analyzed directly on a Becton-Dickinson FACS-Calibur flow cytometer. To analyze, histograms were plotted based on the fluorescence intensity in arbitrary units, and by using BD CellQuest Pro software, the mean fluorescence intensity has been determined (Ismail et al., 2007). The mean γH2AX antibody staining intensity to the isotype control was calculated with the mean fluorescence. The labeled samples were gated according to the control histograms to calculate the percentage of cells stained with γH2AX antibody. Each sample was analyzed for 10,000 cells.
2.8 Statistical analysis
A two-way ANOVA test was done with Tukey's multiple comparisons test for statistical analysis by utilizing GraphPad Prism 8.2 software. These treatments were performed triplicates and the findings were expressed as a mean ± S.D. (standard deviation). As statistically significant, P < 0.05 was considered.
3 Results
3.1 Phytochemical screening
The percentage yield (w/w %) of G. sylvestre aqueous extract was calculated and found to be 13.8%. The results of the preliminary phytochemical analysis of the GSE screening are shown in Table 1 and Table 2. The phenol content was 295.24 ± 1.12 μg/g, flavonoids were 134.63 ± 15.85 μg/g, total antioxidants were 10.14 ± 0.04 and free radical scavenging activity of 62.14 ± 0.32 were detected in GSE.
Phytochemical
Presence (+)/ Absence (-)
Alkaloids
+
Flavonoids
+
Glycosides
–
Saponins
+
Triterpenoids
+
Phenolics
+
Steroids
+
Tanins
+
Qualitative Analysis
Results
Total Phenolics
295.24 ± 1.12
Total flavonoids
134.63 ± 15.85
Total antioxidant activity
10.14 ± 0.04
DPPH free radical scavenging activity
62.14 ± 0.32
3.2 Cytotoxicity in human lymphocytes
When assayed by MTT, G. sylvestre did not show any cytotoxicity in the tested doses in human lymphocytes. In concentrations above 15 μg/mL higher cellular counts than the control was observed, indicating the mitogenic ability of plant extract. The concentration of 15 μg/mL of GSE was the maximum extract dosage not statistically distinct from the control response (data not given) and was thus identified as NOAEL. Uranyl nitrate was toxic to human peripheral blood mononuclear cells in all tested doses (P < 0.05 versus Control). G. sylvestre was observed to be successful in protecting against uranyl nitrate-induced cytotoxicity (Fig. 1).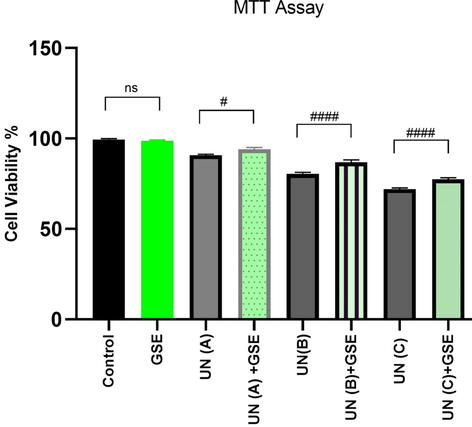
Effect of G. sylvestre extract (GSE) on uranyl nitrate (UN) induced cytotoxicity in human peripheral lymphocytes. A = 0.25 mM, B = 0.50 mM and C = 0.75 mM uranyl nitrate, GSE (NOAEL) = 15 μg/mL. # refers to P values of UN vs UN + GSE group with statistical significance P < 0.05 level. Values given in Table 2.
3.3 Micronuclei frequency in treated human lymphocytes
The micronuclei frequency in cultures treated with uranyl nitrate was significantly higher than control values. An increase in concentrations of uranyl nitrate resulted in increased micronuclei (Fig. 2 and Fig. 3). A significant decrease in micronuclei was observed in cell cultures treated with GSE along with uranyl nitrate (P < 0.05). GSE by itself did not cause any genetic damages (P-value non-significant compared to control). The average number of micronuclei observed per 1000 binucleate cells with treatment doses 0.25 mM, 0.50 mM & 0.75 mM were 40.66 ± 2.08, 49.66 ± 3.03 and 55.33 ± 3.05 respectively.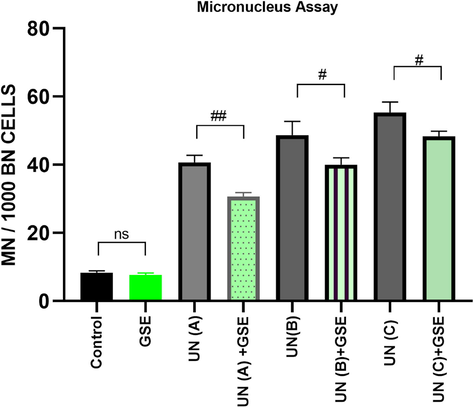
Micronucleus assay results depicting MN (%) in human lymphocytes treated with different concentrations of uranyl nitrate (UN) and G. sylvestre extract (GSE). BN = Binucleate cell, # refers to P values of UN Vs (UN + GSE) group with statistical significance P < 0.05 level. Abbreviations and values as in Table 2.
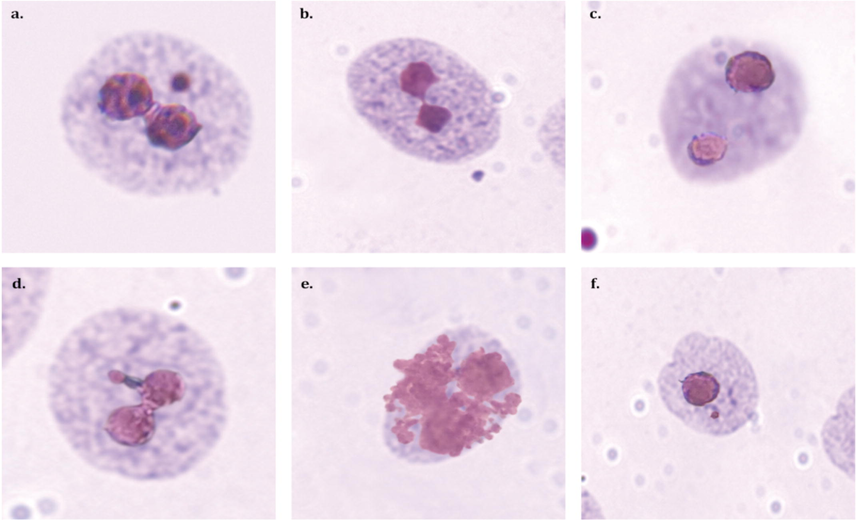
Representative images of micronuclei formation as observed in this study. a.) Binucleate cell (BN) with micronucleus (MN) b.) BN with nucleo-plasmic bridge c.) BN cell d.) BN with nuclear bud e.) Apoptotic cell f.) Mononucleated cell with MN.
3.4 Measurement of DNA damage and repair response
A considerable augmentation of DNA damage response was observed as enhanced phosphorylation of histone H2AX, with increasing doses of uranyl nitrate in human lymphocytes which were statistically significant in all treatment groups (Fig. 4). Treatment with G. sylvestre alone did not result in γH2AX detection signifying no DNA damage in comparison to control (P-value non-significant compared to control). The average number of γH2AX percentages in the cells treated with uranium doses 0.25 mM, 0.50 mM & 0.75 mM were 33.52 ± 3.33, 57.18 ± 2.24and 63.02 ± 1.84 respectively. G. sylvestre was successful in reducing the γH2AX percentages in the treated cell groups with statistically significant protection observed in all three treatment groups (Table 3). summary: a to e is Control Vs treatment; f to j is UN Vs (UN + GSE) a = not significant compared to control group, f = not significant compared to respective UN treatment group. b = significant compared to control group (P < 0.0001) (****), g = significant compared to respective UN treatment group (P < 0.0001) (####) c = significant compared to control group(P < 0.001) (***), h = significant compared to respective UN treatment group (P < 0.001) (###) d = significant compared to control group(P < 0.01) (**), i = significant compared to respective UN treatment group(P < 0.01) (##). e = significant compared to control group (P < 0.05) (*), j = significant compared to respective UN treatment group (P < 0.05) (#).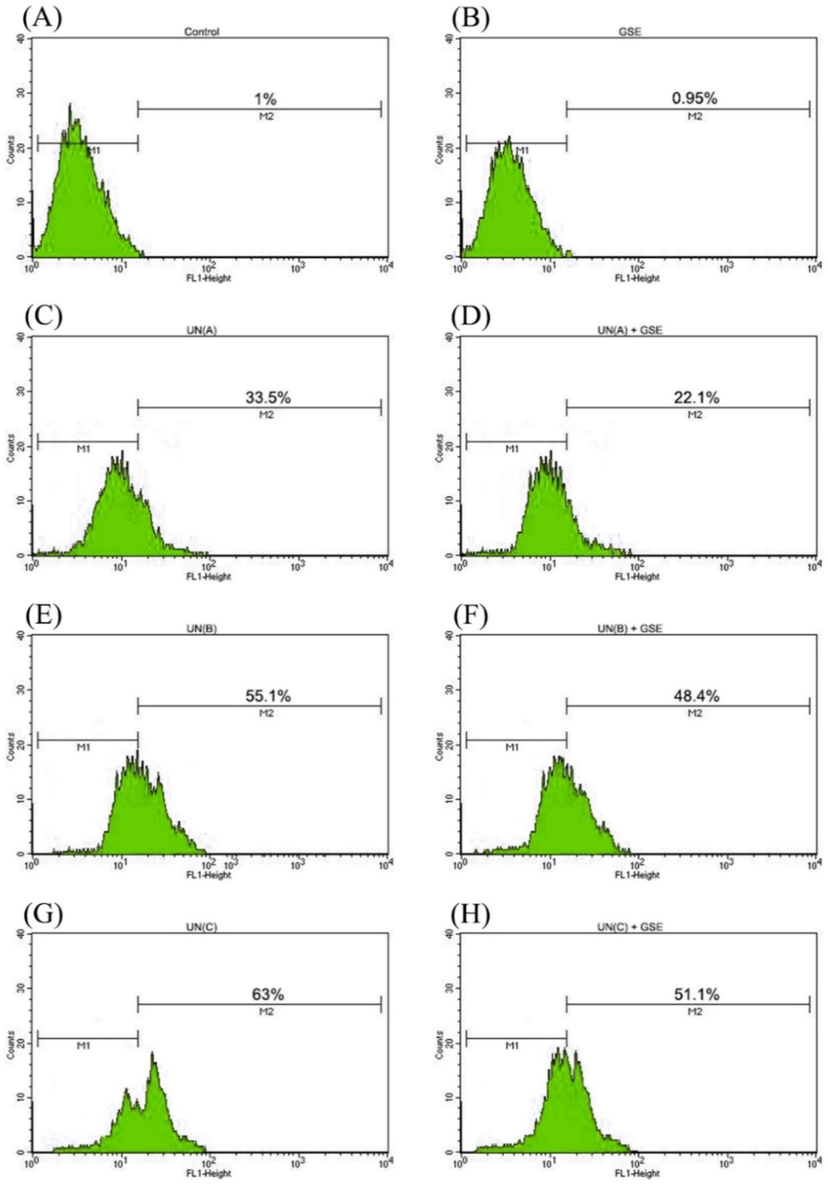
Flow cytometric detection of γH2AX formation induced in human peripheral blood lymphocytes in response to treatments with uranyl nitrate (UN) and G. sylvestre (GSE). M1 refers to cells with basal DNA damage response. M2 defines the percentage of cells with increased DNA damage response represented by enhanced phosphorylation of histone H2AX. Statistical analysis and abbreviations as in Table 2.
Treatment Groups
Cell Viability %
MN/1000BN Cells
γH2AX %
Control
99.40 ± 0.51
8.33 ± 0.57
1.04 ± 0.16
GSE
98.66 ± 0.57a
7.66 ± 0.56a
0.95 ± 0.06a
UN (A)
90.77 ± 0.58b
40.66 ± 2.08b
33.52 ± 3.33b
UN (A) + GSE
94 ± 1.0b,j
30.66 ± 1.15b,i
22.11 ± 1.56b,g
UN (B)
80.40 ± 0.94b
49.66 ± 3.0b
57.18 ± 2.24b
UN(B) + GSE
86.81 ± 1.33b,g
40 ± 2.0b,i
48.43 ± 0.83b,j
UN (C)
72.03 ± 0.62b
55.33 ± 3.05b
63.02 ± 1.84b
UN (C) + GSE
77.40 ± 0.92b,g
48.33 ± 1.52b,j
51.14 ± 1.78b,g
4 Discussion
Plant products have gained attention in research because of the presence of medically important bioactive constituents. The availability of these compounds signifies the role of plant products in healthcare industry (Mousavi et al., 2018). The application of plant products with strong antioxidant activity which is due to the presence of phenolic compounds is considered as a better strategy for preventing oxidative cellular damage (Dhanasekaran, 2019). Our study shows the presence of phytochemicals such as alkaloids, phenolics, flavonoids, saponins which are responsible for the antioxidant activity. The GSE also had sufficient phenol content (295.24 ± 1.12 mg GAE/g extract) and flavonoids (134.63 ± 15.85 mg CAE/g extract). The GSE is an efficient free radical scavenger as shown in Table 2. We observed that G. sylvestre extract was nontoxic to human lymphocytes in vitro in our tested dose. The tested dose of GSE did not result in γH2AX detection signifying no DNA damage in comparison to the control group. The identification of mitogenic ability of the GSE is an added advantage suggesting that G. sylvestre is safe for human lymphocytes and can potentially be used for therapeutic purposes. In a study carried out by Singh et al, 2016 Gymnemic acid, a phytochemical available in Gymenema extracts were found to demonstrate stimulation of lymphoproliferation, both in the presence and absence of mitogens on splenic lymphocytes. They concluded that the Gymnemic acid shows immunomodulatory and lympho proliferation abilities (Singh et al., 2016).
Previous studies of uranium effect on human lymphocytes have been epidemiological with studies carried out in samples from individuals exposed to uranium via accident or working in uranium mines. The casual impact was hard to identify as effects of other toxicants and radon are also involved in uranium miners (Kryscio et al., 2009). The present study involved preliminary investigations of uranyl nitrate effect on human lymphocytes in vitro and dose-dependent increase in cytotoxicity was observed by MTT assay. An exorbitant elevation in micronuclei was observed as a consequence of increased uranyl nitrate concentration. Consistent with our micronuclei results, we observed a high DNA damage response triggered in all uranyl nitrate treated cultures by an increase in γH2AX percentage in human lymphocytes. Similarly, a dose-dependent elevated γH2AX expression in BEP2D cells was found following treatment with uranyl nitrate in a 24 h study (Jin et al., 2017). The present study also demonstrates the genotoxic effects of uranyl nitrate on human lymphocytes using γH2AX staining, confirming their utility as genotoxic biomarkers, a useful tool for biomonitoring (Nikolova et al., 2014). γH2AX may therefore serve as a useful biomarker of uranium exposure in human communities such as individuals working in uranium mines, communities living close to areas of uranium mining, nuclear reactor workers, and those exposed to accidental exposure to uranium.
From the results of the experiments conducted, G. sylvestre was recorded to be successful in protecting cells induced with uranyl nitrate cytotoxicity which can be attributed to the existence of antioxidants and various secondary metabolites. The elevation in the micronuclei got declined when treated with GSE proving its efficacy in rectifying cytotoxicity. Environmental factors and increased oxidative damage by chemicals trigger DNA double-strand breaks which have also led to identifying plant-based therapeutics to counter DNA damage. Previous studies have indicated that G. sylvestre has a protective action against radiation-induced DNA damages due to its free radical scavenging and antioxidant capabilities (Sinha et al., 2018a). Besides being safe by not causing any genetic damage to cells GSE was also successful in rectifying DNA damage by efficiently reducing the percentage of γH2AX in uranyl nitrate-treated cells in all tested doses. Natural antioxidant compounds can be relied for preventing the DNA damage caused by free radical generation (Aryal et al., 2019; De Giffoni De Carvalho et al., 2019). G. sylvestre is rich in antioxidants compounds such as gurmarin, conduritol A, gymnemasins, Gymnema saponins, gymnemosides, kaempferol, quercetin besides containing compounds such as phenols and saponins (Sinha et al., 2018b). These factors have contributed to its role as a drug detoxifying agent and the current study has evidenced its potential in protection from uranium. This suggests that GSE can be a new source of therapeutics against genotoxicity induced by metals such as Uranium as recognized in this study. Our investigations, call for further research to understand minimal uranium risk assessment in human lymphocytes, as well as other cells and organs to advance our knowledge on uranium toxicity to humans.
5 Conclusion
In conclusion, our study demonstrates the genotoxic effects of uranyl nitrate on human lymphocytes using γH2AX detection by flow cytometry. This study also indicates that GSE at 15 μg/mL doesn’t exert any significant changes to the human PBMCs. It is also evident that GSE ameliorates cytotoxicity and genotoxicity induced by uranyl nitrate in human peripheral blood mononuclear cells. GSE can be a source of therapeutics against DNA damage induced by heavy metals.
Acknowledgements
The authors acknowledge Researchers Supporting Project number (RSP2022R465), King Saud University, Riyadh, Saudi Arabia for funding this research. The authors wish to thank the management of SRM Institute of Science and Technology, Kattankulathur, Chennai for providing needed amenities and support for the accomplishment of this work.
Informed Consent
This study passed the Ethics Clearance of the Institutional Ethics Committee, SRM Medical College Hospital and Research Centre. All participants signed informed consent.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Preliminary phytochemical screening and In vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complement. Altern. Med.. 2010;10:1-8.
- [CrossRef] [Google Scholar]
- Micronucleus assay in human lymphocytes after exposure to alloxydim sodium herbicide in vitro. Cytotechnology. 2015;67:1059-1066.
- [CrossRef] [Google Scholar]
- Pretreatment of Gymnema sylvestre revealed the protection against acetic acid-induced ulcerative colitis in rats. BMC Complement. Altern. Med.. 2014;14:1-11.
- [CrossRef] [Google Scholar]
- Potential anticancer properties of bioactive compounds of Gymnema sylvestre and its biofunctionalized silver nanoparticles. Int. J. Nanomedicine. 2014;10:31-41.
- [CrossRef] [Google Scholar]
- Aryal, S., Baniya, M.K., Danekhu, K., Kunwar, P., Gurung, R., Koirala, N., 2019. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, Vol. 8, Page 96 8, 96. Doi: 10.3390/PLANTS8040096.
- B. Aggarwal, B., Prasad, Sahdeo, Reuter, S., Kannappan, R., R. Yadav, V., Park, B., Hye Kim, J., C. Gupta, S., Phromnoi, K., Sundaram, C., Prasad, Seema, M. Chaturvedi, M., Sung, B., 2011. Identification of Novel Anti-inflammatory Agents from Ayurvedic Medicine for Prevention of Chronic Diseases: “Reverse Pharmacology” and “Bedside to Bench” Approach. Curr. Drug Targets 12, 1595–1653. Doi: 10.2174/138945011798109464.
- Peak Exposures in Epidemiologic Studies and Cancer Risks: Considerations for Regulatory Risk Assessment. Risk Anal.. 2019;39:1441-1464.
- [CrossRef] [Google Scholar]
- Council, N.R., 2012. Uranium mining in Virginia: scientific, technical, environmental, human health and safety, and regulatory aspects of uranium mining and processing in Virginia.
- Council, N.R., 1999. Evaluation of guidelines for exposures to technologically enhanced naturally occurring radioactive materials.
- Differential response of human T-lymphocytes to arsenic and uranium. Toxicol. Lett.. 2020;333:269-278.
- [CrossRef] [Google Scholar]
- Medicinal plants from Brazilian Cerrado: Antioxidant and anticancer potential and protection against chemotherapy toxicity. Oxid. Med. Cell. Longev.. 2019;2019:1-16.
- [CrossRef] [Google Scholar]
- Augmented cytotoxic effects of paclitaxel by curcumin induced overexpression of folate receptor-α for enhanced targeted drug delivery in HeLa cells. Phytomedicine. 2019;56:279-285.
- [CrossRef] [Google Scholar]
- Effect of Solvents on Phytochemical Composition and Antioxidant Activity of Cardiospermum halicacabum (L.) Extracts. Pharmacogn. J.. 2020;12(6):1241-1251.
- [CrossRef] [Google Scholar]
- Total Phenolic and Flavonoid Contents of Aqueous Extract of Stinging Nettle and In Vitro Antiproliferative Effect on Hela and BT-474 Cell Lines. Int. J. Mol. Cell. Med.. 2014;3:102.
- [Google Scholar]
- Flieger, J., Flieger, W., Baj, J., Maciejewski, R., 2021. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Mater. 2021, Vol. 14, Page 4135 14, 4135. Doi: 10.3390/MA14154135.
- Metabolism-dependent bioaccumulation of uranium by Rhodosporidium toruloides isolated from the flooding water of a former uranium mine. PLoS One. 2018;13(8):e0201903.
- [Google Scholar]
- Total Phenolic Contents and Antioxidant Activities of Different Extracts and Fractions from the Aerial Parts of Artemisia biennis Willd. Iran. J. Pharm. Res. IJPR. 2014;13:551.
- [Google Scholar]
- Homma-Takeda, S., Numako, C., Kitahara, K., Yoshida, T., Oikawa, M., Terada, Y., Kokubo, T., Shimada, Y., 2019. Phosphorus Localization and Its Involvement in the Formation of Concentrated Uranium in the Renal Proximal Tubules of Rats Exposed to Uranyl Acetate. Int. J. Mol. Sci. 2019, Vol. 20, Page 4677 20, 4677. Doi: 10.3390/IJMS20194677.
- Ismail, I.H., Wadhra, T.I., Hammarsten, O., 2007. An optimized method for detecting gamma-H2AX in blood cells reveals a significant interindividual variation in the gamma-H2AX response among humans. Nucleic Acids Res. 35, e36–e36. Doi: 10.1093/NAR/GKL1169.
- Assessment of Antioxidant Potential, Total Phenolics and Flavonoids of Different Solvent Fractions of Monotheca Buxifolia Fruit. Osong Public Heal. Res. Perspect.. 2013;4(5):246-254.
- [Google Scholar]
- Inhibitory effect of uranyl nitrate on DNA double-strand break repair by depression of a set of proteins in the homologous recombination pathway. Toxicol. Res. (Camb). 2017;6(5):711-718.
- [CrossRef] [Google Scholar]
- Keith, S., Faroon, O., Roney, N., Scinicariello, F., Wilbur, S., Ingerman, L., Llados, F., Plewak, D., Wohlers, D., Diamond, G., 2013. Toxicological Profile for Uranium. Toxicol. Profile Uranium.
- Radon and cancer mortality among underground uranium miners in the Příbram region of the Czech Republic. Am. J. Ind. Med.. 2020;63(10):859-867.
- [Google Scholar]
- Comprehensive review on phytochemicals, pharmacological and clinical potentials of gymnema sylvestre. Front. Pharmacol.. 2019;10:1223.
- [CrossRef] [Google Scholar]
- Kryscio, A., Müller, W.U.U., Wojcik, A., Kotschy, N., Grobelny, S., Streffer, C., 2009. A cytogenetic analysis of the long-term effect of uranium mining on peripheral lymphocytes using the micronucleus–centromere assay. Doi: 10.1080/09553000110070289 77, 1087–1093. Doi: 10.1080/09553000110070289.
- Phytochemical composition and in vitro antioxidant activity of aqueous extract of Aerva lanata (L.) Juss. ex Schult. Stem (Amaranthaceae) Asian Pac. J. Trop. Med.. 2013;6(3):180-187.
- [CrossRef] [Google Scholar]
- Meerloo, J. van, Kaspers, G.J.L., Cloos, J., 2011. Cell Sensitivity Assays: The MTT Assay 237–245. Doi: 10.1007/978-1-61779-080-5_20.
- Mousavi, L., Salleh, R.M., Murugaiyah, V., 2018. Phytochemical and bioactive compounds identification of Ocimum tenuiflorum leaves of methanol extract and its fraction with an anti-diabetic potential. https://doi.org/10.1080/10942912.2018.1508161 21, 2390–2399. https://doi.org/10.1080/10942912.2018.1508161.
- An optimized method for measurement of gamma-H2AX in blood mononuclear and cultured cells. Nat. Protoc.. 2008;3(7):1187-1193.
- [Google Scholar]
- The γH2AX Assay for Genotoxic and Nongenotoxic Agents: Comparison of H2AX Phosphorylation with Cell Death Response. Toxicol. Sci.. 2014;140:103-117.
- [CrossRef] [Google Scholar]
- An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed.. 2012;2(4):320-330.
- [CrossRef] [Google Scholar]
- Rahman, M.M., Habib, M.R., Hasan, M.A., Amin, M. Al, Saha, A., Mannan, A., 2014. Comparative assessment on in vitro antioxidant activities of ethanol extracts of Averrhoa bilimbi, Gymnema sylvestre and Capsicum frutescens. Pharmacognosy Res. 6, 36. Doi: 10.4103/0974-8490.122915.
- Uranium Exposure in American Indian Communities: Health, Policy, and the Way Forward. Environ. Health Perspect.. 2021;129(3)
- [CrossRef] [Google Scholar]
- Gymnemic Acid Stimulates In Vitro Splenic Lymphocyte Proliferation. Phyther. Res.. 2016;30(2):341-344.
- [Google Scholar]
- Radio-protective dosimetry of Pangasius sutchi as a biomarker, against gamma radiation dosages perceived by genotoxic assays. Ecotoxicol. Environ. Saf.. 2018;164:629-640.
- [CrossRef] [Google Scholar]
- Ecotoxicology and Environmental Safety Radio-protective dosimetry of Pangasius sutchi as a biomarker, against gamma radiation dosages perceived by genotoxic assays. Ecotoxicol. Environ. Saf.. 2018;164:629-640.
- [CrossRef] [Google Scholar]
- Uranyl acetate induces hprt mutations and uranium–DNA adducts in Chinese hamster ovary EM9 cells. Mutagenesis. 2005;20:417-423.
- [CrossRef] [Google Scholar]
- Effect of Solvent on the Phytochemical Extraction and GC-MS Analysis of Gymnema sylvestre. Pharmacogn. J.. 2020;12(4):749-761.
- [CrossRef] [Google Scholar]
- Effect of gamma sterilization on Gymnema sylvestre leaf extract fused Polycaprolactone nanofiber for effective wound dressing applications. Mater. Lett.. 2021;300:130145.
- [CrossRef] [Google Scholar]
- Determination of Highest Dose of Ammonia without Effect at Work Environment through the Expression of Interleukin-2 Cell in Rattus Novergicus. Open Access Maced. J. Med. Sci.. 2019;7(6):897-902.
- [Google Scholar]
- Persistence of Genetic Damage in Lymphocytes from Former Uranium Miners. Cytogenet. Genome Res.. 2012;136(4):288-294.
- [CrossRef] [Google Scholar]







