Translate this page into:
Protective effect of coenzyme-10 and piperine against cyclophosphamide-induced cytotoxicity in human cancer HuH-7 cells
⁎Corresponding author. salkahtani@ksu.edu.sa (Saad Alkahtani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cancer is one of the leading health problems worldwide with hepatic cancer being one of the most common cancers. To date, a number of anti-cancer agents have been employed for chemotherapeutic purposes one of which includes cyclophosphamide (CP). Because of the serious side effect of CP, this study aims to investigate the potential protective effect of coenzyme-10 (CoQ10) and piperine (PIP) against cytotoxicity induced by CP in HuH-7 cell line. Based on IC50, 10, 12, 10, and 12 µg/ml of CP, PIP, CoQ10, PIP + CoQ10 were selected for further investigations. MTT and NRU assays results showed the combinations of CP + PIP, CP + CoQ10, CP + PIP + CoQ10 demonstrated a potential cytotoxic effect on HuH-7 cells. Also, these drug combinations induced apoptosis and relative migration rate compared with CP as evaluated by TUNEL and wound healing assays respectively. Furthermore, mitochondrial membrane potential (MMP) indicated the three drug combinations improved the mitochondrial membrane permeability in HuH-7 cells. Finally, upregulation of bcl-2, caspase-3 caspase-8, caspase-9 and p53 genes in HuH-7 cells were observed in the drug combination treatments compared to CP alone, confirming the positive effect of PIP and CoQ10 when combined with CP. In conclusion, current results showed that PIP and CoQ10 have anti-cancerous properties and may be a useful resource for effective remedies in the treatment of liver cancer.
Keywords
cyclophosphamide
Coenzyme-10
Piperine
Liver
Cancer
Apoptosis
- CP
-
cyclophosphamide
- PIP
-
piperine
- CoQ10
-
coenzyme Q-10
Abbreviations
1 Introduction
Cancer is one of the leading health problems worldwide and it is one of the major global causes of death (Sung et al., 2021). It begins with abnormal regulation of specific gene expression that is usually triggered by genetic alterations (Kooti et al., 2017). One of the major hallmarks of cancer cells is their ability to uncontrollably divide (Parkin et al., 2005). Current biological paradigms suggested for cancer is that all cancers are develop from multiple external factors combined with genetic changes (Sonnenschein and Soto, 2008). Saudi Cancer Registry (SCR) offers information on reliable population-based cancer incidence data and the prevalence of cancer in Saudi Arabia varies from one region to another (Mosli et al., 2015; Alsanea et al., 2015; Althubiti and Nour Eldein, 2018). According to World Health Organization (WHO), cancer was responsible for 9.6 million deaths worldwide in 2018, and was roughly the cause 0.16% of deaths in Saudi Arabia (Glorieux and Calderon, 2018). Hepatic cancer has risen nearly 3-fold in cases in Saudi Arabia between 1990 and 2016 (Althubiti & Nour Eldein, 2018). Chronic liver disease, with its complications such as; liver cirrhosis and liver cancer, has been the world’s leading cause of death and morbidity due to late diagnosis and weak medical results (Bommer & Vine, 2014). Investigation of the progression of pathways leading to liver cancer is also essential for deep understanding of the disease and for the most effective therapy (Safety, 2002). CP is an alkylating agent that has been used for treating different types of cancers in both humans and animals (Voelcker, 2020b). In addition to the anti-mitotic and anti-replicative effects of this agent, it has immunosuppressive and immunomodulatory properties (Ahlmann & Hempel, 2016). It is considered a prodrug that is metabolized within target cells and causes hemorrhage, necrosis, and fibrosis of the urinary bladder (Stanton and Legendre, 1986). Recently, studies have been invested into investigating if natural compounds can ameliorate the effects of various mutagens and carcinogens (Lee et al., 2020). Among these natural compounds being considered are flavonoids and alkaloids such as black pepper (Piper nigrum) and long pepper (Piper longum), which are used worldwide in various traditional remedies systems (Selvendiran et al., 2005). Along with the application of PIP as a food preservative, it is used in traditional medicine because of its bioactive effects such as; antioxidant, antimetastatic, antitumor (Zorica et al., 2019). Another study reported that PIP would be used as adjunctive therapy in order to enhance the bioavailability of various chemotherapeutic drugs (Anshuly et al., 2020). CoQ10 is an essential enzymatic cofactor, plays an important role in cellular bioenergetics and the synthesis of mitochondrial adenosine triphosphate (ATP) (Saini, 2011). It has shown beneficial effects on the inflammatory mechanism of multiple human diseases (Zhai et al., 2017) and pro-inflammatory cytokines (Farsi et al., 2019). Preclinical and clinical studies have shown that induced cardiotoxicity by anthracycline drugs such as doxorubicin and daunorubicin - can be prevented by the use of CoQ10 during cancer chemotherapy. Conklin, (2005) suggested that CoQ10 does not interfere with the antineoplastic action of anthracyclines and might even improve their anticancer effects. Apoptosis is a closely regulated multi-step pathway that is necessary for the removal of infected, damaged or defective cells. Disturbance in pathways that control apoptosis can result in cancer, autoimmune, and degenerative disorders (Wimalasena et al., 2015; Pfeffer and Singh, 2018). Taken together, this study undertaken to investigated the potential chemo-protective role of CoQ10 and PIP alone or in combination against CP-induced cytotoxicity in the human liver cancer cell line (HuH-7), focusing on apoptotic cellular events.
2 Materials and methods
2.1 MTT assay
HuH-7 cells were cultivated in DMEM, Sigma (St Louis, MO, USA) supplemented with 10% FBS, and 1% penicillin–streptomycin. MTT assay was performed as described by (Mosmann, 1983). In brief, cells were seeded in 96 wells plate (5 × 104). Cells were exposed to different concentrations (2, 10, 15, 20, and 25 µg/ml) of CP, PIP, CoQ10, and combination of PIP + CoQ10. Treated cells were incubated at 37˚C for 48 hr. 10 µl of MTT solution was added to each well and then incubated for 3–4 hr at 37C°. After incubation, the culture medium was removed and formed formazan crystals were dissolved in isopropanol. The absorbance was measured at 540 nm using a multi-mode Microplate Reader-Gen5™, BioTek Cytation 5™, USA (Mohammed et al., 2021).
2.2 Neutral red uptake (NRU) assay
The NRU assay was done according to the method described by Ali et al., (2011) to confirm the effect of tested compounds. In brief, cells (5 × 104 cells/well) were seeded in 96-well plates and kept in the 5% CO2 incubator for 48 hr at 37 °C. At confluency of 80–85%, cells were exposed to 10 µg/ml of CP, 12 µg/ml PIP, 10 µg/ml CoQ10, 12 µg/ml of PIP + CoQ10, combination of CP + PIP, CP + CoQ10, and CP + PIP + CoQ10 for 48 hr. NRU dye was added (100 μl with DMEM for 3 hr), and after incubation, the cells were washed with fixative and simultaneously dye extractor solution. The OD was taken at 540 nm using a spectrophotometer (Khursheed et al., 2020).
2.3 Wound–healing assay
To further assess the effects of chemical compounds on HuH-7 cells migration, a wound-healing assay was used as previously described by Al-Zharani et al. (2019). Briefly, cells were seeded in 12 wells plate at density of 105 cells/well and allowed to grow until reaching confluent monolayer at for 24hr at 37° C. After incubation media was discarded and a straight scratch was made using a sterile 10-μl pipette tip on the cell's monolayer. Cells were washed with PBS to remove the floating cells and then fresh media was added. Cells were treated with selected concentrations of 10 µg/ml of CP, 12 µg/ml PIP, 10 µg/ml CoQ10, 12 µg/ml of PIP + CoQ10, combination of CP + PIP, CP + CoQ10, and CP + PIP + CoQ10 for 48 hr. Images were captured at 0, 12, 24 and 36 h using an inverted microscope equipped with a digital MC-170 HD camera (Leica, Germany). ImageJ WH_NJ macro software (NIH, USA) was used for image analysis (Luanpitpong et al., 2010).
2.4 Mitochondrial membrane potential (MMP)
The fluorescence dye JC– was used to evaluate the effect of tested compounds on the potential permeabilzation of mitochondrial membrane in HuH-7 cells. Cells were grown on cover slips in 6 well plate and in 96 wells black bottom plate at density 5 × 105 in cultured 10 % medium then incubated in 5% CO2 for 24 h at 37 °C. After incubation, cells were exposed to 10 µg/ml of CP, 12 µg/ml PIP, 10 µg/ml CoQ10, 12 µg/ml of PIP + CoQ10, combination of CP + PIP, CP + CoQ10, and CP + PIP + CoQ10 for 48 hr. Medium was removed, then cells washed with 1X dilution buffer, then stained and incubated with JC-1 dye for 20 min 5% CO2 at 37 °C. For microplate, the monomer JC-1 dye was read at excitation: 475 nm, emission: 530 nm whereas aggregate JC-1 dye excitation: 535 nm, emission: 590 nm. For images, the JC-1 was disposed and cells were washed several times with PBS, cover slips were transferred into microscopic slides and fluorescent images were taken by CRCL, s LSM780 NLO confocal microscope (Maqsood et al., 2020).
2.5 TUNEL assay
TUNEL assay kit-FITC was used to detect the DNA damage. The HuH-7 cells were seeded in 6 wells plate on the center of the cover slip at density of 2 × 104 cells/well for 24 h at 37C°. Cells were treated with the following doses; 10 µg/ml of CP, 12 µg/ml PIP, 10 µg/ml CoQ10, 12 µg/ml of PIP + CoQ10, combination of CP + PIP, CP + CoQ10, and CP + PIP + CoQ10 for 48 hr. Cells were washed with PBS and fixed with 4% paraforomaldehyde for 15 min at 37 °C. The fixative was discarded and cells were washed with PBS once followed by adding 1 ml of 70% alcohol to each well and then incubation for 10 min in the dark. Staining solution (51 µl) was added followed by 60 min incubation at 37 °C. Cells were treated with 10 µl of propidium Iodide/RNase A solution and incubated for 30 min at 37 °C. Cells were re-suspend with 1 ml of wash buffer followed by washing with PBS. Cover slips were shifted to microscopic slides and cells images were captured by a confocal microscope (CRCL’s LSM 780 NLO confocal microscope) (Mohamed et al., 2020, Mohammed et al., 2020).
2.6 Flow cytometry
Cells were seeded in 6 well plate at density of 4 × 105 cells/well in 2 ml media for 24hr at 37C°. Treated cells with each chemical compound alone or in combination were incubated for 48 h. After incubation cells were washed 3 times with PBS and trypsinized with (0.25% trypsin). Cell suspension was collected and added to the media in the centrifuge tubes. Tubes were centrifuged at 1600 rcf for 5 mins. Cells were re-suspended in 500 μl of 1X Binding Buffer. 5 μl of Annexin V-FITC and 5 μl of propidium iodide (PI) were mixed to cells suspensions and incubated for 20 mins in the dark at room temperature. The cells were analysed by Flow Cytometry (Becton-Dickinson Immuno cytometry Systems, Sunnyvale, CA, USA) using FACS Diva 6.1.2 software (Abdullah et al., 2019).
2.7 Gene expression
RNA from treated cells was isolated using RNeasy Mini Kit (Cat No. /ID: 74104), and converted to cDNA using cDNA reverse transcription kit (Revert Aid First Strand cDNA Synthesis Kit; Thermo Fisher Scientific). The concentration and purity of RNA were determined with a Nanodrop 8000 spectrophotometer (Thermo Scientific, USA). To perform RT-PCR, SYBR Green master mix was prepared and 7,500 Fast RT-PCR System (Applied Biosynthesis, Carlsbad, CA) with gene-specific primers was used. The expression level of pro– and anti–apoptotic marker genes were quantified. qPCR was performed in triplicate. All data are expressed as the mean of three independent experiments, calculated by the 2-ΔΔCT method (Saquib et al., 2013).
2.8 Statistical analysis
The present data were analyzed by one-way analysis of variance (ANOVA), and T-test. p Values < 0.05 were considered significant.
3 Results
3.1 MTT assay
MTT assay was conducted to detect the inhibitory effects of each chemical compounds on HuH-7 cells growth. The HuH-7 cells were treated with different concentrations (10, 15, 20, and 25 µg/ml) of CP, PIP, CoQ10, and combination of PIP + CoQ10 for 48 hr. Result showed the (IC50) value of CP, PIP, CoQ10, and combination of PIP + CoQ10 to be 10, 16, 15, and 18 µg/ml respectively (Fig. 1A). Based on these IC50 values, 10 µg/ml of CP, 12 µg/ml PIP, 10 µg/ml CoQ10, 12 µg/ml of PIP + CoQ10 were selected for further investigations. Also, current result showed that the cell viability was significantly decreased in a dose dependent manner (Fig. 1B).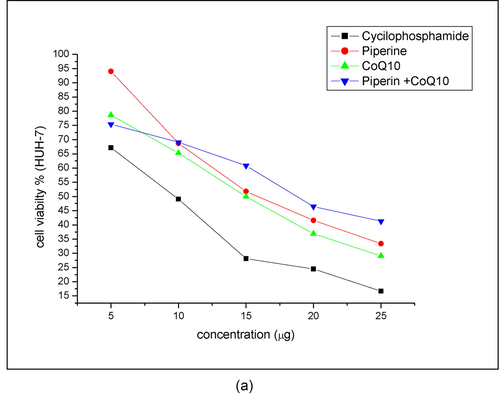
IC50 detection of investigated compounds. HuH-7 cells treated with (5, 10, 15, 20, and 25 μg/ml) of each compound (CP, PIP, CoQ-10, and PIP + CoQ-10) respectively for 48hr as evaluated by MTT assay.
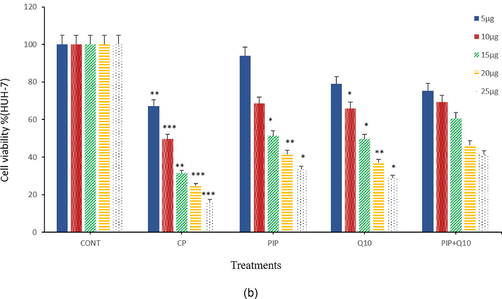
Cell viability of HuH-7 cells after treatment with (5, 10, 15, 20, and 25 μg/ml) of CP, PIP, CoQ-10, and PIP + CoQ-10 respectively for 48hr as evaluated by MTT assay. Each value represents the percentage of cells viability.
3.2 NRU assay
Cytotoxicity of the investigated compounds on the HuH-7 cells was further assessed using NRU test. Present data showed there is significantly higher cytotoxicity in cells treated with; PIP, CP + PIP, CP + CoQ10, and CP + PIP + CoQ10 after 48 hr when compared with control, whereas cytotoxic effect was significantly decreased in cells treated with CoQ10 and PIP + CoQ10 respectively (Fig. 2).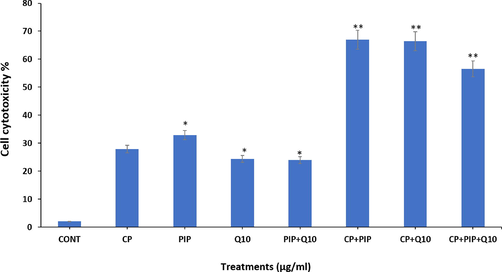
Cytotoxic effect of tested compounds on HUH-7 cells after 48 hr as evaluated by NRU assay. Each value represents the mean SE ± (n = 3), (*p < 0.05, **p < 0.01) compared with control.
3.3 Wound–healing assay
Cell migration was examined by wound healing assay to evaluate the effect of investigated compound on the HuH-7 cells. Findings showed that at 36 h, the untreated HuH-7 cells had migrated into the scratched area, whereas the migration capability of all treated cell groups were significantly inhibited. The closure rate of created wound of HuH-7 treated cells was 0.657% with PIP + CoQ10 at 36 hr among all treatments, followed by the same treatment at 24 hr. In contrast, the widest gap was 0.016% in cells treated with CP + PIP + CoQ10 at 12 hr among all treatments (Fig. 3A, Fig. 3B, Fig. 3C).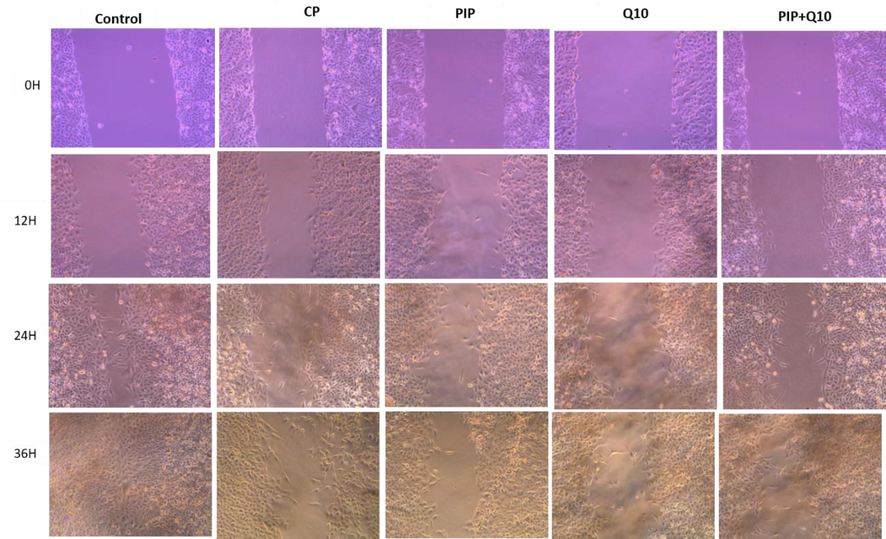
Shows HuH-7 cells migration in wound-healing assays. cells were treated with CP, PIP, CoQ-10, PIP + CoQ-10 for 48 hr and images were captured at 0 and 12, 24, and 36 hr.
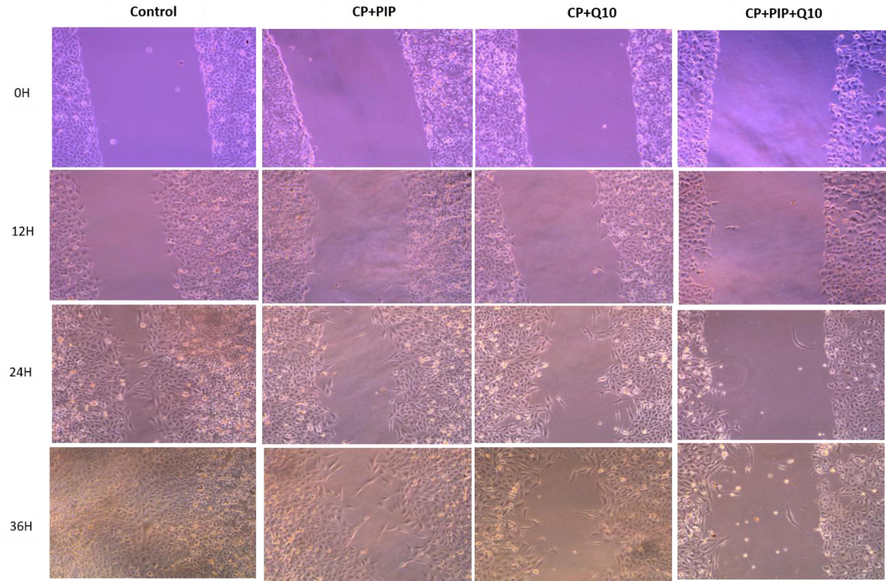
Shows HuH-7 cells migration in wound-healing assays. cells were treated with CP + PIP, CP + CoQ-10, CP + PIP + CoQ-10 for 48 hr and images were captured at 0 and 12, 24, and 36 hr.
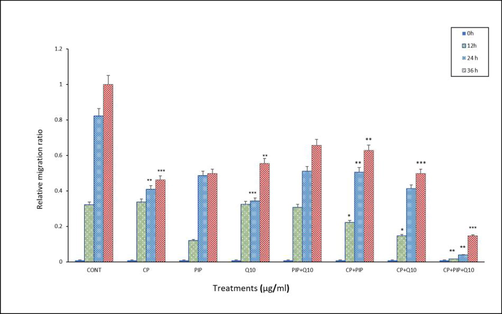
Shows HuH-7 relative migration rate in wound-healing assays. cells were treated with CP, PIP, CoQ-10, PIP + CoQ-10, CP + PIP, CP + CoQ-10, CP + PIP + CoQ-10 for 48 hr and images were captured at 0 and 12, 24, and 36 hr. *p < 0.05, ** p < 0.01, *** p < 0.001. vs control.
3.4 Determination of MMP
The effect of chemical compounds exposure on MMP was evaluated in HuH-7 cells by JC-1 staining method as shown in (Fig. 4A, Fig. 4B and Fig. 4C). First, the potential of MMP was measured according to the ratio aggregate (red)/monomer (green) fluorescence intensity. As shown in the result, there was significant loss of MMP in HuH-7 cells treated with PIP, PIP + CoQ10, CP + PIP, CP + CoQ10, and CP + PIP + CoQ10 compared to untreated cells. To confirm this result, the MMP was also evaluated using fluorescence microscope. The strong MMP was found in control cells and cells treated with CoQ10 as JC-1 dye staining was strongly observed in form of its metabolite J-aggregates, a deep red fluorescence.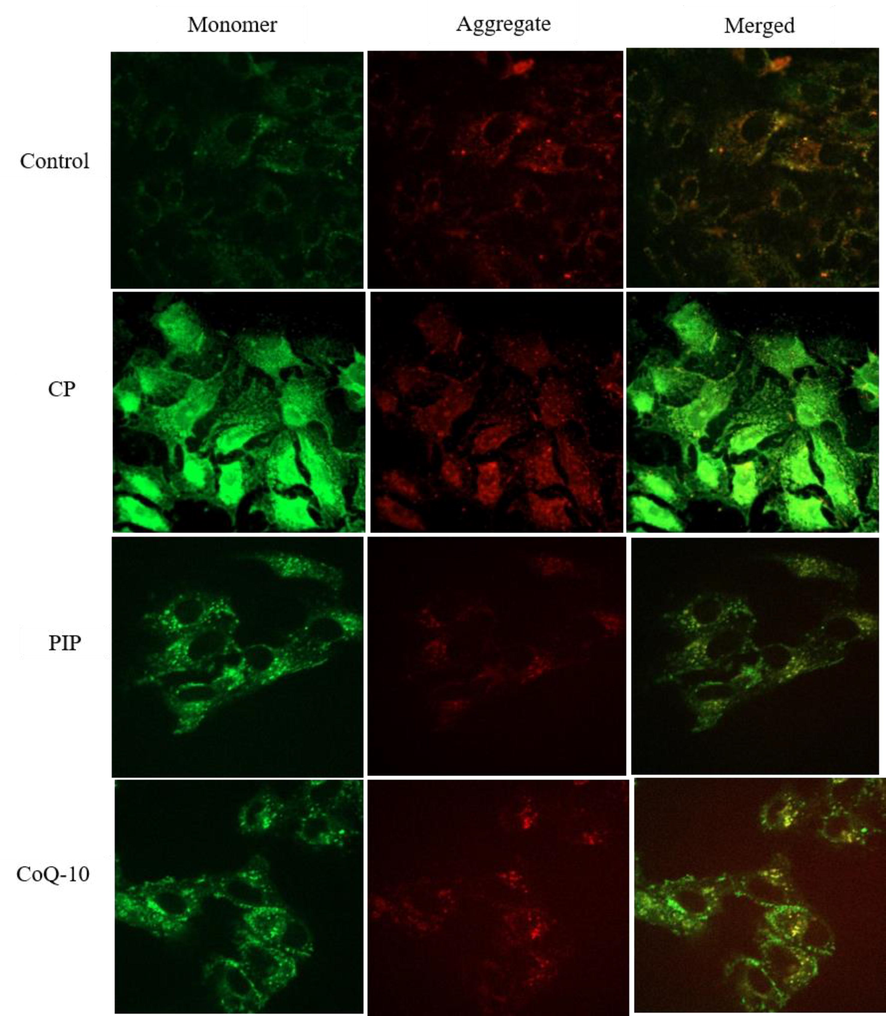
Shows MMP of HuH-7 as evaluated by JC-1. Cells were treated with CP, PIP, CoQ-10 for 48 hr.
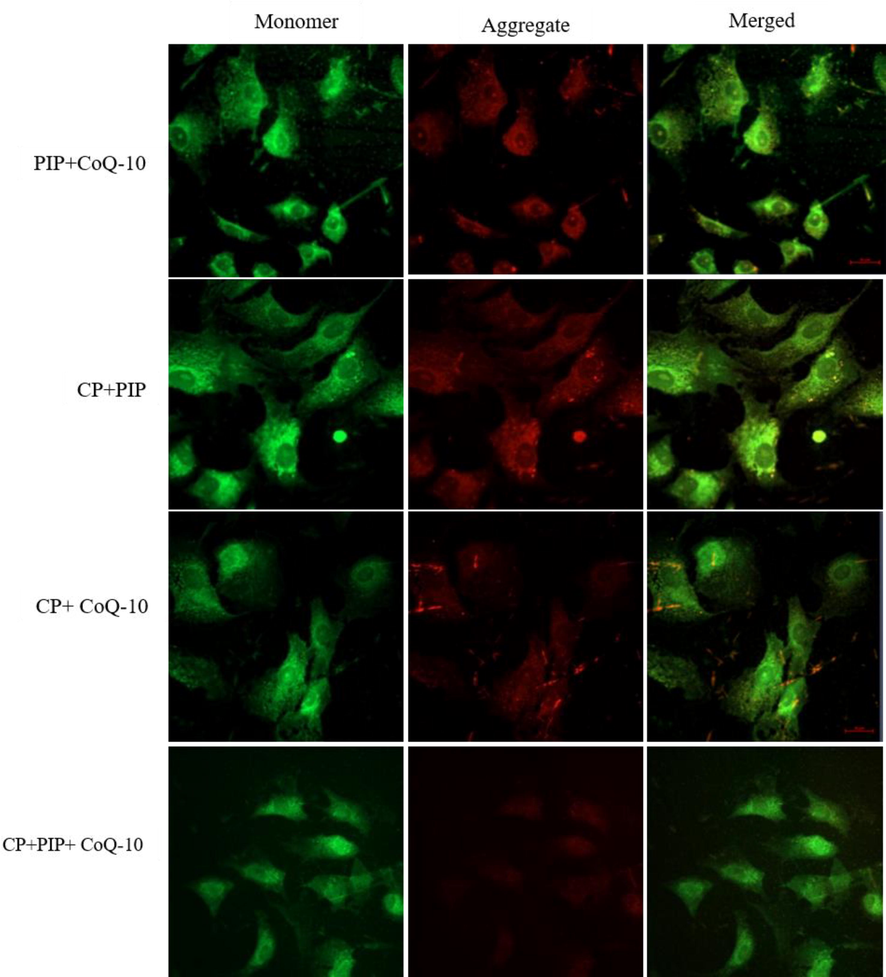
Shows MMP of HuH-7 as evaluated by JC-1. Cells were treated with CP + CoQ-10, CP + PIP, CP + PIP + CoQ-10 for 48 hr.
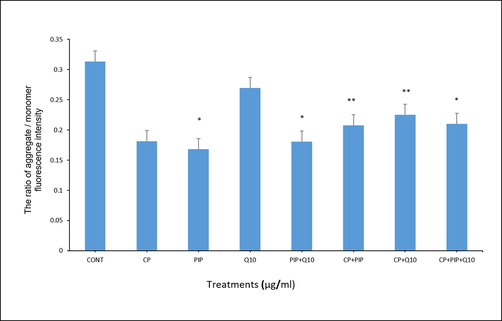
Shows MMP test of HuH-7 cells treated with each compound for 48 hr as evaluated by JC-1. The ratio of aggregate / monomer fluorescence intensity was measured. Data are presented as the mean ± SD of three different experiments. *p < 0.05, ** p < 0.01, *** p < 0.001. vs control.
3.5 TUNEL assay
DNA damage was evaluated after each treatment to illustrate the effect of tested compounds on the apoptotic events in HuH-7 treated cells. The TUNEL assay was conducted to detect apoptotic cells by dUTP labelling of DNA breaks. As shown in (Fig. 5A) the percentage of apoptotic labelled HuH-7 cells with CP, PIP, PIP + CoQ10, and CP + CoQ10 were significantly higher compared with control. Furthermore, apoptosis assessed using fluorescence microscopy revealed that HuH-7 cells treated with CP, PIP, and CoQ10 underwent apoptosis based on the high intensity of green fluorescence corresponding to FITC-dUTP stain which (Fig. 5B and Fig. 5C). Moreover, rate of apoptosis increased after treatment with combination of CoQ10 compared to control.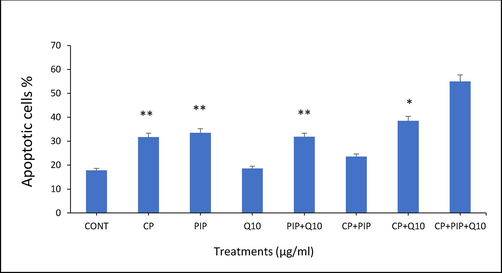
TUNEL assay results showed the percentage of apoptotic HUH-7 cells after treatment with different compounds for 48 hr. Each value represents the mean SE ± (n = 3), (*p < 0.05, **p < 0.01).
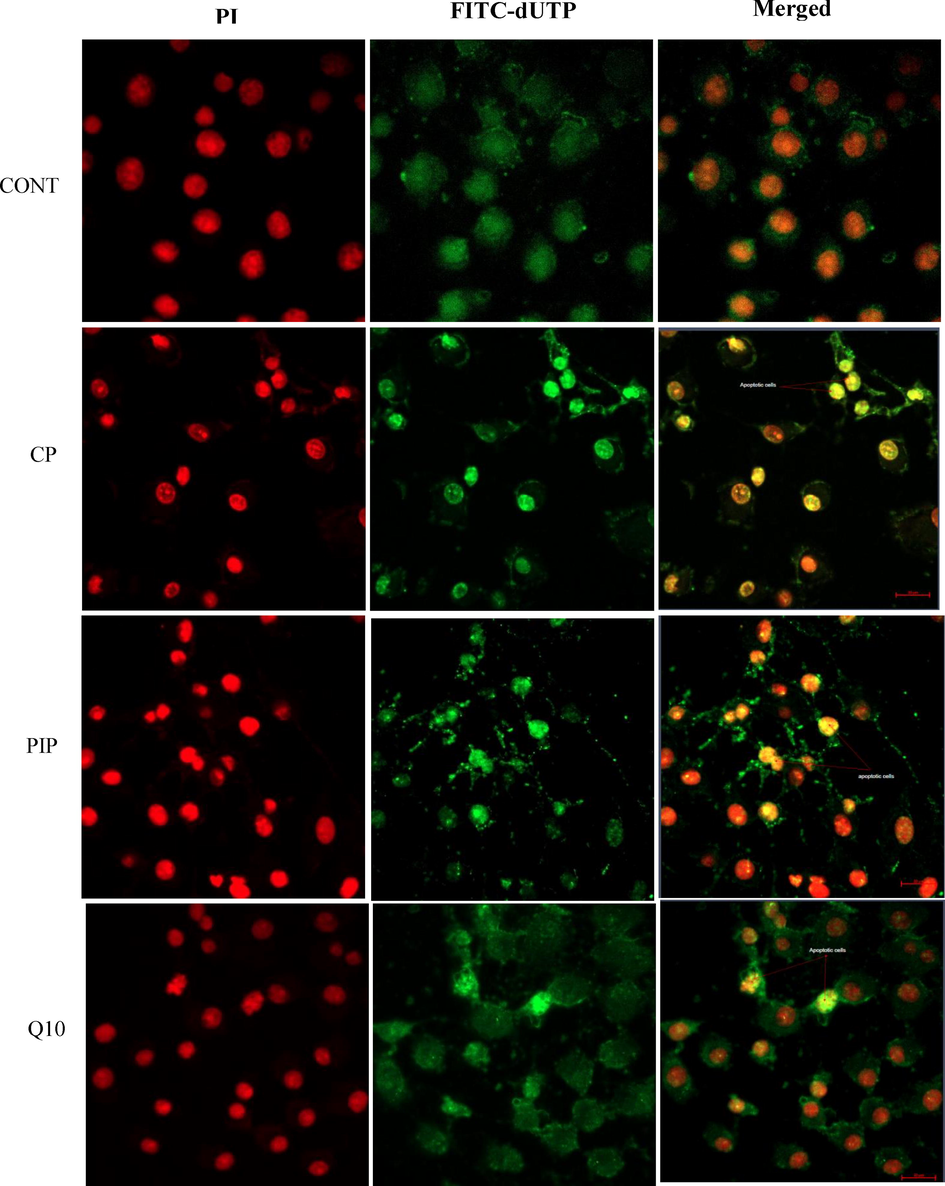
Fluorescence microphotograph of HUH-7 cells showed effect of treatments with CP, PIP and CoQ10 on the apoptotic rate compared to the control.
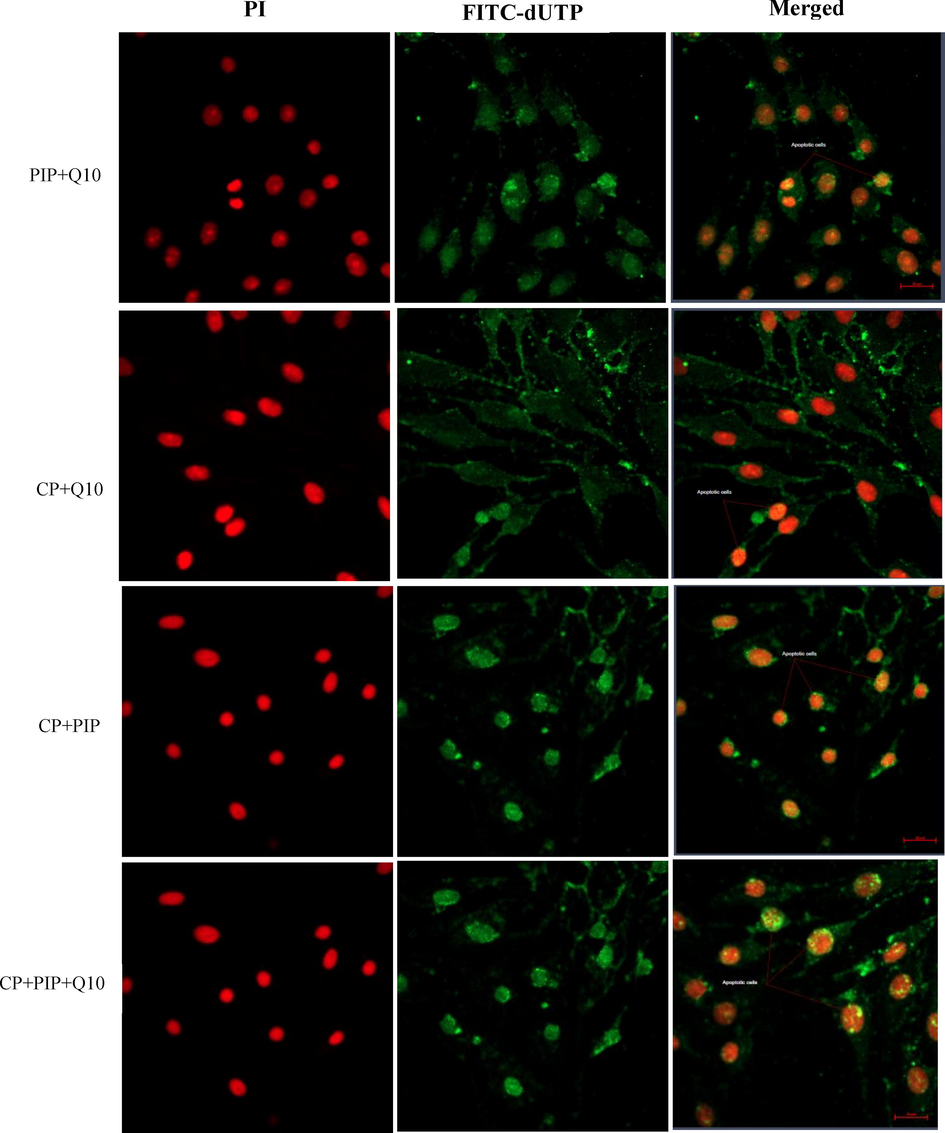
Fluorescence microphotograph of HUH-7 cells showed effect of treatments with combination of PIP + CoQ10 and combination of CP with PIP, CoQ10, and both PIP + CoQ10 on the apoptotic rate compared to the control.
3.6 Flow cytometry
After treatments of the cells with all drug combinations, Annexin V-FITC/PI staining was used to assess apoptosis and necrosis by means of flow cytometry technique. Findings showed that all tested compounds induced elevated rate of apoptosis compared to control (Fig. 6A and Fig. 6B). On the other hand, treatment with CP, PIP, CoQ10, and combination of PIP + CoQ10 demonstrated reduction in necrotic cells compared to control. HuH-7 cells treated with CP + PIP, CP + CoQ10, and CP + PIP + CoQ10 were found to undergo necrosis. However, no significant differences were found in the induction of apoptosis for any of the treatments except with CoQ10. Based on the flow cytometry analysis, more than 97% of HuH-7 control cells were found alive with 1%, 0.2% and 1.8% of cells classified as being in the early, late and necrotic stages respectively.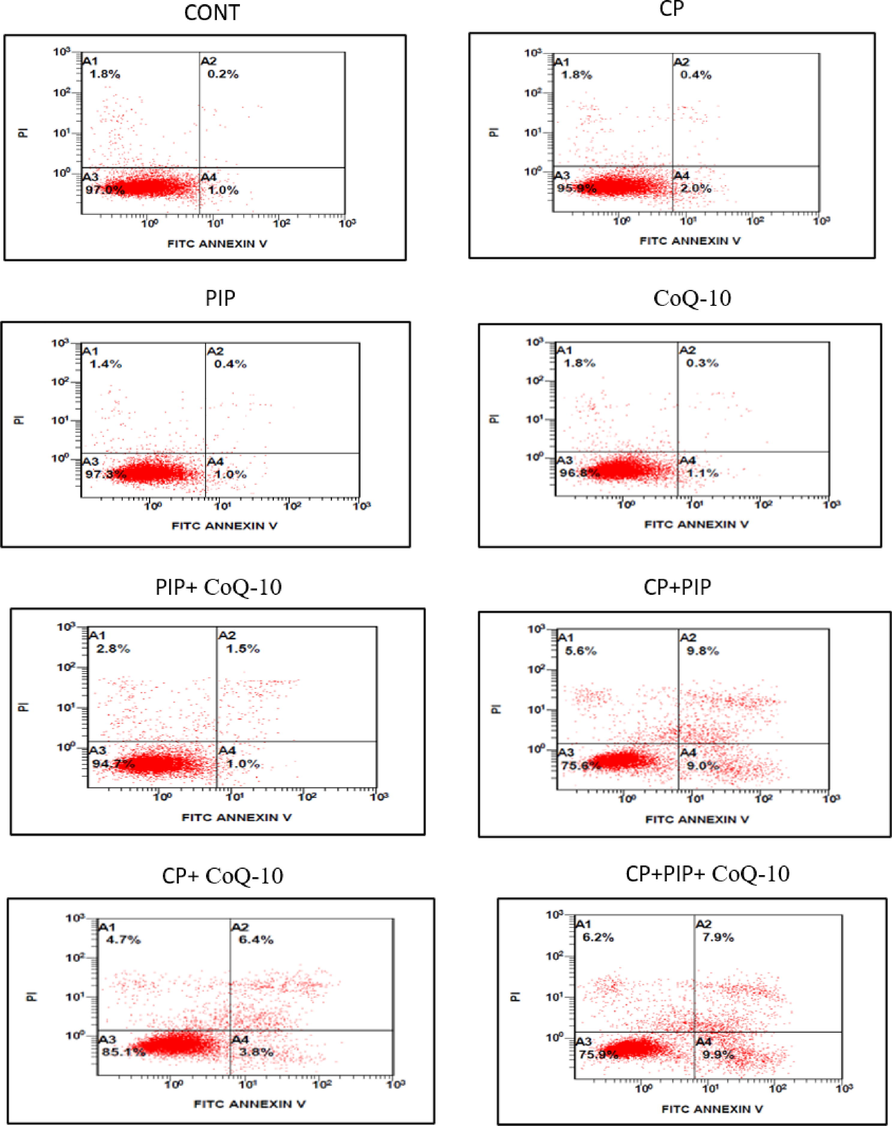
Shows flow cytometric analysis of HuH-7 cells treated with each compound for 48 hr. Combination of CP + PIP, CP + CoQ-10, and CP + PIP + CoQ-10 induced apoptosis.
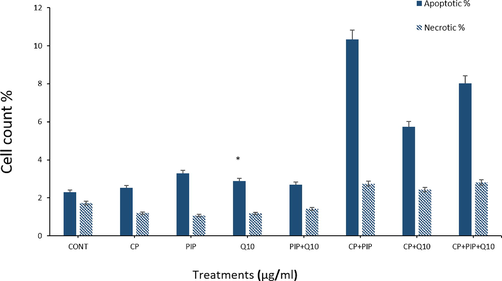
Shows flow cytometric analysis of HuH-7 cells treated with each compound for 48 hr. Combination of CP + PIP, CP + CoQ-10, and CP + PIP + CoQ-10 induced apoptosis. Data are presented as the mean ± SD of three different experiments. *p < 0.05 vs control.
3.7 Gene expression
After exposure, HuH-7 cells to the tested compounds for 48 hr by quantitative real-time PCR to detect the effect of each compound on the level of apoptosis-related genes (caspase, 3, 8, 9, Bax, Bcl-2, and p53). Table 1 shows the specific sequences of used primers. The expression of bcl-2, an anti-apoptotic gene was significantly upregulated in cells treated with each compound (Fig. 7A). In contrast, mRNA expression for Bax as a pro-apoptotic gene was down-regulated after 48 hr (Fig. 7B). Also, treated HuH-7 cells showed significant upregulation in the level of cas-3,8, and 9 with most of tested compounds compared with untreated cells (Fig. 7C, D, and E). Also, the level of p53 was measured and was significantly upregulated with CP only and with combination of each compound (Fig. 7F). Bcl-2 gene exhibited maximal upregulation of 3.65 folds of the control, whereas bax gene exhibited and minimal down-regulation of 0.45 folds. Cells treated with combination of CP and PIP or CoQ10 or both showed clear reduction in the level of bcl-2, cas-3 compared to CP alone (Fig. 7A and C).
Primers
F
R
Tm (°C)
β-actin
5’ TCACCCACACTGTGCCCATCTACGA 3’
5’ AGCGGAACCGCTCATTGCCAATGG 3’
69.1
BCL2
5’ AGGAAGTGAACATTTCGGTGAC 3’
5’ GCTCAGTTCCAGGACCAGGC 3’
62.4
BAX
5’ TGCTTCAGGGTTTCATCCAG 3’
5’ GGCGGCAATCATCCTCTG 3’
58.4
Caspase 3
5’ ACATGGCGTGTCATAAAATACC 3’
5’ CACAAAGCGACTGGATGAAC 3’
58.4
Caspase 8
5’ CTGGTCTGAAGGCTGGTTGT 3’
5’ CAGGCTCAGGAACTTGAGGG 3’
60
Caspase 9
5’ CCAGAGATTCGCAAACCAGAGG 3’
5’ GAGCACCGACATCACCAAATCC3’
64
P53
5’ CCCAGCCAAAGAAGAAACCA 3’
5’ TTCCAAGGCCTCATTCAGCT 3’
58.4
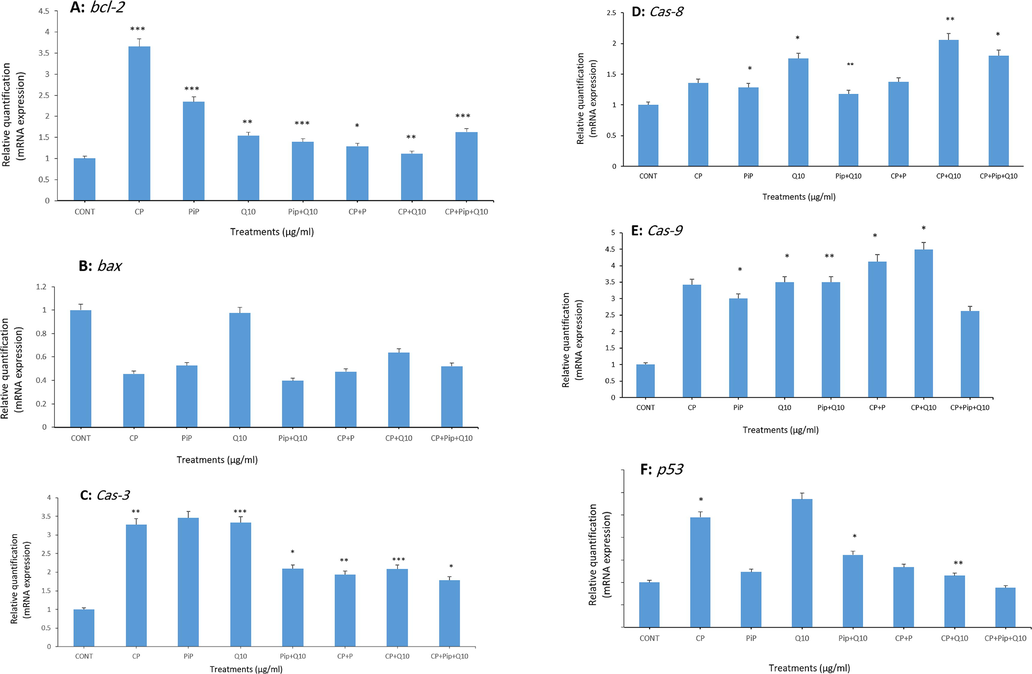
Shows fold change in the expression of apoptosis-related genes in treated HuH-7 cells analyzed by real-time PCR (qPCR). HuH-7 cells were exposed to different compounds for 48 hr. the assessed genes were; bcl-2, bax, cas-3, cas-8, cas-9, and p53 respectively. The results are presented as the mean ± SD of three different experiments. Data represents the mean ± SE. n = 3, (*p < 0.05, **p < 0.01, and ***p < 0.001) compared with control.
4 Discussion
Currently, CP uses for treating different types of cancers in both humans and animals (Voelcker, 2020a). It has anti-mitotic, anti-proliferation, immunosuppressive and other side effects (Ahlmann and Hempel, 2016). Lee and others have investigated whether natural compounds can mitigate the toxic effects of mutagens and they found that flavonoids and alkaloids compounds have antioxidant capacity and other positive effects (Lee et al., 2020). In order to ameliorate the side effects of CP, this study investigated two kinds of natural products; piperine and CoQ10 alone and in combination as potential cancer therapy enhancers in Human HuH-7 cancer cells. Our findings showed that the cell survival was significantly decreased in a dose dependent manner as evaluated by MMT assay. This result was confirmed by NRU test. Dietary constituents used as chemoprotective strategy for control genetic diseases (Selvendiran et al., 2005). PIP is a rich source of many active constituents and other volatile compounds. It has lots of therapeutic uses on human and animal such as; hepatoprotective, anti-inflammatory, anti-oxidant, and anti-carcinogenic (Meghwal and Goswami, 2013; Farsi et al., 2019). Another study, assessed the role of CoQ10, this study illustrated that CoQ10 supplementation significantly promoted the antioxidant capacity to reduce oxidative stress in hepatocellular carcinoma patients (Y. kang Liu et al., 2016). TUNEL assay result showed increased DNA damage after each treatment. We found a significantly higher rate of apoptosis in HuH-7 cells treated with CP, PIP, PIP + CoQ10, and CP + CoQ10 were compared with control. Analysis of apoptotic genes also confirmed this finding as upregulation of the gene expression of caspases; 3, 8, and 9 was observed in these cell groups. On the contrary, bcl-2 gene expression was significantly decreased after all treatments. Activities of caspases and bcl-2 are dissimilar, however, some studies reported that bcl-2 family interacting with other partners to either inhibit or induce cell death and this simultaneous action may explain the high expression of both tested caspases and bcl-2 (Marie and Soane, 2013). Loss function of MMP has been considered as the main factor for the intrinsic pathway in apoptosis involving the release of cytochrome c from the mitochondria, triggering caspase-9 activation and subsequently caspase-3 (Mohamed et al., 2020, Mohammed et al., 2020). Our result showed bax gene expression was down-regulated in the cells treated with all tested compounds. The activation of caspases and expression bcl–2 induces apoptosis via the mitochondrial pathway (Aboul-Soud et al., 2020; Fatima et al., 2021). However, the mitochondrial pathway might not play any role through bax and bcl-2 gene expression in apoptosis. It was reported that no correlation between the role of bax and bcl-2 genes in lymphocytes of patients suffering from depression (Hammad et al., 2019). Furthermore, our finding detected significant upregulation of p53 gene after treatment with CP. In contrast, treatment with CP + PIP, CP + CoQ10, and CP with both PIP + CoQ10 result in reduction of p53 level compared to CP alone. In agreement with our findings, published data reported that p53-indepenedent apoptosis regulate the cell cycle checkpoints and other related regulators that enhance activation of caspase-3 (Liu et al., 2017). As shown in MMT and NRU results, cell proliferation was affected when exposure to CP. This finding was in line with wound healing assay result. HuH-7 cells migration was significantly inhibited after treatment with CP. CP + PIP, and CoQ10 at 24 and 36 hr. In addition, the cell migration was reduced inhibited after treatment with CP + CoQ10 at 12 and 36 hr and with CP + PIP + CoQ10 at 12, 24, and 36 hr. Cancer cell migration is one of the major hallmarks of cancer responsible for metastasis (J. Liu et al., 2017). Thus, present data suggest that taking a combination of PIP and CoQ10 as a food supplement might be useful compounds for targeting cancer cell and reduce the side effect of CP in cancerous patients who are using CP as chemotherapy. However, both compounds triggered apoptosis in HuH-7 cells treated with CP + PIP + CoQ10 as determined by flow cytometric analysis. In last decades, human beings have taken advantage of natural products as an anti-cancer therapeutics. Chemopreventive effect of a combined stigmasterol and palmatine was investigated and it was found that this combined decreased the number of tumors and their size. Moreover, this combined was significantly reduced the serum level of liver enzymes and enhanced the level of oxidative enzymes (Nada et al., 2021). In addyion, Griñan-Lison and other, (2021), investigated the role of antioxidants against breast cancer, with attention to clinical trials, and found that using antioxidants in combination therapy ameliorate the side effects of chemotherapeutic agents (Griñan-Lison et al., 2021).
5 Conclusion
Cells treated with combination of CP + PIP and CP + CoQ10 or both PIP + CoQ10 have demonstrated potential hepatotoxic effects against HuH-7 cells. In addition, three combinations elevated the percentage of apoptotic cells and relative migration rate compared with CP alone. Furthermore, MMP indicated three combinations improved the mitochondrial membrane permeability and exerts apoptosis in HuH-7 cells. Taken together, this study uncovered the anti-cancerous properties of PIP and CoQ10. Thus, we highly recommended taking PIP and CoQ10 as a potential therapeutic supplement along with chemotherapeutic agents for liver cancer and other types of cancer.
6 Availability of data and materials
The data generated or analyzed in this article are online publicly available without request.
7 Authors' contributions
Norah S. AL-Johani, Norah M. Alhoshani and Nada H. Aljarba prepared and conducted the cytotoxicity assays. Mohammed Al-Zharani, Norah S. AL-Johani and Bader Almutairi evaluated the MMP and TUNEL assay. Saad Alkahtani, Norah M. Alhoshani and Daoud Ali measured the HuH-7 cells migration. Saud Alarifi, Daoud Ali assessed cell counts of death cells. Norah S. AL-Johani, Nora Alkeraishan, and Bader Almutairi performed the RNA isolation and cDNA synthesis. Norah M. Alhoshani and Norah S. AL-Johani evaluated the genes expression.
Acknowledgement
This work was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R62), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. Researchers Supporting Project number (RSP-2021/26), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Organophosphorus flame retardant (tricresyl phosphate) trigger apoptosis in HepG2 cells: Transcriptomic evidence on activation of human cancer pathways. Chemosphere. 2019;237(2019):124519
- [Google Scholar]
- Biochemical and molecular investigation of in vitro antioxidant and anticancer activity spectrum of crude extracts of willow leaves salix safsaf. Plants. 2020;9(10):1-23.
- [Google Scholar]
- The effect of cyclophosphamide on the immune system: implications for clinical cancer therapy. Cancer Chemother. Pharmacol.. 2016;78(4):661-671.
- [Google Scholar]
- Apoptotic induction and anti-migratory effects of Rhazya stricta fruit extracts on a human breast cancer cell line. Molecules. 2019;24(21):1-16.
- [Google Scholar]
- UVB-induced apoptosis and DNA damaging potential of chrysene via reactive oxygen species in human keratinocytes. Toxicol. Lett.. 2011;204(2–3):199-207.
- [Google Scholar]
- National Guidelines for Colorectal Cancer Screening in Saudi Arabia with strength of recommendations and quality of evidence. Ann. Saudi Med.. 2015;35(3):189-195.
- [Google Scholar]
- Trends in the incidence and mortality of cancer in Saudi Arabia. Saudi Med. J.. 2018;39(12):1259-1262.
- [Google Scholar]
- Piperine: A comprehensive review of methods of isolation, purification, and biological properties. Med. Drug Discov.. 2020;7:100027
- [Google Scholar]
- Bommer, U. A., & Vine, K. L. (2014). Cancer biology: molecular and genetic basis. January.
- Coenzyme Q10 for prevention of anthracycline-induced cardiotoxicity. Integr. Cancer Therap.. 2005;4(2):110-130.
- [Google Scholar]
- Can coenzyme Q10 supplementation effectively reduce human tumor necrosis factor-α and interleukin-6 levels in chronic inflammatory diseases? A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res.. 2019;148(May):104290
- [Google Scholar]
- Epigallocatechin gallate and coenzyme Q10 attenuate cisplatin-induced hepatotoxicity in rats via targeting mitochondrial stress and apoptosis. J. Biochem. Mol. Toxicol.. 2021;35(4):e22701
- [Google Scholar]
- Catalase down-regulation in cancer cells exposed to arsenic trioxide is involved in their increased sensitivity to a pro-oxidant treatment. Cancer Cell Internat.. 2018;18(1):1-9.
- [CrossRef] [Google Scholar]
- Antioxidants for the treatment of breast cancer: Are we there yet? Antioxidants. 2021;10(2):1-44.
- [Google Scholar]
- Prescribing statins among patients with type 2 diabetes: The clinical gap between the guidelines and practice. J. Res. Med. Sci.. 2019;24(1):1-5.
- [Google Scholar]
- Effective medicinal plant in cancer treatment, part 2: Review study. J. Evid.-Based Complement. Alternat. Med.. 2017;22(4):982-995.
- [Google Scholar]
- Chemical composition and antioxidant capacity of black pepper pericarp. Appl. Biol. Chem.. 2020;63(1):1-9.
- [Google Scholar]
- In vitro anticancer effects of two novel phenanthroindolizidine alkaloid compounds on human colon and liver cancer cells. Mol. Med. Rep.. 2017;16(3):2595-2603.
- [Google Scholar]
- An improved strategy to detect the epithelial-mesenchymal transition process in circulating tumor cells in hepatocellular carcinoma patients. Hep. Intl.. 2016;10(4):640-646.
- [Google Scholar]
- Regulation of lung cancer cell migration and invasion by reactive oxygen species and caveolin-1. J. Biol. Chem.. 2010;285(50):38832-38840.
- [Google Scholar]
- Single and multi–metal oxide nanoparticles induced cytotoxicity and ROS Generation in human breast cancer (MCF–7) Cells. J. Inorg. Organomet. Polym Mater.. 2020;2020(30):4106-4116.
- [Google Scholar]
- Multiple functions of BCL-2 family proteins. Cold Spring Harbor Perspect. Biol.. 2013;5(2):1-22.
- [Google Scholar]
- Expression of TLR-2 in hepatocellular carcinoma is associated with tumour proliferation, angiogenesis and Caspase-3 expression. Pathol.-Res. Pract.. 2020;216(8)
- [Google Scholar]
- Platinum nanoparticles induced genotoxicity and apoptotic activity in human normal and cancer hepatic cells via oxidative stress- 39 mediated Bax/Bcl-2 and caspase-3 expression. Environ. Toxicol. 2020:1-12.
- [Google Scholar]
- The protective effect of Ammi visnaga extract against human hepatic cancer. J. King Saud Univ.. 2021;33:101540
- [Google Scholar]
- A Saudi Gastroenterology association position statement on the use of tumor necrosis factor-alfa antagonists for the treatment of inflammatory bowel disease. Saudi J. Gastroenterol.. 2015;21(4):185-197.
- [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1–2):55-63.
- [Google Scholar]
- Synergistic dose permutation of isolated alkaloid and sterol for anticancer effect on young Swiss Albino Mice. Drug Design Dev. Ther.. 2021;15:4043-4052.
- [Google Scholar]
- Safety, P. (2002). Measures Taken from the Aspect of Nursing Jobs to Prevent Medical Accidents. 45(3), 80–83.
- Zinc ferrite nanoparticles activate IL-1b, NFKB1, CCL21 and NOS2 signaling to induce mitochondrial dependent intrinsic apoptotic pathway in WISH cells. Toxicol. Appl. Pharmacol.. 2013;273(2):289-297.
- [Google Scholar]
- Preliminary study on inhibition of genotoxicity by piperine in mice. Fitoterapia. 2005;76(3–4):296-300.
- [Google Scholar]
- Theories of carcinogenesis: An emerging perspective. Semin. Cancer Biol.. 2008;18(5):372-377.
- [CrossRef] [Google Scholar]
- Effects of cyclophosphamide in dogs and cats. J. Am. Vet. Med. Assoc.. 1986;188(11):1319-1322.
- [Google Scholar]
- Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.. 2021;71(3):209-249.
- [Google Scholar]
- Aldophosphamide-thiazolidine (NSC-613060) an oxazaphosphorine cytostatic that crosses the blood brain barrier. Anticancer Drugs 2020:61-65.
- [Google Scholar]
- The mechanism of action of cyclophosphamide and its consequences for the development of a new generation of oxazaphosphorine cytostatics. Sci. Pharm.. 2020;88(4):1-13.
- [Google Scholar]
- Effects of coenzyme Q10 on markers of inflammation: A systematic review and meta-analysis. PLoS ONE. 2017;12(1):e0170172.
- [Google Scholar]
- Piperine-A major principle of black pepper: A review of its bioactivity and studies. Appl. Sci.. 2019;2019(9):4270.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102009.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







