Translate this page into:
Preparation and characterization of p–n heterojunction CuBi2O4/CeO2 and its photocatalytic activities under UVA light irradiation
*Corresponding author at: LCMIA Laboratory of Inorganic Materials Chemistry and Application, Department of Physical Chemistry, University of the Science and the Technology of Oran (USTO MB), BP 1505 El M’naouar, 31000 Oran, Algeria elaziouti_a@yahoo.com (Abdelkader Elaziouti),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 10 September 2014
Peer review under responsibility of King Saud University.
Abstract
CuBi2O4/CeO2 nanocomposites were synthesized by the solid state method and were characterized by a number of techniques such as X-ray diffraction, scanning electron microscopy and UV–Vis diffuse reflectance spectroscopy. The photocatalytic activity of the samples was investigated under UVA light and assessed using Congo red (CR) dye as probe reaction. The efficiency of the coupled CuBi2O4/CeO2 photocatalyst was found to be related to the amount of added CuBi2O4 and to the pH medium. The CuBi2O4/CeO2 photocatalyst exhibited the high efficiency as a result of 83.05% of degradation of CR under UVA light for 100 min of irradiation time with 30 wt% of CuBi2O4 at 25 °C and pH 7, which is about 6 times higher than that of CeO2. The photodegradation reactions satisfactorily correlated with the pseudo-first-order kinetic model. The mechanism of the enhanced photocatalytic efficiency was explained by the heterojunction model.
Keywords
CuBi2O4/CeO2 heterojunction
Congo red
Photocatalytic activity
Synergy effect
1 Introduction
The heterogeneous photocatalysis of organic pollutants on semiconductor surfaces has attracted much attention as a ‘green’ technique. Up to date, the researches on photocatalysis have mostly focused on TiO2 based photocatalysts with a crystalline modification of anatase (Degussa P25, Hombriat UV-100, Aldrich, etc.) as a result of their high photocatalytic activity and widespread uses for large-scale water treatment (Wang et al., 2006). However, the intrinsic band gap of TiO2 is 3.2 eV, which requires the excitation wavelength <387.5 nm, limited their efficiency under solar light, so that the effective utilization of solar energy is limited to about 4% of total solar spectrum. Therefore, development of a highly efficient, non toxic and chemically stable photocatalyst under visible light irradiation is required. Semiconductor catalysts such as SnO2 (Sangami and Dharmaraj, 2012), CeO2 (Yongchuan et al., 2014), Fe2O3 (Seiji and Toshiyuki, 2009), Bi2O3 (Zhong et al., 2011), Sb2O3 (Aslam et al., 2011), WO3 (Fumiaki et al., 2013) and ZnO (Vora et al., 2009) metal oxides and CdS (Chae et al., 2010), CdSe (Frame et al., 2008), CdTe (Kovalenko et al., 2004), PbS (Wang et al., 2011a) and HgS (Rengaraj et al., 2014) metal chalcogenides have long been investigated for environmental applications. But their practical uses have been constrained by their low photocatalytic activity under solar light, short-term stability against photo- and chemical corrosion as well as potential toxicity.
The lanthanide oxide cerium dioxide (CeO2) has been attracting great interest in the recent years because of its effective technological applications, such as in solid-state electrolytes for electrochemical devices (Mogensen et al., 2000; Yashima et al., 1998), catalysts for three-way automobile exhaust systems (Nikolaou,1999; Ozawa, 1998), abrasives for chemical–mechanical planarization (Feng et al., 2006), sunscreens for ultraviolet absorbents (Imanaka et al., 2003), the adsorption and reaction of formaldehyde (Zhou and Mullins, 2006), oxygen storage capacity (Kakuta et al., 1997), hybrid solar cells (Lira-Cantu and Krebs, 2006), H2S removal (Flytzani-Stephanopoulos et al., 2006) and luminescent materials for violet/blue fluorescence (Morshed et al., 1997). Cubic fluorite cerium dioxide (CeO2), a semiconductor with a relatively narrow band gap of 2.7 and 3.4 eV depending on the technique of preparation (Ozer, 2001), shows promising photocatalytic activity for the degradation of various organic dye pollutants such as Methylene Blue (MB), Methyl Orange (MO) and C.I. Reactive Black 5 (RB5) (Zhang, 2009; Song et al., 2007). CeO2 has also successfully been employed in water splitting for H2 production and phenol and chlorinated phenol photodegradation under UV illumination (Chung and Park, 1996; Valente et al., 2011). Although photocatalytic activity of CeO2 has intensively been investigated, the broad band gap energy and the electronic potential position in the conductance and valence bands of this material seriously limit its further application as a photocatalyst utilizing solar energy (Li et al., 2009). Various strategies in liquid-phase system have been adopted for size-controlled synthesis of various functional nanomaterials, including transition metal doping (Couselo et al., 2008), noble metal deposition (Sasahara et al., 2006), doping non-metallic elements (Geng et al., 2008), doping transition metal surface photosensitization (Mora-Sero et al., 2007) and coupled polycrystallites or colloidal semiconductors (Bian et al., 2008). Thus, improving photocatalytic activity by coupled semiconductor has become a hot topic among researchers.
Thus, the combination of two semiconductors with different band gap level energies has been investigated extensively in the last decade as one of the most effective ways to decrease the frequency of the recombination of electron–hole (e−/h+) pairs. The major characteristic of this technique is to assemble a heterojunction interface between wide and narrow band gap semiconductors with matching energy band potentials. In this way, electric field assisted transportation of charges from one particle to the other through interfaces is favorable for the electron–hole separations in the composite materials, and thus the electron and hole transfer from catalyst to adsorbed substrate can be obtained (Li and Yan, 2009; Liu et al., 2010a,b). The extensive search published on n–n type junction semiconductor systems was mostly focused on CeO2-based photocatalyst materials, such as CeO2/Fe2O3 (Pradhan and Parida, 2010), CeO2/ZnO (Wu et al., 2010), CeO2/CeLnOx (Ln = Pr, Tb, Lu) (Małecka et al., 2007), CeO2/TiO2 (Cai et al., 2009), CeO2/ZrO2 (Ranga and Ranjan Sahu, 2001), CeO2/MnOx (Wu et al., 2011), CeO2/Bi2O3 (Lingzhi and Bing, 2009), H3PW12O40-CeO2/TiO2 and CeO2/TiO2 (Cai et al., 2009), CeO2/CrO (Bhati et al., 2010), CeO2/MCM-41, CeO2/MCM-48 and CeO2/SBA-15 (Pouretedal et al., 2012), CeO2/SiO2 (Mohamed and Aazam, 2012), CeO2/SrTiO3 (Shuang et al., 2008; Song et al., 2007), CeO2/Ag–AgCl (Wang et al., 2011b), CeO2/BiVO4 (Wetchakun et al., 2012), [CeO2, La2O3, C]/TiO2 (Rangel et al., 2012), CeO2/Co (Sabari Arul et al., 2012) and so on. The results showed that nearly all the n–n junction semiconductor materials exhibited better photocatalytic properties than single ones. However, to the best of our knowledge, the use of the p–n type junction semiconductors has not been reported in the literature. Theoretically, when p-type semiconductor and n-type semiconductor are connected to each other, the micro p–n heterojunction semiconductors will be formed; the inner electric field will also be produced in the interface. Once optical excitation occurs, a free electron (e−) and an electronic vacancy (a hole, h+) are formed, separated and migrated effectively in a semiconductor being partially localized on structural defective centers of its crystalline lattice, hence improving the electrical properties of semiconductor system.
In the present study, we have studied the photocatalytic efficiency of a p-CuBi2O4/n-CeO2 system, in which CeO2 (n-type semiconductor) was associated with bismuth cuprite, CuBi2O4 (p-type semiconductors) to form p–n heterojunction composite semiconductors in different mass ratios. Bismuth cuprite (CuBi2O4) was chosen as a sensitizer semiconductor due to its narrow band gap energy of 1.5 eV (Arai et al., 2007; Liu et al., 2010a,b). CuBi2O4 is well-known as an excellent host matrix for luminescent materials due to its low phonon energy, high visible-light responsiveness and adequate thermal stability. It functions as a sensitizer by the absorption of UV light to yield an excited state in the heterojunction composite semiconductors of p-CuBi2O4/n-CeO2, which may increase the probability of light-generated carrier transfer and hence reduces the recombination of photogenerated electrons and holes substantially improving the photocatalytic properties.
So, the aim of this study is to clarify the photocatalytic efficiency of this novel p–n type composite semiconductor p-CuBi2O4/n-CeO2 prepared by a solid state route. The as-prepared p-CuBi2O4/n-CeO2 materials were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and UV–Visible diffuse reflectance spectroscopy (DRS) techniques. The photocatalytic degradation of Congo red (CR) dye under UV light irradiation was investigated over p-CuBi2O4/n-CeO2 photocatalyst at different operating parameters such as, the amount of added CuBi2O4 and pH medium. The experimental data were quantified by applying the pseudo-first order kinetic model. On the basis of the calculated energy band positions and the active species during photocatalytic process, the mechanism of the enhanced photocatalytic activity was discussed through the heterojunction model.
2 Experimental
2.1 Materials and methods
α-Bi2O3 (99.99%), CuO (99.99%) and CeO2 (99.99%) materials were obtained from Aldrich Chemical Company Ltd. Congo red (CR) azoic dye (C.I. 22,020, MW = 696.67 g mol−1, C32H24N6O6S2.2Na, λmax = 497 nm and pKa = 4) and other chemicals used in the experiments (NH4OH and H2SO4) were purchased from C.I.S.A. Espagne.
2.2 Preparation of p-CuBi2O4
The p-CuBi2O4 powder was prepared according to the previously reported procedure (Liu et al., 2006; Chen et al., 1999; Takeo et al., 2007). The stoichiometric proportion mixture of α-Bi2O3 and CuO oxides was previously ground for a period of time in an agate mortar, and then heated at the rate of 5 °C/min in a muffle oven (Linn High Therm) and thermally treated at 750 °C for 72 h in air. After the muffle oven was naturally cooled to room temperature, the black CuBi2O4 powder was ground in the agate mortar and then was collected as the precursor to prepare the CuBi2O4/CeO2 photocatalysts.
2.3 Preparation of CuBi2O4/CeO2 photocatalyst
CuBi2O4/CeO2 nanocomposite photocatalysts were prepared by the solid state technique with the CuBi2O4:CeO2 mass ratio of 5:95, 10:90, 20:80, 30:70, 40:60 and 50:50. The corresponding precursors of CuBi2O4/CeO2 were milled in an agate mortar for 30 min to form the nanosized photocatalysts.
2.4 Characterization
X-ray diffraction patterns of the powders were recorded at room temperature using an automatic D8 Bruker AXS diffractometer with CuKα radiation (λ = 1.5406 Å) over the 2θ collection interval of 10–70° with a scan speed of 10°/min. The mean grain size (dDRX) was assessed using the Debye–Scherrer equation (Cullity, 1956; Pullar et al., 1988; Azàroff, 1968) as follows Eq. (1):
where β is the corrected full-width at half maximum (FWHM) (radian), λ is the X-ray wavelength (1.5406 Å) and θ is the Bragg angle (radian). The lattice constants of the samples calculated from their corresponding XRD pattern data are obtained by Fullprof program. UV–Vis DRS measurements were carried out at room temperature using a Perkin Elmer Lambda 650 spectrophotometer equipped with an integrating sphere attachment. The analysis range was from 200 to 900 nm, and polytetrafluoroethylene (PTFE, Teflon) was used as a reflectance standard. The band gap values were estimated by extrapolation of the linear part of the plot of absorbance versus the wavelength and Eg = 1240/λAbsorp. Edge equation assuming that all the prepared photocatalysts are direct crystalline semiconductors. Scanning electron microscopy observations (SEM) were performed by using Hitachi S-4800N.
2.5 Photocatalytic study measurements
The photodegradation of CR catalyzed by the CuBi2O4/CeO2 samples was investigated under UV-light irradiation. 100 mg of catalyst was suspended in a CR solution (200 mL, 20 mg/L) in quartz cell tube. The suspension pH value was previously adjusted at 7 using NaOH/H2SO4 solutions using (Hanna HI 210) pH meter. Prior to UVA light irradiation, the suspension was stirred with a magnetic stirrer (Speedsafe™ Hanna) for 30 min under dark conditions at 298 K to ensure the establishment of adsorption/desorption equilibrium between the catalyst and CR. The sample was then irradiated at 298 K using 6 W ultraviolet (λ = 365 nm, BLX-E365) photoreactor under continuous stirring. As the reaction proceeded, a 5 mL suspension was taken at 20 min intervals during the catalytic reaction and was centrifuged using centrifuge (EBA-Hettich) at 3500 rpm for 15 min to completely remove photocatalyst particles. The residual RC concentrations during the course of degradation were monitored with a UV mini-1240 Spectrophotometer (Shimadzu UV mini-1240) in the range of 200–800 nm, using 1 cm optical pathway cells.
The effect of initial pH on the photocatalytic degradation of CR was conducted in the pH range of 6–12. The experiments were also performed by varying the amount of CuBi2O4 from 0 to 100 wt%.
The data obtained from the photocatalytic degradation experiments were then used to calculate the degradation efficiency η′ (%) of the substrate Eq. (2):
According to Planck’s Law and some further calculation, we can find that the absorption wavelength of the photoreactor can be done by determining its band gap value Eq. (3):
The photocatalytic degradation efficiency of catalyst for the degradation of CR was quantified by measurement of dye apparent first order rate constants under operating parameters.
Surface catalyzed reactions can often be adequately described by a monomolecular Langmuir–Hinshelwood mechanism, in which an adsorbed substrate with fractional surface coverage θ is consumed at an initial rate given as follows Eq. (4) (Vasanth Kumar et al., 2008):
Thus, a plot of reciprocal of the apparent first order rate constant 1/Kapp against initial concentration of the dye C0 should be a straight line with a slope of 1/K1 and an intercept of 1/K1K2. Such analysis allows one to quantify the photocatalytic activity of catalyst through the specific rate constant K1 (with larger K1 values corresponding to higher photocatalytic activity) and adsorption equilibrium constant K2 (K2 expresses the equilibrium constant for fast adsorption–desorption processes between surface of catalyst and substrates). The integrated form of the above equation (Eq. (5)) yields to the following Eq. (6):
The half-life of dye degradation at various process parameters was raised from Eq. (8).
where half-life time, t1/2, is defined as the amount of time required for the photocatalytic degradation of 50% of CR dye in an aqueous solution by catalyst.
3 Results and discussions
3.1 XRD analysis of (x wt%)CuBi2O4/CeO2 composites
Fig. 1 shows the XRD patterns of the as-synthesized (30 wt%) CuBi2O4/CeO2 composite in comparison with those of precursor CuBi2O4 and pure CeO2. Diffraction peaks of pure CeO2 (Fig. 1a) at 2θ of 28.02°, 33.11°,47.45°, and 56.3°can be indexed as the (1 1 1), (2 0 0), (2 2 0), and (3 1 1) planes of pure fluorite phase CeO2, which is in good agreement with standard value (Fm.3m, JCPDS file No. 34–0394) with lattice constant a = 5.4110 (2) Å. This is in agreement with the reported previous work (Keren, 2011; Truffault, 2010). The diffraction peaks of the Cubi2O4 precursor (Fig. 1b) at 2θ of 28.03°, 29.73°, 30.73°, 32.54°, 33.36° and 46.71° were respectively indexed as (2 1 1), (2 2 0), (0 0 2), (1 0 2), (3 1 0), and (4 1 1) planes of pure tetragonal phase of crystalline Cubi2O4, according to the Joint Committee Powder Diffraction Standards (P42/mnm, JCPDS file No. 42–0334). The lattice constants (a = 8.5004 Å, c = 5.819 Å) were calculated from their corresponding XRD pattern data obtained by Fullprof program. Both precursor CuBi2O4 and pure CeO2 show preferred (0 0 2) crystallographic orientation owing to the preparation route of the sample during the XRD analysis.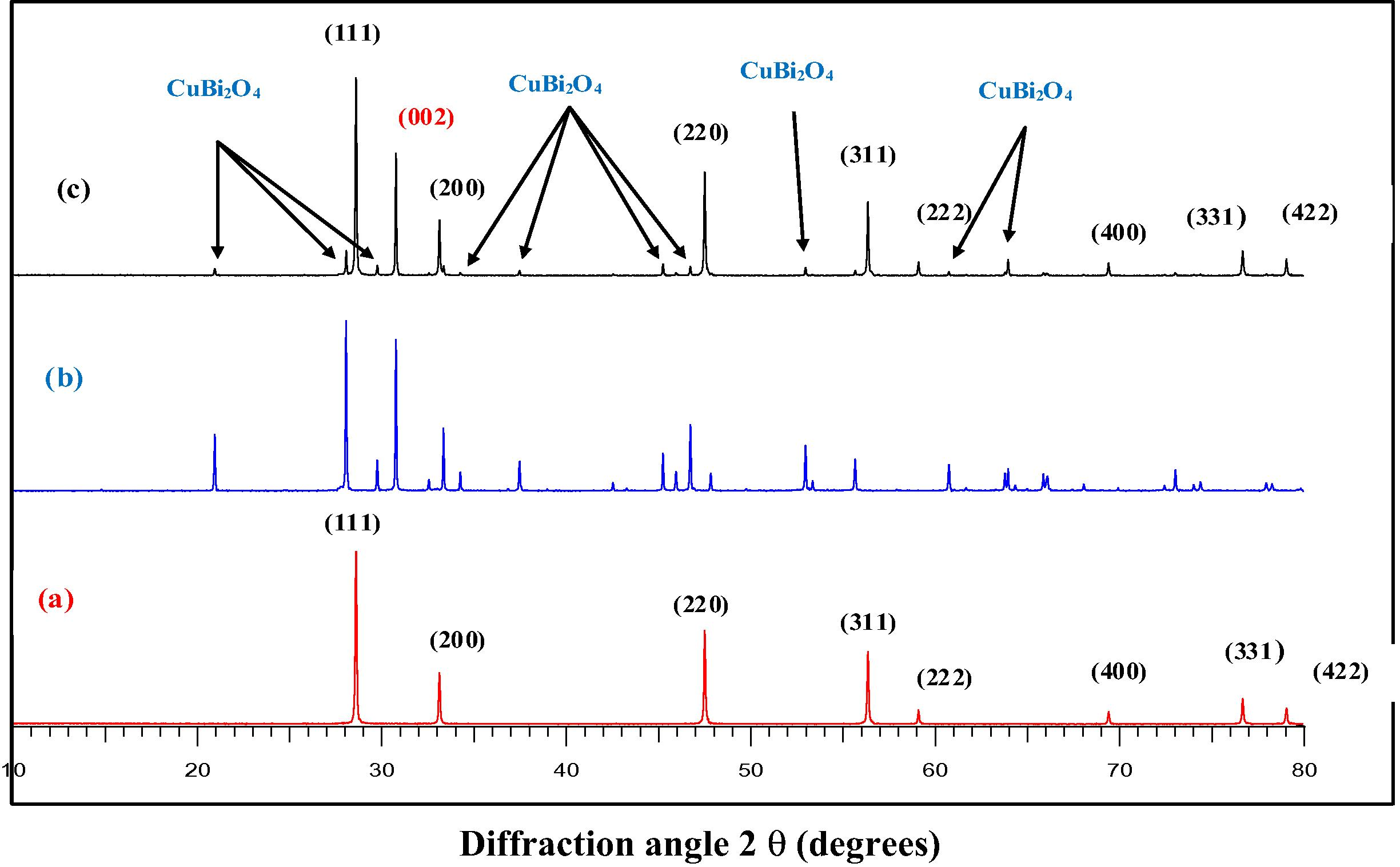
XRD patterns of pure CeO2 (a) precursor CuBi2O4 (b) and the synthesized (30 wt%) CuBi2O4/CeO2 (c).
The crystallite sizes of pure CeO2 deduced from the XRD patterns by calculation of the Scherrer equation showed that crystalline size of the composite, dXRD was calculated to 100 nm.
On the other hand, the XRD patterns of (30 wt%) CuBi2O4/CeO2 composite exhibited characteristic diffraction peaks of both Cubi2O4 and CeO2 crystalline phases. It can be seen from Fig. 1c that at 30 wt% mass concentration of Cubi2O4, the diffraction pattern of the materials was quite similar to that of pure CeO2. This is probably due to the high crystallinity of the CeO2 phase, thus appearing as the dominant peaks in the XRD spectra of the composite sample.
Here, we observe that the XRD patterns (Fig. 2) in the 2θ range from 25° to 40° show that (30 wt%) CuBi2O4/CeO2 sample exhibits broadened peaks with a little shift toward higher intensities. Based on the Scherrer equation, the crystallite size of a sample is inversely proportional to the full-width-half-maximum (FWHM), indicating that a broader peak represents smaller crystallite size (Hu et al., 2006). Thus the presence of Cubi2O4 promotes the crystallinity and a consequent broadening of the diffraction peaks of the (30 wt%) CuBi2O4/CeO2 composite sample.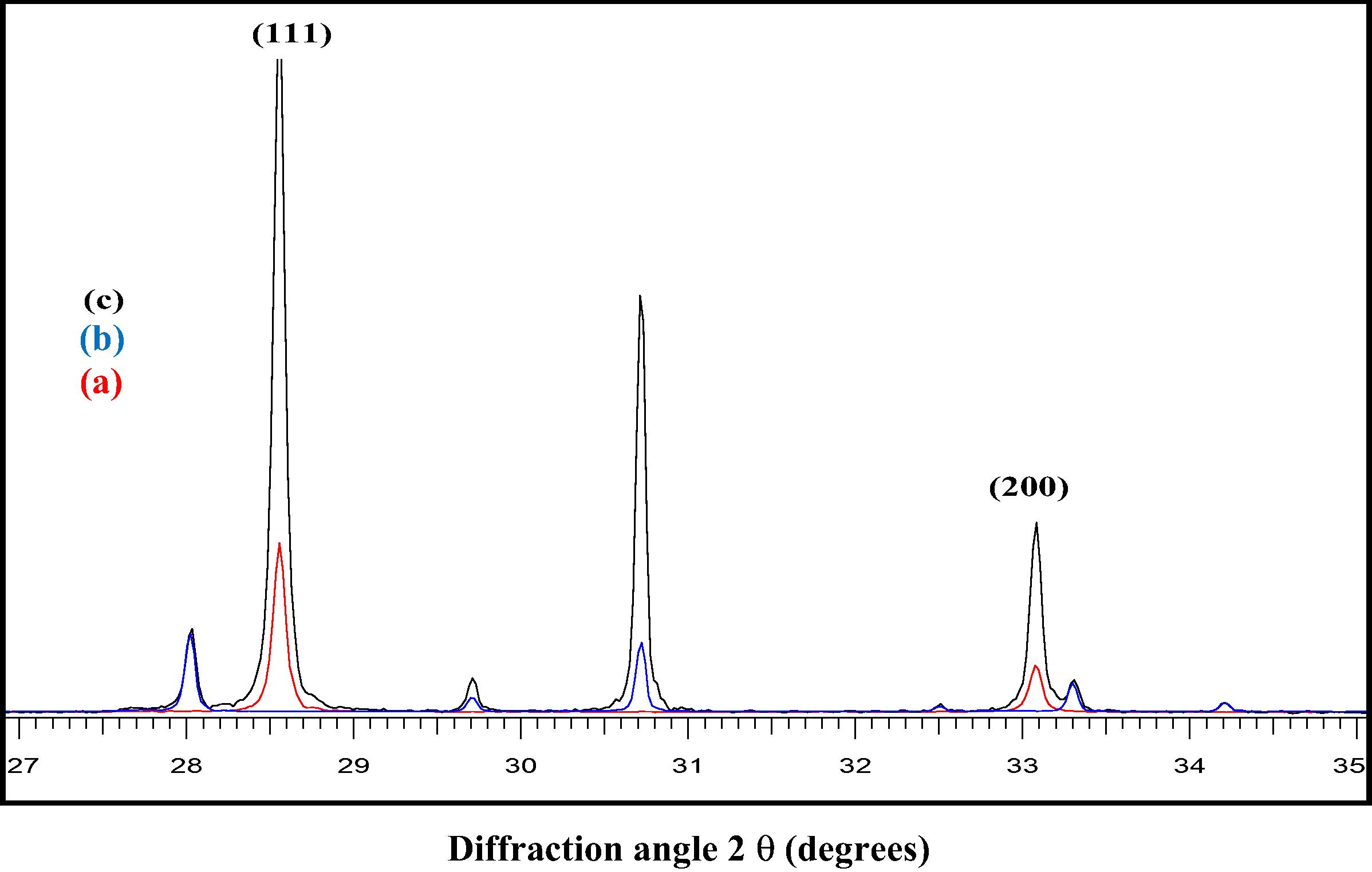
XRD patterns of pure CeO2 (a) precursor CuBi2O4 (b) and the synthesized (30 wt%) CuBi2O4/CeO2 (c) in the 2θ range from 2 ° to 40°.
3.2 SEM analysis
Fig. 3a, illustrates typical SEM images of CuBi2O4 powder synthesized by solid-state reaction of CuO and α-Bi2O3 at 750 °C for 24 h, pure CeO2 and (30 wt%) CuBi2O4/CeO2 composite. It can be seen that, for the CuBi2O4, the appearance is a shapeless sheet, and the particle size of the CuBi2O4 is about 10–20 μm. Fig. 3b shows typical high-resolution SEM image of CuBi2O4 powder to further show the details of the particles. As shown in Fig. 3b, it clearly shows two different crystal shapes on the CuBi2O4 surface, corresponding to two different particle sizes of CuBi2O4. The appearance of CuBi2O4 is a shape sheet and a well-defined tetragonal phase with the crystallite diameter of the CuBi2O4 being 5 μm, whereas groups of smaller particles do not have any specific shape with size up to 500 nm tend to cover the bigger particles. However, pure CeO2 from SEM analysis (Fig. 3c) clearly shows two different spherical-shaped nanoparticle structures on the CeO2 surface, which can be assigned to CeO2 with a particle size in the range of 100 nm and Ce2O3 with approximately 200 nm dimensions, which agrees with the UV–Vis diffuse reflectance of Ceria (see Fig. 4 in the UV–Vis DRS Spectra and Band Gap Energy section). Both nanoparticles are close to each other in the form of chains. The as synthesized (30 wt%) CuBi2O4/CeO2 composite (Fig. 3d) clearly shows the presence of CeO2 nanoparticles deposited onto the CuBi2O4 surface, displaying a particle size of 100–200 nm and strong assembly of the nanoparticles measuring from 200 nm to 1 μm. Such aggregation can be explained by the solid-state synthesis route, which generally requires repeated mechanical mixing process and a high temperature process, often give rise to particle agglomeration with severe loss of effective surface area.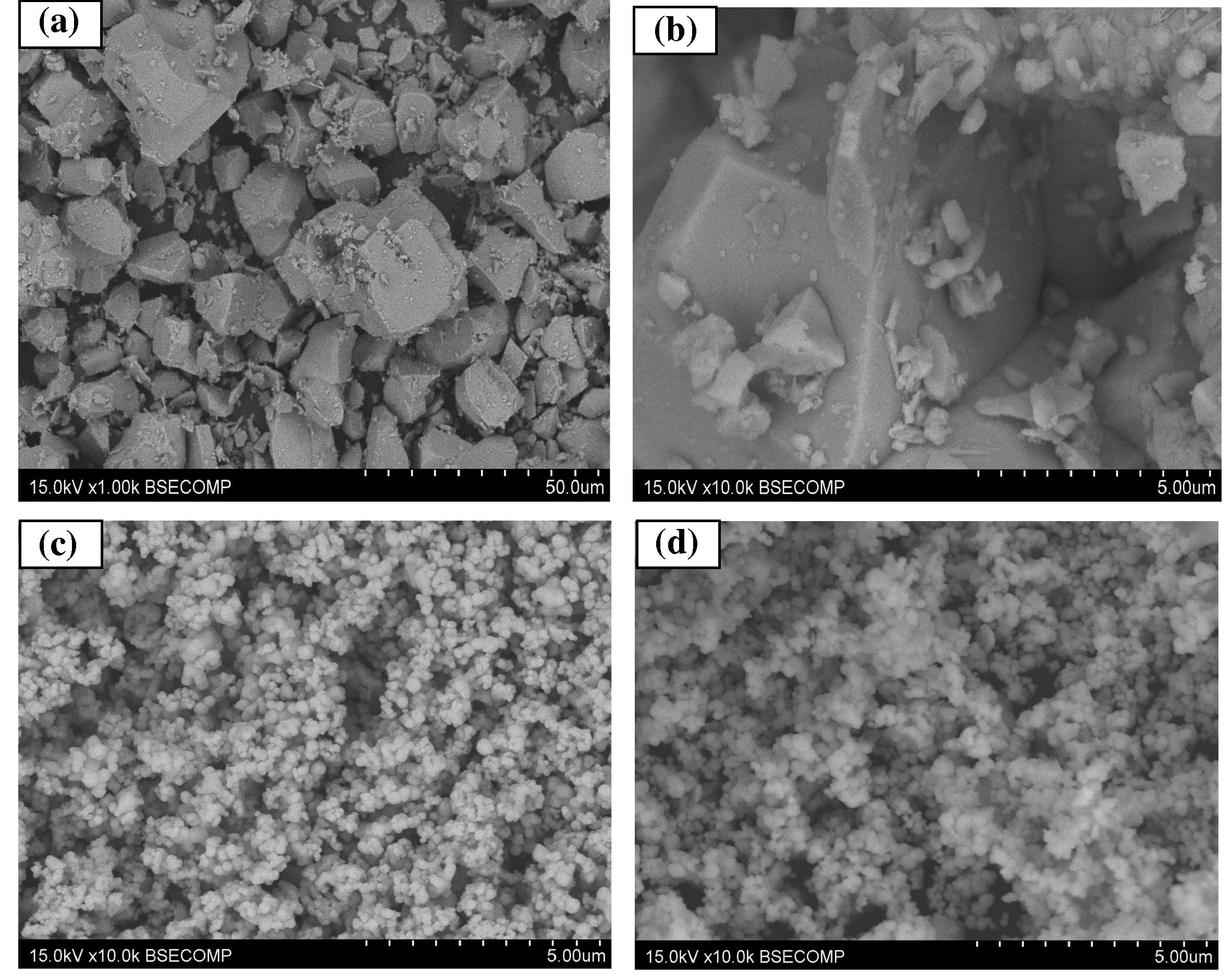
SEM images of (a) low-resolution of CuBi2O4 (b) high-resolution of precursor CuBi2O4 (c) pure CeO2 (d) (30 wt%)CuBi2O4/CeO2 composite.
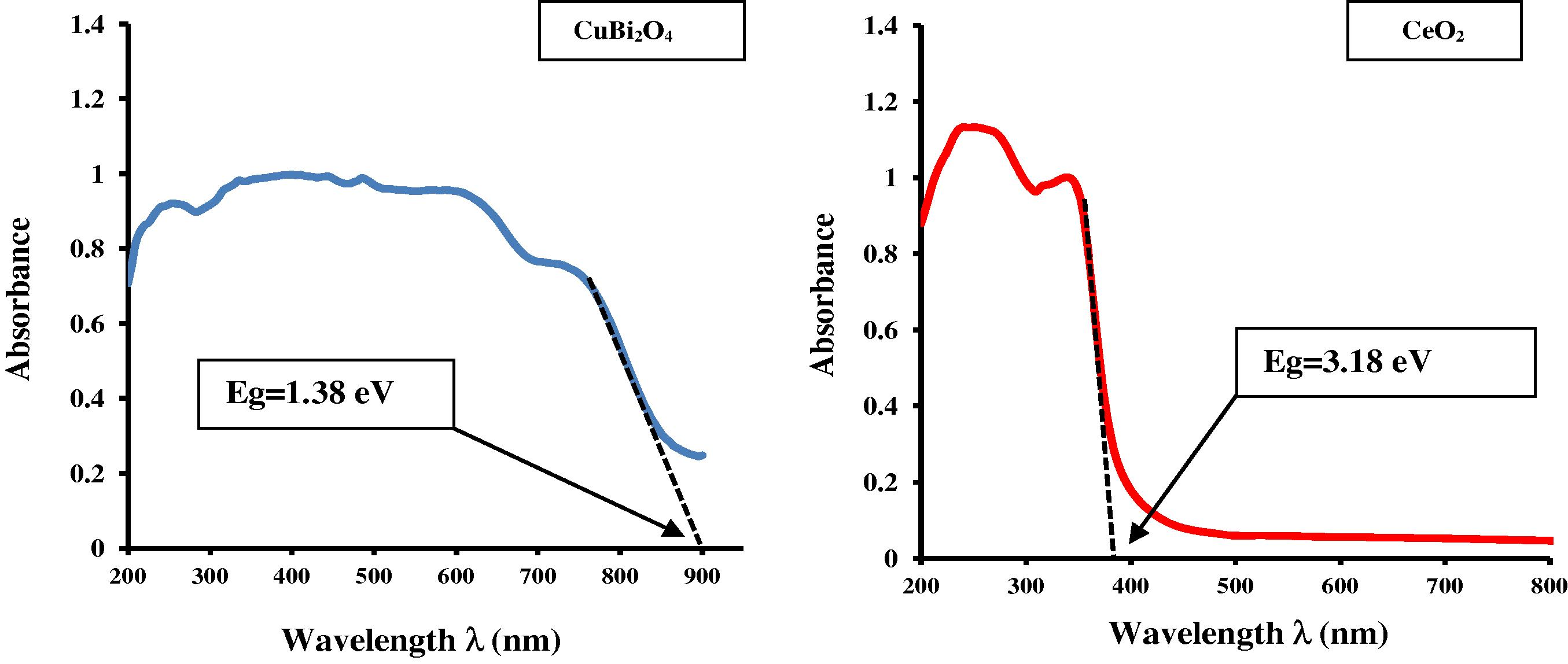
UV–Visible absorbance spectra of pure CeO2 and CuBi2O4 synthesized by solid-state reaction.
3.3 UV–Vis diffuse reflectance spectra and band gap energy
Fig. 4 shows the UV–Vis absorbance spectra of CuBi2O4 synthesized by solid-state reaction at 750 °C for 24 h and pure CeO2. It is clear from the recorded UV–Visible spectrum of CeO2 that two absorption bands are observed in the UV region at 345 and 245 nm. Generally, the absorption of ceria in the UV region originates from the charge-transfer transition between the O 2p and Ce 4f states in O2− and Ce4+. This spectral profile indicates that charge-transfer transition of Ce4+ overlaps with the 4f1 → 5d1 transition of Ce3+ (Lin et al., 2010a,b). The UV–Visible spectrum of CuBi2O4 sample is presented in Fig. 4. It can be seen that it has strong and broad absorption in the range of 200–900 nm. This suggests that the prepared sample absorbs both UV and visible light. Obviously, for CuBi2O4 sample, the broad absorption band observed in the UV–Visible region was attributed to the charge-transfer transition between the O 2p and Cu 3dx2 − y2 states in O2− and Cu2+ respectively (Hahn et al., 2012). Fig. 5 shows UV–Vis diffuse reflectance spectra of a series of photocatalysts (x wt%) CuBi2O4/CeO2. From Fig. 5, it can be seen that the absorption wavelength range of the (x wt%) CuBi2O4/CeO2 photocatalysts is extended greatly toward visible light and its absorption intensity is also increased in comparison with pure CeO2. The red-shift observed in the nanocrystalline CeO2 would be explained by the formation of localized states within the band gap owing to oxygen vacancies and increase in Ce3+ ion concentration (Lu et al., 2009).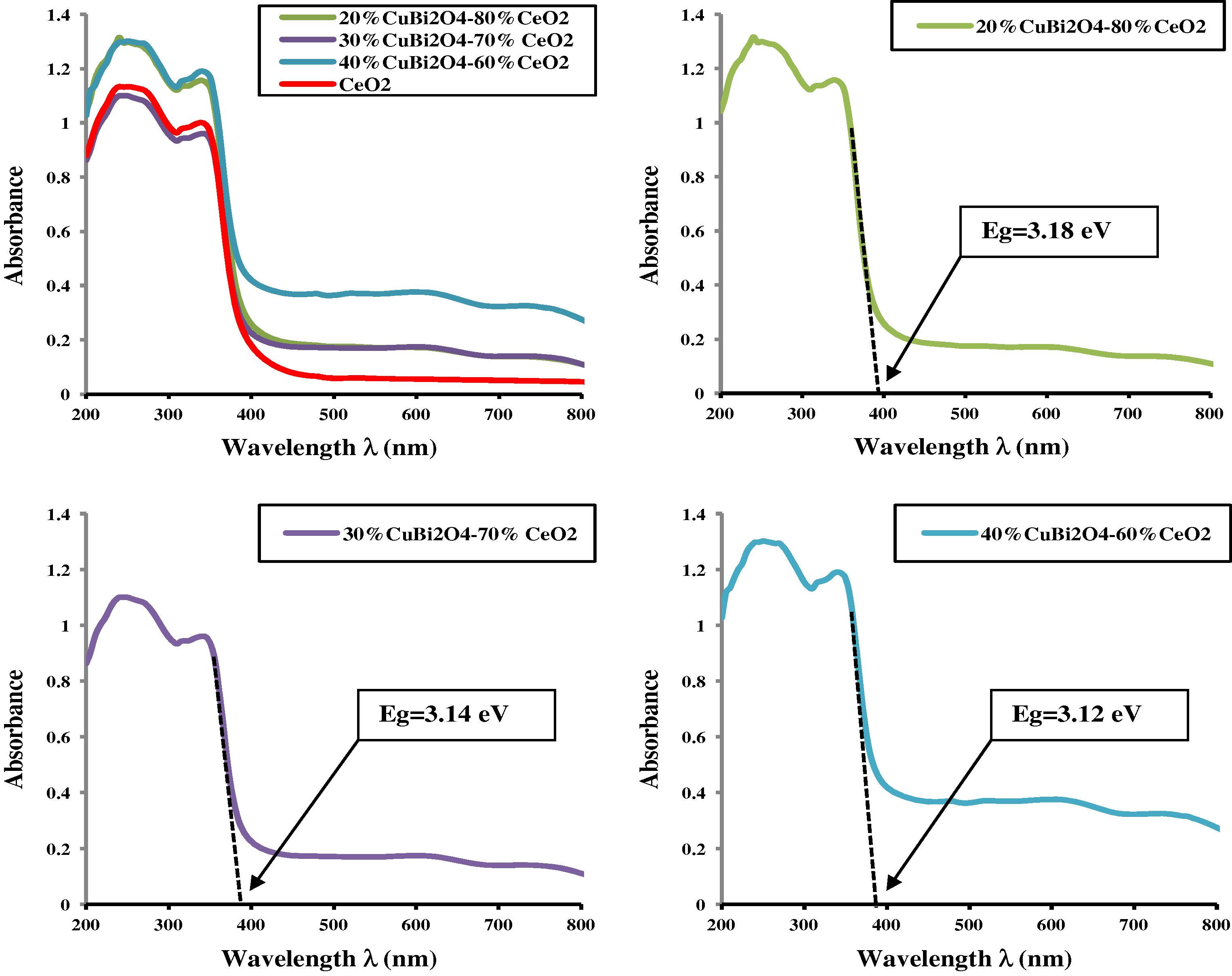
UV–Visible absorbance spectra of a series of (x wt%) CuBi2O4/CeO2 composites (x = 0–40 wt%).
The onset absorption edges and band gap energies of CuBi2O4 particle, CeO2 nanoparticle and (x wt%) CuBi2O4/CeO2 composite are shown in Figs. 4 and 5 respectively. The as-synthesized CuBi2O4 exhibits an absorption onset at 900 nm, which corresponds to band gap energy of 1.38 eV. This value is lower than that reported in the literature (1.5 eV) (Arai et al., 2007). It is clear from the recorded spectrum (Fig. 4) that the pure CeO2 nanocrystalline has two absorption onsets at 390 and 520 nm, which match to band gap energies of 3.18 and 2.38 eV, attributing to CeO2 dioxide and Ce2O3 sesquioxide respectively. These results are in well agreement with values reported in the literature (Xu and Schoonen, 2000; Magesh et al., 2009). The optical properties of the as-synthesized CuBi2O4 and pure CeO2 nanoparticles are reported in Table 1. λ, Wavelength; Ref., Reference.
Systems
λ (nm)
Charge-transfer transition
Band gap Eg (eV)
Experimental
Literature Refs.
CuBi2O4
900
2p6 (O) → 3dx2 − y2 (Cu)
1.38
1.5 (Arai et al., 2007)
CeO2
390
2p6 (O → 4f0 (Ce)
3.18
2.7–3.4 (Ozer, 2001)
Ce2O3
520
4f0 (Ce) → 4f1 (Ce)
2.38
2.40 (Ozer, 2001)
It is widely accepted that electronic transport properties depend on the physical and structural characteristics of photocatalyst, such as crystallite size, morphology, phase structure and amount of CuBi2O4 loaded (Li et al., 2009; Liu et al., 2009; Yu et al., 2008). As reported from the UV–Vis DRS in Fig. 5 and Table 2, for the series of (x wt%) CuBi2O4/CeO2 composites, the band gap energy decreased from 3.18 to 3.12 eV as the amount of CuBi2O4 was increased up to 40% on the CeO2 matrix, suggesting that the physical preparation of composite powders will result in good particle-to-particle connections, especially in cases where there is a high electrical conductivity (Marunsek, 2009). So, the decrease of the band gap energy with an enhanced absorption intensity of the (30 wt%) CuBi2O4/CeO2 composite upon loading the amount of CuBi2O could be ascribed to the homogeneous dispersion of CuBi2O4 within the CeO2 matrix in the bulk of the catalyst and the formation of conducting network at very low temperature.
Amount of CuBi2O4 (%)
Charge-transfer transition 2p6 (O) → 4f0 (Ce)
Charge-transfer transition 4f0 (Ce) → 4f1 (Ce)
λ (nm)
Band gap Eg (eV)
λ (nm)
Band gap Eg (eV)
0
390
3.18
520
2.38
20
390
3.18
495
2.51
30
395
3.14
500
2.48
40
397
3.12
490
2.53
3.4 Photocatalytic activity tests
3.4.1 Effect of pH solution on the photocatalytic activity of (30 wt%) CuBi2O4/CeO2 composite
In order to study the effect of initial pH on the degradation efficiency of (30 wt%) CuBi2O4/CeO2 composite on photodecomposition of CR, experiments were carried out at various pH, ranging from 6 to 12 for avoiding dye aggregation. The results showed that the pH significantly affected the photocatalytic degradation efficiency of both CR. As shown in Fig. 6 and Table 3, for CR, the degradation rate increased from 64.96% to 83.05% as the pH value was increased from 6 to 7, and then decreased to 26.53 at pH = 12. The maximum degradation rate of CR (83.05%) was achieved at pH = 7. For this reason, the pH = 7 was selected for subsequent experiments.![Effect of the pH solution on the photocatalytic redox of CR under UVA-light irradiation ([(30 wt%) CuBi2O4/CeO2] = 0.5 g/L, [CR] = 20 mg/L, T = 298 K, λmax = 365 nm, I = 90 J/cm2 and irradiation time = 100 min).](/content/185/2015/27/2/img/10.1016_j.jksus.2014.08.002-fig6.png)
Effect of the pH solution on the photocatalytic redox of CR under UVA-light irradiation ([(30 wt%) CuBi2O4/CeO2] = 0.5 g/L, [CR] = 20 mg/L, T = 298 K, λmax = 365 nm, I = 90 J/cm2 and irradiation time = 100 min).
pH initial
Adsorption activity η (%)
Photocatalytic activity η′ (%)
2
Dye aggregation
4
6
11.85
64.96
7
17.30
83.05
8
1.73
55.98
10
17.14
29.50
12
9.41
26.54
It is commonly accepted that in photocatalyst/aqueous systems, the potential of the surface charge is determined by the activity of ions (e.g. H+ or pH). A convenient index of the tendency of a surface to become either positively or negatively charged as a function of pH is the value of the pH required to give zero net charge (pHPZC) (Zhang et al., 1998; Yates et al., 1974). pHPZC is a critical value for determining the sign and magnitude of the net charge carried on the photocatalyst surface during adsorption and the photocatalytic degradation process. Most of the semiconductor oxides are amphoteric in nature, can associate Eq. (15) or dissociate Eq. (17) proton. To explain the relationship between the layer charge density and the adsorption, so-called Model of Surface Complexation (SCM) was developed (Fernandez et al., 2002), which consequently affects the sorption–desorption processes as well as the separation and transfer of the photogenerated electron–hole pairs at the surface of the semiconductor particles. In the 2-pK approach we assume two reactions for surface protonation. The zero point charge pH PZC for CeO2 (about 7.5) is approximately identical to that of (30 wt%) CuBi2O4/CeO2 sample, since there is no adsorption of CR ions than the potential determining H+/OH− at the surface of CuBi2O4 particles. This is often the case for pure (“pristine surface”) oxides in water.
When the pH is lower than the pH PZC value, the system is said to be “below the PZC.” Below the PZC, the acidic water donates more protons than hydroxide groups, and so the adsorbent surface is positively charged (attracting anions/repelling cations), according to the following reaction Eqs. (9) and (10):
Conversely, above pH PZC the surface is negatively charged (attracting cations/repelling anions), given by the following reaction Eqs. (11) and (12):
CR contains an azo (–N⚌N–) chromophore and an acidic auxochrome (–SO3H) associated with the benzene structure. CR is also called acidic diazo dye. The pKa value of CR is 4.1, thus CR would be negatively charged at pH range 5.0–10.0 (Ahmad and Kumar, 2010; Zhang et al., 2011). At pH below the pKa value, a dye exists predominantly in the molecular form.
The experimental results revealed that higher degradation rate of CR was observed at pH = 7. Since CR is an anionic dye, its adsorption is mainly performed via electrostatic interactions between the positive (30 wt%) CuBi2O4/CeO2H+ surface (pH > pHPZC) and CR− anionic form (pH > pKa), leading to a maximum value in lower pHPZC (i.e. pH = 7). Thus, the activity of an adsorbent is due to the presence of sulfonated groups (–SO3−). The presence of the tightly physically bonded or close contact interfaces between the two semiconductors, by which the photoinduced charge transfer from one particle to the other through interfaces spatially is available, can lead to a strong photocatalytic redox of CR over the combined catalysts.
At acidic medium (i.e. pH = 6), higher adsorption extent of CR onto (30 wt%) CuBi2O4/CeO2H+ was observed. Such an occurrence could be explained via van der Waals forces, H-bonding and hydrophobic–hydrophobic interactions (Ahmad and Kumar, 2010). Although the electrostatic interaction between the positively charged (30 wt%) CuBi2O4/CeO2H+ surface and CR− anionic dye was detected, the photocatalytic activity of the (30 wt%) CuBi2O4/CeO2 catalyst was significantly reduced. This can be explained by the following causes: Assuming most of the reactions take place at the surface of the catalyst, with decreasing pH medium (i.e. pH = 6), Congo red (CR) has a propensity to aggregate in acidic or highly acidic pH ranges. The proposed mechanisms suggest hydrophobic interactions between the aromatic rings of the dye molecules, leading to a π–π stacking phenomenon. CR decolorization has been limited by the available surface area. Moreover, due to this, only fewer photons reach the surface of the photocatalyst. This results in a decrease in concentration of hydroxyl radicals (•OH) and superoxide (O2•−) radicals, thereby decreasing the photocatalytic activity.
At pH higher than pH PZC value (i.e. pH = 10–12), the total surface of the (30 wt%) CuBi2O4/CeO2 catalyst is negatively charged. Hence due to the electrostatic repulsion forces between the negatively charged (30 wt%) CuBi2O4/CeO2 surface and CR anionic dye, mainly sulfonated groups (–SO3−), affecting strongly the accessibility of the surface reducing species to the CR photocatalytic oxidation/reduction kinetics. But appreciable adsorption extent in this pH range suggested strong involvement of physical forces such as hydrogen bonding, van der Waals force, etc. in the adsorption process (Chatterjee et al., 2007). Thus, the observed degradation is primarily taking place in the solution. Further, under alkaline conditions (high concentration of hydroxide ions), more hydroxyl radical (•OH) formation is possible from the abundant hydroxide ions, which also decline the degradation. There were similar results in the previous reports (Laouedj et al., 2011; Elaziouti et al., 2011; Elaziouti et al., 2012).
3.4.2 Effect of the amount of CuBi2O4 on the photocatalytic activity of (x wt%) CuBi2O4/CeO2
The effect of the amount of CuBi2O4 on photocatalytic degradation of CR was conducted over a range of catalyst amounts from x = 0 to x = 100 wt%. As observed in Fig. 7 and Table 4, it is evident that the photocatalytic redox of CR greatly depends on the amount of CuBi2O4 loaded. The photocatalytic activity increased drastically from 14. 928% to 83.054% as the catalyst amount was raised from x = 0 to x = 30 wt%. On further increase in the CuBi2O4 amount beyond x = 30 wt%, the photocatalytic activity decreased gradually, almost reaching 3.13% at x = 100wt%. The highest photocatalytic activity of (x wt%) CuBi2O4/CeO2 (83.054%) under UVA-light irradiation was achieved within 100 min when the amount of CuBi2O4 loaded x was 30 wt%, which is obviously about 6 times higher than that of pure CeO2 and 28 times superior than that of the synthesized CuBi2O4.![Effect of the amount of CuBi2O4 on the photocatalytic redox of CR under UVA-light irradiation ([Catalyst] = 0.5 g/L, [CR] = 20 mg/L, pH = 7, T = 298 K, λmax = 365 nm, I = 90 J/cm2 and irradiation time = 100 min).](/content/185/2015/27/2/img/10.1016_j.jksus.2014.08.002-fig7.png)
Effect of the amount of CuBi2O4 on the photocatalytic redox of CR under UVA-light irradiation ([Catalyst] = 0.5 g/L, [CR] = 20 mg/L, pH = 7, T = 298 K, λmax = 365 nm, I = 90 J/cm2 and irradiation time = 100 min).
Amount of CuBi2O4 x (%)
Adsorption activity η (%)
Photocatalytic activity η′ (%)
0
8.17
14.92
5
20.84
29.33
10
21.515
39.03
20
13.71
60.98
30
17.30
83.05
40
4.024
75.00
50
17.25
53.53
100
0.00
3.13
On the other hand, both CuBi2O4 on CeO2 precursors showed poor adsorption affinity toward organic pollutant among the CuBi2O4 loadings. Within the range of CuBi2O4 amounts from 0 to 30 wt%, the observed increase in CR decolorization may be due to an increased number of available adsorption and catalytic sites on the surface of (x wt%) CuBi2O4/CeO2 catalyst. So there is an optimum CuBi2O4 content for high dispersion morphology of particles CuBi2O4 on the CeO2 surface with high activity.
The effective electron–hole separation both at the physically bonded interfaces and in the two semiconductors as well as charge defect during the physical mixing method was believed to be mainly responsible for the remarkably enhanced photocatalytic activity of (30 wt%) CuBi2O4/CeO2 in the course of the photocatalytic redox conversion of CR.
But until now, there are no reports about synergistic effect between CeO2 and CuBi2O4 in the (30 wt%) CuBi2O4/CeO2 catalyst under UVA-light excitation. From Fig. 7, it is clear that the photocatalytic activity of CeO2 is drastically increased in the presence of an amount of CuBi2O4 (30 wt%) compared to pure CeO2 and the CuBi2O4 samples. These results strongly suggest the existence of a synergistic effect between CeO2 and the CuBi2O4 in the (30 wt%) CuBi2O4/CeO2 catalyst under UVA light excitation.
A further increase in catalyst amount (i.e. >30 wt%), however, may cause an increase in the overlapping of adsorption sites of CeO2 particles as a result of overcrowding of the CuBi2O4 granule owing to the decrease in screening effect and interfering of light. Furthermore, at higher catalyst amount, it is difficult to maintain a homogeneous suspension due to agglomeration of the particles, which decreases the number of active sites. An exception was observed for (50 wt%) CuBi2O4/CeO2 catalyst sample owing to the overestimating value in the experimental data. Thus, results indicate that an optimized catalyst amount (30 wt%) is necessary for enhancing the decolorization efficiency. An analogous trend was reported in the reduction of Cr2O72- and photocatalytic oxidation of methylene blue orange (MB) using p–n heterojunction photocatalyst CuBi2O4/Bi2WO6 (Liu et al., 2011).
3.4.3 Effect of UVA-light and catalyst
The photocatalytic activities of all three CuBi2O4, CeO2, (30 wt%) CuBi2O4/CeO2 catalysts were assessed by the photocatalytic redox reaction of Congo red (CR) aqueous solution under UVA-light irradiation. Variations of CR reduced concentration (C/C0) versus UVA-light irradiation time (t) over different catalysts under different experimental conditions through UV-A alone, UVA/CuBi2O4, UVA/CeO2, (30 wt%) CuBi2O4/CeO2 and UVA/(30 wt%)CuBi2O4/CeO2 are presented in Fig. 8. Results showed that (30 wt%)CuBi2O4/CeO2 sample exhibited higher photocatalytic performance, as compared to the single phases CuBi2O4 and CeO2. The highest efficiency was obtained, under UVA-light irradiation over (30 wt%)CuBi2O4/CeO2, as a result of 83.05% degradation of CR for 100 min of irradiation time. However, the photocatalytic degradation of CR over single phases CuBi2O4 and CeO2 was only 3.13% and 14.92% respectively. When 20 mg/L of CR along with (30 wt%) CuBi2O4/CeO2 was magnetically stirred for the same optimum irradiation time in the absence of light, lower (21.48%) degradation was observed, whereas, disappearance of dye was negligible (0.49%) in the direct photolysis. On the basis of these results, the high decomposition of CR dye in the presence of (30 wt%) CuBi2O4/CeO2 catalyst is exclusively attributed to the photocatalytic reaction of the combined semiconductor particles under UVA-light irradiation. Thus, such an above occurrence in the present experiment is primarily assigned to the charge defect during the physical mixing method, which is advantageous for the effective electron–hole separation and the suppression of the recombination rate of the photogenerated charge carriers, hence result in an improvement of the probability of light-generated carrier transfer via interfaces spatially available. Thus, enhancing the effectiveness of the photocatalytic redox conversion of CR over (30 wt%) CuBi2O4/CeO2 composite under UV light irradiation. A similar result was reported in the heterojunction semiconductor SnO2/SrNb2O6 with an enhanced photocatalytic activity (Liu and Yu, 2008).![Photocatalytic degradation kinetics of CR at different experimental conditions ([Catalyst] = 0.5 g/L, [CR] = 20 mg/L, pH = 7, T = 298 K, λmax = 365 nm, I = 90 J/cm2 and irradiation time = 100 min).](/content/185/2015/27/2/img/10.1016_j.jksus.2014.08.002-fig8.png)
Photocatalytic degradation kinetics of CR at different experimental conditions ([Catalyst] = 0.5 g/L, [CR] = 20 mg/L, pH = 7, T = 298 K, λmax = 365 nm, I = 90 J/cm2 and irradiation time = 100 min).
3.4.4 Kinetic modeling
The photocatalytic degradation of CR over different experimental conditions is displayed in Table 5. As it can be seen, the straight lines for the entire as-prepared samples of the plots of ln C/C0 versus t with high regression coefficients (R2 = 0.892–0.939), for the pseudo-first-order kinetic model strongly suggest that all the photodegradation systems were a pseudo-first-order model. Exception was observed in the cases of photodegradation and adsorption reactions in the presence of the single phase CuBi2O4 and the combined semiconductors respectively.
Systems
η (%)
η′ (%)
K1 (min−1)
t1/2 (min)
R2 (%)
CR/UV-A
–
0.49
–
–
–
CR/(30 wt%) CuBi2O4-CeO2
21.48
–
–
–
–
CR/CeO2/UVA
8.00
14.92
0.0024
288.811
0.892
CR/(30 wt%) CuBi2O4-CeO2/UVA
17.30
83.05
0.0133
52.116
0.939
CR/CuBi2O4/UVA
0
3.13
0.0002
3465.736
0.203
3.5 Discussion of mechanism
The above analysis shows that the migration direction of the photogenerated charge carrier depends on the band edge positions of the two semiconductors. There are three methods to determine the band edge positions: experiments based on photoelectrochemical techniques, calculation according to the first principle, and predicting theoretically from the absolute (or Mulliken) electronegativity (Kim et al., 1993; Butler and Ginley, 1978; Xu and Schoonen, 2000). The first one is not always easy to handle, and the second one cannot obtain the absolute energy of band edges with respect to vacuum and always has large discrepancies between calculated and measured values. The third one is a simple approach with reasonable results for many oxide photocatalysts (Xu and Schoonen, 2000).
The conduction band edge of a semiconductor at the point of zero charge (pH zpc) can be predicted by Eq. (14):
Catalyst
χ (eV)
λ (nm)
Eg (eV)
E0BC (eV)
E0BV (eV)
CuBi2O4
4.75
900
1.38
−0.44
+0.94
CeO2
5.56
390
3.18
−0.53
+2.65
The as-prepared CuBi2O4 is a p-type semiconductor, which always exhibits good stability under UV–Visible illumination, and CeO2 is determined as an n-type material. Fig. 9 depicts reaction schemes of CuBi2O4 (a) and CeO2 (b) as the p and n type respectively for charge separation for the reductivity/oxidizability improvement model. According to Fig. 9, when the CuBi2O4 and CeO2 photocatalysts are irradiated under UVA (365 nm) light, both catalysts CuBi2O4 and CeO2 can be activated since the band gap energies of CuBi2O4 observed in this study were 3.18 and 1.38 eV respectively.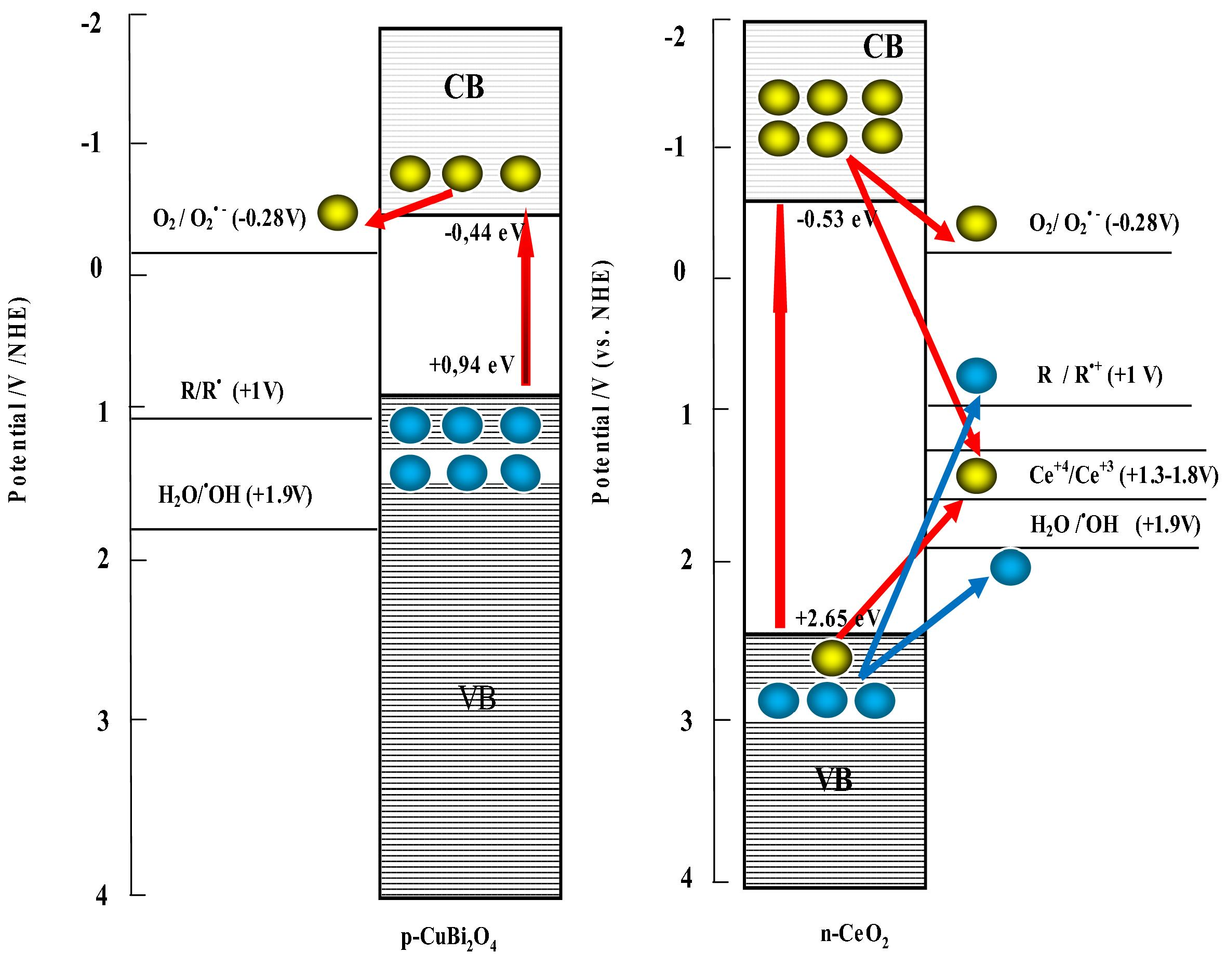
Reaction schemes of CuBi2O4 (a) and CeO2 (b) as the p and n type respectively for charge separation for the reductivity/oxidizability improvement model (electron
 and hole
and hole  ).
).
For the p-CuBi2O4 (Fig. 9a), the electronic potential of the CB of p-CuBi2O4 is around −0.44 eV/NHE which is more negative than that of superoxide radical (E0 (O2/O2•−) = −0.28 V/NHE. This indicated that the electron photoproduced at the CB directly reduced O2 into O2•−. These reduced O2•− can subsequently transfer the charge to the species present in the reaction medium that are preferentially adsorbed onto the p-CuBi2O4 particles. Hence, the superoxide radical (O2•−) reduces the recombination of the charge carriers enhancing the activity in the UVA light. However, the p-CuBi2O4 VB of +0.94 eV/NHE, which is too negative than the potential of hydroxyl radical (E0 (H2O/•OH)) = +1.9 V/NHE. The photogenerated holes in the VB of p-CuBi2O4 are not able to oxidize H2O to •OH radicals.
p-CuBi2O4 powder formed in our laboratory exhibits a black color. The presence of non stoichiometric regions of the nominally p-CuBi2O4 particles or small domains of binary oxide phases of CuxO or BixO, undetected by XRD data, as unstable impurity phases which could be originated from a number of processes such as reduction of the p-CuBi2O4, could be responsible for higher recombination rates. Thus, the result is consistent with the previous study in electrochemical synthesis and characterization of p-CuBi2O4 thin film photocathodes (Hahn et al., 2012). Therefore, CuBi2O4 alone shows negligible photocatalytic activity under UVA light. As a result, less efficient charge-carrier separation, and thus the increment of photocatalytic activity was restricted.
On the other hand, pure CeO2 (Fig. 9b) shows little photocatalytic activity under UVA light. Since the VB of CeO2 is around +2.65 eV/NHE and the CB of CeO2 is around −0.53 eV/NHE, we expect that photogenerated electrons at the CB of CeO2 can directly reduce O2 into superoxide (O2•−) because electronic potential of the CB of CeO2 (−0.53 eV/NHE) is more negative than that of superoxide radical (E0 (O2/O2•−) = −0.28 V/NHE). In contrast, the CeO2 VB of +2.65 eV/NHE is more positive than that of hydroxyl radical (E0 (H2O/•OH)) = +1.9 V/NHE, indicating that the photogenerated holes in the CeO2 can oxidize H2O to •OH radicals and CR dye molecule directly forming the organic cation-radicals (R•+ads). These (O2•−) superoxide and (•OH) and organic cation (R•+ads) radical species can subsequently transfer the charge to the present in the reaction medium. Thus, the superoxide radical (O2•−) suppresses the recombination of the charge carriers enhancing the photocatalytic activity in the UVA light as well. Moreover, the redox potential for one-electron oxidoreduction of cerium Ce+4/Ce3+ (1.3–1.8 V) is more negative than that of CeO2 VB (+2.65 eV/NHE) and more positive than that of CeO2 CB (−0.53 eV/NHE). Hence, the photogenerated electrons at the CB and VB of CeO2 are able to reduce Ce+4 to Ce3+ and can oxidize Ce+3 to Ce4+, respectively, reducing the recombination of the charge carriers.
In a contrast experiment, p-CuBi2O4/n-CeO2 composite exhibits higher activity than phases p-CuBi2O4 and n-CeO2. So we should continue with a further discussion on the mechanism in the photocatalysis. The possible reason for the remarkably enhanced photocatalytic performance of p-CuBi2O4/n-CeO2 in the course of the photocatalytic redox of Congo red can be explained by p–n type heterojunction formation model of the electron–hole separation process under UV light irradiation. The schematic diagram p–n heterojunction formation model is depicted in Fig. 10.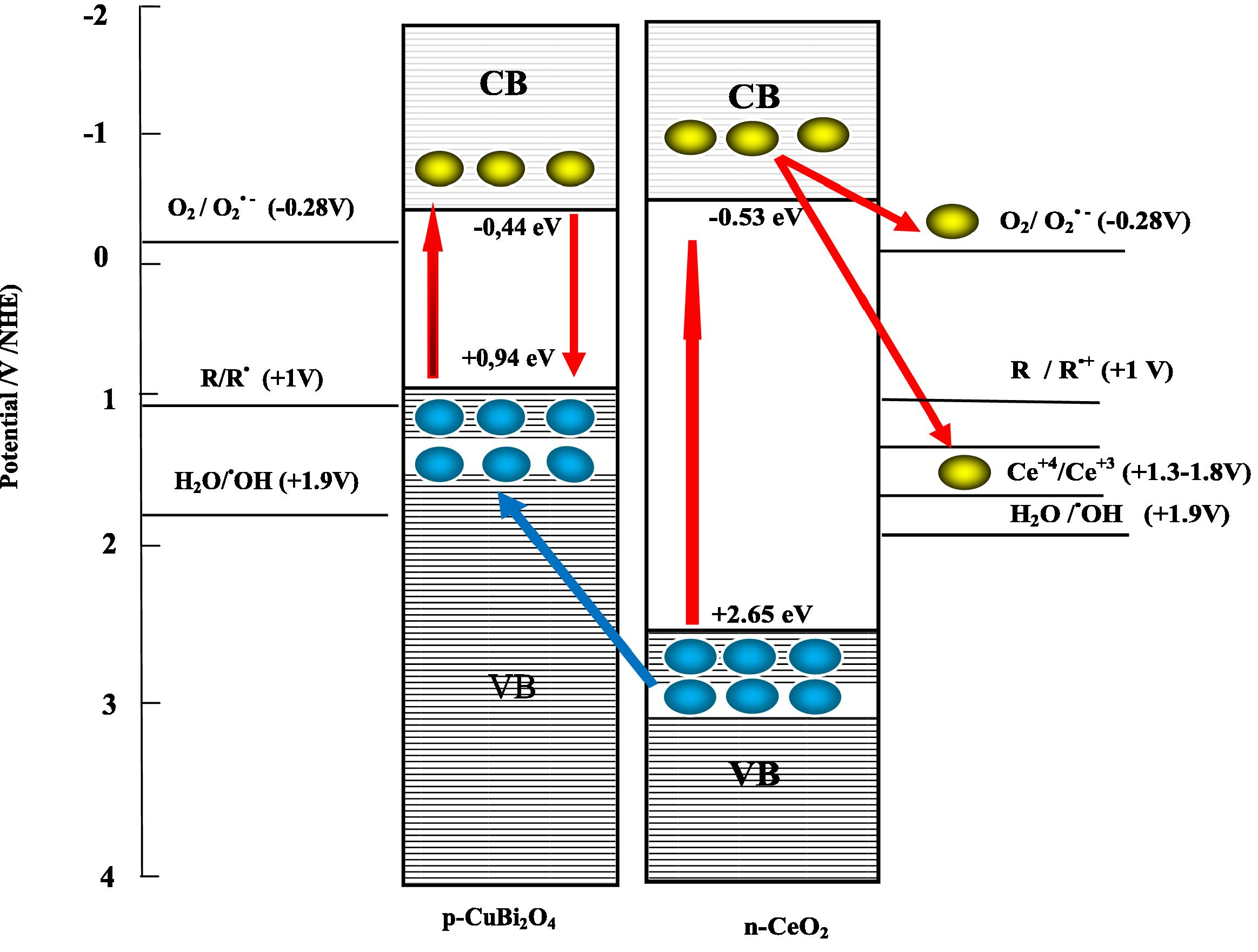
Reaction scheme of CuBi2O4/CeO2 as the p–n type charge separation for the reductivity/oxidizability improvement model (electron
 and hole
and hole  ).
).
CuBi2O4 is a p-type semiconductor, which always exhibits good stability under UV visible illumination, and CeO2 is determined as an n-type semiconductor. The band gap of p-CuBi2O4 was 1.38 eV, which could be excited by photons with wavelengths below 900 nm, whereas n-CeO2 with band gap is about 3.18 eV which can be excited by photons with wavelengths of 390 nm. So at the interfaces of p-CuBi2O4 loaded n-CeO2 composite, a p–n heterostructure would be formed.
According to the band edge position (Table 1), the electronic potential of the CB of n-CeO2 is slightly more anodic than that of p-CuBi2O4, whereas, the hole potential of the VB of n-CeO2, is more positive than that of p-CuBi2O4. Under UVA (λUVA = 355–375 nm → Ehν = 3.30–3.49 eV) light irradiation, the energy of the excitation light was large enough to yield an excited state of both p-CuBi2O4 (λCuBi2O4 = 900 nm → Eg = 1.38 eV) and n-CeO2 (λCeO2 = 390 nm → Eg = 3.18 eV) semiconductors. A part of the photogenerated charge carriers, free electron (e−) and electronic vacancy-a hole (h+), recombines in the bulk of the semiconductors, while the rest transfers in the photocatalyst surfaces being partially localized on structural defective centers of its crystalline lattice.
So, when p-type semiconductor CuBi2O4 and n-type semiconductor CeO2 were connected to each other, p–n heterostructure will be formed between p-CuBi2O4 and n-CeO2, and at the equilibrium the inner electric field will be also produced at the same time in the interface. So a number of micro p–n heterostructure CuBi2O4/CeO2 photocatalysts will be formed after doping p-CuBi2O4 powder into n-CeO2 granule. The electron–hole pairs will be created under UVA light illumination. With the effect of the inner electric field, the holes can transfer from n-CeO2 to p-CuBi2O4 easily, because it is thermodynamically more favorable than to go to H2O (larger driving force). Photogenerated electrons in p-CuBi2O4 will recombine with excess holes that are injected from CeO2. But the electrons cannot move from n-CeO2 to p-CuBi2O4. If electrons are transferred to p-CuBi2O4, the photocatalytic activity would decrease because of recombination (Li et al., 2004). Although the transfer of electrons is feasible for the potential between the two CB, it is blocked because of the inner electric field. So the minor carrier in n-CeO2, which is the control factor of recombination in this n-CeO2 semi-conductor, can transfer out. In this way, the photoinduced electron (e−)–hole (h+) pairs in the two semiconductors were effectively separated by the p–n heterostructure formed in the CuBi2O4/CeO2 catalyst and transferred to the semiconductor/substrate interfaces efficiently, thus the probability of electron–hole recombination was reduced. As a result, the net effect of holes in p-CuBi2O4 surface acting as powerful oxidants Eqs. (15) and (16). The stepwise photocatalytic mechanism is illustrated below:
The photogenerated electrons in the CB of n-CeO2 as well as holes in the VB of p-CuBi2O4 act as powerful oxidants, respectively. The electrons at the CB of n-CeO2 react with the adsorbed molecular O2ads on the p-CuBi2O4/n-CeO2 catalyst sites, reducing it to superoxide anion (•−2ads), hydroperoxide (HO2ads) radicals, hydrogen peroxide (H2O2ads) and hydroxide (•OHads) radicals Eqs. (17)–(19), while the holes at the VB of p-CuBi2O4 are not able to oxidize the CR dye molecule. These processes could be represented in the following equations:
The superoxide anion (O•−2ads) and the hydroxide radicals (•OHads) formed from n-CeO2 on the illuminated p-CuBi2O4/n-CeO2 catalyst surface are highly effective oxidizing agents in the p-CuBi2O4/n-CeO2 mediated photocatalytic oxidation of Congo red Eq. (20).
The primary reason for the observed photocatalytic activity of the p-CuBi2O4/n-CeO2 composites can be attributed to p-CuBi2O4 being less active than n-CeO2. At 30 wt% p-CuBi2O4 loading, the amount of Ce+4/Ce+3 present on the p-CuBi2O4/n-CeO2 composites surface is favorable for faster charge transfer and at the same time allows light to reach the p-CuBi2O4/n-CeO2 surface. A similar trend was reported in the efficient photocatalytic degradation of phenol over Co3O4/BiVO4 composite under Visible Light Irradiation (Mingce et al., 2006).
4 Conclusion
Novel p-CuBi2O4/n-CeO2 photocatalysts with different mass ratios were synthesized via the solid state route. The as-prepared p-CuBi2O4/n-CeO2 catalysts were characterized by XRD, SEM and UV–Vis DRS technique. The photocatalytic activity of the samples was investigated under UVA light and assessed using Congo red (CR) dye as probe reaction. The effect of some parameters such as the amount of p-CuBi2O4 catalyst and pH of the CR dye solution on the photocatalytic activity of the structurally optimized sample; (30 wt%) p-CuBi2O4/n-CeO2; was studied. Results show that (30 wt%) p-CuBi2O4/n-CeO2 catalyst exhibits enhanced photocatalytic activity under UVA-light irradiation. The highest efficiency was observed at 30 wt% of CuBi2O4 content as a result of 83.05% of photoactivity for 100 min under UVA-light at 25 °C. The photocatalytic reactions are most sensitive to the pH medium in the range of 6–12 and maximum efficiency was observed at pH = 7. These results strongly suggest the existence of a synergistic effect between CeO2 and the CuBi2O4 in the (30 wt%) CuBi2O4/CeO2 catalyst. The mechanism of the increased photocatalytic activity of (30 wt%) CuBi2O4/CeO2 catalyst has been discussed by calculated energy band positions. The efficient electron–hole separation process in the p–n heterostructure semiconductors under UVA-light irradiation was considered to be mainly responsible for the obviously improved photocatalytic activity of (30 wt%) CuBi2O4/CeO2 catalyst in the course of the photocatalytic redox conversion of Congo red.
Acknowledgments
We are greatly indebted to the University of Science and Technology of Oran (Mohamed Boudiaf), and the University of Science and Technology of Saida (Moulay Tahar) for their material support. We gratefully acknowledge the support for X-ray diffraction (XRD), scanning electron microscopy (SEM) and UV–Vis diffuse reflectance spectroscopy (DRS) by Mrs. Professor Rose-Noëlle Vannier of the Unit of Catalysis and Solid State Chemistry of Lille 1 University.
References
- Adsorptive removal of Congo red dye from aqueous solution using bael shell carbon. Appl. Surf. Sci.. 2010;257:1628-1633.
- [Google Scholar]
- Efficient complete oxidation of acetaldehyde into CO2 over CuBi2O4/WO3 composite photocatalyst under visible and UV light irradiation. J. Phys. Chem. C. 2007;111:7574-7577.
- [Google Scholar]
- Elements of X-ray Crystallography. New-York: McGraw-Hill; 1968. pp. 331–568
- Photocatalytic degradation of fast green using nanosized CeCrO3. Macedonian J. Chem. Chem. Eng.. 2010;29:195-202.
- [Google Scholar]
- Self assembly of active Bi2O3/TiO2 visible photocatalyst with ordered mesoporous structure and highly crystallized anatase. J. Phys. Chem. C. 2008;112:6258-6262.
- [Google Scholar]
- Prediction of flatband potentials at semiconductor-electrolyte interfaces from atomic electronegativities. J. Electrochem. Soc.. 1978;125:228-232.
- [Google Scholar]
- Photocatalytic performance of TiO2 catalysts modified by H3PW12O40, ZrO2 and CeO2. J. Environ. Sci.. 2009;21:997-1004.
- [Google Scholar]
- Photocatalytic efficiency analysis of CdS nanoparticles with modified electronic states. J. Anal. Sci. Technol.. 2010;1:25-29.
- [Google Scholar]
- Adsorptive removal of Congo red, a carcinogenic textile dye by chitosan hydrobeads: binding mechanism, equilibrium and kinetics. Colloids Surf. A. 2007;299:146-152.
- [Google Scholar]
- Structural transformations of Bi2CuO4 induced by mechanical deformation. J. Appl. Phys.. 1999;5:3155-3158.
- [Google Scholar]
- Water photolysis reaction on cerium oxide photocatalysts. Catal. Today. 1996;30:157-162.
- [Google Scholar]
- Tungsten-doped TiO2 vs pure TiO2 photocatalysts: effects on photobleaching kinetics and mechanism. J. Phys. Chem. C. 2008;112:1094-1100.
- [Google Scholar]
- ZnO-assisted photocatalytic degradation of Congo Red and benzopurpurin 4B in aqueous solution. J. Chem. Eng. Proc. Technol.. 2011;2:1-9.
- [Google Scholar]
- Synthesis, characterization and UV-A light photocatalytic activity of 20 wt% SrO–CuBi2O4 composite. Appl. Surf. Sci.. 2012;258:5010-5024.
- [Google Scholar]
- Converting ceria polyhedral nanoparticles into single-crystal nanospheres. Science. 2006;312:1504-1508.
- [Google Scholar]
- Factorial experimental design of Orange II photocatalytic decolouration. J. Photochem. Photobiol. A: Chem.. 2002;151:213-219.
- [Google Scholar]
- Regenerative adsorption and removal of H2S from hot fuel gas streams by rare earth oxides. Science. 2006;312:1508-1510.
- [Google Scholar]
- First demonstration of CdSe as a photocatalyst for hydrogen evolution from water under UV and visible light. Chem. Commun.. 2008;2008:2206-2208.
- [Google Scholar]
- Effect of particle size on the photocatalytic activity of WO3 particles for water oxidation. J. Phys. Chem. C. 2013;117:22584-22590.
- [Google Scholar]
- Carbon- modified TiO2 nanotubes with enhanced photocatalytic activity synthesized by a facile wet chemistry method. Scr. Mater.. 2008;59:352-355.
- [Google Scholar]
- Electrochemical synthesis and characterization of p-CuBi2O4 thin film photocathodes. J. Phy. Chem. C. 2012;116:6459-6466.
- [Google Scholar]
- Direct synthesis and structure characterization of ultrafine CeO2 nanoparticles. Nanotechnology. 2006;17:5983-5987.
- [Google Scholar]
- Amorphous cerium–titanium solid solution phosphate as a novel family of band gap tunable sunscreen materials. Chem. Mater.. 2003;15:2289-2291.
- [Google Scholar]
- Oxygen storage capacity (OSC) of aged Pt/CeO2/Al2O3 catalysts, roles of Pt and CeO2 supported on Al2O3. Appl. Surf. Sci.. 1997;121(122):408-412.
- [Google Scholar]
- Keren J., 2011. Fabrication and Catalytic Property of Cerium Oxide Nanomaterials. Thesis University of Nebraska – Lincoln.
- Sensitized layered metal oxide semiconductor particles for photochemical hydrogen evolution from nonsacrificial electron donors. J. Phys. Chem.. 1993;97:11802-11810.
- [Google Scholar]
- Spectral, optical, and photocatalytic characteristics of quantum-sized particles of CdTe. Theo. Exp. Chem.. 2004;40:220-225.
- [Google Scholar]
- Photodegradation study of Congo Red in aqueous solution using ZnO/UV-A: effect of pH and band gap of other semiconductor groups. J. Chem. Eng. Proc. Technol.. 2011;2:1-9.
- [Google Scholar]
- CeO2-Bi2O3 nanocomposite: two step synthesis, microstructure and photocatalytic activity. J. Non-Cryst. Solids. 2009;355:776-779.
- [Google Scholar]
- Synthesis of nanosized nitrogen- containing MOx-ZnO (M = W, V, Fe) composite powders by spray pyrolysis and their visible-light-driven photocatalysis in gas-phase acetaldehyde decomposition. Catal. Today. 2004;93(95):895-901.
- [Google Scholar]
- Monoclinic BiVO4 with regular morphologies: hydrothermal synthesis, characterization and photocatalytic properties. Mater. Chem. Phys.. 2009;115:9-13.
- [Google Scholar]
- CeO2–Bi2O3 nanocomposite: two step synthesis, microstructure and photocatalytic activity. J. Non-Cryst. Solids. 2009;355:776-779.
- [Google Scholar]
- Hybrid solar cells based on MEH-PPV and thin film semiconductor oxides (TiO2, Nb2O5, ZnO, CeO2 and CeO2–TiO2): Performance improvement during long-time irradiation. Sol. Energy Mater. Sol. Cells. 2006;90:2076-2086.
- [Google Scholar]
- Cooperative self-construction and enhanced optical absorption of nanoplates-assembled hierarchical Bi2WO6 flowers. J. Solid State Chem.. 2008;181:1048-1055.
- [Google Scholar]
- Composite-hydroxide-mediated approach for the synthesis of nanostructures of complex functional-oxides. Nano Lett.. 2006;6:1535-1540.
- [Google Scholar]
- Fabrication of TiO2/ZnO composite nanofibers by electrospinning and their photocatalytic property. Mater. Chem. Phys.. 2010;121:432-439.
- [Google Scholar]
- Preparation and characterization of p–n heterojunction photocatalyst p-CuBi2O4/n-TiO2 with high photocatalytic activity under visible and UV light irradiation. J. Nanopart. Res.. 2010;12:1355-1366.
- [Google Scholar]
- Preparation, characterisation of p–n heterojunction photocatalyst CuBi2O4/Bi2WO6 and its photocatalytic activities. J. Exp. Nanosci.. 2011;6:102-120.
- [Google Scholar]
- Hydrothermal synthesis of prism-like mesocrystal CeO2. J. Alloys Compd.. 2009;476:958-962.
- [Google Scholar]
- Photocatalytic behavior of CeO2–TiO2 system for degradation of methylene blue. Indian J. Chem.. 2009;48A:480-488.
- [Google Scholar]
- Structure evolution of nanocrystalline CeO2 and CeLnOx mixed oxides (Ln = Pr, Tb, Lu) in O2 and H2 atmosphere and their catalytic activity in soot combustion. Appl. Catal. B. 2007;74:290-298.
- [Google Scholar]
- Efficient photocatalytic degradation of phenol over Co3O4/BiVO4 composite under visible light irradiation. J. Phys. Chem. B. 2006;110:20211-20216.
- [Google Scholar]
- Physical, chemical and electrochemical properties of pure and doped ceria. Solid State Ionics. 2000;129:63-64.
- [Google Scholar]
- Synthesis and characterization of CeO2–SiO2 nanoparticles by microwave-assisted irradiation method for photocatalytic oxidation of methylene blue dye. Int. J. Photoenergy 2012:1-9.
- [Google Scholar]
- Photosensitization of TiO2 layers with CdSe quantum dots: correlation between light absorption and photoinjection. J. Phys. Chem. C.. 2007;111:14889-14892.
- [Google Scholar]
- Violet/blue emission from epitaxial cerium oxide films on silicon substrates. Appl. Phys. Lett.. 1647;70
- [Google Scholar]
- Emissions reduction of high and low polluting new technology vehicles equipped with a CeO2 catalytic system. Sci. Total Environ.. 1999;235:71.
- [Google Scholar]
- Role of cerium-zirconium mixed oxides for car pollution. J. Alloys Compd.. 1998;275(277):886-890.
- [Google Scholar]
- Optical properties and electrochromic characterization of sol-gel deposited ceria films. Sol. Energy Mater. Sol. Cells. 2001;68:391-400.
- [Google Scholar]
- Fabrication of iron-cerium mixed oxide: an efficient photocatalyst for dye degradation. Int. J. Eng. Sci. Technol.. 2010;2:53-65.
- [Google Scholar]
- J. Eur. Ceram. Soc.. 1988;18:1759-1764.
- XRD and UV–Vis diffuse reflectance analysis of CeO2–ZrO2 solid solutions synthesized by combustion method. Proc. Indian Acad. Sci.. 2001;113:651-658.
- [Google Scholar]
- Nanostructured-[CeO2, La2O3, C]/TiO2 catalysts for lignin photodegradation. Sci. Adv. Mater.. 2012;4:573-578.
- [Google Scholar]
- A simple hydrothermal route for the preparation of HgS nanoparticles and their photocatalytic activities. RSC. Adv.. 2014;4:15371-15376.
- [Google Scholar]
- Enhanced photocatalytic activity of cobalt-doped CeO2 nanorods. J. Sol-Gel Sci. Technol.. 2012;64:515-523.
- [Google Scholar]
- UV–Visible spectroscopic estimation of photodegradation of rhodamine-B dye using tin(IV) oxide nanoparticles. Spectrochim. Acta, Part A. 2012;97:847-852.
- [Google Scholar]
- Local work function of Pt clusters vacuum-deposited on a TiO2 surface. J. Phys. Chem. B. 2006;110:17584-17588.
- [Google Scholar]
- Photocatalysis for water oxidation by Fe2O3 nanoparticles embedded in clay compound: correlation between its polymorphs and their photocatalytic activities. J. Mater. Sci.. 2009;44:2890-2898.
- [Google Scholar]
- Mechanism of the photocatalytic degradation of CI reactive black 5 at pH 12.0 using SrTiO3/CeO2 composite as the catalyst. J. Environ. Sci. Technol.. 2007;41:5846-5853.
- [Google Scholar]
- High-throughput screening using porous photoelectrode for the development of visible-light responsive semiconductors. J. Comb. Chem.. 2007;9:574-581.
- [Google Scholar]
- Truffault, L., 2010. Synthesis and Characterisation of Cerium Dioxide – and Iron Oxide Based Nanoparticles for UV Filtration in Sunscreens (Thesis), University of Wollongong.
- Highly efficient photocatalytic elimination of phenol and chlorinated phenols by CeO2/MgAl layered double hydroxides. J. Appl. Catal. B. 2011;102:276-285.
- [Google Scholar]
- Kinetic study of application of ZnO as a photocatalyst in heterogeneous medium. Eur. J. Chem.. 2009;6:531-536.
- [Google Scholar]
- Structure, preparation and photocatalytic activity of titanium oxides on MCM-41. Surf. J. Catal.. 2006;238:13-20.
- [Google Scholar]
- Size-dependent photocatalytic reduction of CO2 with PbS quantum dot sensitized TiO2 heterostructured photocatalysts. J. Mater. Chem.. 2011;21:13452-13457.
- [Google Scholar]
- A highly efficient and stable visible-light plasmonic photocatalyst Ag–AgCl/CeO2. World J. Nano Sci. Eng.. 2011;1:129-136.
- [Google Scholar]
- BiVO4/CeO2 nanocomposites with high visible-light-induced photocatalytic activity. Appl. Mater. Interfaces. 2012;4:3718-3723.
- [Google Scholar]
- Preparation of new sunscreen materials Ce1−xZnxO2−x via solid-state reaction at room temperature and study on their properties. Rarest Met.. 2010;29:149.
- [Google Scholar]
- Textural–structural properties and soot oxidation activity of MnOx–CeO2 mixed oxides. Catal. Commun.. 2011;12:345-348.
- [Google Scholar]
- The absolute energy positions of conduction and bands of selected semiconducting minerals. Am. Mineral.. 2000;85:543-556.
- [Google Scholar]
- Internal distortion in ZrO2–CeO2 solid solutions: neutron and high-resolution synchrotron x-ray diffraction study. Appl. Phys. Lett.. 1998;72:182.
- [Google Scholar]
- Site-binding model of the electrical double layer at the oxide/water interface. J. Chem. Soc. Faraday Trans.. 1974;70:1807-1818.
- [Google Scholar]
- Improving the catalytic activity of CeO2/H2O2 system by sulfation pretreatment of CeO2. J. Mol. Catal. A: Chem. 2014;381:38-45.
- [Google Scholar]
- Effects of pH on the microstructures and photocatalytic activity of mesoporous nanocrystalline titania powders prepared via hydrothermal method. J. Mol. Catal. A: Chem.. 2008;258:104-112.
- [Google Scholar]
- Hydrothermal processing for obtaining of BiVO4 nanoparticles. J. Mater. Lett.. 2009;63:1939-1942.
- [Google Scholar]
- TiO2-assisted photodegradation of dye pollutants: II. Adsorption and degradation kinetics of eosin in TiO2, dispersions under visible light irradiation. Appl. Catal. B: Environ.. 1998;15:147-156.
- [Google Scholar]
- Congo Red adsorption by ball-milled sugarcane bagasse. Chem. Eng. J.. 2011;178:122-128.
- [Google Scholar]
- Photocatalytic activity of Bi2O3 prepared by different precipitants. Adv. Mater. Res.. 2011;239–242:998-1001.
- [Google Scholar]
- Adsorption and reaction of formaldehyde on thin-film cerium oxide. Surf. Sci.. 2006;600:1540-1546.
- [Google Scholar]







