Translate this page into:
Polyphenolic contents and antioxidant potential in Nasturtium officinale
⁎Corresponding author at: Department of Environmental Sciences, COMSATS University Islamabad, Abbottabad Campus 22060 Abbottabad, Pakistan. arshad799@yahoo.com (Arshad Mehmood Abbasi) amabbasi@cuiatd.edu.pk (Arshad Mehmood Abbasi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The present study was conducted with the aim to assess the impact of environmental conditions on polyphenolic content and free radical scavenging ability in the leaves of N. officinale used as food and medicine in the Himalayan region of Pakistan. Samples were gathered from six different sites located in Himalaya, Hindukush, and Karakorum ranges. Polyphenolic profiling, and in vitro antioxidant potential were determied using standard analytical techniques. The samples collected from Abbottabad had significnat levels (p≤0.05) of total phenolic (331.43±41.43 mg GAE/100g DW) and total flavonoid contents (8.45±1.53 mg RutE/100g DW). Whereas, rutin, quercitin and kaempferol were maximum in samples collected from Dir(132.46±0.31, 8.80±0.05 and 0.95±0.04 µg/g, respectively). Likewise, Gallic and caffeic acids were highest in Haripur and Swat samples, whereas ferulic and p-coumaric acids were maximum in samples taken from Dir. The percentage scavenging of DPPH was maximum in samples collectded from Chitral (69.57±3.39%) and that of H2O2 at Dir (57.24±8.05%), whereas samples collected from Abbottabad depicted maximum inhibition of ferric and molybdate ions. Total phenolic and total flavonoids contents exhibited highly significant positive correlations (p≤0.01) with ferric and molybdate ions reduction capacity. Cluster and principal component analyses also depicted significant associations among samples collected from Abbottabad-Haripur, Dir-Swat, and Mansehra-Chilas. Our findings contribute in understanding the antioxidant potential of N. officinale leaves, and highlights the importance of polyphenolic composition and environmental factors in future investigations related to its nutraceutical applications. Further research on detailed phytochemcial profiling, their in vitro and in vivo mechanisms of action could unveil the potential of this plant as a functional food.
Keywords
Phenolics
Flavonoids
Free radicals
Seasonal variation, Watercress
Data Availability Statement
All data is provided in the manuscript; however, the corresponding author may be requested for any additional information.
1 Introduction
Polyphenolics offer a wide range of health benefits to human beings because of their anti-inflammatory, anti-allergic, antiviral, anticancer, antihypertensive, antioxidant, and anticancer properties (Montenegro-Landívar et al., 2021). These compounds are abundant in vegetables, fruits, legumes, grains, spices, medicinal plants, and drinks (Ren et al., 2021). Watercress (Nasturtium officinale L.), belonging to family Brassicaceae is a perennial herb with origins in Europe and Asia. This plant is typically found in freshwater springs, streams, and canals (Klimek-Szczykutowicz et al., 2018). Watercress is eaten as a vegetable, used in different cuisines, soups, meat dishes, pasta, and also as salad (Googoolee et al., 2022). The usage of N. officinale in conventional medicine includes the treatment of hyperglycemia, hypercholesterolemia, bronchitis, hypertension, arthritis, odontalgia, scurvy, and diuresis. And such medicinal properties of this plant are a result of polyphenolics, glucosinolates, isothiocyanates, and various vitamins (Panahi Kokhdan et al., 2021). Furthermore, these secondary metabolites support antioxidant capacity of N. officinale, which reduces cellular lipid peroxidation, superoxide anion, and free radicals’ damage (Mahmood et al., 2021). In Pakistan, fresh leaves of N. officinale are cooked as vegetable, and is consider as a coolong and appetizing agent, and to control consitpation.
Genetic diversity, growing conditions, agricultural activities, harvesting and post-harvesting techniques, processing and analytical techniques, and consuming methods all have a substantial impact on the functional properties of food and medicinal plants (Prabhu et al., 2021; Tuladhar et al., 2021). The nutritional value, phytochemical concentration, and bioactive potential of the same plant species or varieties of same species grown under diverse environmental circumstances may differ considerably. For instance, Bibi et al. (2022), reported substantial variation in polyphenolic contents and free radical secevning potential of onion varieties grown in diverse agro-ecological zones of Pakistan. In this context, present stduy was based on the hypothesis that there may be variation in the polyphenolic contents and bioactive potential of N. officinale which grows naturally in diverse agroecological zones of Pakistan. Our main goal was to determine free radicals’ scavenging potential and polyphenolics profiling in the leaves of N. officinale gathered from various parts of Pakistan.
2 Materials and Methods
2.1 Sampling and extraction
Fresh leaves of N. officinale (≈1.0 kg) were collected from Abbottabad, Haripur, Muzaffarabad (Himalayan), Dir, Swat (Hindukush), and Chilas (Karakorum) regions from March 2022 to September, 2022 at full maturity stage (Figure 1). Samples were identified by expert taxonomists and with the help of Flora of Pakistan (Ali and Qaiser, 1993–2018), and voucher speciemen (CUHA-50), were submitted at COMSATS University herbarium in Abbottabad, Pakistan. Scientific and family names were confirmed by “World flora online”.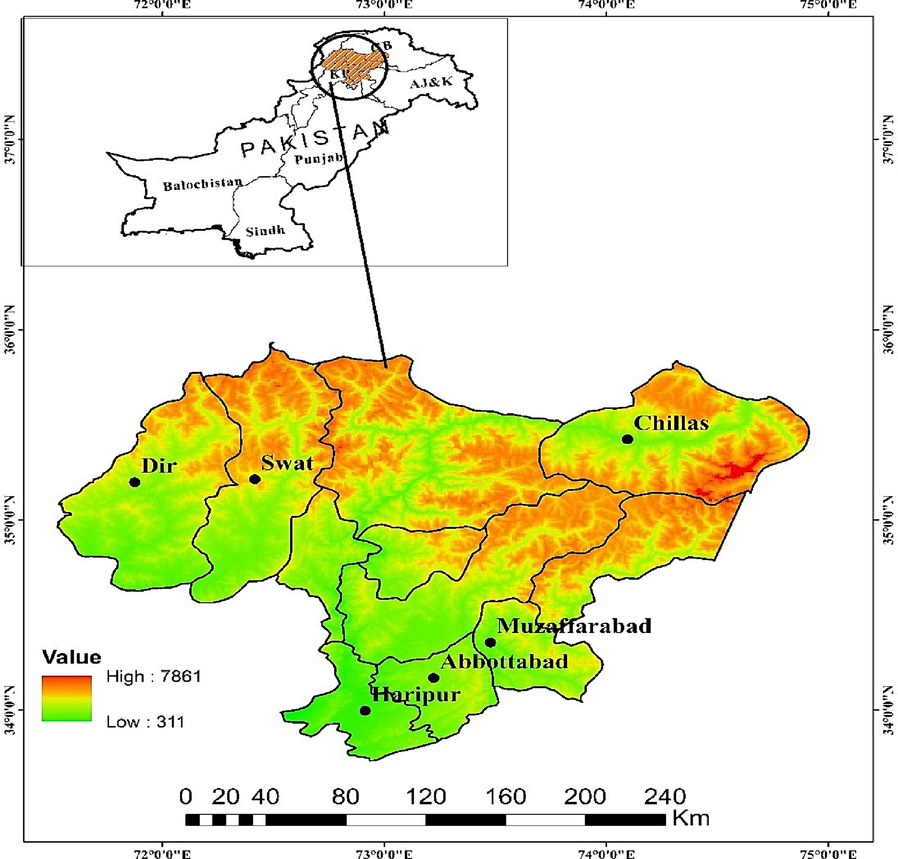
Map indicating collection sites of N. officinale
Fresh leaves were cleaned with distilled water, and kept in shade at room temperature. After drying, leaves were grinded into fine powder and stored at 4°C in refrigerator before extraction. The finely ground powdered material was extracted according to previously established protocol (Abbasi et al., 2015). Briefly, 0.5g of powdered sample was mixed with 10 mL methanol (99.8%), and kept at room temperature for 24 hrs. Afterwards, samples were centrifugated (at 8000 rpm.), and supernatants were combined in labeled flasks. All extracts were stored at 4 °C in refrigerator for subsequent analysis.
2.2 Quantification of polyphenolics
Determination of total phenolic and total flavonoids contents and was carried out as reported earlier by Zhang et al. (2011), using gallic acid and rutin as standards, respectively. Final values of total phenolic were reprted as mg GAE/100g, and that of total flavonoids as mg RutE/100g based on dry weight. Standards curves of gallic acid (GA) and rutin (Rut) are shown in Figure S1 (A & B).
Profiling of phenolic acids and flavonoid compounds by modified method as explained earlier (Abbasi et al., 2015). In HPLC system (UV detector Cecil, UK, and C18 column Waters, USA.), 0.1% formic acid solution was used as mobile phase A, and methnol as mobile phase B at a flow rate of 1 mL/min. Polyphenolics were deected at 280 nm and quantified using the peak area of sample compared to calibration curve of standards, and final values were expressed as ug/g.
2.3 DPPH assay
The scvanging assay of DPPH radical was performed according to the method of He et al., (2018). Briefly, 0.1mM solution of DPPH (2 mL) was mixed with equal volume of plant extract, and incubated in dark for 30 min. Absorbance was measured at 517 nm using UV-spectrophotometer, and ascorbic acid was used as positive control. The percentage inhibition was calculated using the equation:
Where As indicates sample absorbance, Ab shows absorbance of blank (methanol), and Ac indicates control absorbance.
2.4 Ferric ion reduction assay
The reduction of ferric ions was estimated using the method of Hazra et al. (2008). Shortly, plant extract, potassium ferricyanides (0.1%), and phosphate buffer (0.2 M, pH 6.6), were mixed in equal volume (2 mL of each). This mixture was incubated for 20 min bath at 50°C. Then 2 mL of trichloroacetic acid (10%) was added, follwoed by the addition of distilled water and 0.01% ferric chloride. Absorbance was measured at 700 nm. Gallic acid was used as standard with different concentrations of 20, 40, 60, 100, 140, 180 µg/mL (Figure S1 C). Final values were expressed mg GAE/100g.
2.5 Hydrogen peroxide scavenging activity
Previosuly described method of Yahaya et al., (2021), was used to estimate H2O2 radical inhibtion potential. Precisely, 2mL plant extract and same amount of H2O2 (mixture in phosphate buffer pH 7.4) were mixed. This mixture was kept in water bath for 10 min at 25°C, before taking absorbance at 230 nm. Phosphate buffer without H2O2 was used as blank. Percentage scavenging of H2O2 radical was determined using the equation:
Where As indicates sample absorbance, Ab shows absorbance of blank (methanol), and Ac indicates control absorbance.
2.6 Phosphomolybdenium complex assay
The scavenging of molybdate ion was determined by performing Phosphomolybdenium complex antioxidant assay (PMCA) following the method as explained earlier (Prieto et al., 1999). Shortly, leaf extract (2 mL) was blended with 6.6 mL of reagent mixture containing sodium phosphate, ammonium molybdate and sulphuric acid. Reagent and extrcat mixture was kept in water bath for 90 min at 85°C. Methanol was used as blank, and absorbance was taken at 695 nm. Different concentrations of ascorbic acid (20 to 180 µg/mL) were used in Figure S1 (D) to calculate molybdate ions scvenging and results were presented as mgAAE/100g on dry weight base.
2.7 Climate data
Data on climate attributes viz. latitude, longitude, and elevation of each sampling site was taken with the help of Global Positing System (GPS). While temperature, rainfall, and relative humidity data of each location were taken from “Pakistan meteorological department (PMD).”
2.8 Statistical analysis
Data were analyzed using Microsoft Excel 2019; One-way ANOVA and correlation analysis were performed with SPSS-13.0 v. For graphical presentation Sigma Plot 12.1, and OriginPro-2023 were used.
3 Results and discussion
3.1 Variation in total phenolic and flavonoids contents in N. officinale
As illustrated in Figure 2A, N. officinale leaves collected from Abbottabad relatively had the higehst concentration of total phenolic content (331.43±41.43 mg GAE/100g), followed by Haripur and Swat samples (288.27±26.06, and 65.25±4.14 mg GAE/100g, respectively) on dry weight basis with signifcnat variations at p≤0.05. However, the lowest concentration of total phenolic content was in the samples collected from Muzaffarabad (21.00±4.34 mg GAE/100g), which was 68% less than from Abbottabad sample. Because of strong bioatcive potential against free radicals, inflammation, mutagensis and cancer, flavonoids are most preferable phytochemicals in nutraceuticals, pharmaceuticals, and cosmetic industries (Karakaya et al., 2020). Measured levels of total flavonoids content (Figure 2B), were significantly higher in N. officinale leaves collected from Abbottabad and Haripur (8.55±1.53 and 7.18±0.98 mg RutE/100g, respectively). Total flavonoids in studied asmples were relatively lower than reported previously from Iraq (Faizy et al., 2021). However, TPC in N. officinale reported from Iran (Mazandarani et al.. 2013) were lower than our samples.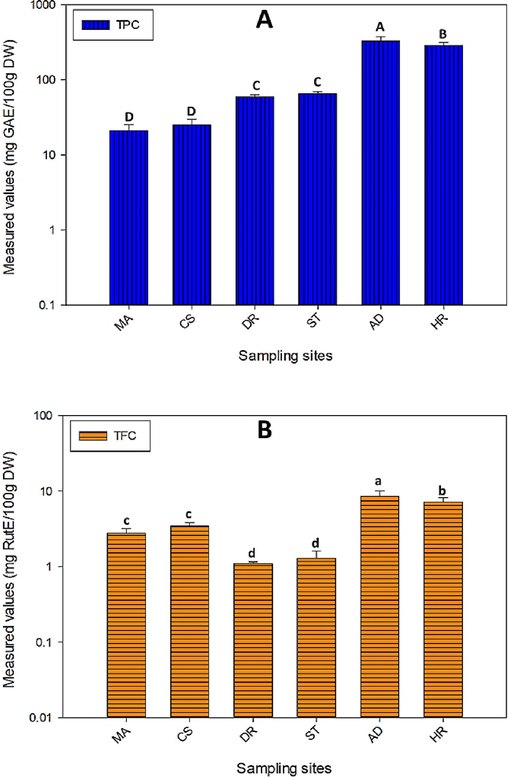
Comparison of TPC (2A) and TFC (2B) in N. officinale collected from different locations. Different letters (A-D/a-d) indicate variation in data at p≤0.05. MA. Muzaffarabad, CS. Chilas, DR. Dir, ST. Swat, AD. Abbottabad. HR. Haripur
It is worth noting that phytochemical components in plants can vary significantly depending on environmental conditions, such as drought, rainfall patterns, temperature changes, and soil characteristics (Prabhu et al., 2021). Therefore, variations in TPC and TFC levels in N. officinale could be attributed to factors like different cultivars, geographical origins, genetic variations in the plant material, as well as analytical methods used. For instance, Prabhu et al. (2021) reported that concentration of phytochemicals may vary in the members of same species, because of their origin, and environmental conditions such as sunlight exposure, temperature, soil composition, and altitude. Additionally, plant's age, genetic variability, and agricultural practices could also contribute to the observed variations in phenolic and flavonoids contents. Likewise, in Pakistan N. officinale grows naturaly in fresh water at different environmental conditions. Therefore, variation in water temperature, pH, concentration of disolved salts and nutrients, composition of hydric soil and rainfall pattern, may effect the production route of polyphenolics (Amelung et al., 1999). Likewise, variation is total phenolic and flavonoids contents in the leaves of N. officinale collected from different areas of Pakistan and those reported from Iraq and Iran (Faizy et al., 2021; Mazandarani et al.. 2013) might be due to difference in the geo-climatic conditions, water properties, genetic variations and analytical techniques used. Such as instrumentation, standards and laboratory conditions may affect concentration of phytochemicals and bioactive potential of the same species.
3.2 Distribution of phenolic and flavonoid compounds in N. officinale
Concentration of phenolic acids and flavonoids in the leaves of N. officinale collected from various location is given in Table 1. The overall increasing trend of phenolic acids and flavonoids at all locations was: RUT ≥ p-CA ≥ GA ≥ FA ≥ QE ≥ CA ≥ KEM. The samples collected from Dir had the highest levels of phenolic acids and flavonoids, while the lowest levels were found in samples of Chilas. Measured levels of FA, p-CA , KEM and RUT were significantly high (p≤0.05) in the leaves of N. officinale collected from Dir, followed by Abbottabad and Haripur. However, the increasing order of CA was: Dir ≥ Swat ≥ Haripur ≥ Abbottabad ≥ Muzaffarabad ≥ Chilas. Gallic acid concentration was maximum at Haripur, and QE was the highest in samples collected from Dir. The differential concentrations of phenolic acids and flavonoids among the samples may attributed to the different structures of these compounds (Lukas et al., 2021). The structure of phenolic acids is known to influence their activity and involvement in plant environmental responses. Therefore, the variations in the levels of these compounds in the N. officinale are likely influenced by their structural differences (Šamec et al., 2011). CA. Caffeic acid, FA. Ferulic acid, PCA. P-coumaric acid, GA. Gallic acid, QE. Quercetin, KEM. Kaempferol, RUT. Rutin
Lacalities
Phenolic acids (ug/g DW)
Flavonoids (ug/g DW)
CA
FA
p-CA
GA
QE
KEM
RUT
Muzaffarabad
1.65±0.06c
5.46±0.15f
12.60±0.06d
12.86±0.04c
6.90±0.06b
0.82±0.11b
113.14±0.50e
Chilas
1.47±0.03d
6.94±0.03d
10.85±0.03f
11.25±0.02e
3.10±0.06e
0.56±0.05c
113.47±0.34e
Dir
2.06±0.08a
8.89±0.08a
15.24±0.12a
13.54±0.09a
8.80±0.10a
0.95±0.08a
132.46±0.62a
Swat
2.10±0.06a
6.72±0.03e
11.24±0.05e
12.24±0.12d
6.34±0.12cd
0.67±0.12c
119.57±1.61d
Abbottabad
1.71±0.05c
7.33±0.05b
14.71±0.03b
13.23±0.05b
6.24±0.07d
0.84±0.06ab
126.52±0.08b
Haripur
1.83±0.04b
7.18±0.03c
13.55±0.05c
13.64±0.09a
6.39±0.06c
0.83±0.07ab
122.80±0.63c
It was observed that the concentrations of certain compounds were relatively lower or higher in N. officinale compared to the reported values for other plant species. Among the phenolic acids, p-CA depicted the highest mean concentration (13.03±5.32 µg/g) at all locations, followed by GA, FA and CA (12.79±5.22, 7.09±2.89, and 1.80±0.73 µg/g, respectively). Comparatively, average concentration of CA in N. officinale (1.80±0.73 ug/g DW), collected from different locations was relatively lower than reported in Brassica juncea from China (Fang et al., 2008), which belongs to the same family “Brassicaceae”. Likewise, measured levels of ferulic acid and p-coumaric acid were less than B. rapa. ssp. pekinensis repprted from Korea (Seong et al., 2016). Mean concentration of GA was 12.79±5.22 µg/g, and the highest levels were at Haripur, Dir and Abbottabad locations. However, these values were relatively lower than reported previously in N. officinale from Poland (Klimek-Szczykutowicz et al., 2020). Whereas, in the case of flavonoids, the highest average concentration was calculated for RUT (121.32±49.53 µg/g), followed by QE and KEM (6.29±2.57 and 0.78±0.32 µg/g) on dry weight basis (Table 1). The contents of flavonoids were relatively high in N. officinale samples collected from Dir, while lowest at Chilas. Comparatively, estimated levels of QE, RUT and KEM were higher than reported earlier in the leaves of N. officinale and some other medicinal and food taxa of Brassicaceae from Iran and Iraq (Arabi et al., 2018; Al-Mashea et al., 2018). It is well established that concentrations of secondary metabolites can vary due to interactions and responses of plants to environmental conditions, genotypic variations among species and varieties, and even individual differences within the same species (Prabhu et al., 2021). Additionally, sunlight duration, temperature, nutrients and water availability, toxins, seasonal variations, pests, pathogens and herbivory influence on the concentration and synthesis of polyphenolics (Bibi et al., 2022). However, the fundamental molecular mechanisms are still not clear enough.
3.3 Free radicals’ scavenging capacity in N. officinale.
These antioxidant molecules function as reductants inside the plant, by denoting hydrogen atoms or electrons to scavenge free radicals effectively (Ahmad et al., 2021). Therefor, ability of plants to produce antioxidants is a key factor in their capacity to combat the oxidative stress and damage to cells and tissues due to free radicals. Results showing scavenging potential of DPPH and H2O2 radicals in the leaves of N. officinale are given in Figure 3. Overall, the percentage inhibition of DPPH varies from 24.81% to 69.57% across different locations. Comparatively, samples collected from Chilas exhibits the highest DPPH scavenging capacity (69.57±3.39%), followed by Swat and Muzaffarabad. Conversly, N. officinale leaves from Haripur exhibited the lowest potential to inhibit DPPH radical. And the percentage variation in the DPPH activity in samples collected from Chilas and Haripur was about 36%. The H2O2 scavenging varies significantly among the locations, with Chilas showing the highest percentage inhibition (57.24±8.05%) and Dir with minimum (7.42±3.92%). However, the variations in H2O2 scavenging potential between the locations, except for Chilas, were not statistically significant (p≥0.05).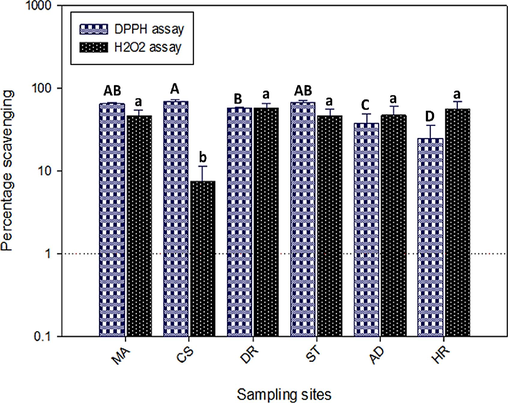
DPPH and H2O2 radicals scavenging potential in N. officinale collected from different locations. Different letters (A-D/a-b) indicate variation in data at p≤0.05. MA. Muzaffarabad, CS. Chilas, DR. Dir, ST. Swat, AD. Abbottabad. HR. Haripur
Relatively, percentage inhibition of DPPH and H2O2 radicals in the leaves N. officinale was higher than reported in the leaves of Brassica rapa from northern Portugal (Fernandes et al., 2016), Brassica oleracea var. capitata and Brassica rapa var. pekinensis from Czech Republic (Šamec et al., 2011), and in other species of Brassica genus of family Brassicaceae cultivated in Spain (Soengas et al., 2012). The observed variations in free radical scavenging activity in the leaves of N. officinale collected from different locations could be attributed to several factors. One primary factor is the presence of bioactive molecules within the plant. The higher DPPH and H2O2 scavenging capacities in watercress leaves could be linked to the abundance of reductants that efficiently donate hydrogen atoms or electrons to neutralize free radicals.
The reduction of ferric ion (Fe+3) and molybdate ion in the leaves of N. officinale as illustrated in Figure 4, provides insights into the antioxidant potential of watercress from different geographic areas. Among these, Abbottabad and Haripur exhibit the highest reduction of both ferric and molybdate ions, indicating potent antioxidant activity in the samples. The ferric ion reduction capacity follows an increasing order of Abbottabad ≥ Haripur ≥ Chilas ≥ Swat ≥ Muzaffarabad ≥ Dir. Similarly, the inhibition of molybdate ions was maximum in samples collected from Abbottabad and Haripur, with significant variations compared to Muzafarabad, Chilas, and Dir. N. officinale collected from Abbottabad and Haripur depicted maximum scavenging of ferric ions (46.57±1.35 and 46.55±1.21 mg GAE/100g DW, respectively). When compared to other species of the same family, N. officinale demonstrates higher ferric ion reducing potential than white and Chinese cabbage (Brassica oleracea var. capitata and Brassica rapa var. pekinensis) grown in the Czech Republic (Šamec et al., 2011). This suggests that watercress could be a more potent source of antioxidants than some other common vegetables belong to same family Brassicaceae. Likewise, inhibition of molybdate ions was maximum in N. officinale collected from Abbottabad and Haripur (1.10±0.20 and 0.96±0.06 mg AAE/100g DW, respectively). These values varied significantly at p≤.05, but there was no significant difference in the samples collected from Muzafarabad, Chilas and Dir.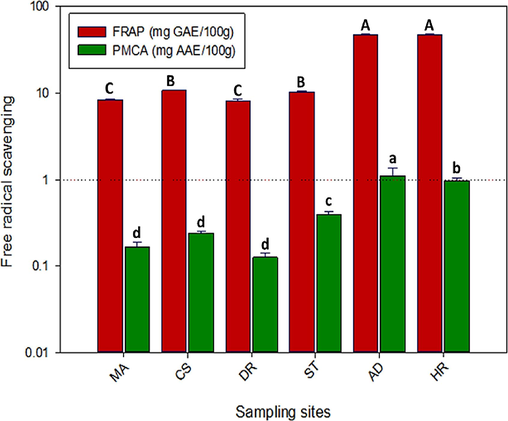
Comparison of ferric and molybdate ions reducing potential in N. officinale collected from different locations. Different letters (A-C/a-d) indicate variation in data at p≤0.05. MA. Muzaffarabad, CS. Chilas, DR. Dir, ST. Swat, AD. Abbottabad. HR. Haripur.
The differential profile of phytochemical compounds at various sites is responsible for the variations in antioxidant ability of N. officinale as well (Thiruvengadam et al., 2016). Because, significant variations were observed in the bioactive potential and phytochemcial comopsition of N. officinale collected from different areas. This observation revealed that geo-environmental factors effect on the production of bioactive molecules responsible for antioxidant activity in the plant (Dastoor et al., 2017). Therefore, understanding the variations in antioxidant ability among different locations can guide the selection of optimal sources for nutraceutical applications. Our findings suggest that N. officinale possesses potent antioxidant properties compared to the other species within the same family. Threfore, N. officinale-derived bioactive molecules may have potential applications in nutraceutical, pharmaceutical, and cosmetic industries in combating oxidative stress and reducing the risk of chronic diseases associated with free radical species. Overall, this study contributes to the growing body of knowledge on the health benefits of N. officinale and paves the way for further research in the field of plant-based antioxidants and nutraceuticals.
3.4 Correlations between polyphenolics and antioxidant activities
The results, presented in Figure 5, reveal several significant associations that provide valuable insights into the role of polyphenolics in the antioxidant potential of plant species. For instance, in the surrent study highly significant (p≤0.01), positive correlations were noted between total phenolic contents in N. officinale leaves with ferric and molybdate ions at r = 0.986 and r = 0.979, respectively. Likewise, total flavonoids also depicted a highly significant (p≤0.01) direct relationship with ferric and molybdate ion reducing potential (r = 0.952 and r = 0.927, respectively). These findings confiem that phenolic compounds, and flavonoids contributes significantly to the antioxidant potential of food and medicinal plants (Karakaya et al., 2020). Interestingly, total phenolic and flavonoids contents exhibited highly significant inverse correlations with DPPH activity (≈92%). This implies that higher levels of phenolic compounds and flavonoids may not contribute as effectively to the scavenging of DPPH radicals. In contrast to the DPPH scavenging, these compounds showed a weak positive association with hydrogen peroxide radical scavenging potential. This suggests that polyphenolics may play a role in neutralizing hydrogen peroxide radicals, albeit to a lesser extent compared to their role in reducing ferric and molybdate ions. The reasons behind this inverse relationship may be complex and warrant further investigation. However, it might be due to the synergistic role of different phytochemcials such as anthocyanins, carotenoids, vitamins, and antioxidant metals (Suleria et al., 2020).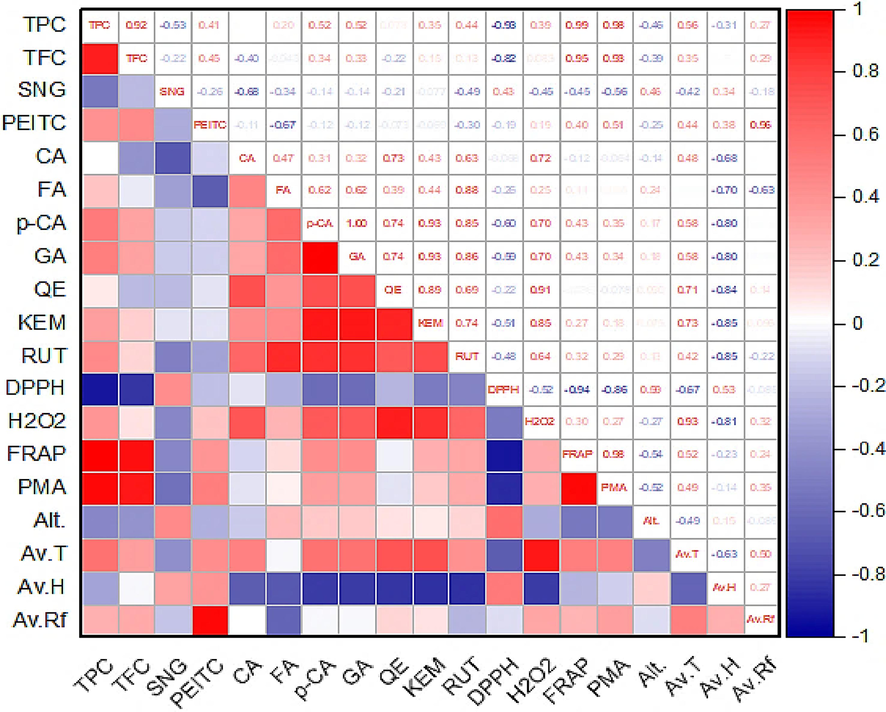
Correlation analysis of polyphenolics and antioxidant activities in N. officinale collected from different locations. Circles in boxes indicate significant associations (p≤0.01 and p≤0.05) among the variables.
The relationships between individual phenolic acids and flavonoid compounds with different antioxidant activities were also evaluated (Figure 5). Notably, GA, QE, and KEM showed significant (p≤0.05), positive interactions with inhibition of hydrogen peroxide in N. officinale leaves. Correspondingly, FA, p-CA, and RUT exhibited positive associations with inhibition of ferric ion, molybdate ion, and hydrogen peroxide radicals. However, these phenolic acids and flavonoid compounds showed negative relationships with DPPH scavenging potential. This suggests that certain phenolic compounds may be more effective in scavenging specific types of radicals, leading to varying relationships with different antioxidant assays.
Correlation analysis revealed that higher levels of the total phenolic contenst in the plant species are associated with increased ferric and molybdate ion reducing capacities, which are indicators of antioxidant potential. Similarly, total flavonoids also show highly significant positive correlations with ferric and molybdate ion reducing potential. Interestingly, the study finds highly significant inverse relationships between total phenolic, total flavonoids and DPPH radical scavenging activity. This inverse correlation implies that while higher levels of polyphenolic compounds positively influence ferric and molybdate ion reducing potential, they are associated with lower DPPH radical scavenging activity. Furthermore, it could indicates that the antioxidant mechanisms of polyphenolics in N. officinale may differ depending on the type of radical species involved.
3.5 Relationships of growing conditions with bioactive potential in N. officinale
Although, complex genetic pathways are involved in the synthesis of various bioactive compounds in plants, but influence of environmental factors could not be underestimate [34]. Furthermore, understanding the impact of environmental factors on the production of secondary metabolites and their bioactive potential can have implications for both ecological and human health perspectives (Dastoor et al., 2017). Therefore, we have also investigated the influence of environmental factors, such as mean minimum and maximum temperature, humidity, rainfall, and altitude on polyphenolic content and antioxidant activities in N. officinale.
On the whole, mean minimum and maximum temperature depicted positive association with polyphenolics and antioxidant activities, except for FA content and DPPH radical scavenging activity (Figure 5) that showed inverse relations. The mean maximum temperature had highly significant (p≤0.01) positive correlation with GA (95.5%), and hydrogen peroxide scavenging potential (91.0%, p≤0.05) collected from different locations. This suggests that higher temperature may favor the production of polyphenolic compounds and enhances the antioxidant potential in watercress leaves. Our findings are compatible with previous reports (Moreira et al., 2020). However, according to Boussaa et al., (2020), antioxidant activities enhance in cold weather. For instance, other workers (Bibi et al., 2022; Wang, 2006) also reported inverse relationships between temperature and polyphenolics. Overall, rain fall usually has no significant association with polyphenolic synthesis in plants (Wang, 2006). Correspondingly, in the present study, humidity, rainfall, and altitude mostly exhibit negative or weak positive associations with polyphenolics and antioxidant activities in the leaves of N. officinale. This indicate that these environmental factors might have a less pronounced effect on the antioxidant capacity of this plant. These findings were similar to previous reports (Bibi et al., 2022; Bernal et al., 2013). However, Boussaa et al. (2020), reported positive correlations between relative humidity and bioactive potential. Similarly, according to Bernal et al. (2013), concentration of phytochemicals and bioactive potential in medicinal plants decrease with increase in altitude. However, in some food and medicinal plants altitude exhibited significantly positive relationships with synthesis of secondary metabolites and antioxidant activities (Mpofu et al., 2006).
The correlation analysis in this study provides valuable insights into the interactions between polyphenolics and antioxidant potential in the leaves of N. officinale collected from different locations. The findings suggest that polyphenolic compounds, including total phenolics and flavonoids, play a significant role in the antioxidant activity of the plant species. However, their impact on different types of radical scavenging activities, such as DPPH and hydrogen peroxide, varies, indicating the complexity of antioxidant mechanisms in N. officinale. Additionally, certain phenolic acids and flavonoid compounds are found to have specific associations with different antioxidant activities. Furthermore, environmental factors, particularly temperature, appear to influence polyphenolic content and antioxidant activities in the plant.
3.6 Cluster and principal component analysis
The statistical techniques provide valuable insights into the grouping patterns and the contribution of specific metabolites to the overall antioxidant activity. The study employs clustering analysis (CA) and principal component analysis (PCA) to explore the relationships between polyphenolic contents and antioxidant potential in the leaves N. officinale. As illsutrated in Figure 6, two main clusters were identified: the first group exhibits a significant association between samples collected from Abbottabad and Haripur. In contrast, the second cluster is subdivided into two groups, with Swat and Dir in one group, and Muzaffarabad and Chilas placed in the other. The clustering of samples may be attributed to the proximity of these sampling sites, which allows for the dispersion of N. officinale seeds through wind and running water. This possibility of seed dispersion among plants in close proximity can lead to genetic similarity and, consequently, similarity in polyphenolic profiles and antioxidant activities (Mpofu et al., 2006).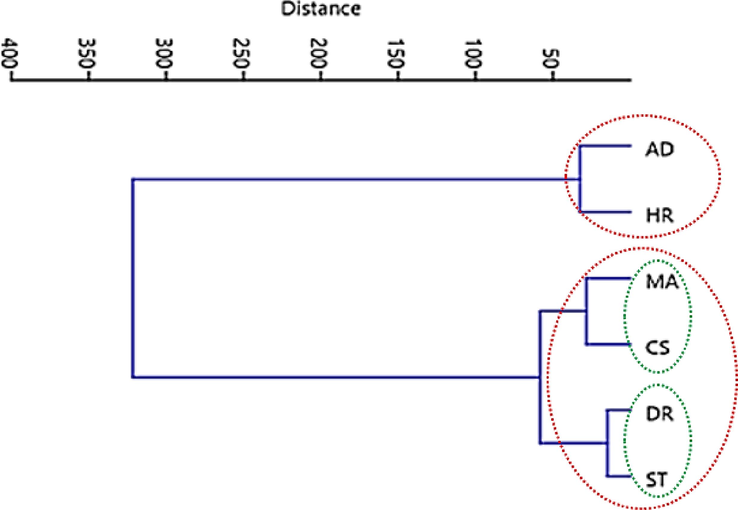
Cluster analysis indicating associations between sampling sites based on polyphenolic contents and antioxidant activities in N. officinale.
The PCA analysis, as shown in Figure 7, further explores the relations among polyphenolic compounds and antioxidant activities in N. officinale. The data is grouped into three main components: PC1, PC2, and PC3. As shown in (Table S1), PC1 accounts for the highest percentage of variance (54.48%), followed by PC2 (30.19%) and PC3 (8.54%). In PC1, GA exhibits the maximum loading value (93.4%), indicating a strong positive association of this phenolic acid with other variables in this component. Similarly, p-CA, RUT, KEM, hydrogen peroxide scavenging activity, TPC, ferric ion reducing activity, and molybdate ion reducing activity also showed high loading values (≥ 60%). This implies that these variables are positively associated with each other and collectively contribute to the antioxidant potential of N. officinale. The findings of PC1 align with the results of the correlation analysis, where TPC and TFC depicted highly significant positive relationships with reduction of ferric and molybdate ions. In PC2, QE, CA, and DPPH activity are grouped together, with QE and CA showing strong positive association (≥ 60%), while DPPH exhibits negative relationships with all metabolites. PC3 primarily involves FA, which shows a loading value of 70.9%. Ferulic acid depicted negative relationships with antioxidant potential in N. officinale but has positive correlations with most of the phenolic acids and flavonoid compounds.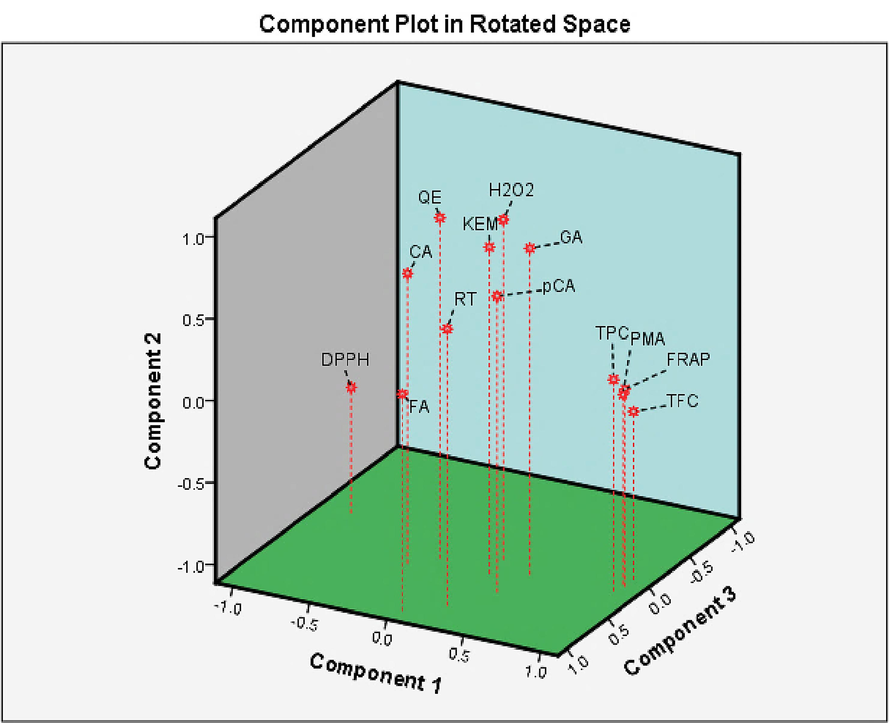
Principal component analysis of polyphenolics and antioxidant activities in N. officinale collected from diverse areas.
The cluster analysis and PCA results provide valuable insights into the diversity of polyphenolic profiles and antioxidant potential in N. officinale samples collected from different locations that suggests the dispersion of seeds as a possible contributing factor. Moreover, it reveals the key metabolites responsible for the observed differences in antioxidant activities and highlights their interrelationships. Understanding the factors that contribute to these variations can have implications for the selection and breeding of plants with enhanced antioxidant properties. Further research could focus on identifying the specific genetic and environmental factors that influence the production of polyphenolic compounds in N. officinale and exploring their potential applications in functional foods and natural antioxidants. Additionally, investigating the impact of different growing conditions on the antioxidant potential of N. officinale could provide valuable information for optimizing its cultivation and utilization as a nutraceutical resource.
4 Conclusions
The present study emphasizes the significance of N. officinale as a potential nutraceutical, and its applications in the food, pharmaceutical, and cosmetic industries. Comparative assessment of polyphenolic content and antioxidant potential provides valuable insights into the variability of bioactivities in the leaves of this plant and highlights the importance of considering geographic variations. The correlation analysis reveals significant associations between polyphenolics and antioxidant activities in N. officinale. Although. Temperature influences the polyphenolic content and antioxidant activities in the leaves of N. officinale, but there was no significnat imapct of humidity, rainfall and altitude which indicates that these environmental factors might have a less effect on the antioxidant capacity of this plant. However, understanding the factors influencing on the synthesis of bioactive compounds in plants can have implications for agricultural, ecological, and human health perspectives. Overall, this study contributes to the understanding of the antioxidant potential of N. officinale and highlights the importance of considering both polyphenolic composition and environmental factors in future investigations related to its nutraceutical applications. Further research on the identification and quantification of the specific bioactive compounds in N. officinale, their mechanism of action and interactions could unveil the full potential of this plant as a functional food, and natural source of antioxidants with possible health benefits.
CRediT authorship contribution statement
Sobia Zaman: Data curation, Formal analysis, Writing – original draft. Raza Ahmad: Conceptualization, Methodology, Project administration, Validation, Writing – review & editing. Manal Abdulaziz Binobead: Funding acquisition, Visualization, Writing – review & editing. Mohamed Ragab Abdel Gawwad: Funding acquisition, Software, Visualization, Writing – review & editing. Mohamed Soliman Elshikh: Funding acquisition, Software, Visualization, Writing – review & editing. Yusufjon Gafforov: Methodology, Visualization, Writing – review & editing. Arshad Mehmood Abbasi: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Software, Supervision, Writing – review & editing.
Acknowledgments
This research was funded by the Researchers Supporting Project number RSPD2024R637, King Saud University, Riyadh, Saudi Arabia
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Comparative Assessment of Phenolic Content and in Vitro Antioxidant Capacity in the Pulp and Peel of Mango Cultivars. Int. J. Mol. Sci.. 2015;16:13507-13527.
- [Google Scholar]
- Phytochemical delivery through nanocarriers: A review. Colloids and Surfaces B: Biointerfaces. 2021;197:111389
- [Google Scholar]
- Flora of Pakistan. Department of Botany. Karachi University; 1993–2018. p. :194-221.
- A comparative study of the phenolic compounds of some Brassicaceae taxa by High-performance liquid chromatography (HPLC) technique. Tikrit J. Pure Sci.. 2018;23(2):45-48.
- [Google Scholar]
- Lignin in particle-size fractions of native grassland soils as influenced by climate.“. Soil Sci. Soc. America J.. 1999;63(5):1222-1228.
- [Google Scholar]
- Development of an eco-friendly approach based on dispersive liquid–liquid microextraction for the quantitative determination of quercetin in Nasturtium officinale, Apium graveolens, Spinacia oleracea, Brassica oleracea var. sabellica, and food samples. New J. Chem.. 2018;42(17):14340-14348.
- [Google Scholar]
- Altitudinal and seasonal changes of phenolic compounds in Buxus sempervirens leaves and cuticles. Plant Physiol. Biochem.. 2013;70:471-482.
- [Google Scholar]
- Variations in Total Phenolic, Total Flavonoid Contents, and Free Radicals’ Scavenging Potential of Onion Varieties Planted under Diverse Environmental Conditions. Foods.. 2022;11:950.
- [Google Scholar]
- Growing Location Affects Physical Properties, Bioactive Compounds, and Antioxidant Activity of Pomegranate Fruit (Punicagranatum L. var. Gabsi). Int. J. Fruit Sci.. 2020;20:S508-S523.
- [Google Scholar]
- Evaluation and comparison of total phenol, total flavonoid, resveratrol and antioxidant capacity in fruits of the species Vitis vinifera, (Pistacia vera), (Sambucus nigra) and (Ilex spinigera) Eco-Phytochem. J. Med. Plants. 2017;5:37-48.
- [Google Scholar]
- Faizy, H. S.;Esmail, L. S.; Mahdi, H. S. Phytochemicals Analysis in Watercress (Nasturtium Officinale) Plant Extracts. In IOP Conference Series: Earth Env. Sci. 2021, 761: 1, 012042. IOP Publishing.
- Fang, Z., Hu, Y., Liu, D., Chen, J., & Ye, X. (2008). Changes of phenolic acids and antioxidant activities during potherb mustard (Brassica juncea, Coss.) pickling. Food Chem. 2008, 108(3), 811-817.
- Evaluation of antioxidant capacity of 13 plant extracts by three different methods: cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. J. Food Sci. Tech.. 2016;53:451-460.
- [Google Scholar]
- Characterizing the cultivation practices and microbiological quality of watercress. J. Agri. Food Res.. 2022;2:100057
- [Google Scholar]
- Antioxidant and free radical scavenging activity of Spondiaspinnata. BMC Complementary Altern. Med.. 2008;8:63.
- [Google Scholar]
- Structural elucidation and antioxidant activity of an arabinogalactan from the leaves of Moringa oleifera. Int. J. Bio. Macromol. 2018:126-133.
- [Google Scholar]
- Identification of non-alkaloid natural compound of Angelica purpurascans (Apiaceae) Angelica purpurascens (Ava-Lall.) Gilli. (Apiaceae) with cholinesterase and carbonic anhydrase inhibition potential. Saudi Pharm. J.. 2020;8:1-14.
- [Google Scholar]
- Chemical composition, traditional and professional use in medicine, application in environmental protection, position in food and cosmetics industries, and biotechnological studies of Nasturtium officinale (watercress)–a review. Fitoterapia. 2018;129:283-292.
- [Google Scholar]
- Phytochemical and Biological Activity Studies on Nasturtium officinale (Watercress) Microshoot Cultures Grown in RITA® Temporary Immersion Systems. Molecules. 2020;25(22):5257.
- [Google Scholar]
- Polyphenol diversity and antioxidant activity of european cistus creticus L. (cistaceae) compared to six further, partly sympatric cistus species. Plants. 2021;10(4):615.
- [Google Scholar]
- Phytochemistry of Allium cepa L. (Onion): An overview of its nutritional and pharmacological importance. Sci. Inq. Rev.. 2021;5(3):41-59.
- [Google Scholar]
- Evaluation of phytochemical and antioxidant activities from different parts of Nasturtium officinale R. Br. Mazandaran. 2013:659-664.
- [Google Scholar]
- Saurina J. Polyphenols and their potential role to fight viral diseases: An overview. Sci. Tot. Env; 2021. p. :149719.
- Optimization of antioxidant activity and bioactive compounds extraction of winter savory leaves by high hydrostatic pressure. High Press. Res.. 2020;40:543-560.
- [Google Scholar]
- Genotype and environmental variation in phenolic content, phenolic acid composition, and antioxidant activity of hard spring wheat. J. Agric. Food Chem.. 2006;54:1265-1270.
- [Google Scholar]
- A narrative review on therapeutic potential of watercress in human disorders. Evidence-Based Comp. Alt. Med. 2021:1-13.
- [Google Scholar]
- Classifications of polyphenols and their potential application in human health and diseases. Int J Physiol Nutr Phys Educ. 2021;6(1):293-301.
- [Google Scholar]
- Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem.. 1999;269:337-341.
- [Google Scholar]
- Effects of GABA on the polyphenol accumulation and antioxidant activities in tea plants (Camellia sinensis L.) under heat-stress conditions. Plant Phy. Biochem.. 2021;159:363-371.
- [Google Scholar]
- Antioxidant potency of white (Brassica oleracea L. var. capitata) and Chinese (Brassica rapa L. var. pekinensis (Lour.)) cabbage: The influence of development stage, cultivar choice and seed selection. Scientia Hort.. 2011;128(2):78-83.
- [Google Scholar]
- Antioxidant capacities and polyphenolics of Chinese cabbage (Brassica rapa L. ssp. Pekinensis) leaves. Food Chem.. 2016;199:612-618.
- [Google Scholar]
- New insights into antioxidant activity of Brassica crops. Food Chem.. 2012;134(2):725-733.
- [Google Scholar]
- Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods. 2020;9:1206.
- [Google Scholar]
- Effects of abscisic acid, jasmonic acid and salicylic acid on the content of phytochemicals and their gene expression profiles and biological activity in turnip (Brassica rapa ssp. rapa) Plant Grow. Reg.. 2016;80:377-390.
- [Google Scholar]
- Role of phenols and polyphenols in plant defense response to biotic and abiotic stresses. Biocontrol Agents Second. Metab. 2021:419-441.
- [Google Scholar]
- Effect of pre-harvest conditions on antioxidant capacity in fruits. Acta Hortic.. 2006;712:299-305.
- [Google Scholar]
- Yahaya, M. F.; Osemeahon, S. A.; Shagal, M. H.; Maitera, O. N.; Dass, P. M.; Yelwa, J. M. (2021). Antimicrobial, antioxidant, cytotoxicity profiles and chemical compositions of ethanolic extracts of Ficus polita and Ficus thonningii plant. J. Res. Chem. 2021, 2(1): 04-10.
- Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J. Agri. Food Chem.. 2011;59(23):12361-12367.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103223.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







