Translate this page into:
Polymeric nanoencapsulation of insect repellent: Evaluation of its bioefficacy on Culex quinquefasciatus mosquito population and effective impregnation onto cotton fabrics for insect repellent clothing
⁎Corresponding author. nchandra40@hotmail.com (Natarajan Chandrasekaran) nchandrasekaran@vit.ac.in (Natarajan Chandrasekaran)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract

Abstract
Diethylphenylacetamide (Bulk-DEPA), an organic insect repellent was subjected to Poly(ethylene glycol) (PEG) polymerization followed by Phase Inversion Temperature (PIT) emulsification method to yield the polymeric nanodroplets of DEPA (Nano-DEPA). The mean hydrodynamic diameter was found to be 149 ± 1.06 nm. The efficacy of Bulk-DEPA and Nano-DEPA was comparatively investigated on the Culex quinquefasciatus mosquito population. The larvicidal bioassay was performed on the 1st, 2nd, and 3rd instar larvae of Culex quinquefasciatus and the median lethal indices (LC50) of was found to be 0.055, 0.208, 1.397 mg/L and 0.023, 0.144, 0.260 mg/L for Bulk-DEPA and Nano-DEPA respectively. The histopathological studies were found to be corroborative with the larvicidal bioassay. The median knockdown indices (KD50) on 2–3 day old sucrose fed adult mosquitoes determined by WHO cone bioassay and was found to be 55.168 and 33.277 mg/L for Bulk-DEPA and Nano-DEPA. The obtained results indicate the improved efficacy possessed by the Nano-DEPA as comparative to Bulk-DEPA even at lower concentrations. Further, the Nano-DEPA was impregnated onto the alginate cross-linked (ACL) and Plain (PL) cotton fabrics, and the Washing resistance index (WRI) was determined. The obtained results indicate the higher WRI possessed by the ACL cotton fabric than the PL cotton fabric. This was owing to the effective physical entrapment of Nano-DEPA onto the alginate matrices, which was further substantiated by high-resolution scanning electron microscopic (HR-SEM) studies. Overall, the present study has emphasized the benefit of formulating Bulk-DEPA into Nano-DEPA to exert higher efficacy on the mosquito population. In addition, study has provided the methodology for the effective impregnation of Nano-DEPA onto the cotton fabrics for the reliable application in long lasting insect repellent clothing.
Keywords
Nanopesticide
Lymphatic Filariasis
WHO cone bioassay
Polymeric nanoencapsulation
Diethylphenylacetamide
Alginate cross-linking
1 Introduction
Mosquitoes cause a severe threat to the world’s population by transmitting several dreadful diseases such as Dengue, Malaria, Yellow fever, Chikungunya, Zika fever, Lymphatic Filariasis (LF) and so on. The modification of the natural environment by humans has raised the disease resurgence and the invasion and proliferation of several dreadful mosquitoes into the human habitats (Nkya et al., 2013). Culex quinquefasciatus is the most predominant species, which thrives and dwells in close proximities to human residences. Culex quinquefasciatus is a principal vector of LF infection, which transmits the filarial nematodes into humans. The transmitted filarial nematodes survive for about 5–8 years inside the human body and produce several millions of microfilariae. These microfilariae or larvae migrates into the lymphatic system and eventually blocks the fluid flow, which led to several human health complications. The acute clinical manifestation includes filarial fever, lymphangitis, lymphadenitis, lymphoedema, epididymo-orchitis, while the chronic infection causes, elephantiasis, hydrocele, chyluria, chylocele, lymph scrotum and the lymphoedema of scrotum and penis.
LF affects about 119 million peoples living in 73 countries worldwide (Ramaiah et al., 2000; Turner et al., 2016). In India, LF is the second predominant disease next to malaria, which accounts for 40% of global prevalence. The disease endemicity was spread across 18 states and Union territories of India, and over 48.11 million peoples were infected by LF (Ramaiah et al., 2000). The recent research efforts to reduce the mosquito prevalence have been highlighted by Hemingway et al. (2006). Even though several strategies are available for controlling the disease transmission, restricting the mosquito population is found to be a prime and effectual choice (Benelli and Mehlhorn, 2016).
Pesticides severed this purpose for several years for curbing the mosquito population and thereby reducing the human-vector contact. Pesticides are a broad spectrum of substances, which broadly include insecticides and insect repellents. Both the insecticides and insect repellents were concomitantly utilized in the mosquito control programmes. However, the continual and prolonged exposure of these conventional pesticidal groups towards the mosquito population has reduced its efficacy in the mosquito population (Hemingway et al., 2000). As realization for the need of novel effective formulation in our previous study, we have formulated PEG polymerized nanoemulsions of DEPA (Nano-DEPA) by the PIT emulsification method (Balaji et al., 2015b). DEPA is a product of Defence Research Development Establishment (DRDE), Gwalior India, is known for its long-lasting repellency over 8 h. Due to which, it has been extensively used in Indian armed forces and paramilitary forces to protect the soldiers from the encounter of several dreadful insects such as mosquitoes, black flies, land leeches, phlebotomine and sand flies (Kalyanasundaram, 1982; Kalyanasundaram and Mathew, 2006). In accordance, the present study was proposed to further explore the effectiveness of Nano-DEPA on Culex quinquefasciatus larvae (1st, 2nd, 3rd instar larval population) and adult mosquito population by larvicidal bioassay and WHO cone bioassay respectively. Further, we have effectively entrapped Nano-DEPA onto the alginate cross-linked (ACL) cotton fabrics. The Washing resistance index (WRI) of Nano-DEPA on ACL cotton fabric and plain (PL) cotton fabric was determined analytically and further extrapolated by HR-SEM studies. The present study is first of its kind in evaluating the efficacy of Nano-DEPA on Culex quinquefasciatus mosquito population and has further provided the insight into the application of Nano-DEPA on insect repellent clothing.
2 Materials and methods
2.1 Materials
Diethylphenylacetamide (Bulk-DEPA) 99.51% (w/w) was obtained from Alkyl Amines Chemicals Limited, India. Polyethylene glycol 400 (PEG), Sucrose, Tween 20, Soya Lecithin 30%, Sodium Chloride (NaCl), Sodium alginate, and Calcium chloride (CaCl2) were purchased from HiMedia Pvt. Ltd. India. Dimethylsulfoxide (DMSO), Dichloromethane, and Isopropanol were obtained from Merck Pvt Ltd. India. WHO polyvinyl chloride cone (WHO Cone) was obtained from Zonal Entomological Team, Vellore, Tamil Nadu, India. The cotton fabric was procured from the local mill without any chemical treatment. The deionized water (Milli-Q) was obtained from BIO-water Purification System (PALL Cascada, USA).
2.2 Nanoformulation of DEPA
The PEG polymerized DEPA nanoemulsion (Nano-DEPA) was formulated as reported earlier by Balaji et al. (2015b). Briefly, the aqueous phase constituted with 0.404% (v/v) of Tween 20 and 0.404% (w/v) of Soya lecithin in 80.97% (v/v) of 10 mM NaCl solution was added into the organic phase consisting 0–9% (v/v) of PEG, 6.47% (v/v) of DEPA and 12.14% (v/v) of dichloromethane. The addition of aqueous phase into the organic phase has resulted in the formation of conventional PEG polymerized DEPA droplets, and this was subjected to PIT emulsification method. The mean hydrodynamic diameter (Z-average) of the Nano-DEPA was determined using 90 Plus Particle Size Analyzer (Brookhaven Instruments Corporation, USA).
2.3 Bioefficacy studies
2.3.1 Mosquito larvae collection
The mosquito larval collection was entirely focussed on the Culex quinquefasciatus habitats. The preliminary species identification of the field-collected mosquito larvae was carried out based on the literature guidelines as reported by Azari-Hamidian and Harbach (2009). The field-collected larval populations were segregated into 1st, 2nd, and 3rd larval instars and directly utilized for the larvicidal bioassay, while, field-collected 4th instar larvae were reared inside the mosquito cage (45 cm × 45 cm × 45 cm) for their emergence into adults. The emerged adult mosquitoes were further confirmed as Culex quinquefasciatus species by ZET, Vellore, Tamil Nadu, India. The cotton wicks dipped in 10% sucrose solution was provided as a food supplement for the emerged adults, and they were utilized for WHO cone bioassay.
2.3.2 Larvicidal bioassay
The larvicidal bioassay was carried out as per the standard protocol of WHO (WHO, 2005). The test concentrations of Bulk-DEPA and Nano-DEPA were prepared accordingly based on the active compound concentration with distilled water. Owing to the poor water miscibility of Bulk-DEPA, the initial stock dilution was made with DMSO, followed by the dilution with distilled water. Negative control (NC) was prepared as a blend of the negative formulation (i.e. similar formulation composition of Nano-DEPA, excluding the active compound (DEPA)) and the higher concentration of DMSO used in the study. A batch of 25 homologue larvae was introduced into the respective test concentrations of Bulk-DEPA and Nano-DEPA. The morbidity of the larvae was determined as their inability to move upon probing. The larvicidal bioassay was carried out until 48 h. The NC was also presumed as a test concentration, and the similar procedure was carried out simultaneously. The complete experiment was performed in triplicates on different days on different larval batches.
2.3.3 Histopathological study
The histopathological studies were performed on the 3rd instar larvae of Culex quinquefasciatus. The larvae were exposed to the corresponding 36 h-LC50 test concentration of Nano-DEPA and Bulk-DEPA. Upon the exposure period (36 h), larvae were isolated and stored in a buffered formalin reagent. Further, the tissues were subjected to dehydration by passing through a graded ethanol series and embedded in the paraffin wax. The longitudinal section of the larval tissues was cut using a microtome (Leica, Germany) and stained with Hematoxylin and Eosin (Almehmadi, 2011). The processed longitudinal sections of the larvae were fixed onto a glass slide and observed under a phase-contrast microscope (Leica DM 2500 microscope, Germany) at 40-x magnification. The captured images were processed using Leica-Application Suite 3.8 software.
2.3.4 WHO cone bioassay
In order to further, extrapolate the efficacy of Bulk-DEPA and Nano-DEPA on the adult mosquito population WHO cone bioassay was carried out on 2–3 day old sucrose fed adult mosquito population The experiment was performed as per the standard WHO protocol (WHO, 2006) with slight modifications. The test concentrations of Bulk-DEPA and Nano-DEPA were uniformly coated onto the inner wall of the respective WHO cone and its substratum. Further, the cone and substratum were shade dried and fixed to a stage inclined at an angle of 60°. A batch of 5 numbers of adult mosquitoes was released from the top of cone and cotton plugged. The repetitive contact of mosquitoes with the applied test concentrations in the respective cones has led to the initiation of contact toxicity and caused Knock down (KD) in the course of time. KD in mosquitoes is remarked as its inability to stand or fly in a coordinated way and eventually falling at the bottom of the cone (WHO, 2013b). The KD was noted for every 10 min up to 60 min. A similar procedure was carried out for the NC simultaneously. The experiment was carried out in triplicate on different days with different batches of adult mosquitoes.
2.4 Nano-DEPA impregnation and evaluation of washing resistance index (WRI)
2.4.1 Preparation of alginate cross-linked fabric
The methodology for the preparation of alginate cross-linked (ACL) cotton fabric was adopted from Bajpai et al. (2012). The Plain (PL) cotton fabric was washed with distilled water for 2–3 times and air-dried. Further, the cotton fabrics were immersed in 4% (w/v) sodium alginate solution for 1 h and hung vertically (4 min) to remove the unbound alginates. Then the alginate-incorporated fabric was transferred into 3% (w/v) CaCl2 solution for 30 min. The presence of Ca2+ ions in CaCl2 solution facilitates the crosslinking between the alginate incorporated fibres by the process of ionotropic gelation (Bajpai and Sharma, 2004). This has resulted in the formation of ACL cotton fabric, which was further air-dried and used for Nano-DEPA impregnation. Alginate was chosen for the Nano-DEPA entrapment due to its harmlessness, biodegradability and a wider application in biomedical textiles (Badwan et al., 1985).
2.4.2 Impregnation of Nano-DEPA onto the ACL and PL cotton fabrics
The commonly used pesticide dosage in the protective clothing for coats, jackets, long-sleeved shirts, and short-sleeved shirts is 0.125 and 0.08 mg/cm2 respectively. In order, Nano-DEPA concentrations of 80 and 125 mg/L were prepared and utilized for the fabric impregnation by the immersion method. A measure of 5 cm2 of the ACL and PL (without alginate impregnation and cross-linking) cotton fabrics was immersed onto 80 and 125 mg/L of Nano-DEPA for 60 min. Further, the fabrics were shade dried at room temperature.
2.4.3 Evaluation of WRI
The washing procedure on ACL and PL cotton fabrics impregnated with Nano-DEPA was carried out as per the protocol reported by Sukumaran et al. (2014). Briefly, the Nano-DEPA impregnated fabrics were introduced into deionized water containing 2 g/L soap in a 250 mL glass beaker. Then the fabrics were stirred at a rate of 155 movements per minute for 10 min using a glass rod. After the completion of this step, fabrics were removed and introduced into a fresh distilled water containing beaker, and the stirring process was performed for 10 min at a similar agitation speed. This step was repeated, and the fabric was shade-dried to complete one cycle (Cycle 1). Totally 5 cycles were carried out sequentially in the subjected fabric.
The active compound (DEPA) present in the fabric, after the completion of every cycle, was determined as per Faulde et al. (2003) with certain modifications. Briefly, 1cm2 of the fabric was cut from different location of ACL and PL cotton fabric before and after washing. The cut fabrics were added to a 5 mL Isopropanol solution and subjected to bath sonication for 20 min. Upon sonication, DEPA concentration in the Isopropanol solution was determined spectrophotometrically at 259 nm (Rakkiyappan et al., 2012). The obtained values were substituted into the standard WHO equation (WHO, 2013a) for determining the Washing resistance index (WRI). where T = Concentration of DEPA released in the Isopropanol solution after washing. n = Number of cycles, i = Concentration of DEPA released in the Isopropanol solution before washing. WRI was expressed in terms of percentage.
2.5 High resolution-scanning electron microscopy (HR-SEM)
The Nano-DEPA impregnated ACL cotton fabrics were air-dried in the sterile environment and subjected to gold sputtering (Cressington 108 Sputter Coaters, England). These fabrics were further observed under FEI Quanta FEG 200 HR-SEM for Nano-DEPA incorporation onto the alginate matrices.
2.6 Statistical data analysis
The larvicidal lethal concentration (LC10, LC50, LC90) and knockdown indices (KD10, KD50, KD90) and their respective slope, intercept, Chi-square values were determined by using Probit statistical analysis program (US EPA, Ver. 1.5) at 95% confidence level (p < 0.05). The P-values, F-values, and significance difference between the corresponding Bulk-DEPA and Nano-DEPA indices were determined using a two-way ANOVA (Graph Pad Prism software 6). WRI were determined from the triplicate values, and the error bars denotes the standard error mean.
3 Results
3.1 Nanoformulation of DEPA
The addition of the aqueous phase into the organic phase spontaneously emulsifies the system and led to the formation of conventional DEPA droplets (in micrometer range). These droplets have exhibited steric stability with the formulation composition of 9% (v/v) PEG, 6.47% (v/v) DEPA, 12.14% (v/v) DCM, 0.40% (v/v) Tween 20, and 0.40% (w/v) Soy lecithin in 80.97% (v/v) 10 mM NaCl (Balaji et al., 2015b). Further, subjecting the system to PIT emulsification followed by subsequent cooling has resulted in the formation of PEG polymerized DEPA nanodroplets (Nano-DEPA). The obtained droplets were hydrodispersive with the Z-average of 149 ± 1.06 nm (Fig. 1).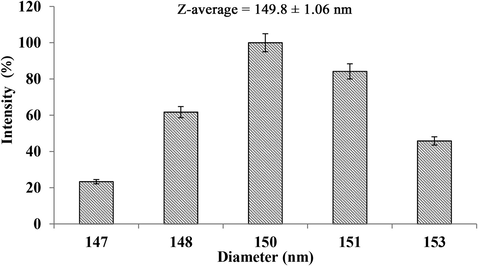
The mean hydrodynamic diameter (Z-average), size distribution and intensity percentage of Nano-DEPA in Milli-Q water.
3.2 Bio-efficacy studies
3.2.1 Larvicidal bioassay
The larval batches exposed to NC have not displayed any mortality, implying the nonlethal effect of NC on the larvae, while for the larvae exposed to the test concentration of Bulk-DEPA and Nano-DEPA, the larval mortality tends to increase with time and concentration. The 48 h-LC50 of Bulk-DEPA for 1st, 2nd, and 3rd instar of Culex quinquefasciatus larvae were found to be 0.055, 0.208, and 1.397 mg/L (Tables 1–3), while, 48 h-LC50 of Nano-DEPA for 1st, 2nd, and 3rd instar larvae were found to be 0.023, 0.144, and 0.260 mg/L. The obtained results indicate the improved efficacy of Nano-DEPA than the Bulk-DEPA on the Culex quinquefasciatus larval population. BD – Bulk-DEPA, ND – Nano-DEPA, LC – Lethal Concentration, LCL – 95% Lower Confidential Limit, UCL – 95% Upper confidential Limit, S.E – Standard Error, Degree of freedom is 1 between the corresponding hour and percentile of Bulk-DEPA and Nano-DEPA indices. BD – Bulk-DEPA, ND – Nano-DEPA, LC – Lethal Concentration, LCL – 95% Lower Confidential Limit, UCL – 95% Upper confidential Limit, S.E – Standard Error. Degree of freedom is 1 between the corresponding hour and percentile of Bulk-DEPA and Nano-DEPA indices. LC – Lethal Concentration, LCL – 95% Lower Confidential Limit, UCL – 95% Upper confidential Limit, S.E – Standard Error. Degree of freedom is 1 between the corresponding hour and percentile of Bulk-DEPA and Nano-DEPA indices.
Time (h)
BD/ND
LC10 (mg/L)
(LCL-UCL)
P value
F value
LC50 (mg/L)
(LCL-UCL)
P value
F value
LC90 (mg/L)
(LCL-UCL)
P value
F value
Slope
Intercept
Chi-Square value
36
BD
0.017 ± 0.000****
(0.006–0.032)<0.0001
1250
0.191 ± 0.003****
(0.126–0.288)<0.0001
3560
2.133 ± 0.119****
(1.295–5.187)<0.0001
330.4
1.20 ± 0.15
5.85 ± 0.13
4.20 ± 0.21
ND
0.005 ± 0.000****
(0.001–0.011)0.049 ± 0.001****
(0.029–0.078)0.485 ± 0.046****
(0.325–1.0951.25 ± 0.17
6.62 ± 0.19
7.58 ± 4.02
48
BD
0.0035 ± 0.000ns
(0.001–0.008)–
2
0.055 ± 0.004***
(0.030–0.097)0.0004
122.1
0.870 ± 0.267*
(0.620–2.911)0.0235
12.70
0.99 ± 0.14
6.22 ± 0.15
5.19 ± 0.16
ND
0.003 ± 0.000ns
(0.001–0.006)0.023 ± 0.00***
(0.012–0.039)0.195 ± 0.021*
(0.134–0.436)1.34 ± 0.20
7.17 ± 0.27
6.82 ± 1.59
Time (h)
BD/ND
LC10 (mg/L)
(LCL-UCL)
P value
F value
LC50 (mg/L)
(LCL-UCL)
P value
F value
LC90 (mg/L)
(LCL-UCL)
P value
F value
Slope
Intercept
Chi-Square value
36
BD
0.056 ± 0.001****
(0.021–0.108)<0.0001
800
0.871 ± 0.054***
(0.597–1.583)0.0001
219.4
13.505 ± 1.407***
(6.469–61.771)0.0004
121.2
1.06 ± 0.15
5.54 ± 0.32
1.98 ± 0.77
ND
0.036 ± 0.000****
(0.016–0.062)0.303 ± 0.005***
(0.213–0.440)2.529 ± 0.087***
(1.575–5.5881.37 ± 0.16
5.70 ± 0.13
2.10 ± 0.23
48
BD
0.009 ± 0.000*
(0.002–0.021)0.0132
18
0.208 ± 0.003****
(0.124–0.344)<0.0001
252.1
5.048 ± 0.231***
(2.442–18.826)0.0001
231.0
0.91 ± 0.13
5.61 ± 0.12
8.48 ± 0.25
ND
0.010 ± 0.000*
(0.003–0.022)0.144 ± 0.004****
(0.092–0.226)1.940 ± 0.174***
(1.176–5.169)1.11 ± 0.14
5.92 ± 0.13
9.23 ± 1.03
Time (h)
BD/ND
LC10 (mg/L)
(LCL-UCL)
P value
F value
LC50 (mg/L)
(LCL-UCL)
P value
F value
LC90 (mg/L)
(LCL-UCL)
P value
F value
Slope
Intercept
Chi-Square value
36
BD
0.370 ± 0.014****
(0.172–0.627)<0.0001
1140
3.765 ± 0.201****
(2.364–9.675)<0.0001
427.2
38.288 ± 2.621***
(14.683–330.915)0.0006
93.17
1.26 ± 0.23
4.24 ± 0.12
3.51 ± 0.36
ND
0.035 ± 0.000****
(0.011–0.073)0.778 ± 0.037****
(0.505–1.465)17.050 ± 1.677***
(7.292–97.353)0.94 ± 0.14
5.08 ± 0.11
3.30 ± 0.70
48
BD
0.195 ± 0.006****
(0.090–0.304)<0.0001
1721
1.397 ± 0.199**
(1.091–2.624)0.0013
65.26
10.297 ± 3.166*
(6.653–45.250)0.0396
9.050
1.38 ± 0.20
4.71 ± 0.11
0.46 ± 0.20
ND
0.019 ± 0.000 ****
(0.007–0.037)0.260 ± 0.004**
(0.165–0.385)3.556 ± 0.138*
(1.857–8.756)1.13 ± 0.14
5.67 ± 0.12
4.41 ± 0.22
3.2.2 Histopathological study
The histopathological study was performed in order to further extrapolate the toxic influence of Bulk-DEPA and Nano-DEPA on the larval body. In order 3rd instar, larvae were exposed to NC, 36 h-LC50 concentration of Bulk-DEPA (3.765 mg/L) and 36 h-LC50 concentration of Nano-DEPA (0.778 mg/L) for 36 h respectively. The larvae exposed to NC have not shown any lesions on the larval sections and further revealed the presence of well-structured epithelial cells (EC), peritrophic membrane (PM) and midgut content (MC) (Fig. 2A). These indicate the non-lethal effect of NC on the larvae, while, for the larval batches exposed to 36 h-LC50 of Nano-DEPA and Bulk-DEPA have displayed damage on EC, PM, and MC (Fig. 2B and C), implying the toxic inference of active compound (DEPA) on the larval body.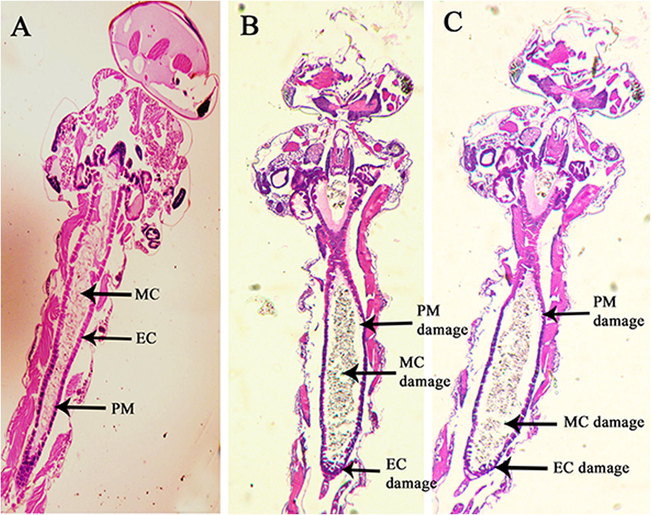
The longitudinal section of 3rd instar Culex quinquefasciatus larvae treated with (A) NC, (B) 36 h-LC50 (7.904 mg/L) of Bulk-DEPA, (C) 36 h-LC50 (0.700 mg/L) of Nano-DEPA. NC – Negative control, EC – Epithelial cells, PM – Peritrophic membrane, MC – Midgut content.
3.2.3 WHO cone bioassay
As larvicidal bioassay has shown convincing results for the improved efficacy of Nano-DEPA, exploring its efficacy on the adult mosquitoes is vital, as the adult mosquitoes majorly involve in disease transmission. In order to investigate the efficacy of Nano-DEPA on adult mosquito population, WHO cone bioassay was carried out as mentioned in the experimental part. The release of adult mosquitoes from the top of cone onsets their interaction with the test concentration. The repetitive contact of mosquitoes with the test concentration during their rest and flight influences toxicity in their body. In the course of time, mosquitoes display uncoordinated flight and resting movement and led to KD. The exhibition of KD directly implies the toxic potential of active compound on the adult mosquitoes. The KD of mosquitoes exposed to the test concentration of Bulk-DEPA and Nano-DEPA were found to be time and concentration dependent. The exhibition of mosquito KD is higher in the Nano-DEPA test concentration than the Bulk-DEPA test concentration. The KD50 concentration (60 min) of Bulk-DEPA and Nano-DEPA were found to be 55.168 and 33.277 mg/L respectively (Table 4), while, the mosquito population exposed to the NC, has not displayed any KD, implying the non-lethality. BD – Bulk-DEPA, ND – Nano-DEPA, KD – Knock Down, LCL – 95% Lower Confidential Limit, UCL – 95% Upper Confidential Limit, S.E – Standard Error. Degree of freedom is 1 between the corresponding hour and percentile of Bulk-DEPA and Nano-DEPA indices.
Time (min)
BD/ND
LC10 (mg/L)
(LCL-UCL)
P value
F value
LC50 (mg/L)
(LCL-UCL)
P value
F value
LC90 (mg/L)
(LCL-UCL)
P value
F value
Slope
Intercept
Chi-Square value
10
–
–
–
–
–
–
–
–
–
–
–
–
–
20
BD
70.104 ± 1.375***
(27.322–89.760)0.0001
231.7
125.283 ± 2.486****
(96.423–164.336)<0.0001
381.50
223.894 ± 4.492****
(164.062–624.061)<0.0001
511.60
5.08 ± 1.59
−15.79 ± 6.49
1.02 ± 0.12
ND
39.986 ± 2.437***
(13.356–54.149)77.933 ± 2.360****
(51.306–97.274)152.036 ± 0.064****
(114.963–299.573)4.21 ± 1.13
−11.36 ± 4.47
1.04 ± 0.25
30
BD
51.977 ± 0.625**
(16.136–72.271)0.0014
61.34
109.683 ± 2.509****
(78.673–146.411)<0.0001
261.7
231.477 ± 7.802****
(158.701–716.907)<0.0001
334.3
4.01 ± 1.22
−11.16 ± 4.93
2.94 ± 0.61
ND
39.396 ± 2.184**
(14.587–52.419)71.581 ± 2.190****
(47.357–88.096)130.141 ± 0.745****
(100.412–226.694)4.73 ± 1.24
−13.19 ± 4.89
2.07 ± 0.46
40
BD
41.920 ± 0.203****
(12.638–60.957)<0.0001
3377
93.318 ± 2.161****
(63.358–121.504)<0.0001
379.5
207.809 ± 8.618**
(142.552–539.625)0.0017
55.53
3.77 ± 1.09
−9.94 ± 4.36
2.28 ± 0.07
ND
20.176 ± 0.488****
(03.293–34.552)55.75 ± 1.663****
(27.961–76.254)154.051 ± 5.460**
(101.867–392.168)2.92 ± 0.82
−5.90 ± 3.23
2.18 ± 0.17
50
BD
34.972 ± 0.208****
(12.209–51.327)<0.0001
569.8
73.278 ± 1.484****
(47.873–92.880)<0.0001
260.1
153.648 ± 7.136**
(110.407–285.752)0.0019
53.25
4.13 ± 1.10
−10.95 ± 4.34
2.41 ± 0.31
ND
20.115 ± 0.855****
(04.289–32.192)47.765 ± 1.674****
(24.485–64.066)113.448 ± 3.126**
(78.739–226.305)3.38 ± 0.91
−7.38 ± 3.51
4.36 ± 0.66
60
BD
26.651 ± 0.550****
(08.432–39.499)<0.0001
250
55.168 ± 1.513***
(33.012–71.090)0.0002
164.2
114.202 ± 3.904***
(82.485–200.465)0.0010
75.79
4.09 ± 1.06
−10.27 ± 4.12
3.29 ± 0.48
ND
12.870 ± 1.103****
(01.046–22.977)33.277 ± 1.884***
(10.816–47.453)86.113 ± 2.362***
(56.116–195.160)3.00 ± 0.91
−5.51 ± 3.45
3.23 ± 0.34
3.3 Nano-DEPA impregnation and evaluation of WRI
The WRI on PL cotton fabric and ACL cotton fabric was determined as mentioned in the experimental part. The WRI tends to decrease with the increase in washing cycles. The WRI-Cycle 5 of PL cotton fabric impregnated with 80 and 125 mg/L was found to be 42.29% and 60.18%. Similarly, WRI-Cycle 5 of ACL cotton fabric impregnated with 80 and 125 mg/L was found to be 49.18%, and 78.47% (Fig. 3). The obtained results indicate the higher WRI possessed by ACL cotton fabric than the PL cotton fabric. This signifies the effective entrapment of Nano-DEPA onto the ACL cotton fabric than the PL cotton fabric. Further HR-SEM observation of Nano-DEPA impregnated ACL cotton fabric have displayed the uniform surface distribution of Nano-DEPA embedded onto the alginate matrices (Fig. 4C and D).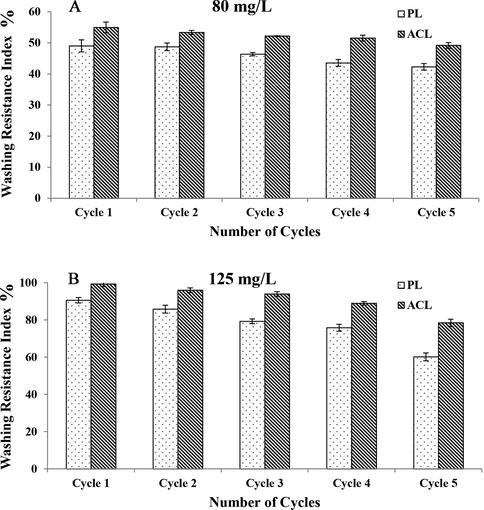
Washing resistance index (WRI) of Plain (PL), and Alginate crosslinked (ACL) cotton fabric impregnated with (A) 80 mg/L and (B) 125 mg/L of Nano-DEPA respectively.
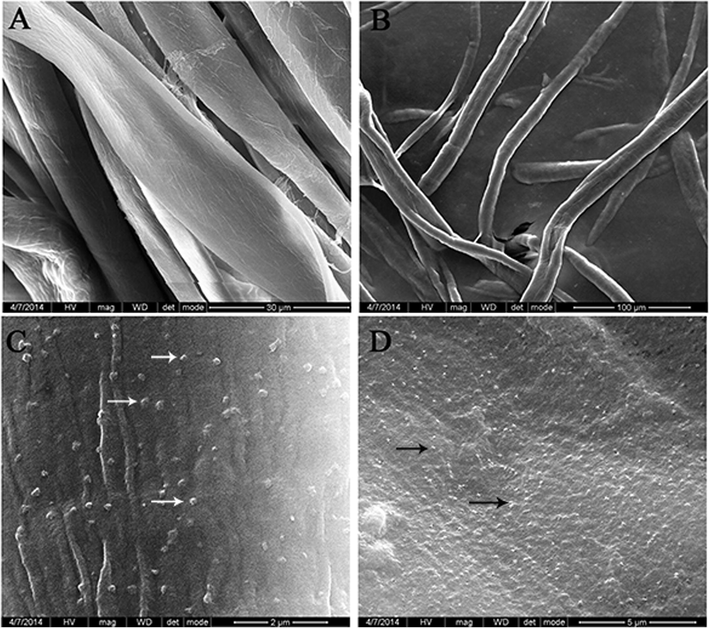
HR-SEM micrographs of (A) Plain (PL) cotton fabric, (B) Alginate impregnated cotton fabric, (C) Alginate cross-linked (ACL) cotton fabric impregnated with 80 mg/L and (D) 125 mg/L of Nano-DEPA. The arrow marks indicate the entrapped Nano-DEPA on the fabric.
4 Discussion
The development of resistance in mosquitoes is a devastating factor, which hinders the efficacy. The mosquito resistance mechanisms such as selection pressure and cross-resistance were majorly influenced due to the continual exposure of conventional pesticidal groups (Kumar et al., 2011; Selvi et al., 2005). For instance, Culex pipiens has evolved the anatomical resistance barrier by the development of thicker cuticles due to the prolonged exposure of fenthion, an organophosphorus pesticide (Stone and Brown, 1969). As to overcome resistance, several research groups have formulated inorganic nanopesticides possessing mosquito ovicidal, larvicidal and pupicidal efficacy (Kah and Hofmann, 2014; Benelli et al., 2017). The majority of the inorganic nanopesticides are formulated from the plant-based systems as to reduce the environmental toxicity (Benelli, 2016a,b,c). In spite of this, they possess the major drawback of environmental uncertainty and toxicity (Kah et al., 2013).
In contrast to the above formulation, Nano-DEPA formulated in the present study is a nanoencapsulated form of conventional pesticides (DEPA) with similar environmental certainty. Moreover, Bulk-DEPA being a poorly water miscible form, it requires a toxic organic polar solvent matrix for their solubilization. These solvent matrices exert several drawbacks such as active compound sedimentation, spray equipment’s corrosion, and environmental toxicity. In contrast to this, Nano-DEPA formulated in the present study is a hydrodispersive form, which does not require any organic polar solvents and the required dosage concentration can be easily prepared with water (a cost effective non-corrosive safer solvent system). Moreover, several drawbacks during the formulation and application procedures can be avoided to a greater extent. The hydrodispersive property of Nano-DEPA was attributed due to the polymeric-surfactant micellization over the hydrophobic DEPA droplets. This aids in effective distribution of the active compound over a vast area in logged water fields. Moreover, Nano-DEPA being a nanocolloidal form it could resist the sedimentation in the field conditions, which is beneficial for retaining its efficacy over a longer period. This sustains the efficacy of the active compound and reduces the higher dosage and repetitive application. These advantages make the Nano-DEPA cost-effective and reliable formulation.
The results of larvicidal bioassay extrapolate the improved efficacy of Nano-DEPA towards the Culex quinquefasciatus larval population, as comparative to Bulk-DEPA. The improved efficacy possessed by Nano-DEPA was due to its nanometric form with improved penetration efficacy (Balaji et al., 2015b). Moreover, the presence of surfactant micellization in Nano-DEPA plays a major role in protecting the active compound (DEPA) from the encounter of detoxifying enzymes in the larval gut region. These enhanced features of Nano-DEPA attribute in improving the dosage availability inside the larval body, owing to this higher degree of lethality was exerted even at lower exposure concentrations. Balaji et al. (2017) and Sugumar et al. (2014) have reported similar results as the nanoemulsion system tend to exert superior efficacy on the mosquito larval population. Besides, there no apparent reports on mosquito resistance development towards the insect repellents such as DEPA until date, which improves their feasible application in the mosquito control programmes.
The histopathological studies on the larval body also propound the higher efficacy of Nano-DEPA to exert lethality on the larval body even at lower exposure concentrations (i.e. the LC50 of Nano-DEPA is lower than the Bulk-DEPA). This was due to the improved active compound availability and penetration efficacy of Nano-DEPA on the larval body. The increased lesions caused by Nano-DEPA in the larval midgut regions were due to the presence of numerous microvilli, which involve in the active adsorption of compounds (Alves et al., 2010). Kumar et al. (2013) and Balaji et al. (2017) have reported similar findings upon exposing the mosquito larvae to nanometric permethrin and nanometric deltamethrin. Overall, the histopathological study was found in agreement with the larvicidal bioassay.
Even though Nano-DEPA exerts superior toxicity on the larval population, controlling the adult mosquito population is vital, as they are prime disease carriers. In order, WHO cone bioassay was performed on the adult mosquito population. The KD count directly implies the toxic influence of the active compound (DEPA) on the mosquito population. The KD provoked by insect repellents was due to the exertion of contact irritancy or excito repellency attributed upon direct mosquito tarsal contact with toxicants (Deletre et al., 2016). The KD50 values of Nano-DEPA were found to be lower than the Bulk-DEPA KD50 values. This was due to the larger surface area, hydrodispersive nature, improved penetration efficacy, and the higher affinity of the nanoemulsion system towards adult insects (Nenaah, 2014; Werdin Gonzalez et al., 2014). Balaji et al. (2015a) and Balaji et al. (2017) have reported the similar findings upon exposing nanometric permethrin and nanometric deltamethrin towards the Culex quinquefasciatus mosquito population. In addition, improved repellency efficacy of the citronella oil nanoemulsion was reported by Sakulku et al. (2009).
As to further explore the feasible application of pesticides in insect repellent clothing, Sukumaran et al. (2014) have incorporated permethrin onto the army uniform and performed the washing studies. In the similar fashion, the Nano-DEPA was impregnated onto the cotton fabric and the WRI was determined. The higher WRI possessed by ACL cotton fabric implies the efficient impregnation and entrapment of Nano-DEPA. This was due to the presence of alginate crosslinking between the yarns. The ionic bonding between the alginate was executed by the Ca2+ ions (Ouwerx et al., 1998). The crosslinking persuade a two-dimensional planar model in the sequence of “egg-box” array (Al-Musa et al., 1999). Thus, the resulted ACL fabrics possessed a dense, insoluble gelling matrix on the surface with the closely bonded yarns. Also, the presence of PEG micellization over the DEPA molecules attributes stronger intermolecular interaction with the alginates in ACL cotton fabric (Wang et al., 2007). Due to which, Nano-DEPA discharge from the ACL cotton fabric was resisted during the washing procedure. Mihailović et al. (2010) have also reported the similar findings, as the polyester fabric modified with alginate has exerted improved laundering durability. In general, the woven textiles such as cotton fabric possesses more complex geometry due to the varying fabric weave and yarn structure. The pore volume distribution was found to be bimodal, where the smaller pores and larger pores correspond to the interfibre and interyarn spaces respectively (Miller and Tyomkin, 1994).The increased pore size in the fabric provokes a higher capillary flow of the insect repellent applied on the fabric surface. This poses a higher insect repellent exposure to the human skin and causes skin irritation and health hazards (Briassoulis et al., 2001). However, the exposure hazard of the insect repellent can be very much reduced in the entrapment methodology employed in the present study, in which Nano-DEPA impregnation was performed over the alginate matrix. This reduces the fluid movement and blocks the dermal exposure. Moreover, Nano-DEPA being a hydrodispersive formulation, the dermal irritation aroused due to the toxic solvents could be excluded. In addition to this, the presence of alginate matrices in ACL cotton fabric could exert controlled release diffusion mechanism of the active compound with sustained release (Badwan et al., 1985; Specos et al., 2010). This prolongs the efficacy of the Nano-DEPA impregnated ACL cotton fabric over a longer period and ensures the reliability in long lasting protective clothing.
5 Conclusions
The control over the mosquito population is demanding for the well-being of human health. The need for alternative pesticides has become the inevitable choice to curb the mosquito population and mosquito-borne disease transmission. In accordance, the present study describes the formulation of Nano-DEPA by PEG polymerization, followed by the PIT emulsification method. The formulated Nano-DEPA have shown improved bioefficacy against the Culex quinquefasciatus (Lymphatic Filariasis vector) larvae and adult mosquitoes even at lower exposure concentrations. Further, the study has provided the methodology for effective impregnation of Nano-DEPA onto the cotton fabric mediated by the alginate crosslinking. This was evident from the higher WRI in ACL cotton fabric than the PL cotton fabric. Besides, the hydrodispersive nature of Nano-DEPA improves the benignity in the environment and humankind. Overall, the present study has stipulated the future prospect of employing efficacious nanometric insect repellents for the control and protection from the dreadful disease causing mosquito population.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
We thank Defence Research & Development Organization – Life Sciences Research Board (DLS/81/48222/LSRB-250/BTB/2012), Government of India for the financial support and VIT University, Vellore for providing lab facilities. We acknowledge Dr. K. Gopalarathinam, Senior Entomologist, ZET, Vellore for his immense contribution to the entomological studies.
References
- Larvicidal, histopathological and ultra-structure studies of Matricharia chamomella extracts against the rift valley fever mosquito Culex quinquefasciatus (Culicidae: Diptera) J. Entomol. 2011;8:63-72.
- [Google Scholar]
- Evaluation of parameters involved in preparation and release of drug loaded in crosslinked matrices of alginate. J. Control Release. 1999;57:223-232.
- [Google Scholar]
- Alterations in the fat body and midgut of Culex quinquefasciatus larvae following exposure to different insecticides. Micron. 2010;41:592-597.
- [Google Scholar]
- Keys to the adult females and fourth-instar larvae of the mosquitoes of Iran (Diptera: Culicidae) Zootaxa. 2009;2078:1-33.
- [Google Scholar]
- A sustained release drug delivery system using calcium alginate beads. Drug Dev. Ind. Pharm.. 1985;11:239-256.
- [Google Scholar]
- Copper nanoparticles loaded alginate-impregnated cotton fabric with antibacterial properties. J. Appl. Polym. Sci.. 2012;126:E319-E326.
- [Google Scholar]
- Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym.. 2004;59:129-140.
- [Google Scholar]
- The environmentally benign form of pesticide in hydrodispersive nanometric form with improved efficacy against adult mosquitoes at low exposure concentrations. Bull. Environ. Contam. Toxicol.. 2015;95:734-739.
- [Google Scholar]
- Nanoformulation of poly(ethylene glycol) polymerized organic insect repellent by PIT emulsification method and its application for Japanese encephalitis vector control. Coll. Surf. B. 2015;128:370-378.
- [Google Scholar]
- Environmental benignity of a pesticide in soft colloidal hydrodispersive nanometric form with improved toxic precision towards the target organisms than non-target organisms. Sci. Total Environ.. 2017;579:190-201.
- [Google Scholar]
- Green synthesized nanoparticles in the fight against mosquito-borne diseases and cancer—a brief review. Enzyme Microb. Technol.. 2016;95:58-68.
- [Google Scholar]
- Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review. Parasitol. Res.. 2016;115:23-34.
- [Google Scholar]
- Plant-mediated synthesis of nanoparticles: a newer and safer tool against mosquito-borne diseases? Asian Pac. J. Trop. Biomed.. 2016;6:353-354.
- [Google Scholar]
- Nanoparticles for mosquito control: challenges and constraints. J. King Saud Univ.. 2017;29:424-435.
- [Google Scholar]
- Declining malaria, rising of dengue and Zika virus: insights for mosquito vector control. Parasitol. Res.. 2016;115:1747-1754.
- [Google Scholar]
- Toxic encephalopathy associated with use of DEET insect repellents: a case analysis of its toxicity in children. Hum. Exp. Toxicol.. 2001;20:8-14.
- [Google Scholar]
- Prospects for repellent in pest control: current developments and future challenges. Chemoecology. 2016;26:127-142.
- [Google Scholar]
- Contact toxicity and residual activity of different permethrin-based fabric impregnation methods for Aedes aegypti (Diptera: Culicidae), Ixodes ricinus (Acari: Ixodidae), and Lepisma saccharina (Thysanura: Lepismatidae) J. Med. Entomol.. 2003;40:935-941.
- [Google Scholar]
- The innovative vector control consortium: improved control of mosquito-borne diseases. Trends Parasitol.. 2006;22:308-312.
- [Google Scholar]
- Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol.. 2000;45:371-391.
- [Google Scholar]
- Nanopesticides: state of knowledge, environmental fate, and exposure modeling. Crit. Rev. Environ. Sci. Technol.. 2013;43:1823-1867.
- [Google Scholar]
- Nanopesticide research: current trends and future priorities. Environ. Int.. 2014;63:224-235.
- [Google Scholar]
- A preliminary report on the synthesis and testing of mosquito repellents. Indian J. Med. Res. 1982;76:190.
- [Google Scholar]
- N, N-Diethyl Phenylacetamide (DEPA): a safe and effective repellent for personal protection against hematophagous arthropods. J. Med. Entomol.. 2006;43:518-525.
- [Google Scholar]
- Multiple insecticide resistance/susceptibility status of Culex quinquefasciatus, principal vector of bancroftian filariasis from filaria endemic areas of northern India. Asian Pac. J. Trop. Dis.. 2011;4:426-429.
- [Google Scholar]
- Distinctive effects of nano-sized permethrin in the environment. Environ. Sci. Pollut. Res.. 2013;20:2593-2602.
- [Google Scholar]
- Functionalization of polyester fabrics with alginates and TiO2 nanoparticles. Carbohydr. Polym.. 2010;79:526-532.
- [Google Scholar]
- Liquid porosimetry: new methodology and applications. J. Colloid Interface Sci.. 1994;162:163-170.
- [Google Scholar]
- Chemical composition, toxicity and growth inhibitory activities of essential oils of three Achillea species and their nano-emulsions against Tribolium castaneum (Herbst) Ind. Crops Prod.. 2014;53:252-260.
- [Google Scholar]
- Impact of environment on mosquito response to pyrethroid insecticides: facts, evidences and prospects. Insect Biochem. Mol. Biol.. 2013;43:407-416.
- [Google Scholar]
- Physico-chemical properties and rheology of alginate gel beads formed with various divalent cations. Polym. Gels Netw.. 1998;6:393-408.
- [Google Scholar]
- Study on encapsulation of diethyl phenyl acetamide in calcium-alginate microsphere for enhanced repellent efficacy. Int. J. Polym. Mater.. 2012;61:1154-1163.
- [Google Scholar]
- The economic burden of lymphatic filariasis in India. Parasitol. Today. 2000;16:251-253.
- [Google Scholar]
- Characterization and mosquito repellent activity of citronella oil nanoemulsion. Int. J. Pharm.. 2009;372:105-111.
- [Google Scholar]
- Resistance development and insecticide susceptibility in Culex quinquefasciatus against selection pressure of malathion and permethrin and its relationship to cross-resistance towards propoxur. Trop. Biomed.. 2005;22:103-113.
- [Google Scholar]
- Microencapsulated citronella oil for mosquito repellent finishing of cotton textiles. Trans. R. Soc. Trop. Med. Hyg.. 2010;104:653-658.
- [Google Scholar]
- Mechanisms of resistance to fenthion in Culex pipiens fatigans Wied. Bull. World Health Organ.. 1969;40:401.
- [Google Scholar]
- Nanoemulsion of eucalyptus oil and its larvicidal activity against Culex quinquefasciatus. Bull. Entomol. Res.. 2014;104:393-402.
- [Google Scholar]
- Knockdown and repellent effect of permethrin-impregnated army uniform cloth against Aedes aegypti after different cycles of washings. Parasitol. Res.. 2014;113:1739-1747.
- [Google Scholar]
- The health and economic benefits of the global programme to eliminate lymphatic filariasis (2000–2014) Infect. Dis. Poverty. 2016;5:54.
- [Google Scholar]
- Alginate/polyethylene glycol blend fibers and their properties for drug controlled release. J. Biomed. Mater. Res. A.. 2007;82A:122-128.
- [Google Scholar]
- Essential oils nanoformulations for stored-product pest control - characterization and biological properties. Chemosphere. 2014;100:130-138.
- [Google Scholar]
- World Health Organization, 2005. Guidelines for laboratory and field testing of mosquito larvicides. Communicable disease control, prevention and eradication, WHO pesticide evaluation scheme.
- World Health Organization, 2006. Guidelines for testing mosquito adulticides for indoor residual spraying and treatment of mosquito nets.
- World Health Organization, 2013a. Guidelines for laboratory and field-testing of long-lasting insecticidal nets.
- World Health Organization, 2013b. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes.







