Translate this page into:
Pinostrobin attenuated cadmium instigated cardiotoxicity in albino rats: A biochemical, inflammatory, apoptotic and histopathological examination
⁎Corresponding author. raifaisal764@gmail.com (Muhammad Faisal Hayat)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cadmium (Cd) is a noxious and non-biodegradable heavy metal which instigates various organ toxicities such as cardiac injuries. Pinostrobin (PSB) is a potent dietary bioflavonoid, which shows various pharmacological potentials. The current research was designed to evaluate the ameliorative effects of PSB against Cd elicited cardiac dysfunction in rats. Twenty-four albino rats were apportioned into four equal groups viz. control, Cd (5 mg/kg), Cd (5 mg/kg) + PSB (40 mg/kg) and PSB (40 mg/kg) only treated group. It was observed that Cd intoxication reduced catalase (CAT), glutathione reductase (GSR), superoxide dismutase (SOD), glutathione peroxidase (GPx), activities and glutathione S-transferase (GST) contents while escalating the levels of reactive oxygen species (ROS), hydrogen peroxide (H2O2) and malondialdehyde (MDA). Furthermore, Cd exposure escalated the levels of cardiac injury markers such as creatine phosphokinase (CPK), creatine kinase-myocardial band (CK-MB), troponin I and lactate dehydrogenase (LDH). Besides, the levels of inflammatory cytokines nuclear factor- κB (NF-κB), interleukin 1beta (IL-1ß), tumor necrosis factor- α (TNF-α), interleukin-6 (IL-6) levels and cyclooxygenase-2 (COX-2) activity were upregulated in Cd intoxicated group. Similarly, Caspase-3, Bax and Caspase-9 levels were augmented, and Bcl-2 levels were reduced after Cd administration. In addition, the histopathological examination revealed a notable cardiac tissue impairment in the Cd exposed group. Nonetheless, PSB treatment significantly (p < 0.05) recovered the abovementioned Cd-induced impairments. Therefore, the current study revealed that PSB might be a promising ameliorative agent to ameliorate Cd instigated cardiac damages.
Keywords
Pinostrobin
Cadmium
Cardiotoxicity
Oxidative stress
Inflammation
1 Introduction
Cd is considered as one of the most lethal heavy metals and designated a carcinogenic substance that instigates severe health risks to animals and humans (Al Olayan et al., 2020). Cd enters the body predominantly by ingestion, inhalation or dermal contact (Nduka et al., 2019). Moreover, Genchi et al. (2020) elucidated that Cd is extensively used in the manufacturing of various products including batteries, pigments, plastics, cigarette, metal coatings and polyvinyl chloride. People exposed to Cd during various operations such as mining, metal ores, production of nickel–cadmium (Ni-Cd) batteries and electronic devices. Owing to low rate of excretion, Cd accumulates in different organs & instigates various organ damages such as hepatotoxicity, cardiotoxicity and nephrotoxicity (Pi et al., 2015; Oyinloye et al., 2016; Dong, 2014).
The heart is one of the vital organs of body that performs pivotal role in living organisms (Peate, 2021). Cd disrupts the cardiovascular system (CVS) by disrupting cytochrome P450 as well as antioxidant balance in the body. When Cd reaches the cardiac tissues, it prompts oxidative stress as well as DNA damage which ultimately disregulates the normal gene transcription (Guo et al., 2020). The exposure to Cd disruptes the antioxidant defense system that involves neutralization of free radicals by lowering ROS production and protecting the macromolecules (proteins, DNA & lipids) from damage (Jan et al., 2015). Furthermore, Cd intoxication instigates peripheral arterial disease, increased vascular intima media thickness, morphological lesions, disorders of cardiac muscles & myocardial infarction that ultimately leads to congenital heart disease & cardiovascular disease (CVD) (Ray et al., 2023).
Flavonoids are secondary metabolites of plants that are extensively used against various disorders (Juca et al., 2020). Pinostrobin (PSB) is a bioflavonoid which demonstrated various pharmacological abilities including anti-oxidative (Hidajati et al., 2018), antifungal (Kanchanapiboon et al., 2020), anticancer (Sun et al., 2020), antiapoptotic (Jadaun et al., 2019) antiparasitic (Vechi et al., 2021) & anti-inflammatory (Patel et al., 2014). However, the cardioprotective potential of PSB is yet to be reported. Consequently, the current trial was executed to ascertain the ameliorative effect of PSB against Cd-instigated heart damages in rats.
2 Materials & methods
2.1 Chemicals
Cd & PSB were bought from Sigma-Aldrich (Germany).
2.2 Animals
Rats (n = 24) having weight approximately (200–220 g) and age (16–18 weeks) were used as model animals during the current investigation. Steel cages were used to accommodate rats in the animal house of University of Agriculture, Faisalabad. All the experimental animals were acclimatized to the laboratory condition (12 h day/night period, standard temperature 22-25⁰C) for 7 days before the execution of trial. Rats were provided standard feed & tap water. European Union of Animal Care & Experimentation (CEE Council 86/609) protocols were followed to handle the experimental animals.
2.3 Experimental layout
Albino rats (n = 24) were apportioned into 4 groups (n = 6). The group 1st was considered as control group. The 2nd group was exposed to Cd (5 mg/kg). The 3rd group was exposed to PSB (40 mg/kg) + Cd (5 mg/kg) while the 4th group was exposed to PSB (40 mg/kg) only. After the completion of experiment (4 weeks), the rats were anesthetized by using ketamine (60 mg/kg) and xylazine (5 mg/kg), beheaded and the blood samples were collected in sterile syringes (heparin containing). Serum samples obtained from blood were homogenized and centrifuged (3000 rpm) for 15 min. Heart was removed and sliced into two equally divided parts. One part was stored in zipper bags & retained at −20 ⁰C for further biochemical examination, while the other half was fixed in 10 % formalin for histopathology.
2.4 Biochemical analysis
Chance & Maehly (1995) approach was used to evaluate the CAT activity. The method described by Kakkar et al. (1984) was used to assess SOD levels. The method of Carlberg & Mannervik (1975) was employed to measure GSR activity. GST activity was assessed using the methods developed by Couri & Abdel-Rahman (1979). The technique of Jollow et al., 1974 was employed to evaluate GSH content. MDA level was quantified by using the strategy of ohkawa et al., (1979). The ROS level was assessed by following the method of Hayashi et al. (2007). The technique developed by Pick & Keisari (1981) was used to quantify H2O2 levels.
2.5 Evaluation of cardiac injury biomarkers
The cardiac injury markers such as CK-MB (Catalog No. MBS2515061), troponin I (CSB-E08594r), LDH (CSB-E11324r) and CPK (Catalog no. E4608-100) were determined via utilizing ELISA kits in consistent to the instructions of manufacturer.
2.6 Evaluation of inflammatory indices
For the assessment of inflammatory indices IL-1β (CSB-E08055r), IL-6 (CSB-E04640r), NF-κB (CSB-E13148r), TNF-α (CSB-E07379r) & COX-2 (CSB-E13399r) levels were carried out by using standardized ELISA kits (TX, Houston, Cusabio Technology Llc, USA).
2.7 Apoptotic markers assessment
For the evaluation of Caspase-9 (CSB-E08863r), Bax (CSB-EL002573RA), Caspase-3 (CSB-E08857r) & Bcl-2 (CSB-E08854r) standard ELISA kits were used according to the guidelines provided by the manufacturers (Cusabio Technology Llc, USA).
2.8 Histological examination
The cardiac samples were kept in formalin (10 %) solution and dehydrated by higher grades of ethanol. Then tissues were carefully kept in paraffin wax. Then thin slices (4–5 µm) of paraffin coated tissues were sliced with rotary microtome & stained by using stain “Hematoxylin-Eosin”. Slides were carefully observed by using compound microscope (Nikon, Japan) and microphotographs were taken by MoticTM camera (5 megapixels).
2.9 Statistical evaluations
All the data were illustrated as Mean ± SEM. The Tukey’s test & ANOVA (one way interaction) were used to statistically analyze the data by using Graph pad Prism 5. The significance level was kept at p < 0.05.
3 Results
3.1 Effects of PSB on antioxidant enzymes activity
Antioxidant enzymes SOD, GSR, CAT, GSH, GST & GPx activities were markedly (p < 0.05) reduced in Cd intoxicated group in relation to control rats. Nonetheless, PSB supplementation substantially (p < 0.05) escalated antioxidant enzyme activities in Cd + PSB administered animals as compared to Cd intoxicated animals. Furthermore, no substantial variation was observed in these activities among the rats of control & PSB only supplemented group (Table 1). Values having different superscripts are significantly (p < 0.05) different from the other groups.
Parameters
Groups
Control
Cd
Cd + PSB
PSB
CAT (U/mg protein)
12.88 ± 1.47a
5.06 ± 0.29c
8.06 ± 0.62b
12.79 ± 1.50b
SOD (U/mg protein)
10.39 ± 0.85a
4.57 ± 0.36c
7.82 ± 0.36b
10.32 ± 0.93a
GSR (nM NADPH oxidized/min/mg tissue
9.34 ± 0.22a
3.26 ± 0.27c
6.40 ± 0.46b
9.39 ± 0.25a
GPx (U/mg protein)
31.28 ± 2.28a
8.90 ± 0.83c
18.72 ± 1.43b
31.39 ± 2.39a
GSH (U/mg protein)
19.59 ± 1.72a
6.17 ± 0.41c
13.67 ± 1.00b
19.64 ± 2.15a
GST (U/mg protein)
41.91 ± 1.63a
14.61 ± 1.49c
32.86 ± 1.78b
42.03 ± 2.01a
MDA (nmol/g)
0.74 ± 0.07c
5.86 ± 0.51a
2.34 ± 0.20b
0.72 ± 0.07c
ROS (nmol/g)
0.44 ± 0.13c
7.40 ± 0.44a
2.33 ± 0.21b
0.41 ± 0.14c
H2O2 (µM/min/ mg protein)
1.38 ± 0.19b
6.54 ± 0.66a
2.34 ± 0.28b
1.36 ± 0.19b
3.2 Effects of PSB on oxidant profile
The levels of ROS & MDA were markedly (p < 0.05) increased in Cd provided rats in compliance to control group. On the other hand, PSB administration remarkably (p < 0.05) decreased the ROS & MDA levels in Cd + PSB supplemented animals as compared with Cd exposed animals. Furthermore, ROS and MDA contents in only PSB supplemented rats were close to control group (Table 1).
3.3 Effects of PSB on cardiac function biomarkers
The levels of CPK, LDH, CK-MB & troponin I was markedly (p < 0.05) elevated in Cd administered animals in comparison to control group animals. Contrarily, Cd + PSB supplementation notably (p < 0.05) reduced the levels of abovementioned cardiac injury biomarkers as compared to Cd administered rats. Moreover, no discrepancies were noted among the control & only PSB supplemented rats (Table 2). Values having different superscripts are significantly (p < 0.05) different from the other groups.
Parameters
Groups
Control
Cd
Cd + PSB
PSB
LDH (mg/dl)
15.97 ± 0.82c
57.72 ± 1.31a
25.95 ± 1.41b
15.91 ± 0.67c
CPK (mcg/L)
126.34 ± 4.67c
354.78 ± 12.72a
227.41 ± 7.68b
125.26 ± 4.46c
CK-MB (ng/mL)
29.53 ± 2.93c
91.13 ± 2.65a
48.07 ± 2.83b
28.70 ± 3.55c
Troponin (pg/ml)
0.60 ± 0.08c
3.84 ± 0.09a
1.62 ± 0.10b
0.59 ± 0.08c
3.4 Effects of PSB on inflammatory biomarkers
The levels of IL-1β, NF-κB, COX-2, IL-6 & TNF-α were substantially (p < 0.05) augmented in Cd provided animals in relation to control group. Nonetheless, these levels were notably (p < 0.05) reduced in co-treated (Cd + PSB) animals as compared to Cd exposed animals. Furthermore, PSB (only) supplemented animals expressed these levels close to untreated group (Table 3). Values having different superscripts are significantly (p < 0.05) different from the other groups.
Parameters
Groups
Control
Cd
Cd + PSB
PSB
NF-κB (ng/g tissue)
18.96 ± 1.68c
84.15 ± 2.18a
35.77 ± 1.55b
18.52 ± 1.36c
TNFα (ng/g tissue)
8.23 ± 0.28c
26.25 ± 1.66a
15.74 ± 1.74b
8.11 ± 0.19c
IL-1ß (ng/g tissue)
27.55 ± 2.03c
76.20 ± 2.42a
42.24 ± 2.36b
27.30 ± 2.07c
IL-6 (ng/g tissue)
6.26 ± 0.27c
51.71 ± 1.84a
18.80 ± 1.72b
6.21 ± 0.28c
COX-2 (ng/g tissue)
16.85 ± 1.51c
55.81 ± 1.24a
28.90 ± 2.11b
16.71 ± 1.63c
3.5 Effects of PSB on apoptotic profile
Cd intoxication notably (p < 0.05) upsurged Caspase-9, Caspase-3 & Bax, while downregulating the levels of Bcl-2 in Cd exposed animals as compared to control animals. However, the exposure to PSB + Cd markedly (p < 0.05) decreased Caspase-9, Caspase-3 & Bax while escalating Bcl-2 levels as compared to Cd exposed animals. Furthermore, the rats of control as well as PSB only supplemented group showed no variation in their mean values (Table 4). Values having different superscripts are significantly (p < 0.05) different from the other groups.
Parameters
Groups
Control
Cd
Cd + PSB
PSB
Bax (pg/mL)
1.84 ± 0.08b
6.74 ± 0.53a
2.62 ± 0.34b
1.82 ± 0.09b
Caspase-3 (ng/mL)
1.58 ± 0.21c
14.26 ± 0.46a
3.18 ± 0.39b
1.54 ± 0.23c
Caspase-9 (pg/mL)
2.16 ± 0.16c
22.85 ± 1.61a
4.82 ± 0.35b
2.13 ± 0.17c
Bcl-2 (pg/mL)
17.25 ± 1.33a
6.26 ± 0.50c
11.60 ± 1.28b
17.43 ± 1.41a
3.6 Effect of PSB on cardiac histopathology
Cd intoxication led to various histopathological disruptions in cardiac tissues such as myocardial damage, focal necrosis, vacuolization of cytoplasm, myofibril disarray, interstitial fibrosis, inflammation, and alterations in cellular morphology in comparison to control group. Nevertheless, PSB supplementation remarkably (p < 0.05) mitigated abovementioned histopathological disruptions prompted by Cd exposure. However, control and PSB only supplemented group showed (Fig. 1).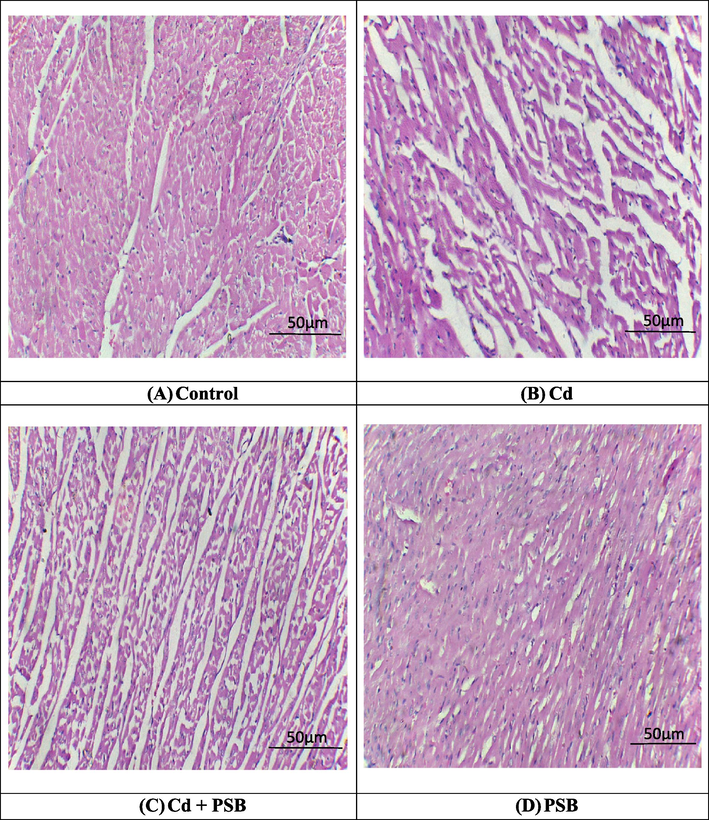
Histopathological analysis of heart tissues. H&E stain; 40X (A) Control group exhibited normal architecture of heart tissues (B) Cd exposed group demonstrated fibrosis, inflammation as well as large interstitial spaces (edema) (C) Cd + PSB group showed a remarkable recovery in contrast to Cd exposed group (D) Only PSB supplemented group showed normal morphology of cardiac tissues as in control group.
4 Discussion
Cd is one of the most prevalent contaminants with a continuous increasing concentration because of agricultural and industrial activities, causing unavoidable hazards to humans (Wang et al., 2023). Setia et al. (2020) documented that Cd penetrates the body through skin, gastrointestinal & respiratory tract. It is reported that Cd exposure induces various damages such as nephrotoxicity, bone disease, infertility, liver toxicity as well as cardiac damages (Mitra et al., 2022). In cardiac tissues, Cd exposure generates oxidative stress, inflammation, cardiomyocyte apoptosis and histological damage (Alpsoy et al., 2014). Recent literature shows that antioxidants can prevent the body from adverse effects of Cd intoxication. PSB is a poly-phenolic compound that is effective in scavenging free radicals & well-known for its broad range of pharmacological properties (Athapaththu et al., 2023).
Our investigation elucidated that Cd intoxication reduced the activities of antioxidant enzymes while augmenting the levels of oxidative stress markers in cardiac tissues of treated animals. Furthermore, excessive generation of ROS reduced the activities of antioxidant enzymes thereby impairing cellular defense system (Ahmad et al., 2023). Ighodaro and Akinloye (2018) elaborated that SOD transforms superoxide radicals into H₂O₂, whereas CAT utilizes the oxygen as a cofactor and stimulates the degradation of H2O2 into H₂O & O2. GPx reduces oxidative stress by scavenging the hydrogen peroxide. GSH also protects the cells from oxidative stress (OS) by lowering the levels of H2O2 and other peroxides. GST participates in cellular detoxification of cytotoxic and genotoxic substances as well as in defending tissues against oxidative damage. (Hayes et al., 1999). Similarly, escalated levels of MDA indicate the onset of lipid peroxidation due to excessive generation of reactive oxygen species (Ijaz et al., 2022). Elsayed et al. (2022) reported that flavonoids can reduce the levels of OS by increasing the activity of antioxidant enzymes. In the current investigation PSB supplementation restored the balance between antioxidant and prooxidants which demonstrates its ROS neutralizing abilities.
Oral administration of Cd led to a remarkable upsurge in the level of cardiac function markers (CK-MB, CPK, LDH & troponin). Troponin, CPK, and CK-MB are the diagnostic biomarkers of cardiotoxicity due to their serum catalytic activity and tissue specificity (Zhang et al., 2015). A previous investigation elucidated that Cd is a cardio-toxic compound having the capability to destroy directly cardiomyocytes & release these cardiac damage biomarkers into the blood stream. Various types of heart injury, including myocarditis, heart failure & myocardial infarction have been associated with elevated levels of these enzymes (Saleh et al., 2017). However, PSB administration notably lowered the level of these enzymes that might be attributed to its cardio-protective ability.
In the current investigation, exposure to Cd instigated an augmentation in the levels of IL-6, TNF-α, NF-κB, IL-1β & COX-2 activity. NF-kB, a cytoplasmic protein complex which is associated with elevated levels of ROS during various disorders (Ali et al., 2022) and its activation ultimately trigger the production of aforementioned inflammatory markers (Somade et al., 2019). Furthermore, COX-2 is an adaptive enzyme that stimulates the complete inflammation state (Kumar et al., 2022). Therefore, inflammatory responses in the cell can be blocked by preventing the activation of NF-κB. However, PSB supplementation reduced the levels of inflammatory biomarkers which is attributed to anti-inflammatory property of PSB.
Cd administration induced a substantial upsurge in the levels of pro-apoptotic markers while downregulating the anti-apoptotic markers. Danial and Korsmeyer (2004) elaborated that Bax works as pro-apoptotic protein that mediates various events of apoptosis while Bcl-2 functions antagonistic to Bax. An escalation in Bax levels & decline in Bcl-2 alters the mitochondrial membrane permeability & triggers the eviction of Cytochrome c into cytoplasmic matrix that in turn activates Caspase-3 to mediate apoptotic pathways (Santana, 2018). Caspase-3 belongs to the cysteine protease family which splits the cellular proteins & making structural variations that led to apoptosis (Hofmann, 2020). However, administration of PSB lowered the levels of pro-apoptotic proteins while elevating the levels of anti-apoptotic markers owing to its anti-apoptotic properties.
The present research demonstrated that Cd intoxication induced adverse histopathological damages in the architecture of cardiac tissues such as focal necrosis, perivascular and interstitial fibrosis, myofibril disarray, alterations in cellular morphology and increased levels of matrix metalloproteinases (MMPs). Our outcomes matched with the investigations of Chou et al. (2023) who stated that Cd exposure instigated various histopathological damages in heart including focal necrosis, myofibril disarray, disorganized sarcomere structures and interstitial & perivascular fibrosis. However, the supplementation of PSB significantly restored all the histopathological damages in the cardiac tissues of rats that might be ascribed to its anti-inflammatory, anti-oxidative & anti-apoptotic nature.
5 Conclusion
Taken together, the current research exposed that Cd intoxication prompted OS in cardiac tissues and disturbed the heart functional enzymes, inflammatory, apoptotic, and biochemical markers along with histopathological profile. However, PSB supplementation significantly restored all the cardiac damages provoked by Cd. As a result, it can be assumed that PSB might be used as a potential therapeutic drug in future to cure cardiac damage. However, further clinical investigations are indispensable to evaluate the efficacy of PSB to cure cardiac damages in humans.
Acknowledgement
The authors are grateful to the Researchers Supporting Project (RSPD2023R984), King Saud University, Riyadh, Saudi Arabia for the support.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ameliorative Effects of Rhamnetin against Polystyrene Microplastics-induced Nephrotoxicity in Rats. Pak. Vet. J.. 2023;43:623-627.
- [CrossRef] [Google Scholar]
- Nrf2 Activation and NF-Kb & caspase/bax signaling inhibition by sodium butyrate alleviates LPS-induced cell injury in bovine mammary epithelial cells. Mol. Immunol.. 2022;148:54-67.
- [CrossRef] [Google Scholar]
- Protocatechuic acid mitigates cadmium-induced neurotoxicity in rats: Role of oxidative stress, inflammation and apoptosis. Sci. Total Environ.. 2020;723:137969
- [CrossRef] [Google Scholar]
- Protective effects of onion extract on cadmium-induced oxidative stress, histological damage, and apoptosis in rat heart. Biol. Trace Elem. Res.. 2014;159:297-303.
- [CrossRef] [Google Scholar]
- Pinostrobin suppresses the α-melanocyte-stimulating hormone-induced melanogenic signaling pathway. Int. J. Mol. Sci.. 2023;24:821.
- [CrossRef] [Google Scholar]
- Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem.. 1975;250:5475-5480.
- [CrossRef] [Google Scholar]
- Cadmium exposure induces histological damage and cytotoxicity in the cardiovascular system of mice. Food Chem. Toxicol.. 2023;175:113740
- [CrossRef] [Google Scholar]
- Effect of chlorine dioxide and metabolites on glutathione dependent system in rat, mouse and chicken blood. J. Environ. Pathol. Toxicol.. 1979;3:451-460.
- [Google Scholar]
- Protective effect of proanthocyanidins in cadmium induced neurotoxicity in mice. Drug Res.. 2014;65:555-560.
- [CrossRef] [Google Scholar]
- Ameliorating effect of lycopene and N-acetylcysteine against cisplatin-induced cardiac injury in rats. Pak. Vet. J.. 2022;42:107-111.
- [CrossRef] [Google Scholar]
- The effects of cadmium toxicity. Int. J. Environ. Res. Public Health. 2020;17:3782.
- [CrossRef] [Google Scholar]
- Cadmium induced cardiac inflammation in chicken (Gallus gallus) via modulating cytochrome P450 systems and Nrf2 mediated antioxidant defense. Chemosphere. 2020;249:125858
- [CrossRef] [Google Scholar]
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res. Genet. Toxicol. Environ. Mutagen.. 2007;631:55-61.
- [CrossRef] [Google Scholar]
- Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Rad. Res.. 1999;31:273-300.
- [CrossRef] [Google Scholar]
- Hidajati, N., Tukiran, T., Setiabudi, D.A., Wardana, A.P., 2018, December. Antioxidant activity of palmitic acid and pinostrobin from methanol extract of Syzygium litoralle (Myrtaceae). Int. Conf. Sci. Technol. ICST2018, 183-187. Atlantis Press. 10.2991/icst-18.2018.39.
- The evolutionary origins of programmed cell death signaling. ColdSpring Harb. Perspect. Biol.. 2020;12:a036442
- [CrossRef] [Google Scholar]
- First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med.. 2018;54:287-293.
- [CrossRef] [Google Scholar]
- Hepatoprotective Potential of Genkwanin Against Aflatoxin B1-Induced Biochemical, Inflammatory and Histopathological Toxicity in Rats. Pak. Vet. J.. 2022;42:499-504.
- [CrossRef] [Google Scholar]
- Pinostrobin inhibits proliferation and induces apoptosis in cancer stem-like cells through a reactive oxygen species-dependent mechanism. RSC Adv.. 2019;9:12097-12109.
- [CrossRef] [Google Scholar]
- Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci.. 2015;16:29592-29630.
- [CrossRef] [Google Scholar]
- Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151-169.
- [CrossRef] [Google Scholar]
- Flavonoids: biological activities and therapeutic potential. Nat. Prod. Res.. 2020;34:692-705.
- [CrossRef] [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Ind. J. Biochem. Biophys.. 1984;21:130-132.
- [Google Scholar]
- Boesenbergia rotunda extract inhibits Candida albicans biofilm formation by pinostrobin and pinocembrin. J. Ethnopharmacol.. 2020;261:113193
- [CrossRef] [Google Scholar]
- Novel alantolactone derivative AL-04 exhibits potential anti-inflammatory activity via modulation of iNOS, COX-2 and NF-κB. Cytokine. 2022;158:155978
- [CrossRef] [Google Scholar]
- Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter toxicity. J. King Saud Univ. Sci.. 2022;34:101865
- [CrossRef] [Google Scholar]
- Health risk assessment of cadmium, chromium and nickel from car paint dust from used automobiles at auto-panel workshops in Nigeria. Toxicol. Rep.. 2019;6:449-456.
- [CrossRef] [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [CrossRef] [Google Scholar]
- Cardioprotective and antioxidant influence of aqueous extracts from Sesamum indicum seeds on oxidative stress induced by cadmium in wistar rats. Pharmacogn. Mag.. 2016;12:S170-S174.
- [CrossRef] [Google Scholar]
- Pinostrobin and Cajanus lactone isolated from Cajanus cajan (L.) leaves inhibits TNF-α and IL-1β production: In vitro and in vivo experimentation. Phytomedicine. 2014;21:946-953.
- [CrossRef] [Google Scholar]
- The heart: an amazing organ. Br. J. Healthcare Assistant. 2021;15(2):72-77.
- [CrossRef] [Google Scholar]
- SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin. Autophagy. 2015;11:1037-1051.
- [CrossRef] [Google Scholar]
- Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages—induction by multiple nonphagocytic stimuli. Cell. Immunol.. 1981;59(2):301-318.
- [Google Scholar]
- Endothelial dysfunction and its relation in different disorders: Recent update. Health Sci. Rev.. 2023;7:100084
- [CrossRef] [Google Scholar]
- Biochemical and histopathological changes of subacute cadmium intoxication in male rats. Environ. Sci. Pollut. Res.. 2017;24:25475-25481.
- [CrossRef] [Google Scholar]
- Apoptosis and cell cycle aberrations in epithelial odontogenic lesions: An evidence by the expression of p53, Bcl-2 and Bax. Med. Oral Patol. Oral Cir. Bucal.. 2018;23:e120.
- [Google Scholar]
- Impact assessment of metal contamination in surface water of Sutlej River (India) on human health risks. Environ. Pollut.. 2020;265:114907
- [CrossRef] [Google Scholar]
- Activation of NF-kB mediates up-regulation of cerebellar and hypothalamic pro-inflammatory chemokines (RANTES and MCP-1) and cytokines (TNF-α, IL-1β, IL-6) in acute edible camphor administration. Sci. Afr.. 2019;5:e00114.
- [Google Scholar]
- The pharmacokinetics, tissue distribution, metabolism, and excretion of pinostrobin in rats: ultra-high-performance liquid chromatography coupled with linear trap quadrupole orbitrap mass spectrometry studies. Front. Pharmacol.. 2020;11:574638
- [CrossRef] [Google Scholar]
- Antiparasitic activity of two Brazilian plants: Eugenia mattosii and Marlierea eugeniopsoides. Nat. Prod. Res.. 2021;35:4876-4880.
- [CrossRef] [Google Scholar]
- Heavy metal (loid) s in agriculture soils, rice, and wheat across China: Status assessment and spatiotemporal analysis. Sci. Total Environ.. 2023;882:163361
- [CrossRef] [Google Scholar]
- Circulating miR-499 are novel and sensitive biomarker of acute myocardial infarction. J. Thorac. Dis.. 2015;7:303-308.
- [CrossRef] [Google Scholar]
Further reading
- Therapeutic Effect of Oroxylin A Against Bisphenol A-induced Kidney Damage in Rats: a Histological and Biochemical Study. Pak. Vet. J.. 2022;42:511-516.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.103074.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







