Translate this page into:
Phytochemicals from Corchorus olitorius methanolic extract induce apoptotic cell death via activation of caspase-3, anti-Bcl-2 activity, and DNA degradation in breast and lung cancer cell lines
⁎Corresponding author at: Department of Clinical Pharmacy, College of Pharmacy, Najran University, Najran, Saudi Arabia. dr.aliresearch19@gmail.com (Ali Mohamed Alshabi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The main objective of the present research is to provide GC–MS and LC-MS phytochemcial profiling of Corchorus olitorius along with Flow cytometry based mechanistic investigation of cytotoxic activity of its extracts against breast and lung cancer cell lines.
Methods
The Soxhlet extraction method was used in the sequential extraction of C. olitorius. The phytochemical profiling was done using LC-MS and GC–MS techniques, and total phenolic and flavonoid content was determined. The cytotoxicity was studied against non-cancerous L929 cells and cancerous MCF-7 and A549 cell lines using MTT assay, followed by flow cytometry based molecular mechanisms study through anti-Bcl-2 assay, Caspase-3, and DNA fragmentation (TUNEL assay).
Results
The C. olitorius methanolic extract showed the phenolic compounds and flavonoids as a major constituents with 699 µg GAE/g of dw and 1361.50 µg QE/g of dw, respectively. The GC–MS and LC-MS results confirmed the presence of active phytochemicals. Toxicity study against L929 cell line revealed that methanol extract proved to be less toxic with a higher IC50 value i.e. 227.84 µg/ml. Cell viability MTT assay for methanol extract against MCF-7 and A549 cell lines revealed significant results with IC50 values of 20 µg/ml and 12.45 µg/ml, respectively. The flow cytometry based molecular marker studies showed that the extract successively induced early and late apoptosis in tested breast and lung cancer cells through activation of Caspase-3 with inhibition of Bcl-2 protein and induced cell death through DNA damage.
Conclusion
Collectively, these findings show that the methanol extract of C. olitorius inhibits breast cancer and lung cancer cell lines with significant cytotoxic activity. Thus, C. olitorius would be a promising source of chemopreventive agents that warrant further investigation to find lead compounds with cancer chemotherapeutic potential.
Keywords
Corchorus olitorius
Breast cancer
Lung cancer
Apoptosis
Bcl-2
Caspase-3
Saudi Arabia
1 Introduction
According to data from the Global Cancer Statistics for the most frequent malignancies, breast and lung cancers were the most frequent cancers worldwide, accounting for 12.5 % and 12.2 % of all new cases diagnosed in 2020 (Sung et al., 2021). In Saudi Arabia, there is an increase in the number of new cancer cases among females in 2020, with breast cancer and thyroid cancer accounting for 29 % and 14.3 %, respectively. Similarly, among the males, colorectum cancer and non-hodgkin lymphoma accounted for 19.3 % and 8 % newly diagnosed cases, respectively (WHO, 2020). In that order, breast, colorectal, prostate, brain, lymphoma, kidney, and thyroid cancers are the most prevalent in Saudi Arabia (Alqahtani et al., 2020). Given the rising global burden, cancer prevention and treatment is one of the twenty-first century's most pressing public health issues.
Medicinal plants with bioactive substances and biological selectivity are vital sources of novel treatment interventions for various medical severe conditions. Various natural dietary products have been shown to play a role in cancer prevention and treatment and are deemed a strategy (Benzie, 2000). The unique characteristics of nutraceuticals have piqued the interest of researchers to explore the plant kingdom for promising leads in the management of various diseases. Nutraceuticals, particularly those rich with phytochemicals like curcumin, lycopene, quercetin, β-carotene, genistein, and others, have chemopreventive properties, as epidemiologic and animal model studies have already demonstrated (Salami et al., 2013). In our ceaseless quest for functionally novel and biologically active plant-derived products from nature (Alshabi et al., 2020; Alshabi et al., 2021a; Alshabi et al., 2021b; Al-Qahtani et al., 2017), we decided to undertake a detailed investigation on plants from the Najran region of Saudi Arabia. Corchorus olitorius, a shrub in the Malvaceae family, is one such plant reported to possess several medicinal properties.The leaves are diuretic, demulcent, tonic, and febrifuge and treat gonorrhoea, chronic cystitis, and dysuria. A cold infusion of the leaves is an appetizer and strengthens the gut. The seeds have a purgative effect (Islam, 2013). The seed and leaf extracts were cytotoxic to multiple myeloma ARH-77 cell line (İşeri et al., 2013). Furthermore, an ethanol extract of C. olitorius significantly reduced cell viability in human hepatocellular carcinoma (HepG2) cells (Li et al., 2012). The aqueous extract of C. olitorius has been reported to have a cytotoxic effect against human melanoma (A-375), pancreatic cancer (SUIT-2), and gastric cancer (AGS) cell lines (Tosoc et al., 2021). In addition, C. olitorius possesses potent antioxidant potential, attributed to its polyphenols and flavonoid content (Ben Yakoub et al., 2018). Thus, given that C. olitorius is a widely consumed culinary plant in Saudi Arabia, we thought it worthwhile to investigate the indigenous grown C. olitorius plant for its qualitative and quantitative analysis of phytochemical content, and to evaluate the cytotoxic properties of its different fractions against non-cancerous fibroblast cells (L929) and cancerous breast cancer (MCF7) and lung cancer (A549) cell lines by MTT assay.
2 Materials and methods
2.1 Plant material and extraction
The whole plant of C. olitorius was obtained in March 2021 from an authentic supplier in Najran local market, Saudi Arabia, and identified by an expert pharmacognosist, Dr Mohammed A. A. Orabi, Associate Professor of Pharmacognosy, Pharmacy College, Najran University, Najran, Saudi Arabia, and a herbarium specimen (CorchL-03–2021) was deposited in the Department of Pharmacognosy, College of Pharmacy, Najran University.
In the current study, the Soxhlet extraction method was used in the sequential extraction with the solvents hexane, ethyl acetate, methanol, and distilled water, based on their increasing order of polarity. Around 100 g dry plant sample of C. olitorius was extracted with solvents. Approximately 1000 ml of the extraction solvent was used. The extracted plant material was dried before being extracted with the successive solvents.
2.2 Phytochemical analysis
Phytochemical screening was performed for detection of presence and absence of tannins, flavonoids, steroids, terpenoids, alkaloids, glycosides, phenols, anthraquinones, based on previously prescribed procedures (Roghini and Vijayalakshmi, 2018).
2.3 Estimation of total phenolic and flavonoid content
Using a spectrophotometric method, the plant extract's total phenolic compounds was determined (Singleton et al., 1999). Gallic acid (GA) calibration curves were created in the 20–100 μg/ml range. Finally, the concentrations of phenolics were converted to Gallic acid equivalents:μg GAE/g of dry weight (dw) of the extract. The total phenolic content was calculated from the standard calibration curve (Y = 0.001X + 0.113).
Further, aluminum chloride colorimetric assay was used to determine total flavonoid content (Zhishen et al. 1999). 20–100 µg/ml standard quercetin was developed. A 10 ml volumetric flask containing 4 ml double distilled water was added to 1 ml of each quercetin concentration in methanol. At time zero, 0.3 ml of 5 % sodium nitrite, 0.3 ml of 10 % AlCl3, and 2 ml of 1 M sodium hydroxide were added. Immediately, 2.4 ml double-distilled water was added to the mixture and thoroughly stirred. The pink color mixture's absorbance was measured at 510 nm against a quercetin-free blank. The calibration curve was plotted using average quercetin absorbance values. The concentrations of flavonoids were converted to quercetin equivalents: μg QE/g dw of the extract (Y = 0.265x − 0.152).
2.4 Gas chromatography-mass spectrometry (GC–MS) profiling
GC/MS analysis was performed for the methanol extract of C. olitorius using GCMS-QP2010S model. The extract was administered in the sample injector of GC–MS apparatus in a 1 µl aliquot. The column temperature was initially set at 80 °C, while the injector was set at 260 °C, and the flow temperature was set to increase at a rate of 10 °C/min throughout the process. In case of GC, ion source temperature and interface temperature was 200 °C and 280 °C respectively. Solvent cut time was set to 6.50 min with relative detector scan mode. In case of MS, start time and end time was 7 min and 50 min, respectively, under scan mode with 0.50 sec time. Scan speed was 1000 and start m/z and end m/z was 50 and 500 respectivley. The mass spectra of the components were compared to those in the NIST mass spectral library to identify them (Davies, 1990).
2.5 Liquid chromatography-mass spectrometry (LC-MS) profiling
LC-MS-8040 (Shimadzu) model was used to analyze and identify different chemical constituents in the methanol extract of C. olitorius. The HPLC was coupled with mass spectra. A C18 column was used with a mobile phase methanol/water (80:20, v/v). The mobile phase constant flow rate was maintained at a rate 0.2 ml/min. The Electron Spray Ionization probe was used as detector mode, a 3 µl of the sample was used for the analysis (Hanafi et al., 2018). In case of MS the ESI (electron spray ionization) probe was used with M + 1 and M−1 ionization variation.
2.6 In-vitro cytotoxicity of C. Olitorius extracts
Cytotoxicity assay was performed by following the standard procedure of MTT. In the current study, different solvent extracts of C. olitorius were taken in various concentrations (50, 100, 150, 200 and 250 µg/ml) for treating on the non-cancerous cell line, lung cancer (A549) and breast cancer (MCF-7) cell lines. IC50 value was determined with the basis of cell viability percentage from the flowing equation (van Meerloo et al., 2011).
2.7 Flow cytometry based apoptosis study
In the present study, early and late apoptosis detection and measurement were performed by using IC50 concentration of methanol extract of C. olitorius against MCF-7 and A549 cells with 24 hrs duration from the instructions provided in the performing kit. After incubation (24 h), analysis was done using FlowJoX 10.0.7 software (Crowley et al., 2016).
2.8 Caspase-3 activity
Flow cytometry was used to assess the activation of caspase-3 in MCF-7 and A549 cells by C. olitorius methanolic extract (Moraes et al., 2013). After treatment with test sample at its IC50 concentrations. Fluorescence was measured after incubation for 24 h (Acquisition: 7. Cytomics FC500 Flow cytometer, Beckman Coulter, USA).
2.9 Anti Bcl-2 activity
Flow cytometry was used to quantify the level of Bcl-2 protein in treated MCF-7 and A549 cells by C. olitorius methanolic extract (Moraes et al., 2013). After treatment with test sample at its IC50 concentrations, cell preparations was analyzed within 30 min by flow cytometry using FlowJo X 10.0.7 software.
2.10 DNA fragmentation analysis
The apoptotic DNA degradation induced by methanol extract of C. olitorius was performed by the TUNEL assay kit (The APO-DIRECT™ Kit) (Kyrylkova et al., 2012). Finally, using flow cytometry, the TUNEL-positive cells percentage was calculated using Flowzo 10.1 Acquisition: 7 Cytomics FC500 Flow cytometr, (Beckman Coulter, USA).
2.11 Statistical analysis
The data were analyzed using a one-way ANOVA followed by Dunnett's test using GraphPad Prism Version 5. p < 0.05 was chosen as the level of statistical significance. The results are presented as the mean standard deviation for three separate tests (n = 3).
3 Results
3.1 Phytochemical analysis and quantification
The phytochemical analysis revealed the presence of flavonoids, terpenoids, phenols and saponins in the methanolic and aqueous extracts (Table.1). The quantification results showed that the methanol extract exhibited the higher phenolic (699 µg GAE/g of dw) and flavonoid (1361.50 µg QE/g dw) content than the aqueous extract which showed lower phenolic (421.6 µg GAE/g of dw) and flavonoid (1147.54 µg QE/g dw) content. +: Present, −: Absent.
Tests
Hexane
Ethyl acetate
Methanol
Aqueous
Alkaloids
–
–
–
–
Flavonoids
–
–
+
+
Glycosides
–
–
–
–
Phenols
–
–
+
+
Saponins
–
–
+
+
Tannins
–
–
–
–
Terpenoids
–
–
+
+
Steroids
–
–
–
–
Anthraquinones
–
–
–
–
3.2 GC–MS analysis
Methanol extract of C. olitorius have shown the presence of 1-Tridecene, 1-Tetradecanol, Tetradecane methyl arachate, (Cis)-2-Nonadecene, Cyclopropane octanoic acid, Linolenic acid methyl ester, Phytol acetate, dibromo-stigmasterol acetate, methyl commate-D (Table 2 and Fig. 1).
Sl.no
Peak No
Percentage (%)
Compound name
Retention time
Base m/z
Nature
Uses
1
1
3.07
1-tridecene
16.256
55.10
Aliphatic hydrocarbon
Used in pharmaceutical industry
2
2
4.30
1-tetradecanol
21.226
55.10
Fatty alcohol
Used in cosmetics
3
3
2.68
Tetradecane
21.389
57.10
Alkane hydrocarbon
Used in organic synthesis
4
4
5.39
Methyl arachate
28.417
74.05
Methyl ester
–
5
5
1.19
2-nonadecene
29.675
97.10
Alkane hydrocarbon
Used in pharmaceutical industry
6
6
6.85
Cyclopropane octanoic acid
31.638
67.00
Methyl ester fatty acid
Antibacterial
7
7
12.74
Linolonic acid methyl ester
31.770
79.10
Methyl ester fatty acid
Cosmetics, flavour and fragrance
8
8
8.46
Phytol acetate
31.989
71.10
Aromatic compound
Detergent, cosmetics and fragrance
9
9
10.67
Dibromostigmastirol acetate
40.733
81.10
Steroid ester
Antioxidant
10
10
44.64
Methyl comate-D
43.874
218.25
Triterpine glycosides
Anti-inflammatory and antimicrobial
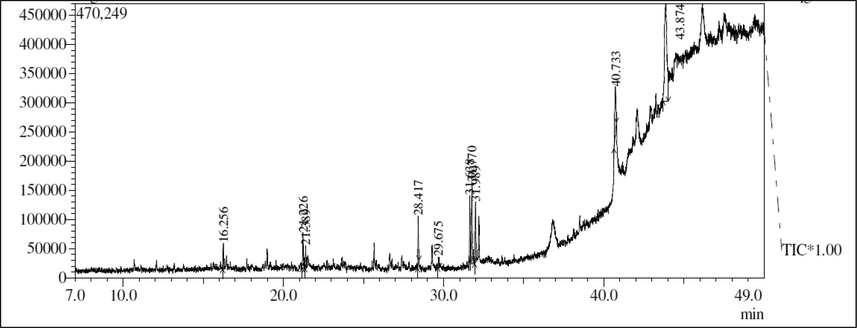
GC–MS profiling of methanol extract of C. olitorius.
3.3 LC-MS analysis
The results of the LC-MS analysis of methanol extract revealed the presence of 5-(3-Furan-2-yl-acryloyl)-2,2-dimethyl-4,6-dioxo-cyclohexanecarboxylic acid methyl ester and 1-(8-Quinolinyl)-beta-carboline as major compounds (Table 3 and Fig. 2, S14). These compounds have the chemical nature of esters and alkaloids.
Sl.no
Structure
Compound name
Retention time
Nature
Uses
1
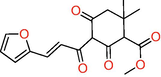
5-(3-Furan-2-yl-acryloyl)-2,2-dimethyl-4,6-dioxo-cyclohexanecarboxylic acid methyl ester
9.538
Ester
Industrial application
2
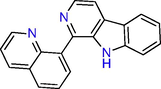
1-(8-Quinolinyl)
-beta-carboline
12.136
Alkaloid
Induce brain function and antioxidant
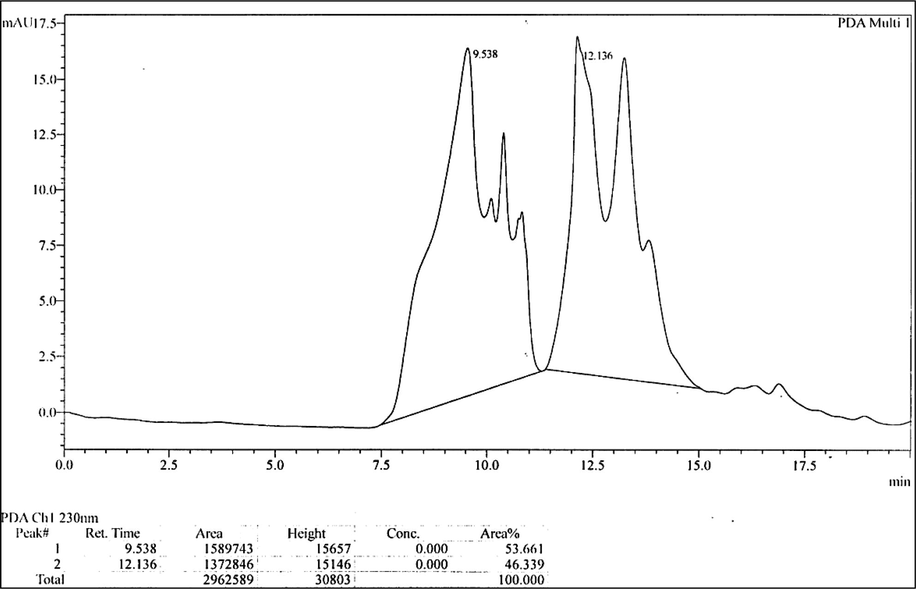
LC-MS analysis of methanol extract of C. olitorius.
3.4 In-vitro cytotoxicity
In this assay, the different concentrations (50, 100, 150, 200 and 250 µg/ml) of the hexane, ethyl acetate, methanol and aqueous extracts were used to treat L929 cell line to check the percentage of viability. The results revealed that the ethyl acetate extract had shown a high level of toxicity among all tested extracts. The results are shown in Table 4. The morphological effects of MTT assay for each solvent extract are provided as Supplementary Files (Figures S1–S4). The values have been expressed in terms of percentage cell viability. Data are demonstrated as mean ± SEM (n = 3) at P < 0.05. One-way ANOVA thereafter by Dunnett’s test is performed to compare means. *P < 0.001, compared to untreated group (100 % cell viability).
Concentration in µg/ml
Percentage (%) of cell viability
Hexane
Ethyl acetate
Methanol
Aqueous
50
85.26 ± 0.007*
89.72 ± 0.013*
91.70 ± 0.003*
90.27 ± 0.009*
100
75.61 ± 0.006*
81.75 ± 0.007*
76.92 ± 0.023*
84.60 ± 0.007*
150
67.64 ± 0.02*
63.98 ± 0.016*
65.99 ± 0.006*
73.45 ± 0.006*
200
61.90 ± 0.01*
45.66 ± 0.009*
53.71 ± 0.017*
67.64 ± 0.009*
250
48.99 ± 0.01*
31.70 ± 0.007*
47.89 ± 0.01*
56.30 ± 0.016*
For Standard Cisplatin (15 µg/ml) % of Cell viability = 72.46 ± 0.01
3.5 In-vitro cytotoxicity against MCF-7 and A549 cell lines
At the initial concentration of hexane, ethyl acetate, methanol, and aqueous extracts, the cell viability in lung cancer was observed to be 80.91 ± 0.008, 77.19 ± 0.003, 45.97 ± 0.003 and 59.06 ± 0.004 % (Table 5). In the case of breast cancer, it was found to be 71.86 ± 0.0145, 51.64 ± 0.0075, 45.70 ± 0.001 and 68.51 ± 0.001 % (Table 6). Further, the IC50 value of test samples was calculated from the standard calibration curve. In the case of hexane, ethyl acetate, methanol and aqueous extracts for breast cancer, it was observed to be 182.47, 55.18, 20.0, and 126.58 %, respectively. In the case of lung cancer, it was found to be 245.15, 227.46, 12.45, and 79.47 %, respectively. The IC50 details have been shown in Table 7. The values have been expressed in terms of percentage cell viability. Data are demonstrated as mean ± SEM (n = 3) at P < 0.05. One-way ANOVA thereafter by Dunnett's test is performed to compare means. *P < 0.001, compared to untreated group (100 % cell viability). The values have been expressed in terms of percentage cell viability. Data are demonstrated as mean ± SEM (n = 3) at P < 0.05. One-way ANOVA thereafter by Dunnett's test is performed to compare means. * P < 0.001, compared to untreated group (100 % cell viability).
Concentration (µg/ml)
Percentage (%) of cell viability
Hexane
Ethyl acetate
Methanol
Aqueous
50
80.91 ± 0.008 *
77.19 ± 0.003 *
45.97 ± 0.003 *
59.06 ± 0.004 *
100
75.03 ± 0.009 *
68.30 ± 0.006 *
38.47 ± 0.010 *
43.93 ± 0.004 *
150
66.44 ± 0.006 *
63.08 ± 0.015 *
35.17 ± 0.004 *
31.99 ± 0.008 *
200
59.42 ± 0.006 *
55.76 ± 0.007 *
29.83 ± 0.005 *
24.00 ± 0.006 *
250
47.17 ± 0.006 *
45.07 ± 0.005 *
21.60 ± 0.009 *
15.48 ± 0.001 *
For Standard Cisplatin (15 µg/ml) % of Cell viability = 57.08 ± 0.008
Concentration (µg/ml)
Percentage (%) of cell viability
Hexane
Ethyl acetate
Methanol
Aqueous
50
71.86 ± 0.01 *
51.64 ± 0.007 *
45.76 ± 0.001 *
68.51 ± 0.001 *
100
61.92 ± 0.01 *
43.35 ± 0.01 *
37.69 ± 0.002 *
52.58 ± 0.005 *
150
53.07 ± 0.01 *
39.83 ± 0.01 *
21.09 ± 0.014 *
41.64 ± 0.01 *
200
46.20 ± 0.01 *
33.29 ± 0.006 *
15.10 ± 0.001 *
36.92 ± 0.001 *
250
41.64 ± 0.01 *
28.57 ± 0.002 *
11.26 ± 0.005 *
27.36 ± 0.006 *
For Standard Cisplatin (15 µg/ml) % of Cell viability = 29.72 ± 0.012
Test sample
IC50 (µg/ml)
L929
MCF-7
A549
Hexane extract
253.67
182.47
245.15
Ethyl acetate extract
191.29
55.18
227.46
Methanol extract
227.84
20
12.45
Aqueous extract
294.03
126.58
79.47
Cisplatin (standard)
10.34
2.50
13.13
The morphological study also correlates with MTT results. In both treated MCF-7 and A549 cell lines, the test samples have shown changes in the cellular morphology compared to the untreated cells. In the untreated cells, the morphology was clear, and they were in more numbers without any intracellular spaces. Whereas, in test samples treated cells, as the concentration was increased, the cell number gradually decreased, and there was an observation of intracellular spaces between the cells (Supplementary file: Figures S5–S12).
3.6 Detection of early and late apoptosis
The results revealed that there was no sign of apoptosis in the case of untreated cells, whereas, in the case of the test treated MCF-7 cells, around 4.18 % and 5.72 % of cells showed the positive result for the early and late apoptosis, respectively (Figure S15). Similarly, in the case of standard drug cisplatin-treated cells, it was observed to be 1.96 % and 8.65 %. The study in lung cancer A549 cells revealed that the methanol extract was inducing apoptosis similar to the standard drug cisplatin. In the case of methanol extract-treated cells, early apoptosis-positive cells were 4.55 %, and late apoptosis cells were 11.30 %, in total, 15.85 % of cells were positive for apoptosis (Figure S16).
3.7 Caspase-3 assay
The results revealed that in the untreated group, around 6.24 % of cells showed positive for caspase-3 in MCF-7, and in lung cancer it was 4.94 %, it might be due to the natural properties of cells to have programmed cell death, and in cancer cells, this mechanism of programmed cell death can be seen but at a lower rate. Compared to the untreated, in the test sample and standard drug cisplatin, this percentage of caspase-3 positive cells increased. In the case of methanol extract treated breast cancer MCF-7, around 25.60 % of cells, and in lung cancer A549, 11.30 % of cells were positive for caspase-3 presence. In the case of standard drug cisplatin for breast cancer MCF-7, it was found to be 51.10 %, and it was 16.20 % for lung cancer (Figures S17 and S18).
3.8 Anti Bcl-2 inhibition assay
The results revealed that in the case of breast cancer MCF-7 untreated cells, a minor degree of apoptosis was observed, which showed the high-level Bcl-2 cells (89.40 %) and low-level Bcl-2 cells (6.28 %); and 92.20 % high level Bcl-2 positive cells and 7.12 % low level Bcl-2 positive cells were observed in lung cancer A549. Compared to the untreated in both test sample and standard drug cisplatin-treated group of cells, there was a change in the percentage of cells for low Bcl-2 and high Bcl-2. In the test sample treated for breast cancer MCF-7, low Bcl-2 positive cells were 14.70 %, and high BCL-2 positive cells were 76.00 %. Similarly, for lung cancer A549, low Bcl-2 positive cells were 12.40 %, and high Bcl-2 positive cells were 86.20 %. But in the case of standard drug cisplatin treated group of cells, significant changes in the Bcl-2 level i.e. for breast cancer MCF-7, low Bcl-2 was seen in 36.90 % of cells and high-level Bcl-2 seen in 52.20 % of cells. Similarly, for lung cancer A549, low-level Bcl-2 was seen in 35.60 % of cells, and 59.90 % of cells showed positive for the high level of Bcl-2 (Figures S19 and S20).
3.9 DNA damage study by TUNEL assay
The results revealed that in the untreated group of cells of breast cancer MCF-7 and lung cancer A549, around 5.99 % and 3.11 % of cells showed DNA damage. It might be due to the small percentage of cells undergoing apoptosis. In the test sample treated for breast cancer MCF-7 and lung cancer A549, around 16.50 % and 12.60 % of cells have shown DNA damage, whereas, in the case of standard drug cisplatin-treated for breast cancer MCF-7 and lung cancer A549, DNA damage was observed in 25.90 % and 13.70 % of cells, respectively (Figures S21 and S22).
4 Discussion
For thousands of years, medicinal plants have been used to cure disease because they contain a vast and diverse array of organic compounds that can produce a definite physiological action on the human body (Cai et al., 2004; Abdelwahab et al., 2010). In the present study methanol extract of C. olitorius has shown the presence of phenolics and flavonoids as a principal phytochemical constituent, which is consistent with previous studies (Patil and Jain, 2019; Biswas et al., 2020).
In the present study, by observing the cell viability and initial observation, it was clear that the methanol extract has shown significant results and proved to be more effective against both breast cancer MCF-7 and lung cancer A549 cell lines.
The MTT assay results revealed morphological features with abnormalities such as apoptotic bodies, cell shrinkage, membrane blabbing, and cell turgidity. All these features were the features of the cells undergoing apoptosis. MTT and microscopic study results show that the test samples might be inducing apoptosis in both breast cancer MCF-7 and lung cancer A549 cell lines. Overall results show that among all tested solvent extracts, the methanol extract of C. olitorius has shown very promising results in breast and lung cancer cell lines with a relatively lower IC50 value. It has also shown less toxicity in the tested non-cancerous cell line. Further, the methanol extract was subjected to higher studies to identify pathway and components through flow-cytometry and GC–MS analysis. The GC–MS and LC-MS result showed the presence of several compounds. These reported compounds are known to have several applications used in the pharmaceutical industry, flavouring agents, cosmetics, detergents, and organic synthesis.
Redox imbalance causes oxidative stress. In this situation, free radicals can destroy tissue. ROS are created by aerobic metabolism in the mitochondrial respiratory chain and other metabolic processes. It contributes to the onset and progression of malignancies. ROS influences signaling pathways, including growth factors and mitogenic pathways, and governs numerous biological functions, including cell proliferation. This drives uncontrolled cell growth, which favors tumor development and initiates carcinogenesis (Nourazarian et al., 2014). As a result, antioxidant compounds that reduce oxidative stress are becoming increasingly popular as a cancer treatment option (Singh et al., 2018). Phytochemical analysis of C. olitorius has indicated a significant concentration of phenolic compounds, which are very well known antioxidants (Cai et al., 2019; Gülçin et al., 2004). Thus, any compound that counteracts oxidative stress is believed to be an effective cancer treatment strategy, as revealed in this study.
Several distinct characteristics define apoptosis (programmed cell death): chromatin condensation and fragmentation, internucleosomal DNA cleavage, membrane blebbing, caspase activation, and phosphatidylserine translocation from the inner to the outer layer of the plasma membrane (Talib and Mahasneh, 2010). As a result, inducing apoptosis is one of the most effective cancer treatment protocols (Reed, 2002). During the early stages of apoptosis, phosphatidyl-serine (P.S.) on the outer layer of the plasma membrane serves as a recognition site for phagocytes (Gordaliza, 2007; Fadok et al., 1992). The calcium-dependent protein Annexin V can bind to the exposed phosphatidyl-serine (P.S.) on the membrane's exterior layer (Demchenko, 2013). P.S. is located on the inner surface of the cell membrane in a normal cell, making it inaccessible to Annexin-V. It is translocated to the outer cell membrane early in apoptosis and binds to Annexin-V. P.S. translocation is a permanent process. In the present study, the total apoptosis by standard drug showed 10.61 % of cells undergoing apoptosis, and in the case of the test sample, it was found to be 9.90 %. The apoptosis study in lung cancer A549 cells revealed that the methanol extract was inducing apoptosis in a similar way to the standard drug cisplatin. In the case of untreated cells, no noticeable apoptotic cells were observed, but in the case of methanol extract-treated cells, early apoptosis-positive cells were 4.55 %, and late apoptosis cells were 11.30 %, in total, 15.85 % of cells were positive for apoptosis. Whereas, standard drug-treated cells showed early apoptosis (8.25 %) and late apoptosis (12.00 %), and in total, 20.25 % of cells were positive for apoptosis. Our findings corroborate with the findings of Moraes et al., 2013.
The activation of caspase initiates the final stage of apoptosis (Soung et al., 2008). The cleavage of several caspase triggers apoptosis. Understanding the effects of caspase cleavage can help us better understand cell death and other biological processes (Shao et al., 2007). The increase in caspase-3,-8, and-9 activities in treated MCF 7 suggests that extrinsic and intrinsic routes were used to activate caspase-3 at this dose (IC50). In the present study, the test sample and standard drug cisplatin, increased the percentage of caspase-3 positive cells. Caspase-mediated apoptosis may activate either the death receptor (extrinsic) or mitochondrial (intrinsic) pathways, or both. Caspase, a class of cysteine proteases that cause cleavage of cellular protein, are one of the primary measures that play a vital part in apoptosis, necrosis, and inflammation (Fadok et al., 1992; Alnemri et al., 1996). After apoptosis, caspase-3 is the final product that is produced. Caspases-3 detection in treated cells can be used as a confirmatory test for apoptosis.
Bcl-2 family members are important regulators of cell death and survival. In cells, Bcl-2 and Bcl-xL operate as apoptotic inhibitors. Vernodalin therapy lowered expression of the pro-survival/anti-apoptotic proteins Bcl-2 and Bcl-xL, showing that Bcl-2 family proteins are important for breast cancer cell survival. Shimizu et al., 1996 found that Bc1-2 and Bcl-xL are important in keeping mitochondria from losing function during apoptosis and some forms of necrotic cell death. Bcl-2 is an anti-apoptotic protein that prevents cells from committing suicide. In the present study, the level of Bcl-2 protein observed in the cells was taken as a parameter to identify the nature of the test sample in inducing apoptosis in cancer cells. The results were compared to the standard drug and untreated group of cells. Both test sample and standard drug have shown anti-Bcl-2 protein activity, which promotes apoptosis compared to the untreated cells. Unlike other previously identified oncogenes that act by increasing cellular proliferation, overexpression of Bcl-2 was found to block cell death and promote cell survival (Vaux et al., 1988). In the present study, the test sample and standard drug have shown a reduction of Bcl-2 protein level in both treated breast cancer MCF-7 and lung cancer A549.
The TdT (Terminal deoxynucleotidyl transferase) dUTP Nick-End Labeling (TUNEL) assay identifies apoptotic cells that undergo substantial DNA damage during the end stages of apoptosis. In the present study, test sample treated for breast cancer MCF-7 and lung cancer A549, around 16.50 % and 12.60 % of cells have shown DNA damage, whereas, in the case of standard drug cisplatin-treated for breast cancer MCF-7 and lung cancer A549, DNA damage was observed in 25.90 % and 13.70 % of cells, respectively. Flow cytometry is a popular approach for profiling cellular protein expression in a heterogeneous mixed population of cells in suspension without having to separate them physically (Adan et al., 2017). The death of cells in apoptotic cells could be caused by DNA damage.
5 Conclusion
Overall results showed the C. olitorius methanol extract has significant cytotoxic activity in inhibiting breast cancer MCF-7 and lung cancer A549 cell lines. Flowcytometry results confirm that the molecular mechanisms of the test drug were by inducing apoptosis successively through DNA damage in both breast cancer MCF-7 and lung cancer A549 cells. In addition, the potent cytotoxic activity of C. olitorius was similar to the standard drug cisplatin. Thus, C. olitorius is a promising source of chemopreventive agents that should be further explored to identify lead compounds with cancer chemotherapeutic potential. Further studies are warranted to extrapolate the results from the present study to clinical trials.
Funding
Authors would like to acknowledge the support of the Deputy for Research and Innovation- Ministry of Education, Kingdom of Saudi Arabia, for this research through a grant (NU/IFC/ENT/01/006) under the Institutional Funding Committee at Najran University, Kingdom of Saudi Arabia.
Acknowledgements
The authors would like to acknowledge the support of the Deputy for Research and Innovation- Ministry of Education, Kingdom of Saudi Arabia, for this research through a grant (NU/IFC/ENT/01/006) under the Institutional Funding Committee at Najran University, Kingdom of Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Phenolic content and antioxidant activities of Goniothalamusumbrosus extracts. Int. J. Natural Product Pharmaceutical Sciences. 2010;1:1-6.
- [Google Scholar]
- Flow cytometry: basic principles and applications. Crit. Rev. Biotechnol.. 2017;37(2):163-176.
- [Google Scholar]
- Epidemiology of cancer in Saudi Arabia thru 2010–2019: a systematic review with constrained meta-analysis. AIMS public health.. 2020;7(3):679-696.
- [CrossRef] [Google Scholar]
- Hibiscus Sabdariffa L. extract ameliorates the diabetic late complications: cardioprotective and nephroprotective effect in streptozotocin-induced diabetic rats. Int. J. Green Pharm.. 2017;11(4):S896.
- [Google Scholar]
- Nootropic and neuroprotective effects of ethanol extract of VateriaIndica L bark on scopolamine-induced cognitive deficit in mice. Trop. J. Pharm. Res.. 2020;19:587-594.
- [CrossRef] [Google Scholar]
- Caffeine modulates pharmacokinetic and pharmacodynamic profiles of pioglitazone in diabetic rats: Impact on therapeutics. Saudi Med. J.. 2021;42(2):151-160.
- [Google Scholar]
- Nootropic and neuroprotective effects of ethanol extract of hibiscus Sabdariffa L. on scopolamine- induced cognitive deficit in mice. Current Topics In Nutraceutical Research.. 2021;19:345-352.
- [Google Scholar]
- Flavonoids, phenols, antioxidant, and antimicrobial activities in various extracts from Tossa jute leave (Corchorus olitorus L.) Ind. Crops Prod.. 2018;118:206-213.
- [CrossRef] [Google Scholar]
- Evolution of antioxidant defence mechanisms. Eur. J. Nutr.. 2000;39(2):53-61.
- [CrossRef] [Google Scholar]
- Comparison of phytochemical profile, mineral content, and in vitro antioxidant activities of Corchorus capsularis and Corchorus olitorius leaf extracts from different populations. J. Food Qual.. 2020;2020:1-14.
- [CrossRef] [Google Scholar]
- Extraction and antioxidant activity of total triterpenoids in the mycelium of a medicinal fungus Sanghuangporus sanghuang. Sci. Rep.. 2019;9:7418.
- [CrossRef] [Google Scholar]
- Quantitation of apoptosis and necrosis by Annexin V binding, propidium iodide uptake, and flow cytometry. Cold Spring Harbor protocols.. 2016;2016(11)
- [CrossRef] [Google Scholar]
- Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and carbowax 20M phases. J. Chromatogr. A.. 1990;503:1-24.
- [CrossRef] [Google Scholar]
- Beyond annexin V: fluorescence response of cellular membranes to apoptosis. Cytotechnology. 2013;65(2):157-172.
- [Google Scholar]
- Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol.. 1992;148:2207-2216.
- [Google Scholar]
- Natural products as leads to anticancer drugs. Clin. Transl. Oncol.. 2007;9(12):767-776.
- [Google Scholar]
- Antioxidant activity of Saponins isolated from Ivy: α-Hederin, Hederasaponin-C. Hederacolchiside-E and Hederacolchiside-F. Planta Med.. 2004;70:561-563.
- [CrossRef] [Google Scholar]
- Phytochemical screening, LC-MS studies and antidiabetic potential of methanol extracts of seed shells of Archidendronbubalinum (Jack) IC Nielson (JulangJaling) from lampung. Indonesia. Pharmacognosy Journal.. 2018;10(6s):s77-s82.
- [Google Scholar]
- Corchorus olitorius (jute) extract induced cytotoxicity and genotoxicity on human multiple myeloma cells (ARH-77) Pharm. Biol.. 2013;51:766-770.
- [CrossRef] [Google Scholar]
- Biochemistry, medicinal and food values of Jute (Corchorus Capsularis L. and C. olitorius L.) Leaf: A review. Int. J. Enhanc. Res. Sci. Technol. Eng.. 2013;2(11):35-44.
- [Google Scholar]
- Detection of apoptosis by TUNEL assay. methods in molecular biology (Clifton. N.J.). 2012;887:41-47.
- [CrossRef] [Google Scholar]
- Induction of apoptosis by ethanolic extract of Corchorus olitorius leaf in human hepatocellular carcinoma (HepG2) cells via a mitochondria-dependent pathway. Molecules. 2012;17:9348-9360.
- [CrossRef] [Google Scholar]
- Organopalladium compound 7b targets mitochondrial thiols and induces caspase-dependent apoptosis in human myeloid leukemia cells. Cell Death Dis.. 2013;4(6)
- [Google Scholar]
- Roles of oxidative stress in the development and progression of breast cancer. Asian Pac. J. Cancer Prev.. 2014;15:4745-4751.
- [CrossRef] [Google Scholar]
- Extraction, Qualitative and quantitative determination of secondary metabolites of Corchorus olitorius. J. Drug Deliv. Ther.. 2019;9:252-255.
- [Google Scholar]
- Phytochemical screening, quantitative analysis of flavonoids and minerals in ethanolic extract of citrus paradisi. International Journal of Pharmaceutical Sciences & Research.. 2018;11:4859-4864.
- [Google Scholar]
- Use of nutraceuticals for prevention and treatment of cancer. Iran. J. Pharm. Res.. 2013;12:219-220.
- [Google Scholar]
- The Caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J. Biol. Chem.. 2007;282(50):36321-36329.
- [Google Scholar]
- Bcl-2 blocks loss of mitochondrial membrane potential while ICE inhibitors act at a different step during inhibition of death induced by respiratory chain inhibitors. Oncogene. 1996;13(1):21-29.
- [Google Scholar]
- Antioxidants as precision weapons in war against cancer chemotherapy induced toxicity – Exploring the armoury of obscurity. Saudi Pharm. J.. 2018;26:177-190.
- [CrossRef] [Google Scholar]
- Singleton, V.L., Orthofer, R., Lamuela-Raventós, R.M. 1999. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Oxidants and Antioxidants Part A; Elsevier. 1999(299), 152–178. ISBN 9780121822002.
- Mutational analysis of caspase 1, 4, and 5 genes in common human cancers. Hum. Pathol.. 2008;39(6):895-900.
- [Google Scholar]
- Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., Bray, F., 2021. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians. 71(3), 209–249. https://doi.org/10.3322/caac.21660.
- Antiproliferative activity of plant extracts used against cancer in traditional medicine. Scientiapharmaceutica. 2010;78(1):33-46.
- [Google Scholar]
- Anticancer effects of the Corchorus olitorius aqueous extract and its bioactive compounds on human cancer cell lines. Molecules. 2021;26:6033.
- [CrossRef] [Google Scholar]
- Cell sensitivity assays: the MTT assay. methods in molecular biology (Clifton. N.J.). 2011;731:237-245.
- [CrossRef] [Google Scholar]
- Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335(6189):440-442.
- [Google Scholar]
- WHO. The Global Cancer Observatory (Saudi Arabia). Available at: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/viewer.html?pdfurl=https%3A%2F%2Fgco.iarc.fr%2Ftoday%2Fdata%2Ffactsheets%2Fpopulations%2F682-saudi-arabia-fact-sheets.pdf&clen=260465&chunk=true.
- The determination of ûavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem.. 1999;64:555-559.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102238.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







