Translate this page into:
Phytochemical, pharmacological and in silico studies on Teucrium stocksianum Bioss
⁎Corresponding authors at: Department of Chemistry, College of Science, King Khalid University, P.O. Box 9004, Abha 61413, Saudi Arabia. irfaahmad@gmail.com (Ahmad Irfan), rashadhej@gmail.com (Rashad Mehmood)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A new triol-based compound, teucriol (1) and eleven others (2–12), were first time isolated from the ethanol extract of Teucrium stocksianum Bioss using bioassay guidelines, and their structures were established using various spectroscopic techniques. Antibacterial, antifungal, insecticidal, cytotoxic, phytotoxic, and antioxidant properties of ethanol extract fractions and a novel isolate were tested. When compared to the standard ceftriaxone, the extract, fractions, and novel isolate demonstrated good antibacterial potential against Streptococcus pneumonia, Staphylococcus aureus, Klebsiella pneumonia and Escherichia coli bacteria, while all were inert against Fusarium solani, Candida albicans, Candida glabrata, Microsporum Canis and Aspergillus flavus fungi, and Tribolium castaneum and Rhyzopertha dominica insects. All fractions showed promising antioxidant activity. Density functional theory was used to optimize the ground state geometry of teucriol (1) at the B3LYP/TZ2P level. The global molecular descriptors, electronic characteristics, molecular electrostatic potential, and Hirshfeld surface analyses were studied to investigate the biological activity of the investigated molecule.
Keywords
Teucrium stocksianum
Pharmacological evaluation
Isolation
Structure elucidation
Density functional theory
1 Introduction
Plants, their extracts and pure isolates have been applied for the treatment of numerous human ailments, diseases and to improve the human health from ancient (Dasilva et al., 2002). The plants (powder/extract) or their pure isolates have medicinal potential based on the presence of bioactive phytochemicals to relieve the various illness (Iqbal and Hamayun 2004). According to World Health Organization (WHO), in the United States 25% of the total medicine used are obtained from natural sources (Süntar 2020). Based on plants/herbs medicinal importance, the researchers screen the medicinal plants for various biological potentials and isolate the bioactive candidates systematically. In view of this scenario, an investigation was planned for pharmacochemical and simulation studies on an indigenous medicinal species Teucrium stocksianum Bioss (Lamiaceae). The larger plant family (Lamiaceae) comprises 180 genera and 3500 species, from annual to perennial shrubs, herbs and sub-shrubs, well distributed around the globe (Li, 2012). The main genera of the Lamiaceae (Teucrium) comprises herbaceous plants having 340 species, extensively dispersed in Iran, Arabian Peninsula, North Africa, Pakistan and within Mediterranean zone. So far, only four species of this genus have been discovered in Pakistan, including Teucrium stocksianum Boiss (Shah et al., 2012). The plant T. stocksianum is found in Northern Oman, United Arab Emirates (UAE), Pakistan (Ahmad et al., 2002) and hilly areas of Iran (Mojab et al., 2003). This is persistent aromatic herb with height 10–30 cm, with grayish-white leaves along with sessile flowers. The previous phytochemical study showed that T. stocksianum contains potent biologically active compounds such as alkaloids, tannins, flavonoid, phenolic compounds, and some essential oils (Shah et al., 2012). The T. stocksianum has been utilized as folk medicine to cure from various ailments such as anti-inflammatory, anti-diabetic, gastrointestinal complications (Radhakrishnan et al., 2001) and treatment of feet syndrome (Barkatullah et al., 2009). Its extracts are used for cytoprotective, anti-ulcerogenic (Islam et al., 2002), hypertensive, blood purifier, epileptic (Ahmad et al., 2002), throat pain (Iqbal and Hamayun 2004), diarrhea, cough, jaundice and for treatment of abdominal pain (Rahim et al., 2012). These precious therapeutic potential of T. stocksianum pointed out the presence of bioactive phytochemicals.
A variety of descriptors are required to highlight the quantitative structure–activity relationship (QSAR) to investigate the nature of interactions, phytochemical activity, and active site evaluation (Abdalla et al., 2021, Khan et al., 2021, Mohapatra et al., 2021, Sahu et al., 2021). We present herein the pharmacological evaluation of crude ethanol extract, subfractions and new isolate. Further bio guided isolation, and characterization give new alcohol and eleven new source phytochemicals from this species. Moreover, to discover the fascinating pharmacological and biological potentials, it is crucial to highlight the essentials molecular descriptors including Hirshfeld analysis, electron affinity (EA), ionization potential (IP), frontier molecular orbitals (FMO), and molecular electrostatic potential (MEP). Thus, we have computed these descriptors by density functional theory (DFT) and discussed properties of interests.
2 Experimental
2.1 General experimental procedures
The polarimeter (JASCO DIP-360) was used for optical rotations whereas Melting points (MP) were determined with the aid of Gallenkamp apparatus. Hitach UV-3200 and Shimadzu FTIR-8900 spectrometer were employed for UV and IR spectral measurement respectively. The 1D-NMR spectra (1H- along with -13C) were taken down on Bruker AM-400/500 spectrometers with the presence of deuterated solvents. The determination of 2D-NMR (HMQC, COSY, HMBC, NOESY) peaks with the aid of above same models. For all NMR spectra the ppm chemical shifts (δ) along with coupling in Hz. The Mass spectra (MSEl/MSHREI) were calculated with electron impact (EI) mode on Finnigan MAT-112/ 113 spectrometers. The E. Merck silica gel (size; 70–230 mesh) employed for various used column chromatography techniques. The pre-coated silica gel G-25-UV254 plates (E. Merck) used for the purpose of TLC followed by UV detection at 254/366 nm along with spraying with Ce(SO4)2 in 10% H2SO4 for UV inactive phytochemicals.
2.2 Collection and identification
The Teucrium stocksianum (whole, 12 kg) was collected from, Ziarat Valley, Baluchistan, Pakistan and recognized by Plant Taxonomist, Prof. Dr. RB Tareen, University of Baluchistan, where voucher specimen No. 1310BUH was placed in the herbarium.
2.3 Extraction, fractionation and purification
The shade dried T. stocksianum whole plant (12 kg) was crushed into fine powder and extracted with ethanol (15 L × 4, each 7 days). The obtained combined extract (EtOH) was evaporated at a low pressure to produce greenish residue. It was dissolved further within distilled H2O (0.5 L) and successively partitioned with n-hexane (7 × 3.0 L; 980 g), dichloromethane (DCM) (6 × 3.0 L; 443 g), ethyl acetate (AcOEt) (4 × 3.0 L; 70 g), n-butanol (7 × 3.0 L; 535 g) and water (907 g), respectively. The ethyl acetate sub-portions (45 g) subjected to CC on silica gel and elution was attained with n-hexane-DCM, DCM, DCM- EtOAC, EtOAC-MeOH. Using the above solvents in order of increasing solvent polarity, 13 sub-portions (A-M) were collected. The sub-portion-A was acquired from n-hexane:DCM (7.0:3.0), chromatographed over silica gel, eluted with n-hexane:DCM (7.5:2.5) to obtain1-dotriacontanol (12). The sub-portion-B was acquired by eluting n-hexane: DCM (6.0:4.0) to achieve binary mixture. The 2H-chromen-2-one (8) and stearic acid (11) was obtained by further CC using solvent system n-hexane: DCM (6.5:3.5 and 6.0:4.0). The n-hexane:DCM (5.0:5.0) elution gave C sub fraction (mixture), further chromatographed over adsorbent silica gel and eluted with n-hexane:DCM (4.5:5.5 and 4.0:6.0) to give benzoic acid (9) and cinnamic acid (7), respectively. The D subfraction was obtained with n-hexane:CH2Cl2 (3.0:7.0), was a single compound with lingering of impurities, whose further CC separation affoard salicylic acid (10). The G subfraction affoards ursolic acid (2) after chromatographed over silica gel with eluent as DCM:EtOAc (6.0:4.0). The H subfraction was obtained with CH2Cl2:MeOH (9.9:0.1), was a semi pure compound, followed with same system CC afford teucriol (1) 8 mg as pure compound. The I subfraction was obtained with CH2Cl2:MeOH (9.5:0.5), further same mobile phase CC was afford pure apigenin (3). The binary mixture J subfraction attained with CH2Cl2:MeOH (9.4:0.6), was rechromatographed over silica gel and eluted with CH2Cl2:MeOH (9.6:0.4) to obtain compound4. The elution with CH2Cl2:MeOH (9.3:0.7) gave K subfraction, was a mixture, rechromatographed over silica gel and eluted with CH2Cl2:MeOH (9.4:0.6) to afford luteolin (5). The subfraction was obtained with CH2Cl2:MeOH (9.0:1.0), rechromatographed over silica gel, eluted by CH2Cl2:MeOH (9.1:0.9) to obtain apigetrin (6).
2.4 Teucriol (1)
White amorphous, [α]20D (c 0.3, MeOH): −28.5, UV (CH3OH) λmax nm (logε): 202 (4.1), 204 (3.4), 193 (4.2), IR νmax (KBr) cm−1: 3406 (OH), 3021 (CH3), 1280 (O-C). 1H/13C NMR (CD3OD, 300/75 MHz) see Table 1; EI-MS (70 e/v) (rel. Int %) m/z: 190.1 ([M]+, 6), 172.1 ([M−H2O]+, 9), 154.1 ([M−2H2O]+, 15), 136.1 (20); HR-EI-MS: m/z 190.1561 [M]+ (calcd for C10H22O3, 190.1569).
Carbon No.
δC
Multiplicity
δH
1
61.1
CH2
3.60 (m)
2
41.1
CH2
1.60 (m)
3
30.7
CH
1.60 (m)
4
29.6
CH2
1.32 (m)
5
35.4
CH2
1.35 (m)
6
73.8
C
---
7
79.8
CH
3.20
8
25.0
CH3
1.1
3-Me
19.9
CH3
0.90 (d, J = 6.35)
6-Me
25.6
CH3
1.2
2.5 Pharmacological evaluation assays
The ethanol extract, its fractions and new isolate of T. stocksianum were subjected to measure various pharmacological potential through reported protocol and strains were selected based on availability at the time of potential measurement. Antibacterial potential was determined against Streptococcus pneumonia, Staphylococcus aureus, Klebsiella pneumonia and Escherichia coli by agar well diffusion method. In vitro antifungal potential against Fusarium solani, Candida albicans, Candida glabrata, Microsporum Canis and Aspergillus flavus was assessed through agar tube dilution method. Insecticidal activity was appraised against insects viz Tribolium castaneum and Rhyzopertha dominica by impregnated filter paper test. Cytotoxicity, phytotoxicity and DPPH radical scavenging activity were also measured. The detail procedures including computational details (Imran and Irfan 2020, Irfan et al., 2020a,b, Imran et al., 2021) are given in supplementary material.
2.6 Computational details
The optimization of structural geometry for teucriol (1) was achieved by DFT at B3LYP/TZ2P method. Then electronic properties, various descriptors, MEP and Hirshfeld analysis was performed to shed some light on the biological activity nature of newly isolated compound. All these calculations were performed by ADF software (see supporting information).
3 Results and discussion
The dried Teucrium stocksianum Bioss ethanol extract was suspended into H2O within glass separating funnel and consecutively subdivided into n-hexane, dichloromethane, EtOAc, n-butanol and H2O-soluble sub-portions. The extract in its crude form along with various sub-fractions, and new isolate were biological screened for antibacterial, antifungal, antioxidant, cytotoxic/brine shrimp lethal and phototoxic activities. The column chromatography (CC) was employed with adsorbent as silica gel within EtOAc fractions to obtain a new compound 1 (Teucriol (1)) and other phytochemicals 2–12 (Fig. 1), respectively. Computational studies were also conducted on new isolate.
Structures of the phytochemicals 1–12.
Compound 1 was attained in the form of white amorphous powder. The HR-EIMS of 1 demonstrated molecular ion peak at m/z 190.1561 that is in agreement with molecular formula (M.F) as C10H22O3. Its UV spectra exhibited absorption maxima (λmax) at 204, 202 and 193 nm whereas IR spectra exhibited absorptions at 3406, 3021 and 1280 cm−1, demonstrating the presence of OH, CH3 and O-C functionalities. 13C NMR (BB and DEPT) (Table 1) spectra displayed total 10 carbon signals which comprise two methines, four methylenes, three methyls, and one quaternary carbon. Oxygen bearing methylene and methine carbons showed signals at δ 61.1 and δ 79.8, correspondingly. The quaternary carbon resonated at δ 73.8 and its downfield shifting indicating its attachment with an oxygen atom. The methyl group attached to methine carbon resonated at δ 19.9, whereas the methyl group attached with the oxyquaternary carbon was appeared at δ 25.5. In the 1H NMR (CD3OD, 300 MHz) spectrum (Table 1), protons of methylene moiety showed signal at δ 3.60, revealing their attachment the oxygen bearing carbon. Oxygen bearing methine proton showed signal at 3.20 whereas methyl bearing methine proton resonated at δ 1.60.
In the important 1H–1H COSY correlations, methylene protons at δ 3.60 (H-1) exhibited correlation at δ 1.59 (H-2) and methine proton at δ 1.60 (H-3) demonstrated correlation at 1.32 (H-4) and δ 0.90 (3-Me). In the important HMBC correlations (Fig. 2), oxymethylene protons at δ 3.60 (H-1) showed 2J and 3J correlations at 31.1 (C-2) and 30.7 (C-3), respectively. The proton of oxymethine (δ 3.20 (H-7)) showed 2J correlations at 73.8 (C-6) and 25.0 (C-8) and 3J correlations at 35.4 (C-5) and 25.6 (6-Me). The methine proton (δ 1.60 (H-3)) showed 2J correlations at 29.6 (C-4) and 19.9 (3-Me) and 3J correlations at 35.4 (C-5). EIMS spectrum of teucriol 1, confirmed the existence of three –OH groups in the molecule as it showed signals at m/z 172.1, 154.1, 136.1 by successive loss of three water molecules. For the stereochemistry, H-7 showed NOESY correlation with H-3, confirmed that both chiral carbons have protons on the same side through comparison with the reported citronellol geometry (Uesato et al., 1982, Nakamura et al., 2008, Sun et al., 2012), it is confirmed that the methyl at C-3 and hydroxyl at C-7 are below the plane. Based on all of the spectral suggestions, teucriol 1 was confirmed as (3S,6R,7S)-3,6-dimethyl-1,6,7-octanetriol (teucriol 1), which is shown in Fig. 2.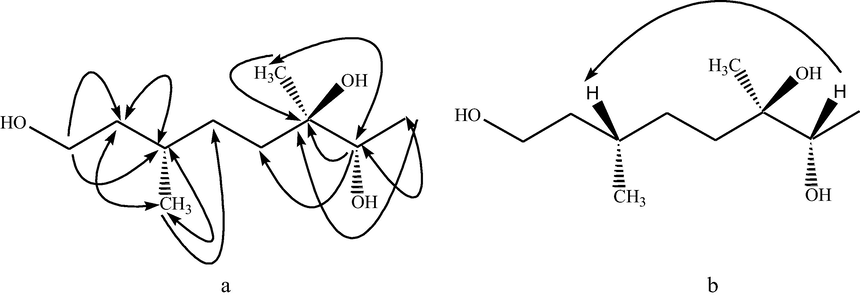
Important HMBS (a) and NOESY correlations of teucriol (1).
All the other isolated compounds 2–12 were elucidated through their spectroscopic data information (IR, UV, MS, and NMR). With the help of spectroscopic information and physical constants with the literature comparison, these compounds were confirmed as ursolic acid (2) (Seebacher et al., 2003), apigenin (4′, 5, 7-trihydroxyflavone) (3), 3ʹ,4ʹ,7-trihydroxy-5,6-dimethoxyflavone (4), 3′,4′,5,7-tetrahydroxyflavone (luteolin) (5) (Chaturvedula and Prakash 2013), apigenin 7-O-β-D-glucopyranose (6) (Takeda et al., 1993), cinnamic acid (7), 2H-chromen −2-one (8), benzoic acid (9), 2-hydroxy benzoic acid (salisylic acid) (10), stearic acid (11) (Cornforth and Henry 1952) and 1-dotriacontanol (12).
3.1 Pharmacological evaluation
The in vitro pharmacological evaluation of T. stocksianum Bioss crude extract, all its fractions and new isolate were carried out for antimicrobial (antibacterial and antifungal), cytotoxic, phytotoxic, insecticidal and antioxidant DPPH scanvenging activities. The antibacterial activity results exhibited that crude extract and all its fractions and new isolate showed remarkable antibacterial potentials against all under examined bacteria Staphylococcus aureus, Streptococcus pneumonia, Escherichia coli and Klebsiella pneumonia when compared with the standard ceftriaxone (Table 2). Extract, fractions and pure isolate, except aqueous fraction, showed better potent potential against S. aureus, E. coli and K. pneumonia as compared to standard ceftriaxone, and all showed excellent potential against S. pneumonia but slightly less than the standard. In antifungal activity, crude extract and all fractions, and new isolate showed inactivity against all under investigation fungi Candida albicans, Fusarium solani, Candida glabrata, Aspergillus flavus, and Microsporum canis, further all of them also showed inactivity against Tribolium castaneum and Rhyzopertha dominica insects (Table 2). Crude extract and fractions, and new isolate are also almost inactive in cytotoxicity and phytotoxicity (Table 3). Based on potent antibacterial potential and inactivity in cytotoxicity, extract, fractions and pure isolate can be excellently used against bacterial infections. In DPPH scavenging antioxidant activity, all fractions showed promising potential (Fig. 3). The antioxidant potential was measured at three different concentrations 500 µg, 400 µg and 100 µg, and this potential is linked with concentration i.e. on increasing concentration the potential is also increased. The n-hexane fraction showed the best antioxidant potential which is 35 % at the highest and n-butane fraction showed the lowest antioxidant potential.
Test Organism
Crude extract
n-Hexane
CH2Cl2
AcOEt
n-Butanol
H2O
Compd1
Standard
Bacteria
Inhibition in mm
Ceftriaxone
Staphylococcus aureus
14 ± 0.17
11 ± 0.16
7 ± 0.32
9 ± 0.34
10 ± 0.48
35 ± 0.87
8 ± 0.25
30 ± 0.27
Streptococcus pneumonia
12 ± 0.25
12 ± 0.61
12 ± 0.33
11 ± 0.19
11 ± 0.44
16 ± 0.50
9 ± 0.17
9 ± 0
Escherichia coli
13 ± 0.44
12 ± 0.19
14 ± 0.13
14 ± 0.23
12 ± 0
16 ± 0.50
13 ± 0.44
15 ± 0.83
Klebsiella pneumonia
15 ± 0.26
7 ± 0.15
14 ± 0.10
14 ± 0.11
16 ± 0.8
20 ± 0.6
12 ± 0.26
14 ± 0.17
Fungi
% Inhibition
Miconozale
Candida albicans
+
+
+
+
+
+
+
100
Candida glabrata
+
+
+
+
+
+
+
100
Fusarium solani
+
+
+
+
+
+
+
100
Microsporum canis
+
+
+
25
20
40
+
100
Aspergillus flavus
+
+
+
+
+
+
+
Amphotericin B (1 0 0)
Insects
% Mortality
Permetharin
Tribolium castaneum
+
+
+
+
+
+
+
100
Rhyzopertha dominica
+
+
+
+
+
+
+
100
Cytotoxicity activity
Phytotoxicity activity
No. of survivor shrimps from 30
% Growth regulation
Extracts
Concentration of samples
Concentration of samples
1000 μg/mL
100 μg/mL
10 μg/mL
1000 μg/mL
100 μg/mL
10 μg/mL
Crude
26
29
30
25
0
0
n-Hexane
27
29
30
5
0
0
CH2Cl2
22
29
30
10
0
0
AcOEt
23
26
28
5
0
0
n-Butanol
25
29
30
90
0
0
Aqueous
22
28
30
80
0
0
Compd 1
25
28
29
4
0
0
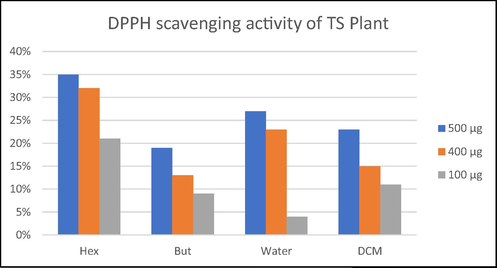
Antioxidant activity of various fractions of ethanolic extract of T. stocksianum.
3.2 Electronic properties
The FMOs of newly isolated derivative including highest occupied (HOMOs/HOMOs-1) and lowest unoccupied molecular orbitals (LUMOs/LUMOs + 1) with employing B3LYP/TZ2P level are presented in Fig. 4. The HOMO-1 orbitals can be established on C14, C15, C16, O32 that is more substituted side, HOMO is at O1, C3, the LUMO is localized on H33, H35, with some charge on O32, O34, C19, C26 whereas LUMO + 1 is localized on H2, O1, C3. The comprehensible intra-molecular charge transfer (ICT) was noted from H → L, H-1 → L and H-1 → L + 1 significantly. The antioxidant talent is also interlinked with HOMO spatial scattering which may illuminating the most possible sites (O1, C3) within newly isolated phytochemical that can be confronted by free radicals ultimately exposed the reactive behavior of this compound as antioxidant which can perform better biological activity. Moreover, better antioxidant drugs might also be effective against COVID19. The energies of FMOs and energy gaps are tabulated in Table 4. The Au and Al work functions (W) are 5.10 and 4.08 eV, accordingly (Imran et al., 2020, Irfan et al., 2020a,b). The injection barrier of the hole / electron (HIE / EIE) is being investigated as (HIE = −W of metal − (EHOMO) and (EIE = − ELUMO − (W of metal) from phytochemicals to Al electrode, i.e., teucriol (1) (4.03 eV = −0.05 − (−4.08)) > ascorbic acid (2.92 eV = −1.16 − (−4.08)) > Quercetin (2.09 eV = −1.99 − (−4.08)) while for Au as teucriol (1) (5.05 eV = −0.05 − (−5.10)) > ascorbic acid (3.94 eV = −1.16 − (−5.10)) > quercetin (3.11 eV = −1.99 − (−5.10)). HIE is estimated as: teucriol (1) (3.42 eV = −4.08 − (−7.50) > ascorbic acid (2.63 eV = −4.08 − (−6.71) > quercetin (1.70 eV = −4.08 − (−5.78) for Al-electrode whilst teucriol (1) (2.40 eV = −5.10 − (−7.50) > ascorbic acid (1.61 eV = −5.10 − (−6.71) > quercetin (0.68 eV = −5.10 − (−5.78) for Au. It can be indicated by a healthy electron injection for Al as a right electrode while for hole Au might be appropriate.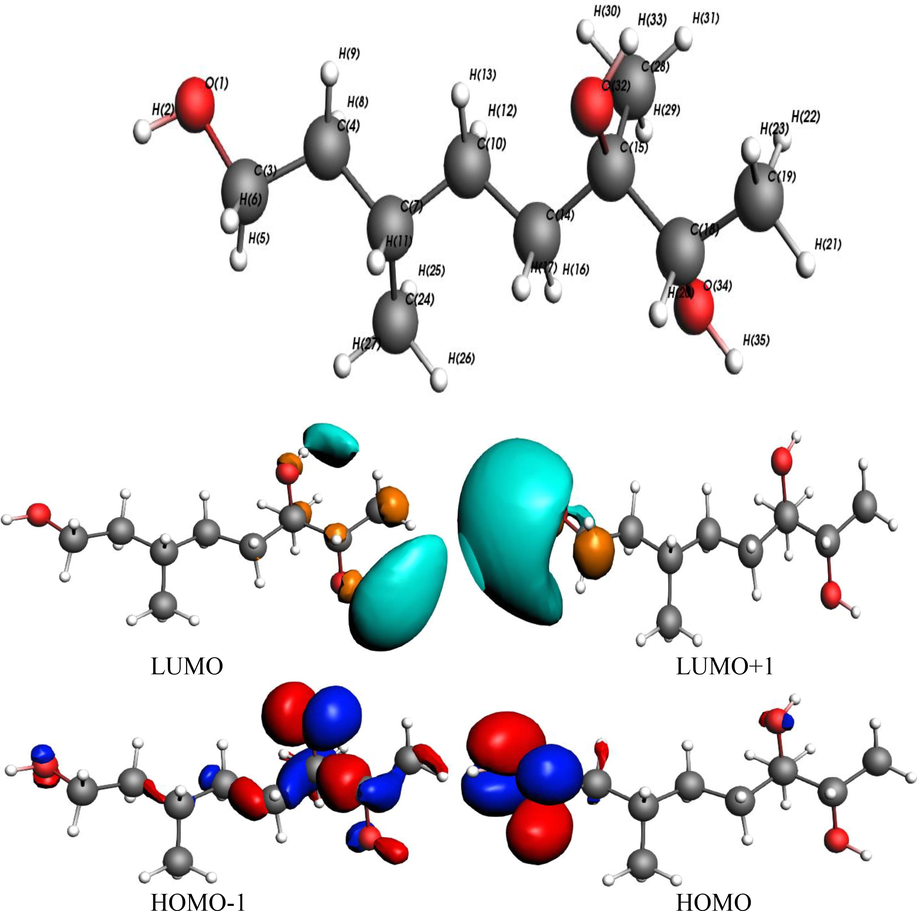
FMOs density of teucriol (1) along with numbering scheme (contour value = 0.035).
Parameters
Ascorbic acid
Quercetin
teucriol (1)
EHOMO
−6.71
−5.78
−7.50
EHOMO-1
−8.03
−6.54
−7.57
ELUMO
−1.16
−1.99
−0.05
ELUMO+1
−0.45
−0.91
0.24
ΔEHOMO –LUMO
5.55
3.79
7.45
ΔEHOMO–1−LUMO+1
7.58
5.63
5.53
Potential (µ)
−4.17
−3.97
−3.77
Electron affinity (EA)
1.16
1.99
0.05
Ionization potential (IP)
6.71
5.78
7.50
Softness (S)
1.31
1.60
1.00
Hardness (η)
2.54
1.81
3.72
Electronegativity (χ)
4.17
3.97
3.77
Electrophilic index (ω)
3.42
4.35
1.91
Other essential parameters i.e., Global chemical reactivity descriptors (GCRD) are good to apprehend the reactivity. The attained HOMO and LUMO energy values are used to measure various GCRD parameters including chemical hardness (η), chemical potential (µ), softness (S), electronegativity (χ), and electrophilicity index (ω), see Table 4 (computational details within SI). The compound η value is unified (Geerlings et al., 2003, Vektariene et al., 2009) and it show the electron affinity to depart from its electronic surroundings. The obtained η value signify the magnitude of the barricade of electronic cloud for deformation. The available value of ω refers to the.
The multi-color MEP maps are significant to foresee the charged sphere of investigated compound. The MEP surfaces for teucriol (1) along with studies standard drugs are depicted in various shape color segments, see Fig. 5. The red and blue color indicates the most potent components that may be auspicious for nucleophilic and electrophilic attacks. The negative potential was observed on the -O atoms while positive on -H of –OH. The Hirshfeld population evaluation scheme by which we can study molecular density into atomic density helpful to understand the biological activities of the compounds. This approach does no longer require a connection with basis sets or their respective locations but is centered totally on a specific mathematical and physical footing. The benefit of this technique is that, while the molecular deformation density is converged to the real solution, the calculated charges will certainly converge. Earlier it became confirmed that the Hirshfeld charges reveal where electrophilic and nucleophilic attacks will ideally ensue, however the charge quantity correlates with experimental nucleophilicity and electrophilicity. Such correlations are feasible, due to the fact nucleophilicity (electrophilicity) measures the potential of a nucleophile (electrophile) to donate (accept) electrons, so traditional chemical knowledge implies that atomic charges must be direct and trustworthy. The nucleophilicity and electrophilicity are established based on the reacting model. Various categories acquire various reacting styles, so their scales are consequently unlike (Zhou, et al., 2014). Hirshfeld approach was showed appropriate for atomic charges establishment (Guerra et al., 2004). The Hirshfeld charges are demonstrated in Fig. 5. The negative charge oxygen atoms IS −0.235 (O1), −0.215 (O32) and −0.214 (O34) while positive charge on hydrogen atoms 0.157 (H35), 0.155 (H2) and 0.151 (H33). These results showed that O1 might be favorable site for electrophilic while H35 for the nucleophilic attacks.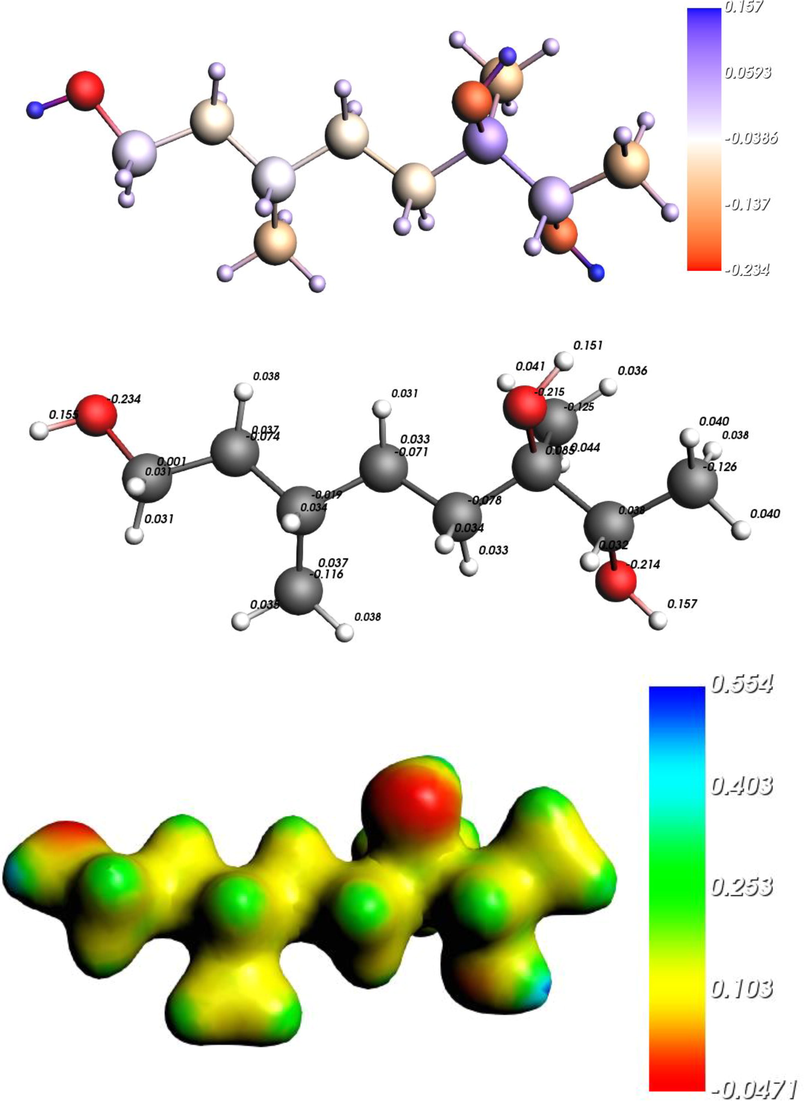
Molecular electrostatic potential surfaces (bottom), Hirshfeld charges (center) Hirshfeld charges color by atoms (top) of teucriol (1).
4 Conclusion
One new and eleven phytochemicals have isolated as a new source from T. stocksianum Bioss through bioassay guidance. Extract and fractions have remarkable antibacterial potential against E. coli, K. pneumonia and Streptococcus, moderate potential against S. aureus, promising antioxidant potential, and did not show cytotoxicity and phytotoxicity. It concludes that this plant is not toxic and may be used to prevent from above mentioned bacteria and may also be used as antioxidant. It was further shown that with a healthy electron injection Al can become the ideal electrode whilst for a hole injection Au would be appropriate. Hirshfeld analysis for newly isolated compound shown that O1 can be a good for electrophilic while H35 for nucleophilic attack. To develop a drug from this plant, further study is required. It is expected, this study will helpful for further study.
Acknowledgements
The authors express their gratitude to ICCBS, H. E. J. Research Institute of Chemistry, University of Karachi, Karachi, Pakistan on providing research facilities for this work. We extend appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through research groups program under grant number R.G.P. 2/30/43.
Declaration of Competing Interest
None.
References
- In silico studies on phytochemicals to combat the emerging COVID-19 infection. J. Saudi Chem... 2021;25(12)
- [Google Scholar]
- Ethnobotanical studies of plants of Charkotli Hills, Batkhela District, Malakand, Pakistan. Front. Biol.. 2009;4(4):539-548.
- [Google Scholar]
- 107. The presence of cis- and trans-3-hydroxystachydrine in the fruit of Courbonia virgata. J. Chem. Society (Resumed). 1952:597-601.
- [CrossRef] [Google Scholar]
- Conceptual density functional theory. Chem. Rev.. 2003;103(5):1793-1874.
- [CrossRef] [Google Scholar]
- Voronoi Deformation Density (VDD) Charges: Assessment of the Mulliken, Bader, Hirshfeld, Weinhold, and VDD Methods for Charge Analysis. J. Comput. Chem.. 2004;25(2):89-210.
- [Google Scholar]
- Carbonic anhydrase and cholinesterase inhibitory activities of isolated flavonoids from Oxalis corniculata L. and their first-principles investigations. Ind. Crops Prod.. 2020;148:112285.
- [CrossRef] [Google Scholar]
- Quantum chemical and experimental exploration of biological activity and inhibitory potential of new acylated oligosaccharides from Pistacia integerrima J. L. Stewart ex Brandis. Iran. J. Chem. Chem. Eng. 2020
- [CrossRef] [Google Scholar]
- Coumaronochromone as antibacterial and carbonic anhydrase inhibitors from Aerva persica (Burm.f.) Merr.: experimental and first-principles approaches. Zeitschrift für Naturforschung C.. 2021;76(1-2):71-78.
- [Google Scholar]
- Studies on the Traditional Uses of Plants of Malam Jabba Valley, District Swat, Pakistan. Ethnobotanical Leaflets.. 2004;2004:15.
- [Google Scholar]
- Quantum chemical, experimental exploration of biological activity and inhibitory potential of new cytotoxic kochiosides from Kochia prostrata (L.) Schrad. J. Theoret. Comput. Chem.. 2020;19(05):2050012.
- [Google Scholar]
- Exploration of electronic nature and intrinsic mobility of 10-(1,3-dithiol-2-ylidene)anthracene based organic semiconductor materials. Optik. 2020;224:165530.
- [CrossRef] [Google Scholar]
- Effect of Teucrium stocksianum on Gastric Ulceration and Secretion in Rats. Pharm. Biol.. 2002;40(3):216-220.
- [Google Scholar]
- Molecular structure simulation of (E)-2-(butan-2-ylidene) hydrazinecarbothioamide using the DFT approach, and antioxidant potential assessment of its complexes. J. King Saud Univ.-Sci.. 2021;33(2)
- [Google Scholar]
- Li X-W, H. I. C., 2012. Flora of China. Science Press (Beijing) and Missouri Botanical Garden Press. 17 50.
- Repurposing benzimidazole and benzothiazole derivatives as potential inhibitors of SARS-CoV-2: DFT, QSAR, molecular docking, molecular dynamics simulation, and in-silico pharmacokinetic and toxicity studies. J. King Saud Univ.-Sci.. 2021;33(8)
- [Google Scholar]
- Essential Oil of the Aerial Parts of Teucrium Stocksianum Boiss. subsp. Stocksianum (Lamiaceae) from Iran. J. Medicinal Plants.. 2003;2(6):49-54.
- [Google Scholar]
- Bioactive Constituents from Chinese Natural Medicines. XXVIII. Chemical Structures of Acyclic Alcohol Glycosides from the Roots of <i>Rhodiola crenulata</i>. Chem. Pharm. Bull.. 2008;56(4):536-540.
- [CrossRef] [Google Scholar]
- Analgesic and Anti-Inflammatory Activities of Teucrium stocksianum. Pharm. Biol.. 2001;39(6):455-459.
- [Google Scholar]
- Rahim, G., R. Qureshi, G. M., et al., 2012. Preliminary phytochemical screening and ethnomedicinal uses of Teucrium stocksianum from Malakand Division. J. Med. Plants Res. 6 704-707.
- An efficient synthesis towards the core of Crinipellin: TD-DFT and docking studies. J. Saudi Chemical Society.. 2021;25(2)
- [Google Scholar]
- Complete assignments of 1H and 13C NMR resonances of oleanolic acid, 18α-oleanolic acid, ursolic acid and their 11-oxo derivatives. Magn. Resonance Chem.. 2003;41(8):636-638.
- [CrossRef] [Google Scholar]
- Analysis of chemical constituents and antinociceptive potential of essential oil of Teucrium Stocksianum bioss collected from the North West of Pakistan. BMC Complementary Alternative Medicine.. 2012;12(1)
- [CrossRef] [Google Scholar]
- Terpene Glycosides from the Roots of Sanguisorba officinalis L. and Their Hemostatic Activities. Molecules.. 2012;17(7):7629-7636.
- [Google Scholar]
- Importance of ethnopharmacological studies in drug discovery: role of medicinal plants. Phytochem Rev.. 2020;19(5):1199-1209.
- [CrossRef] [Google Scholar]
- Malonylated flavonoids and blue flower colour in lupin. Phytochemistry.. 1993;34(2):421-423.
- [CrossRef] [Google Scholar]
- Studies on Monoterpene Glucosides and Related Natural Products. XLV. Synthesis of <SUP>13</SUP>C-Labeled Acyclic Monoterpenes for Studies on the Mechanism of the Iridane Skeleton Formation in the Biosynthesis of Iridoid Glucosides. Chem. Pharm. Bull.. 1982;30(3):927-940.
- [CrossRef] [Google Scholar]
- A theoretical approach to the nucleophilic behavior of benzofused thieno [3, 2-b] furans using DFT and HF based reactivity descriptors. ARKIVOC.. 2009;7:311-329.
- [CrossRef] [Google Scholar]
- Hirshfeld Charge as a Quantitative Measure of Electrophilicity and Nucleophilicity: Nitrogen-Containing Systems. Acta Phys. Chim. Sin.. 2014;30(11):2055-2062.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.101969.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







