Translate this page into:
Phytochemical characterization, antibacterial, and anti-biofilm efficacy of Mangifera indica seed kernel: A preliminary study using in vitro and in silico approaches
⁎Corresponding authors. sadhaon@gmail.com (Subramaniam Sadhasivam), sadhaofficial@buc.edu.in (Subramaniam Sadhasivam), geldar1949@gmail.comeldar.gasimo (Eldar K. Gasimov) v@amu.edu.az (Eldar K. Gasimov)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Biofilm-formation is one of the most important virulent factors in the pathogenesis of urinary tract infections. Biofilm is a complex microbial population protected by exopolysaccharides (EPS), which presents with adaptive resistance against antimicrobials. In the current study, we aimed to evaluate the efficacy of a methanolic extract of Mangifera indica seed kernels (MISK) against uropathogenic bacteria collected from urine samples. In total, n = 21 uropathogenic Escherichia coli (UPEC) strains were identified from n = 30 urine samples. Among the n = 21 strains, n = 17 E. coli strains were biofilm-producers in different in vitro model systems (tube-based method, microtiter-plate based method, Congo Red Agar, EPS production). Uropathogens were identified by traditional microbiological methods. MISK is a potential natural source of phytochemicals such as carbohydrates, proteins, stearic and linoleic acids. In the present study, 50 μg/mL and 100 μg/mL of methanolic extract of MISK exhibited prominent antibacterial and anti-biofilm activity against the clinical UPEC strains. In addition, qualitative phytochemical characterization of methanolic extract of MISK showed the presence of bioactive compounds such as tannins, saponins, flavonoids, terpenoids, alkaloids, carbohydrates, polyphenols, and glycosides. Moreover, the characterization of the methanolic extract by UV–VIS, FTIR, and GC–MS analysis confirmed the presence of a phenolic profile, namely mangiferin (1,3,6,7-tetrahydroxyxanthone-C2 -β-D-glucoside). Furthermore, in silico analysis was also investigated to validate that mangiferin can bind to the specific active sites of CAMP LpxC in E. coli. Based on the collective findings of our in vitro and in silico analyses, mangiferin may be the principal compound responsible for the anti-bacterial and anti-biofilm effects of the extract.
Keywords
Urinary tract infections
Escherichia coli
Antimicrobial
Anti-biofilm
Mangiferin
Phytochemicals
In vitro
In silico
Screening
1 Introduction
Urinary tract infections (UTIs) represent a major factor of morbidity worldwide, being one the most common reasons to seek out a primary care physician (Wagenlehner et al., 2022). There are around 120–150 million cases of UTIs worldwide annually, making them the third most common bacterial disease after infections of the respiratory and gastrointestinal tract, respectively (Javed et al., 2021). Bacteriuria has a J-shaped prevalence distribution, with a greater incidence among the very young and a steady rise with age in both men and women. Women are affected at a substantially higher frequency (especially regarding uncomplicated UTIs) than males until the age of 60 years and later (Foxman, 2010). A UTI episode occurs at least once during the lifetime of 40% of women and 12% of men (Ahsan et al., 2021). Additionally, in patients with advanced age or existing underlying conditions, the rate of complicated UTIs (cUTI) or recurrence may increase considerably (Nicolle, 2005).
The most common causative agents associated with UTIs are Escherichia coli, Klebsiella pneumoniae, the Citrobacter-Enterobacter-Serratia group, and the Proteus-Providencia-Morganella group among Gram-negative bacteria (Flores-Mireles et al., 2015; Khusro et al., 2018; Gajdács et al., 2021), while among Gram-positives, Enterococcus spp. (E. faecalis and E. faecium) and Staphylococcus saprophyticus are the most commonly implicated (Adeghate et al., 2016). Nevertheless, uropathogenic E. coli (UPEC), a Gram-negative, facultative anaerobic, peritrichous rod may be considered as the most frequent etiological agent, as it is the cause of 50% of nosocomial UTIs that affect all age groups as well as 95% of community-acquired UTIs (Terlizzi et al., 2017). The pathogenesis of UPEC may be divided into two stages: firstly, bacterial adhesion to the urogenital epithelium and formation of the protective biofilm. In this first phase, UPEC interacts with glycosylated surface proteins (uroplakins) via fimbriae (Matuszewski et al., 2016). In the second phase – following the formation of a mature biofilm – components of the cellular and humoral immune system, and antimicrobials are inhibited from reaching these microorganisms embedded in the biofilm (Chatterjee et al., 2014). Biofilms allow bacterial populations to adhere to different inanimate and in vivo habitats, protecting them from severe environmental conditions and toxic xenobiotics, such as antimicrobials (Aarti et al., 2018; Craft et al., 2019). Furthermore, biofilm-formation is also essential to allow bacteria to withstand the shear forces associated with normal urine flow, allowing them to remain in the urinary system (González-Villalobos et al., 2021). The presence of biofilm-like intracellular bacterial communities (IBCs) allows UPEC to colonize the bladder and resist evacuation, playing an essential role in pathogenicity and ascending UTIs (Javed et al., 2021). In addition to the protections provided by biofilm, antibiotic treatment of UTIs is worsened by the emergence of multidrug-resistant (MDR) bacteria, which present a significant clinical issue (Shakya et al., 2021).
Several medicinal plant extracts are frequently utilized as alternative supplementary medicines to prevent or treat certain illnesses, including UTIs (Shaheen et al., 2019). Numerous plant extracts were widely reported to have antimicrobial and anti-biofilm activity (Prasathkumar et al., 2022) and thus, may be used to treat UTIs (Elamary et al., 2020; Omokhua-Uyi et al., 2020). Mangifera indica L. (mango), belonging to the Anacardiaceae family, is the most traditionally and commercially in terms of tropical fruit crop worldwide. M. indica is the world's second most traded tropical fruit, the fifth most produced, widely known as the “king of fruits”, and is consumed by all people of all ages. Aside from direct consumption, more than half of the mangoes are used to make juice, nectar, puree, squash, slices, jam, and pickles, among other things (Mwaurah et al., 2020). The seeds account for 20 percent to 60 percent of the total fruit mass, while the kernel located inside the seed accounts for 45 percent to 75 percent of the seed's weight and is released as a significant waste into the environment. Globally, 123,000 metric tonnes of mango seeds are released per year, which is mainly regarded as agro-industrial waste (Reddy et al., 2016). The recovery and use of valuable compounds from M. indica by-products is a significant economic and environmental issue.
Traditional medicines described the use of M. indica extracts to treat diabetes, bronchitis, diarrhoea, asthma, renal, scabies, respiratory illnesses, and syphilis. Earlier, Kumar et al. (2021) reported that M. indica leaf extracts have anticancer, anti-diabetic, anti-oxidant, hepatoprotective, antidiarrheal, and lipid-lowering properties. The prominent biological properties such as antioxidant, antitumour, and antimicrobial activities of kernel extract of M. indica were also reported (Abdel-Aty et al., 2018; Nakpanich et al., 2017; Raju et al., 2019). Thus, the valorization of M. indica based by-products as traditional medicines may be an effective method for their utilization. Therefore, the present study aimed to collect urine samples from males and females affected by UTIs, and to confirm the most common pathogen associated with UTI as E. coli, and to identify the strains with the highest biofilm-producing capacity. Following this, the antibacterial and anti-biofilm activity of M. indica seed kernel (MISK) extract against E. coli was tested using traditional in vitro methods. Lastly, the primary bioactive compound associated with biological properties of MISK was identified to be mangiferin, and hence, an in silico activity study was also performed to validate its efficacy against E. coli biofilms.
2 Materials and methods
2.1 Materials
All the reagents and chemicals used during the experiments were purchased from Hi-Media PVT LTD. Glassware were purchased from Borosil Glass Works Limited, Mumbai-400 018 (India), and plastic wares were procured from M/S Tarsons Products PVT LTD (New Delhi–110001).
2.2 Sample collection
Urine samples were provided by patients affected by with UTIs and were collected at government hospitals in Srirangam, Trichy, Tamil Nadu, India. Overall, 10 male and 20 female patients, between the ages of 25 and 70 provided n = 30 urine samples over the course of a month, each of which underwent traditional microbiologic analysis.
2.3 Isolation and identification of urinary pathogens
Urine samples were inoculated into a selective and differential medium, such as Eosin Methylene Blue (EBM) agar, MacConkey agar, and Blood agar, using a sterile standard wire loop. The plates were macroscopically examined for shape and size of the colony formation as presumptive identification after 24 h of incubation at 37 °C (Wagenlehner et al., 2022). Microscopy and biochemical testing were used to identify a selection of colonies from a selective and differential medium (Koneman et al., 1994). Uropathogens were identified based on the results from biochemical tests.
2.4 Antibiotic susceptibility tests
The antibiotic sensitivity of all isolated microorganisms were tested in accordance with Bauer et al., (1966). The disc diffusion assay was employed to evaluate the susceptibility of the abovementioned isolates. The interpretation of the results was performed based on the recommendations of Clinical and Laboratory Standards Institute (CLSI) standards (CLSI, 2019). Classification of the isolates as a multidrug resistant (MDR) was based on the guidelines of Magiorakos et al. (2012).
2.5 Assessment of biofilm-formation by the microtitre plate method (MTP)
A microtiter-plate experiment, based on crystal violet (CV) staining method, was conducted to evaluate the biofilm-forming capacity of the relevant bacteria in various nutritional environments, pH, and temperatures (Kırmusaoğlu, 2019). After incubation, the test organisms were cultured in a microtitre well-containing the broth (Nutrient Broth, Sabouraud Dextrose Broth [SDB], De Man, Rogosa and Sharpe [MRS] medium). To remove loosely attached cells, the microtitre well was cleaned three times with sterile distilled water. The microtitre plates first underwent air drying, followed by 45 min in a 60 °C oven. After the wells were dried, 100 μL of 1% CV was added to the wells and left at room temperature for 15 min (Ramos-Vivas et al., 2019). The plates were rinsed five times with sterile distilled water to get rid of the unabsorbed stain.
2.6 Biofilm-formation assay by the tube method (TM)
A loopful of bacterial culture was inoculated in test tubes with 10 mL of Nutrient Broth containing 1% glucose, after which the tubes were incubated the 24 h at 37 °C. After the incubation, the tubes were decanted, washed with phosphate buffer saline (pH 7.3), and dried. A 0.1% CV solution was then applied to the tubes. The excess stain is removed with distilled water and tubes were dried in an upturned arrangement. When a visible thick film lined the tube's wall and bottom, the isolate was considered positive for biofilm formation (Kırmusaoğlu, 2019).
2.7 Biofilm-formation assay by the Congo red agar method (CRA)
CRA medium was prepared for the phenotypic confirmation of biofilm formation, as described previously, with 37 g/L brain heart infusion broth, 50 g/L sucrose, 10 g/L agar, and 8 g/L Congo red indicator. The test organisms were inoculated on CRA plates and incubated for 24 h at 37 °C (Kırmusaoğlu, 2019). Isolates were then assessed for their colony-morphologies: isolates were considered as biofilm-producers, if they presented black colonies with a dry consistency, while negative if they presented with red colonies with smooth, round and shiny surfaces.
2.8 Exopolysaccharide (EPS) extraction
The overnight bacterial cultures were centrifuged at 10,000 rpm for 20 min at 4 °C to eliminate bacterial cells. The supernatant was then collected into a fresh vial and precipitated with chilled absolute ethanol and incubated at 4 °C for overnight; following this, another centrifugation step occurred at 10,000 rpm for 20 min at 4 °C, after which, the supernatant was decanted. The pellet containin exopolysaccharides (EPS) was dried at room temperature. EPS was assessed by the phenol–sulphuric acid method, described previously (Felz et al., 2019).
2.9 Collection of the plant material
Mangifera indica fruits were collected from the Thathachariar garden, Tiruchirappalli, Tamil Nadu, India. The seed kernel was carefully separated by using a sterile knife, and the seed kernel identity was confirmed by Dr. John Britto, Director, Rapinat herbarium, St. Joseph’s College, Thiruchirapalli, Tamil Nadu, India. An electric grinder was used to grind the dried seed kernel into a fine powder. The seed kernel powder was kept in a sterile container for future studies.
2.10 Extraction of MISK
In a Soxhlet extractor, 100 g of seed kernel powder was added with 1500 mL of solvent such as methanol, and the extraction was carried out for 48 h. After finishing the extraction, the extract was distilled off and the dried extract was obtained and analyzed by UV and FTIR, GC–MS methods (Shinde et al., 2014).
2.11 Antibacterial study of plant extract against uropathogenic E.coli
The known quantity of extracts was dissolved in a 1:1 ratio of DMSO:methanol and it was diluted with an equivalent amount of phosphate-buffered saline (PBS pH 7.3). The extracts were sterilized by using a 0.22 µm pore size Sartorius syringe filter. 20 µg − 100 µg of extracts were loaded with sterile discs of 6 mm in diameter (Whatmann MM) and were dried. Disks loaded with solvent were used as a negative control. The standardized antibiotic-loaded discs (Hi-Media) were used as the positive control. The antibacterial assay of seed kernel extracts was determined by the disc diffusion method (Kebede et al., 2021).
2.12 Study of the effect of plant extracts on EPS formation
The 40 μL of the test sample was dispensed in microtitre culture plates and 50 and 100 μL/mg concentrations of methanolic extract seed kernel were added to the wells and incubated at 37 °C for 24 h. After incubation, the unattached cells were decanted and washed with PBS (pH 7.3), followed by an air dry and stained with 0.1% CV (Xiao et al., 2007).
2.13 Phytochemical analysis of the methanolic extract of MISK
Freshly produced seed kernel extracts were examined for the presence of phytoconstituents using conventional methods (Rajan et al., 2011).
2.14 UV–Vis analysis of methanolic extract of MISK
The methanolic extract of MISK was centrifuged at 3000 rpm for 10 min before being filtered via Whatmann No.1 filter paper. The solution was diluted at a ratio of 1:10 in the same solvent. The extract was analyzed with a Perkin Elmer Spectrophotometer for spectral analysis (Dhivya et al., 2017).
2.15 FTIR analysis of methanolic extract of MISK
The methanolic extract MISK's infrared spectrum was acquired using Fourier Transform Infrared Spectroscopy from Perkin Elmer Spectrum GX FTIR, USA. 5 mg of methanolic extract of MISK was mixed with 150 mg of potassium bromide (KBr) and pressed into tablet form to make sample discs. Infrared spectra were collected with a resolution of 0.2 cm over the 4000–500 cm−1 range.
2.16 GC–MS
The evaluation of phytoconstituents in the methanolic extract of MISK was carried out using GC–MS equipment (Thermo Scientific Co.) Thermo GC-TRACE ultra ver.: 5.0, Thermo MS DSQ II, with the following experimental conditions: DB 5 - MS Capillary Standard Non-Polar Column, dimension: 30Mts, ID: 0.25 mm, Film thickness: 0.25 m. The mobile phase flow rate (carrier gas: He) was set to 1.0 mL/min. The temperature program was 70 °C raised to 260 °C at 6 °C/min in the gas chromatography section, and the insertion volume was 1 L.
2.17 In silico analysis
2.17.1 Protein preparation
The protein structure of LPXC E. coli (protein ID: 3P3G) with Kollmann charge was assigned side chains, and was tuned for H2 bonding through the addition of hydrogens. The protein that had its energy reduced was then archived in PDB format. Nonpolar H2 were combined, AutoDock atom, AD4 and Gasteiger charges was assigned, and then the results were saved in protein.pdbqt format using MGLTools-1.4.6 (Morris et al., 1998) for future analyses.
2.17.2 Ligand preparation
ChemSketch (Chem Sketch) was used to draw the ligand structures, which were then optimized with 3D. The 2D structure of mangiferin was then transformed into a 3D ű structure using the open Babel format molecule converter (Guha et al., 2006) and kept in PDB format for AutoDock compatibility. To exchange ligand.pdb files to ligand.pdbqt files, MGLTools-1.4.6 (The Sripps Research Institute) was used.
2.17.3 Docking protocol
TMGLTools-1.4.6 has been used to create the grid variable data (protein.gpf) and scalar data (ligand.dpf). Grid spacing of 0.375 Å and 90 X 60 X 60 grid points in xyz were used to create receptor grids. Co-crystallized ligand plot types have been derived using autogrid4, and the grid box was centered. Macromolecule docking was carried out using a Lamarckian Genetic Algorithm and an empirical free energy function, with a starting population of 250 randomly disseminated individuals, a utmost of 106 energy analyses, a mutation rate of 0.02, and a intersect rate of 0.80. For each ligand, 100 separate docking runs was carried out. Results with positional RMSD differences of 2.0 Å were grouped together and were represented by the outcome with the lowest free energy of binding.
2.18 Statistical analysis
The data were presented as means ± standard deviations [SD], with each experiment performed in triplicate. One-way ANOVA was used for inferential statistics; statistical analyses were performed by SPSS version 12.0. (SPSS, Inc., Chicago, IL). p values < 0.05 were considered statistically significant.
3 Results and discussion
3.1 Isolation and identification of urinary pathogens
Table S1 shows that clinical isolates from the urinary tract are common. In total, n = 30 urine samples were collected from various age groups of male and female patients. A possible pathogen was isolated from urine samples based on colony morphology seen on selective media. The growth patterns, microscopic features, and biochemical parameters were used to identify five clinical isolates (Koneman, 1994). Depending on the nation's various biographical, geographical, and socioeconomic conditions, the incidence and species-distribution of these organisms may show considerable variation (Sherry et al., 2018). In the present study, clinical pathogens like E. coli, Klebsiella, Staphylococcus, and Pseudomonas were isolated, as summarized in Table S2. Out of 10 samples taken from male patients, E. coli (60%) was the most common isolate in the sample. Similarly, E. coli was more prevalent than other bacteria out of the 20 samples of female patients from the various age groups. Due to the anatomical features of the urethra, uncomplicated UTIs largely affects female patients; these findings confirm the previous results of Pardeshi, (2018). Out of 20 samples overall, E. coli was present in 75%, Staphylococcus in 15%, and Pseudomonas species in 10%, respectively. To investigate the antibiotic sensitivity assay and biofilm activity, a high level of E. coli was primarily isolated from both male and female patients. These finding are closely related to the results of other studies (Thattil et al., 2018).
3.2 Antibiotic susceptibility of the isolates
Before assessing the MISK's antimicrobial activity on relevant urinary pathogens, the antibiotic sensitivity patterns of isolated E. coli was evaluated. Results showed that ampicillin, cephalosporins, and novobiocin were ineffective against the isolated bacteria. According to the study's findings, some of the isolated strains of E. coli were MDR (Table S3). According to Siedelman et al. (2012) and Walters et al. (2012), MDR microorganisms have a considerably high prevalence in community-acquired infections, reaching rates as high as 70–80%.
3.3 Biofilm-formation assays and EPS extraction
One of the main virulence factors of UPEC is the formation of the protective biofilm. When intestinal bacteria reach the bladder, they adhere to the uroepithelium using their fimbriae, and in later stages form a biofilm that can reach the renal tissue, causing pyelonephritis (Khan et al., 2011; Behera et al., 2008). All n = 21 E. coli strains were tested for their biofilm-producing capacities, and several tests are available to determine which E. coli strains were biofilm-producers. These tests included microtiter-based plate assays, tube-based test, and the CRA plate method. On the CRA plate, the biofilm-producing E. coli have generated black colonies, while the potent biofilm-producing strains were easily identifiable when using the tube method, which is less sensitive but more robust when it comes to strong-biofilm producers.
The extent of biofilm-formation may be strongly influenced by the in vitro and in vivo conditions (Fernández-Barat et al., 2018). Table 1 displays the various nutritional environments, pH, and temperatures that were utilized to study the biofilm-producing activity of the isolates. Out of three nutritional environments, isolates cultured in the Nutrient Broth showed the highest biofilm activity in 96 h; an OD value of 2.25 was measured, which was higher than in other nutritional environments (in SDB broth: 1.622, and MRS broth: 1.684). Table 2 shows the high amount of EPS production in the different nutritional environments tested using the phenol–sulphuric acid method. The Nutrient Broth environment (OD value: 2.29) showed a high amount of EPS production, compared to the other environments (SDB: 1.006), MRS: 1.021)). pH is another significant factor, which may affect biofilm formation among urinary pathogens (Kincses et al., 2021). E. coli is able survive in a neutral pH environment, indicating that pH 7 and 8 was the critical condition to produce the biofilm and EPS concentration at 96 h. The other pH ranges did not have a significant effect on biofilm formation of the tested isolates. Different temperature conditions were also relevant in the context of biofilm formation. The 30 °C and 37 °C were highly responsible for the biofilm formation and EPS compared to the other temperatures tested. Donalin (2002) also revealed that temperature was an important parameter when it comes to biofilm formation. The EPS are the building blocks of the biofilm matrix, creating a hydrated viscous layer, protecting the bacteria from desiccation and immune cells. The matrix may additionally support the bacteria's defense mechanisms against harmful chemicals like biocides and reactive oxygen species (Gambino et al., 2016). The biofilm environment may also interrupt nutrients, enzymes, and even signaling pathways from washing out, allowing them to concentrate and build more favorable microenvironments (Redfield et al., 2002; Starkey et al., 2004; Welch et al., 2002). All of these matrix characteristics may help pathogenic bacteria develop phenotypic (“adaptive”) resistance. Biofilm-formation in E. coli is a critical factor in causing chronic infections (Anderson et al., 2003; Justice et al., 2004). EPSs like poly-1,6-N-acetylglucosamine, LPS, cellulose, capsules and colanic acid are the ones that are most frequently found in the composition of this matrix. Out findings presented in Tables 1 and 2 are also supported by the aforementioned claims. The various environmental factors significantly influenced the development of the biofilm, and high levels of EPS production were produced after 96 hrs. Values are expressed as the mean ± SD (n = 3). Statistical significance was considered at P < 0.05 in all cases. **denotes P < 0.01 and *denotes as P < 0.05. Values are expressed as the mean ± SD (n = 3). Statistical significance was considered at P < 0.05 in all cases. **denotes P < 0.01 and *denotes as P < 0.05.
Sample number
Days
Nutrients
pH
Temperature
Nutrient
SDB
MRS
7
8
9
10
25 °C
30 °C
37 °C
45 °C
1
I
2.148 ± 0.005
1.112 ± 0.02*
1.271 ± 0.008
1.131 ± 0.08
1.089 ± 0.01
0.125 ± 0.05*
0.022 ± 0.01
1.171 ± 0.01
1.298 ± 0.05
1.554 ± 0.08
1.323 ± 0.02
2
II
2.183 ± 0.006
1.201 ± 0.05
1.318 ± 0.006*
1.142 ± 0.01
1.365 ± 0.02*
0.534 ± 0.12
0.026 ± 0.08*
1.187 ± 0.05
1.523 ± 0.05
1.568 ± 0.01
1.220 ± 0.05
3
III
2.241 ± 0.009
1.559 ± 0.08
1.424 ± 0.010
1.901 ± 0.01
1.450 ± 0.12
0.749 ± 0.05
0.035 ± 0.01
1.194 ± 0.08*
1.632 ± 0.02*
1.773 ± 0.05
1.139 ± 0.02*
4
IV
2.250 ± 0.015**
1.622 ± 0.12**
1.684 ± 0.013
1.951 ± 0.01*
1.593 ± 0.05
0.983 ± 0.12
0.078 ± 0.12
1.153 ± 0.01
1.764 ± 0.05
1.822 ± 0.01*
1.046 ± 0.05
5
V
1.109 ± 0.006 *
0.072 ± 0.01
0.069 ± 0.007**
0.098 ± 0.08**
0.792 ± 0.02**
0.076 ± 0.01**
0.007 ± 0.05**
0.943 ± 0.02**
0.747 ± 0.12**
0.832 ± 0.05**
0.532 ± 0.08**
Sample
Days
Nutrients
pH
Temperature
Nutrient
SDB
MRS
7
8
9
10
25 °C
30 °C
37 °C
45 °C
1
I
2.153 ± 0.015
1.891 ± 0.006
1.424 ± 0.009
1.564 ± 0.006
1.090 ± 0.006
0.190 ± 0.006
0.055 ± 0.006
1.166 ± 0.006
1.685 ± 0.009
1.835 ± 0.015**
1.612 ± 0.006**
2
II
2.199 ± 0.009
2.125 ± 0.009
1.786 ± 0.007*
1.782 ± 0.015
1.419 ± 0.009
0.805 ± 0.015
0.059 ± 0.006**
1.088 ± 0.006**
1.747 ± 0.006*
1.760 ± 0.007
1.679 ± 0.005
3
III
2.239 ± 0.007*
2.171 ± 0.007*
1.806 ± 0.006
1.930 ± 0.009
1.839 ± 0.007*
0.834 ± 0.009
0.062 ± 0.015
1.053 ± 0.009
1.943 ± 0.015
1.931 ± 0.006
1.845 ± 0.009
4
IV
2.291 ± 0.006
2.215 ± 0.009
1.892 ± 0.009
1.971 ± 0.007*
1.986 ± 0.006**
0.995 ± 0.009*
0.093 ± 0.006
0.931 ± 0.005
1.882 ± 0.006
1.939 ± 0.006*
1.900 ± 0.015
5
V
1.046 ± 0.007**
1.036 ± 0.015**
1.02 ± 0.007**
0.087 ± 0.009**
0.632 ± 0.007
0.085 ± 0.006**
0.006 ± 0.006*
0.043 ± 0.007*
0.439 ± 0.005**
0.499 ± 0.009
0.332 ± 0.006*
3.4 Antibacterial activity of methanolic extract of MISK
The large “chemical space” of substances found in natural sources, such as plants and herbal material are well-known. Recently, there has been great attention to the isolation and characterization of plant extracts and secondary metabolites as potential antimicrobial agents for infectious diseases (Jalal et al., 2021; Spengler et al., 2022). Mangifera indica seed kernel shows effective antibacterial activity at 100 µL/mg concentrations. It produced 13.0 ± 0.1 mm, 17.0 ± 0.1 mm and 19.0 ± 0.15 mm zone of inhibitions against biofilm-producing E. coli. Table S4 shows the 50 µL/mg concentration of MISK to reduce the biofilm ability of the E. coli at 96 h. Gradually it will affect the E. coli in different environmental conditions, e.g., the 24 h in Nutrient Broth showed an 0.11 OD value, followed by the 96 h extract, ultimately reducing the growth of E. coli, with an OD value of 0.008. This indicates that the 50 µL/mg concentration of MISK decreased the E. coli growth at 96 h. The different pH and temperature conditions stated the same results for UPEC. M. indica seed kernel contained some phenolic compounds that proven to be useful to eradicate biofilm-formation. This could be due to the specific phytochemicals present in these extracts. One of the earlier studies suggested that MISK contained steroids, terpenes, polyphenols, and phenolic compounds (Rajan et al., 2011). Consequently, the MISK Soxhlet-extract that includes the particular compound may be used as a prospective source of therapeutic compound that works to prevent UPEC from forming a biofilm. Similarly, Bua et al. showed that Austroeupatorium inulifolium essential oils possessed significant antibacterial activity against biofilm-forming pathogens such as S. aureus, S. epidermidis, and Mycobacterium tuberculosis (Bua et al., 2018).
3.5 Antibiofilm activity and EPS extraction of MISK
Tables 3 and 4 indicated the level of EPS production in UPEC in the presence of the 50 µL/mg concentration of MISK; based on our findings, EPS concentration was significantly reduced at 96 h, as the EPS is one of the important factors, as it will induce biofilm formation and provide stability to the biofilm structure. Our result shows the EPS level was reduced considerably at 96 h. Table 5 shows the antibiofilm activity of Soxhlet-extract on MISK in 100 µL/mg concentration. It shows the good antibiofilm effect against the UPEC isolates. Different nutrient, pH, and temperature environments also showed noteworthy activity against E. coli. MISK reduces the biofilm-forming activity at 72 h in the different nutritional environments (Nutrient Broth OD: 0.005), SDB (OD: 0.009), MRS (OD: 0.012), the same results as indicated by the other condition like pH and temperature. pH 7 shows good antibiofilm activity in this condition, as the MISK completely reduced the growth of E. coli at 48 h when compared to the other pH conditions. Temperatures 30 °C and 37 °C show the complete arrest of E. coli growth at 48 h compared to other conditions. EPS concentration was completely arrested by using Soxhlet-extract of MISK in 48 h. It indicates the 100 µL/mg concentration of MISK was very effective against biofilm-forming UPEC (Table 6). The interaction of plant extracts and antibiotics with synergistic activity against microbial pathogens has been reported numerous times previously. For example, Donadu et al. (2021) reported that essential oils of Ruta graveolens showed excellent anti-biofilm activity and synergism with other antibiotics. Likewise, Abraham et al., (2012) also reported that some of the plants extracts inhibit biofilm formation. The Soxhlet-extract has phenolic compounds, which presumably have important roles in the anti-biofilm efficacy. According to Shan et al. (2007), phenolic compounds play a major role in the antimicrobial properties of many plant extracts. Values are expressed as the mean ± SD (n = 3). Statistical significance was considered at P < 0.05 in all cases. **denotes P < 0.01 and *denotes as P < 0.05. Values are expressed as the mean ± SD (n = 3). Statistical significance was considered at P < 0.05 in all cases. **denotes P < 0.01 and *denotes as P < 0.05. Values are expressed as the mean ± SD (n = 3). Statistical significance was considered at P < 0.05 in all cases. **denotes P < 0.01 and *denotes as P < 0.05. Values are expressed as the mean ± SD (n = 3). Statistical significance was considered at P < 0.05 in all cases. **denotes P < 0.01 and *denotes as P < 0.05.
Sample number
Days
Nutrients
pH
Temperature
Nutrient
SDB
MRS
7
8
9
10
25 °C
30 °C
35 °C
45 °C
1
I
0.110 ± 0.015
0.109 ± 0.005*
0.108 ± 0.005*
0.122 ± 0.006
0.151 ± 0.007
0.153 ± 0.015*
0.160 ± 0.005*
0.156 ± 0.007*
0.12 ± 0.005*
1.064 ± 0.006
1.093 ± 0.015
2
II
0.093 ± 0.005*
0.099 ± 0.015
0.100 ± 0.005
0.120 ± 0.015*
0.133 ± 0.005*
0.149 ± 0.015
0.1510.007
0.130 ± 0.005
0.110.007
0.839 ± 0.005**
1.062 ± 0.005**
3
III
0.015 ± 0.005
0.045 ± 0.015
0.098 ± 0.015
0.087 ± 0.006**
0.095 ± 0.006
0.098 ± 0.005
0.110 ± 0.015**
0.108 ± 0.015
0.095 ± 0.006
0.120 ± 0.007
1.03 ± 0.015
4
IV
0.008 ± 0.006**
0.005 ± 0.007**
0.087 ± 0.007**
0.051 ± 0.005
0.065 ± 0.007**
0.065 ± 0.005
0.095 ± 0.007
0.098 ± 0.005**
0.05 ± 0.005**
0.009 ± 0.007*
0.081 ± 0.006*
Sample number
Days
Nutrients
pH
Temperature
Nutrient
SDB
MRS
7
8
9
10
25 °C
30 °C
35 °C
45 °C
1
I
0.095 ± 0.001
0.093 ± 0.002*
0.085 ± 0.002*
0.125 ± 0.001
0.084 ± 0.002*
0.157 ± 0.001
0.097 ± 0.001*
0.106 ± 0.002*
0.094 ± 0.001*
0.080 ± 0.002
0.074 ± 0.002
2
II
0.026 ± 0.002
0.053 ± 0.002
0.065 ± 0.002
0.103 ± 0.002
0.065 ± 0.001
0.094 ± 0.002*
0.045 ± 0.002
0.087 ± 0.001
0.042 ± 0.002
0.062 ± 0.001
0.069 ± 0.002
3
III
0.053 ± 0.001
0.043 ± 0.001
0.053 ± 0.002*
0.007 ± 0.001**
0.008 ± 0.001
0.043 ± 0.001
0.017 ± 0.001
0.052 ± 0.001**
0.039 ± 0.002
0.018 ± 0.001**
0.021 ± 0.002
4
IV
0.002 ± 0.001**
0.007 ± 0.001**
0.031 ± 0.001
0.000 ± 0.00
0.001 ± 0.001**
0.009 ± 0.001**
0.004 ± 0.001**
0.009 ± 0.001
0.006 ± 0.001
0.001 ± 0.001
0.009 ± 0.002**
Sample number
Days
Nutrients
pH
Temperature
Nutrient
SDB
MRS
7
8
9
10
25 °C
30 °C
37 °C
45 °C
1
I
0.070 ± 0.001*
0.069 ± 0.002
0.098 ± 0.001
0.051 ± 0.001*
0.054 ± 0.001
0.095 ± 0.001
0.062 ± 0.002
0.193 ± 0.002*
0.184 ± 0.002*
0.132 ± 0.002
0.156 ± 0.002*
2
II
0.051 ± 0.002
0.056 ± 0.001**
0.065 ± 0.002
0.033 ± 0.002
0.047 ± 0.002**
0.069 ± 0.002**
0.051 ± 0.002
0.102 ± 0.001
0.062 ± 0.001**
0.001 ± 0.001**
0.013 ± 0.001
3
III
0.005 ± 0.002**
0.009 ± 0.001
0.012 ± 0.001**
0.000
0.001 ± 0.001
0.006 ± 0.001
0.002 ± 0.001**
0.052 ± 0.001-**
0.000
0.000
0.001 ± 0.001**
Sample number
Days
Nutrients
pH
Temperature
Nutrient
SDB
MRS
7
8
9
10
25 °C
30 °C
37 °C
45 °C
1
I
0.045 ± 0.001*
0.056 ± 0.001*
0.081 ± 0.001*
0.036 ± 0.001*
0.039 ± 0.001*
0.068 ± 0.001*
0.088 ± 0.001*
0.056 ± 0.001*
0.045 ± 0.001*
0.040 ± 0.001*
0.125 ± 0.001*
2
II
0.002 ± 0.001**
0.026 ± 0.001**
0.055 ± 0.001**
0.002 ± 0.001**
0.005 ± 0.001*
0.024 ± 0.001**
0.032 ± 0.001*
0.025 ± 0.001**
0.012 ± 0.001**
–
0.002 ± 0.001**
3.6 Phytochmical analysis
According to the preliminary phytochemical screening, the Soxhlet-extract contained alkaloids, terpenoids, carbohydrates, flavonids, phenolic compounds, and tannins. This test is necessary to determine the primary type of phytochemicals present in the extract. This study suggested the biological activity of phenolic compounds like flavonoids, terpenoids, and tannins (Table S5). It is necessary to identify the unidentified compounds using various physiochemical spectroscopic techniques, including FT-IR, UV–VIS, and GC–MS. Thus, we characterized the mangiferin using the aforementioned spectroscopic techniques, and the outcomes were evaluated against previous literature reports (Prakash et al., 2014).
3.7 UV–visible analysis
Numerous different molecules are found in plants that are directly or indirectly involved in photosynthesis and may also give the plants their colour. Because they absorb UV-A and UV-B rays and have antioxidant properties, natural substances derived from plants are also being investigated as potential sunscreen resources. There is compelling evidence that the accretion of UV light-absorbing phenolic and flavonoids in the epidermal tissue of plants, which have outstanding antioxidant and photo-protecting properties, is triggered by DNA-damaging UV light. The present result exhibited a phenolic compound in the UV region, indicating a potential active compound. The π-π* transition lies in the UV region. The electronic spectra of the compound are characterized by the π-π* transitions (λ = 238, 256 nm), was in agreement with the reported bands for the xanthenes nucleus in mangiferin (Jo et al., 2016; Gomez-Zaleta et al., 2006).
3.8 FT-IR analysis
Fig. 1 shows the FT-IR analysis of MISK. The spectrum contains strong absorption at 1653 cm−1, indicating the presence of a carbonyl group (C = O). O–H stretching was observed at 3414 cm−1. The stretching of (C = C), (C-O), (Ar-H) and (C-O-C) were observed at 1436, 1208, 3005 and 1024 cm-1, respectively.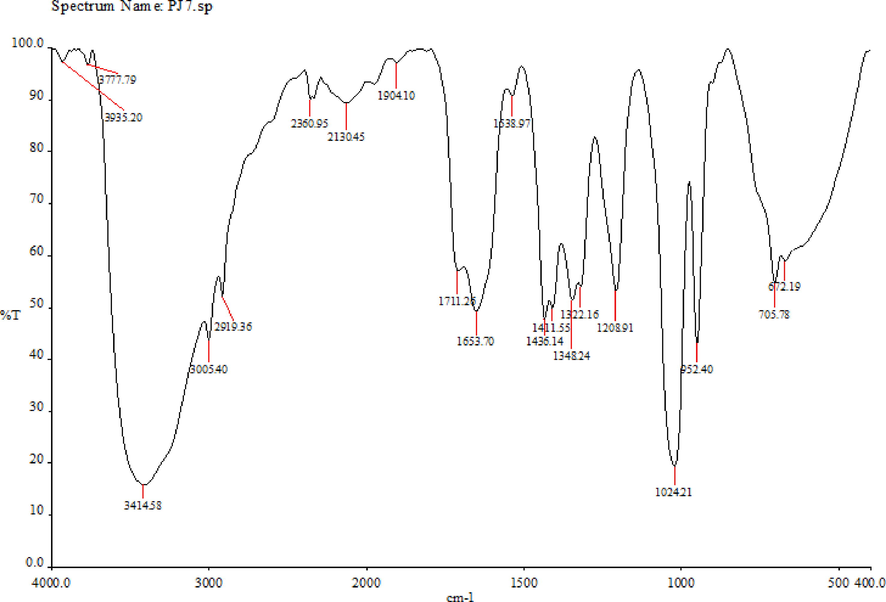
FT-IR analysis of the methanolic extract of Mangifera indica seed kernels (MISK).
3.9 GC–MS analysis
GC–MS was recorded in a Thermo GC-Trace Ultra Ver:5.0, Thermo MS DSQ II and the mass spectra shown in Fig. 2. For mangiferin, the parent ion peak [M + H]+ comes at m/z 423 and the product ion peak comes at m/z 357, followed by the fragments peak at m/z 405, 387, 369, 357, 327, 303, and 273, respectively. In the recorded mass spectra of mangiferin, similar m/z peaks were observed. This points to the presence of mangiferin in the tested extract (Suryawanshi 2007; Patil et al., 2019). All the above spectroscopic methods possibly suggests that compound present in the Soxhlet-extract was mangiferin and that this compounds had a critical role in the antibiofilm activity of the extract against the UPEC isolates tested.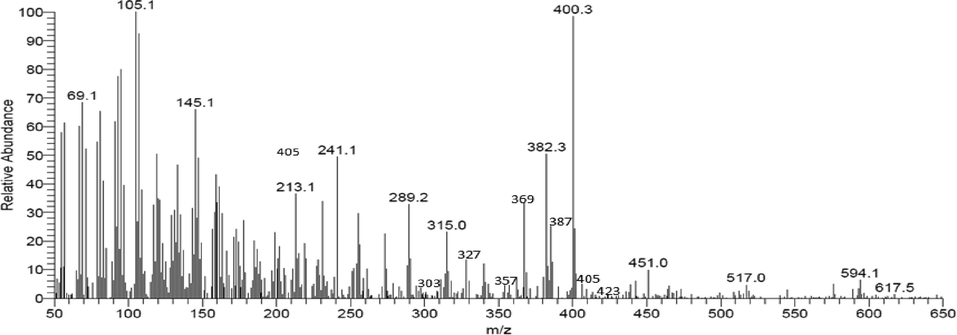
GC–MS analysis of the methanolic extract of Mangifera indica seed kernels (MISK).
Compounds with one or more aromatic rings and hydroxyl groups are known as phenolics. Due to the different hydroxylation patterns and stereochemistry at the three chiral centres, there is considerable diversity in their structure. The current FT-IR peaks showed aromatic rings with hydroxyl groups, which supported this conclusion. The findings of this study showed phenolic compounds that inhibit biofilm and the reduction of microbial populations on epithelia and mucosa, which might cause infection. Due to an inadequate penetration, drug inactivation, variable of strains, or the physiological condition of microbes in the biofilm, inhibiting the adhesion of planktonic microbial cells to an adsorbent is smoother than the ability to inhibit the association of a previously founded biofilm (Nassima et al., 2019).
The chemical nature of the analysis, assay method, choice of standards, and presence of interferences all have an impact on how much phenolic compounds are present in plant extract. By using GC–MS, the purified phenolic acids' chemical structures were verified (Khoddami et al., 2013). The M. indica Soxhlet-extract displays phenols like mangiferin (1,3,6,7-tetrahydroxyxanthone-C2 -β-D-glucoside).
3.10 In silico analysis
To carry out our in silico analyses, mangiferin must be docked into the binding pocket of LpxC E. coli. Our docking simulation produced a target protein structure that was quite similar to our observations. Mangiferin had a strong interaction with LpxC, and had a strong inhibitory effect. The amino acid residues LEU 18, MET195, SER211, and LYS239 were found in the active site of LpxC E.coli and played an important role. Fig. 3 shows the amino acid residues ARG841 and LEU797 were involved in interactions with mangiferin the active site of CAMP LpxC. The length of the hydrogen bond formed 1.880 Å and 1.976 Å. The IC50 values of this compound was determined as 26 µmol, and a low docking score (-7) (Table 7). Rath et al., 2016 already revealed that lpxC catalyses the initial stages of the lipid biosynthesis. During our in silico analyses, mangiferin was shown to bind the specific active sites of CAMP LpxC (Fig. 3). The blocking of LpxC leads to an increase in the permeability of antibacterial agents and reduces the abundance of LPS in outer membrane of Gram-negative bacteria, like E. coli. The present study shows that mangiferin is one of the major compounds that could lead to the eradication of biofilm formation and to an arrest in EPS formation.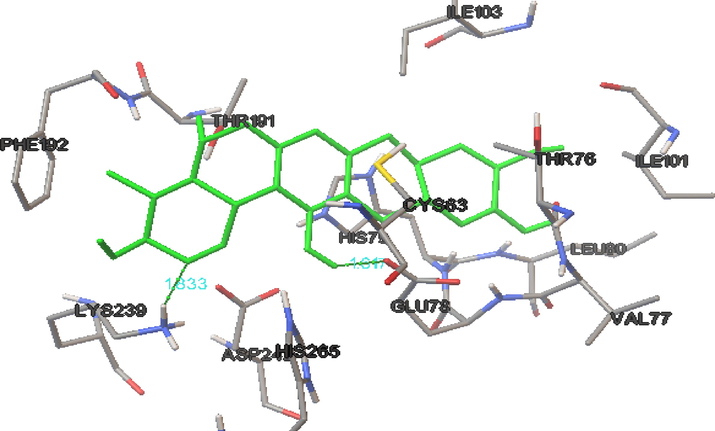
The binding intraction of biofilm-producing uropathogenic E.coli LpxC protein with mangiferin.
Compound name
Docking score
IC50 value (µmol)
H-Bond interaction
Distance
1
Mangiferin
−7
26
LYS239 N–H…O
GLU78 O–H…O1.833
1.647
4 Conclusions
M. indica seed kernel is rich in phytochemicals and secondary metabolites, and it has been widely used in alternative medicine in the treatment of UTIs. The present preliminary in vitro and in silico study has revealed that 50 µL/mg and 100 µL/mg concentrations of M. indica seed kernel have shown considerable anti-biofilm activity against UPEC in various nutritional and environmental conditions. In addition, based on the collective findings of our in vitro and in silico analyses, mangiferin may be the principal compound responsible for the anti-bacterial and anti-biofilm effects of the extract. Although more research is needed to establish the exact mechanisms underlying the anti-biofilm capability of M. indica seed kernel at the molecular level, and to assess their biological functions for their safety in the development of a novel herbal product for clinical applications.
Acknowledgement
Dr. Kamaraj Prabhu acknowledges Department of Biotechnology, Srimad Andavan Arts And Science College (Autonomous), Tiruchirappalli, for providing the required facilities to carry out the research. The author, Dr. Subramaniam Sadhasivam acknowledges DBT, India for the financial support provided by the Ramalingaswami Re-entry fellowship (Order No.BT/RLF/Re-entry/55/2013). M.G. was supported by the János Bolyai Research Scholarship (BO/00144/20/5) of the Hungarian Academy of Sciences. The research study was supported by the ÚNKP-22-5-SZTE-107 New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund. The authors like to thank Taif University, Taif, Saudi Arabia, for their support (Taif University Researchers Supporting Project number: TURSP-2020/80)
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- In vitro investigation on probiotic, anti-Candida, and antibiofilm properties of Lactobacillus pentosus strain LAP1. Arch. Oral Biol.. 2018;89:99-106.
- [Google Scholar]
- Phenolic-antioxidant capacity of mango seed kernels: therapeutic effect against viper venoms. Rev. Bras. farmacogn.. 2018;28:594-601.
- [CrossRef] [Google Scholar]
- Investigation of the potential antibiofilm activities of plant extracts. Int. J. Pharm. Pharm. Sci.. 2012;4:282-285.
- [Google Scholar]
- Does Staphylococcus saphrophyticus cause acute cystitis only in young females, or is there more to the study? a one-year comprehensive study done in Budapest. Hungary. Acta Microbiol. Immunol. Hung.. 2016;63:57-67.
- [Google Scholar]
- Determination of the efficacy of azithromycin on biofilm-forming uropathogenic Escherichia coli isolated from urinary tract infection samples. Dhaka Univ. J. Biol. Sci.. 2021;30:1-11.
- [CrossRef] [Google Scholar]
- Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105-107.
- [CrossRef] [Google Scholar]
- Bauer, A.W. Kirby M.M, Sherris, J.C.T., 1966. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45, 493.
- Parasites in patients with malabsorption syndrome: a clinical study in children and adults. Dig. Dis. Sci.. 2008;53:672-679.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of Austroeupatorium inulaefolium (HBK) against intracellular and extracellular organisms. Nat. Prod. Res.. 2018;32:2869-2871.
- [CrossRef] [Google Scholar]
- Biofilms on indwelling urologic devices: microbes and antimicrobial management prospect. Ann. Med. Health Sci. Res.. 2014;4:100-104.
- [Google Scholar]
- Clinical Laboratory Standards Institute (CLSI): M100: Performance Standards for Antimicrobial Susceptibility Testing. ISBN 978-1-68440-033-1.
- Methicillin-resistant Staphylococcus aureus (MRSA): antibiotic-resistance and the biofilm phenotype. Med. Chem. Comm.. 2019;10:1231-1241.
- [Google Scholar]
- Screening of phytoconstituents, UV-VIS Spectrum and FTIR analysis of Micrococca mercurialis (L.) Benth. Int. J. Herb. Med.. 2017;5:40-44.
- [Google Scholar]
- Colombian essential oil of Ruta graveolens against nosocomial antifungal resistant Candida strains. J. Fungi. 2021;7:383.
- [CrossRef] [Google Scholar]
- Efficacy of Acacia nilotica aqueous extract in treating biofilm-forming and multidrug resistant uropathogens isolated from patients with UTI syndrome. Sci. Rep.. 2020;10:1-14.
- [CrossRef] [Google Scholar]
- Chemical characterization methods for the analysis of structural extracellular polymeric substances (EPS) Water Res.. 2019;157:201-208.
- [CrossRef] [Google Scholar]
- Assessment of in vivo versus in vitro biofilm formation of clinical methicillin-resistant Staphylococcus aureus isolates from endotracheal tubes. Sci. Rep.. 2018;8:e11906.
- [Google Scholar]
- Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol.. 2015;13:269-284.
- [CrossRef] [Google Scholar]
- The epidemiology of urinary tract infection. Nat. Rev. Urol.. 2010;7:653-660.
- [CrossRef] [Google Scholar]
- Interplay between phenotypic resistance to relevant antibiotics in gram-negative urinary pathogens: a data-driven analysis of 10 years’ worth of antibiogram data. Life. 2021;11:e1059.
- [Google Scholar]
- UV/vis, 1H, and 13C NMR spectroscopic studies to determine mangiferin pKa values. Spectrochim. Acta - A: Mol. Biomol. Spectrosc.. 2006;64:1002-1009.
- [CrossRef] [Google Scholar]
- Isolation and characterization of novel bacteriophages as a potential therapeutic option for Escherichia coli urinary tract infections. Appl. Microbiol. Biotechnol.. 2021;105:5617-5629.
- [CrossRef] [Google Scholar]
- The Blue Obelisk—interoperability in chemical informatics. J. Chem. Inf. Model.. 2006;46:991-998.
- [CrossRef] [Google Scholar]
- Identification of a novel therapeutic target against XDR Salmonella Typhi H58 using genomics driven approach followed up by natural products virtual screening. Microorganisms. 2021;2021(9):2512.
- [CrossRef] [Google Scholar]
- Phylogenetic group B2 expressed significant biofilm formation among drug-resistant uropathogenic Escherichia coli. Libyan J. Med.. 2021;16:1845444.
- [CrossRef] [Google Scholar]
- Degradation products of mangiferin by gamma irradiation with inhibitory effects on NO production. Biosci. Biotechnol. Biochem.. 2016;80:2022-2024.
- [CrossRef] [Google Scholar]
- Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci.. 2004;101:1333-1338.
- [CrossRef] [Google Scholar]
- Antimicrobial activities evaluation and phytochemical screening of some selected medicinal plants: a possible alternative in the treatment of multidrug-resistant microbes. PLoSOne. 2021;16:e0249253.
- [Google Scholar]
- Detection of biofilm formation in Staphylococcus aureus. does it have a role in the MRSA infections. Trends Med. Res.. 2011;6:116-123.
- [Google Scholar]
- Techniques for analysis of plant phenoliccompounds. Molecules. 2013;18:2328-2375.
- [CrossRef] [Google Scholar]
- Antagonistic trait of Staphylococcus succinus strain AAS2 against uropathogens and assessment of its in vitro probiotic characteristics. Microb. Pathog.. 2018;118:126-132.
- [Google Scholar]
- The relationship between antibiotic susceptibility and pH in the case of uropathogenic bacteria. Antibiotics. 2021;10:e1431.
- [Google Scholar]
- Kırmusaoğlu, S., 2019. The methods for detection of biofilm and screening antibiofilm activity of agents. Antimicrobials, antibiotic resistance, antibiofilm strategies and activity methods, 1-17
- Koneman, E.W., Allen, S.D., Janda, W.M., Schreckenberger, P.C., Winn, W.C., 1994. Introduction to diagnostic microbiology. J.B. Lippincott company, 527, 544
- Mango (Mangifera indica L.) leaves: nutritional composition, phytochemical profile, and health-promoting bioactivities. Antioxidants. 2021;10:299.
- [CrossRef] [Google Scholar]
- Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect.. 2012;18:268-281.
- [Google Scholar]
- Uroplakins and their potential applications in urology. Cent. Eur. J. Urol.. 2016;69:252-257.
- [Google Scholar]
- Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem.. 1998;19:1639-1662.
- [CrossRef] [Google Scholar]
- Physicochemical characteristics, bioactive compounds and industrial applications of mango kernel and its products: a review. Compr. Rev. Food Sci. Food Saf.. 2020;19:2421-2446.
- [CrossRef] [Google Scholar]
- Antioxidant activities and stability of seed Kernel extracts from mango (Mangifera indica linn.) cultivated in Northern Thailand. Chiang Mai J. Sci.. 2017;44:573-583.
- [Google Scholar]
- Antimicrobial and antibiofilm activities of phenolic compounds extracted from Populus nigra and Populus alba buds (Algeria) Bra. J. Pharma. Sci.. 2019;55
- [CrossRef] [Google Scholar]
- Complicated urinary tract infection in adults. Can. J. Infect. Dis. Med. Microbiol.. 2005;16:349-360.
- [Google Scholar]
- Extracts of Gomphrena celosioides Mart as potential treatment for urinary tract infections against antibiotic resistant β-lactamase producing uropathogens. S. Afr. J. Bot.. 2020;132:502-510.
- [CrossRef] [Google Scholar]
- Prevalence of urinary tract infections and current scenario of antibiotic susceptibility pattern of bacteria causing UTI. Indian J. Microbiol. Res.. 2018;5:334-338.
- [CrossRef] [Google Scholar]
- Extraction of curcuminoids from Curcuma longa: comparative study between batch extraction and novel three phase partitioning. Prep. Biochem. Biotechnol.. 2019;49:407-418.
- [CrossRef] [Google Scholar]
- Antioxidant activity of common plants of Northern Tamil Nadu. India. Int. J. Pharm. Pharm. Sci.. 2014;6:128-132.
- [Google Scholar]
- Anti-pathogenic, anti-diabetic, anti-inflammatory, antioxidant, and wound healing efficacy of Datura metel L. leaves. Arab. J. Chem.. 2022;15:104112.
- [CrossRef] [Google Scholar]
- Pharmacognostical and phytochemical studies of Mangifera indica seed kernel. J. Pharm. Res.. 2011;4:4272-4275.
- [Google Scholar]
- In-vitro studies on antitumour and antimicrobial activities of Methanolic kernel extract of Mangifera indica L. cultivar Banganapalli. Biomed. Pharmacol. J.. 2019;12:357-362.
- [CrossRef] [Google Scholar]
- Biofilm formation by multidrug resistant Enterobacteriaceae strains isolated from solid organ transplant recipients. Sci. Rep.. 2019;9:e8928.
- [Google Scholar]
- Drug target identification and elucidation of natural inhibitors for Bordetella petrii: an insilico study. Genom. Inform.. 2016;14:241-254.
- [CrossRef] [Google Scholar]
- Utilization of mango kernel oil for the rhamnolipid production by Pseudomonas aeruginosa DR1 towards its application as biocontrol agent. Bioresour. Technol.. 2016;221:291-299.
- [CrossRef] [Google Scholar]
- Plasma brain natriuretic peptide concentration: impact of age and gender. J. Am. Coll. Cardiol.. 2002;40:976-982.
- [CrossRef] [Google Scholar]
- Therapeutic potential of medicinal plants for the management of urinary tract infection: a systematic review. Clin. Exp. Pharmacol. Physiol.. 2019;46:613-624.
- [Google Scholar]
- High multidrug resistance in urinary tract infections in a tertiary hospital, Kathmandu. Nepal. Pub. Health Action. 2021;11:24-31.
- [Google Scholar]
- The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol.. 2007;117:112-119.
- [CrossRef] [Google Scholar]
- Emerging Gram negative resistance to last-line antimicrobial agents fosfomycin, colistin and ceftazidime-avibactam—epidemiology, laboratory detection and treatment implications. Expert Rev. Anti Infect. Ther.. 2018;16:289-306.
- [Google Scholar]
- Isolation of mangiferin from different varieties of mangifera indica dried leaves. Int. J. Sci. Eng. Res.. 2014;5:928-934.
- [Google Scholar]
- Risk factors for community-and health facility–acquired extended-spectrum β-lactamase–producing bacterial infections in patients at the University of Minnesota Medical Center. Fairview. Am. J. Infect. Control. 2012;40:849-853.
- [CrossRef] [Google Scholar]
- Evaluation of the antimicrobial and antivirulent potential of essential oils isolated from Juniperus oxycedrus L. ssp. macrocarpa aerial parts. Microorganisms. 2022;10:758.
- [CrossRef] [Google Scholar]
- A sticky business: the extracellular polymeric substance matrix of bacterial biofilms. Microbial Biofilms. 2004;174–191
- [CrossRef] [Google Scholar]
- Simultaneous estimation of mangiferin and four secoiridoid glycosides in rat plasma using liquid chromatography tandem mass spectrometry and its application to pharmacokinetic study of herbal preparation. J. Chromatogr. B. 2007;858:211-219.
- [CrossRef] [Google Scholar]
- UroPathogenic Escherichia coli (UPEC) infections: virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front. Microbiol.. 2017;8:e1566.
- [Google Scholar]
- Prevalence of UTI in different age groups in a tertiary care hospital and their antibiogram. Int. J. Contemp. Med.. 2018;5:3-6.
- [Google Scholar]
- A global perspective on improving patient care in uncomplicated urinary tract infection: expert consensus and practical guidance. J. Glob. Antimicrob. Res.. 2022;18:18-29.
- [Google Scholar]
- Kinetics of uropathogenic Escherichia coli metapopulation movement during urinary tract infection. MBio. 2012;3:e00303-e00311.
- [CrossRef] [Google Scholar]
- Motion tracking: No silver bullet, but a respectable arsenal. IEEE Comput. Graph. Appl.. 2002;22:24-38.
- [CrossRef] [Google Scholar]
- Effects of Nidus vespae extract and chemical fractions on glucosyl transferases, adherence and biofilm formation of Streptococcus mutans. Arch. Oral Biol.. 2007;52:869-875.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102688.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







