Translate this page into:
Phylogenetic analyses and pathogenic diversity of Meloidogyne graminicola of rice (cv. BRRI Dhan28) from different agro-ecological zones of Bangladesh
⁎Corresponding author. atiq.ppath@bau.edu.bd (Md. Atiqur Rahman Khokon)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Rice root-knot caused by Meloidogyne graminicola is a major yield-limiting factor in all rice growing countries around the world, which is often ignored in Bangladesh due to its subtle and obscure above ground symptoms. The current study proposes a comprehensive diagnosis of rice root-knot nematode at the molecular level and analyzes the pathogenic diversity of M. graminicola population of different AEZs. Rice seedlings of a popular variety (BRRI Dhan28) showing galls in the roots were collected from thirty Agro-Ecological-Zones (AEZs) of Bangladesh. For molecular identification, nuclear DNA was collected from single second-stage juvenile and internal transcribed spacer (ITS) was used as a universal primer. Molecular identification and phylogenetic analyses revealed that all nematode populations of galled rice seedlings from different locations belong to M. graminicola. The nematode populations constitute six phylogenetic clades. Nematode population of phylogenetic clade II showed the highest no. of galls (178 ± 1.67), although galling index was higher and similar in clade I, clade-II, clade-III and clade-VI. The highest percent reduction in both vegetative and yield-contributing traits were recorded in clade-II. A negative correlation was found in galling incidence and all growth and yield parameters. Comparative hatching of M. graminicola from different locations reveals that the pathogenicity of M. graminicola depends on the genetic variability of the nematode population, but not on the hatched juveniles. It might be concluded that the rice root-knot nematodes (M. graminicola) are prevalent in all agro-ecological zones of Bangladesh, despite the most pathogenic groups being frequent in AEZ- 1, 6, 7, 10, 18, 19, 21 and appropriate management initiatives are therefore crucial for maximizing rice yield.
Keywords
Hatching
Meloidogyne graminicola
Pathogenicity
Phylogenesis
Rice
1 Introduction
Rice (Oryza sativa L.) ensures the nutritional security of more than half of the world’s human population. In Bangladesh, 75–80 % of cultivable land is occupied for producing 390.95 lakh metric tons of rice (BBS, 2024). In three seasons, namely aus, aman, and boro, indigenous, high-yielding, and hybrid rice varieties are cultivated every year in Bangladesh. A considerable yield reduction in rice is primarily attributed to plant parasitic nematodes (PPN) along with other 100 pests (Ali et al., 2021). Rice is the primary host of more than 200 Plant Parasitic Nematodes (PPN) species (Narasimhamurthy et al., 2023), which significantly reduces yield. In Bangladesh, 16–20 % yield loss is reported in low land rain-fed rice cultivation, while in India, 16–32 % under irrigated conditions and 11–73 % under flooded conditions are also reported (Narasimhamurthy et al., 2023, Tian et al., 2018).
Four species of Meloidogyne occur only on upland rice; Meloidogyne graminicola (Bangladesh, USA, India, Nepal, Myanmar, Laos, Thailand, Philippines, South East Asia, Vietnam, and South Africa), Meloidogyne arenaria (Nigeria, Egypt, South Africa), Meloidogyne salasi (Costa Rica and Panama), M. oryzae in Surinam of irrigated rice, Meloidogyne incognita (Costa Rica, Cuba, Egypt, Ivory Coast, Nigeria, South Africa, and Japan) and Meloidogyne javanica (Brazil, Egypt, Comoro Island, Nigeria, and Ivory Coast were reported by Narasimhamurthy et al., (2023). According to various studies (Golden and Birchfield 1965; Pokharel et al., 2010), Meloidogyne graminicola is considered the most destructive from all rice nematode species and is also classified as a quarantine pest. Furthermore, 98 different hosts, including grains, vegetables, and grasses commonly seen in rice fields, support nematodes (Singh et al., 2022). The stationary endoparasite M. graminicola has also adapted to waterlogged conditions.
In Bangladesh, nemic diseases of rice and their negative impact on rice yield are always ignored due to a lack of awareness among the rice growers and proper knowledge of identification. Identification of rice root-knot nematodes (M. graminicola) especially at the species level requires ample morphological data and below-ground symptoms like ‘hook-like’ galls produced on the tip of rice roots. Molecular identification especially employing nuclear ribosomal DNA (rDNA) and mitochondrial DNA (mtDNA) has been largely used for quick and accurate identification (McClure et al., 2012; Htay et al., 2016). Additionally, genetic diversity assessment using DNA sequences is effective for screening resistant germplasms against specific nematode populations (Hesar et al., 2011). The correct identity of nematodes genetic variation among the population and pathogenic diversity is imperative for developing management strategies, utilizing resistant cultivars, and tracking population movement (Das et al., 2021; Adam et al., 2007).
Previous studies (Man Luo et al., 2020; Cabasan et al., 2017) reported that DNA extracted from multiple second-stage juvenile nematodes has been utilized as a traditional approach for the identification of RKN. DNA extracted from individual second-stage juvenile nematodes has been reported by Jabbar et al. (2021), which requires less time and chemical reagents. In depth, identification of rice root-knot nematodes at the molecular level and information about root-knot nematode species diversity in Bangladesh is lacking. This research was aimed at molecular identification, assessing the genetic variation and pathogenic diversity of rice root-knot nematodes of a popular rice variety (BRRI Dhan28) grown in different agro-ecological zones of Bangladesh.
Throughout the entire experimentation, we collected the nema-infested rice seedlings from across the country maintaining 30 agro-ecological zones and inoculated them in previously growing rice seedlings in pot for maintaining generations to work with these in the future. Then, the nematodes were identified at the molecular level and constructed phylogenetic tree. Based on the phylogenetic groups we conducted hatching experiments and analyzed the pathogenicity to reveal the variability.
2 Materials and methods
2.1 Sampling and maintenance of nematode population
RKN-infested rice plants were collected from 90 rice-growing areas in Bangladesh, consisting of 30 agro-ecological zones (AEZs) (Supplementary table 1). Rice plants exhibiting above-ground symptoms, viz., characteristic hook-shaped galls, were considered for sampling (Narasimhamurthy et al., 2023, Leidy et al., 2021). RKN-infested rice plants were carefully uprooted, and galls were collected and immediately transferred to the laboratory for the study. For future experiments, the nematode generations were maintained in the potting soil by culturing rice plants in a net house. Then, twenty characteristic galls were separated with scissors from the roots and inoculated in the root zone area of the previously planted 26-day-old rice seedlings (BRRI dhan28). Altogether, 30 pots were maintained for 30 AEZ nematodes. Before inoculation, the soils of the pot were sterilized with 5 % formalin, and all necessary nutrients were incorporated. Regular watering and weeding were also done. The roots of inoculated plants were examined after 30 days for gall formation (Bellafiore et al., 2015).
2.2 DNA extraction, polymerase chain reaction, and gel documentation
Genomic DNA was extracted from single second-stage juveniles (J2) nematode of different AEZs following the protocol described by Das et al., 2021 with modifications. At first, characteristic galls of each AEZ were kept in water for hatching. The next day freshly hatched single live J2 was taken on the slide with a drop of distilled water and cut into several pieces under stereo-binocular microscope with a sterile needle then transferred into a PCR tube which was previously filled with 20 µl of warm lysis buffer (Singh et al., 2023) and incubated at −20⁰ C for 20 min and added 1 µl of proteinase K. Afterwards, it was incubated at 65⁰c for 1 hr and at 95⁰c for 10 mins. The stock solution of crude DNA was kept at −20⁰ C for future purposes.
The crude DNA was used as a template and amplified using universal primers (Supplementary table 2). The amplification reaction for PCR involved preparing a 25 μL PCR mix. This mix was created by combining 12.5 μL master mix (Promega, Madison, WI, USA), 9.5 μL of nuclease-free water, 1 μL each of 10 μM forward and reverse primers, and 1 μL of the corresponding template DNA. The designated part of the Internal Transcribed Spacer (ITS) was amplified using the forward primer 18S and the reverse primer 26S (Supplementary table 2). DNA amplification was carried out using a thermal cycler (Techne, UK) according to the instructions provided in Supplementary table 3.
2.3 Nucleotide sequencing and phylogenetic analyses
For electrophoresis6μL of PCR mix from each sample was loaded into a 1 % agarose gel and stained with Ethidium Bromide (0.5 mg/ mL) for visualization. The PCR products that were chosen were randomly put into the gel in a random sequence. The process of electrophoresis was carried out using the Tris-Borate-EDTA (TBE) buffer at 100 V for 27 min. The DNA bands were stained and images were captured using ultraviolet (UV) light with the Gel View Master device (Dynamica Scientific Ltd). The size of PCR products was evaluated by comparing them to the molecular weight marker ladder of 100 bp (Fanelli et al., 2017). Based on the sequence of the primers, the amplicon size was expected to be visualized at 800 bp. The partial sequence was done by a commercial sequence provider (Invitrogen) in Shanghai, China. The same primers were utilized for sequence reaction those were used in the PCR reaction.
The partial sequence of the nematode population using universal primer and the sequences of the closest relatives of NCBI GenBank using BLAST homology were aligned to construct the phylogenetic tree (Supplementary table 4). Hirschmennielia oryzae was considered as outgroup (Htay et al., 2016; Fanelli et al., 2017). Phylogenetic tree was constructed based on the neighbour-joining method to identify the closest neighbour of the existing nematode population. A distance-based tree was also constructed to understand any genetic diversity among the nematode population collected from different AEZs of Bangladesh. The MEGA 11 software was exploited to construct the phylogenetic tree.
2.4 Pathogenicity test
The nematode populations belonging to different phylogenetic clades were subjected to pathogenicity test in a susceptible rice cultivar BRRI Dhan28. Plastic pots (16 cm x 25 cm) were filled with 6 kg sterilized sandy loam soil. Previously raised rice seedlings (25 days old) were transplanted in the puddle of pots. Six seedlings were initially transplanted and a week later single seedlings were kept in each pot. Nematodes were selected randomly from each phylogenetic clade for inoculation. To collect freshly hatched nematodes, galls were put in a hatching tube (Das et al., 2021; Khokon et al., 2009) and filled with tap water. Freshly hatched 3000 s stage juveniles (J2) were incorporated (1500 J2s at 15 DAT followed by 1500 J2s after two days of first application) at the base of seedlings by making holes (5 cm) for ensuring adherence of nematodes to the roots. Seedlings without nematode inoculation were considered as the control treatment. Fertilization and irrigation were maintained accordingly. The inoculated plants were examined after 35 days for gall formation. The experiments were laid out following a completely randomized design (CRD). The responses of the nematodes from every phylogenetic clade were recorded at the vegetative stage (fresh root and shoot weight, Plant height and root length) and yield contributing traits were(number of tillers /plants, panicles/plant, panicle length, spikelet's /panicle, percentage of filled and unfilled grain /panicle, weight of 100 grains /plant and grains weight/plant) were recorded. After harvest galling index was examined for assessing the aggressiveness of nematodes (Mukesh et al., 2024; Das et al., 2021; Hussey and Jansen, 2002).
2.5 Hatching test
Hatching ability of M. graminicola galls collected from different agro-ecological zones of Bangladesh was done in a hatching tube following the non-linear model where non-linear least-square fitting was exploited (Das et al., 2021; Khokon et al., 2009). Ten uniform and well-developed galls having typical hook-like structures were randomly chosen from each phylogenetic clade. The hatching was done in tap water. Total numbers of hatched juveniles were counted under stereo-binocular microscope each week. The galls were again immersed in tap water. The hatching was continued for 5 weeks until the minimum number of juveniles were found under the microscope. For each treatment, three replicates were maintained following a CRD design.
2.6 Statistical analyses
The statistical analyses were conducted using Statistix 10, SAS, and MS Excel. One-way analysis of variance (ANOVA) was conducted to determine the significance of mean difference of populations of M. graminicola for pathogenicity and hatching. Data on pathogenicity testing were analysed using a generalized linear model for analysis of variance (ANOVA) and fitting a Poisson distribution. The Tukey's HSD test was conducted at a significance level of 5 % to identify any significant differences between the means.
3 Results and discussion
3.1 Phenotypic expression of nema-infested rice seedlings
Rice nursery beds were visited in 30 agro-ecological zones of Bangladesh to examine the nematode- infested seedlings for detecting any visible abnormalities in a rice variety BRRI Dhan28. The infested seedling showed stunted growth, chlorosis having severely affected root system. Characteristic hook-shaped galls were observed in different intensities. These galls did not show any external egg masses on the roots. Unlike other Meloidogyne spp. rice root-knot nematode laid egg within the root cortex which is generally inconspicuous. These symptoms are similar that was reported by Leidy et al., 2021. These findings indicated that rice root-knot nematodes are well distributed in all AEZs of Bangladesh.
3.2 Molecular identification and diversity of RKN of rice
For molecular identification, a modified method was followed for extracting genomic DNA from single second- stage live juvenile. Universal molecular marker (Internal Transcribed Spacer – ITS: 18SF/26SR) were used (Supplementary table 2) for Polymerase Chain Reaction (PCR) and visualized by gel electrophoresis in ethidium bromide (Fig. 1A, 1B). A characteristic amplicon of 800 bp was observed during gel electrophoresis. Individually hatched juveniles from every AEZ were identified using universal primers (Supplementary table 2). Out of 30 AEZs, quality DNA was effectively extracted from 26 AEZs which were then utilized for PCR. The PCR data indicated that all nematode populations from different locations belong to the nematode genus Meloidogyne. The molecular study further reveals that rice root-knot nematode is frequent in BRRI Dhan28 in almost all AEZs of Bangladesh. The PCR product of respective AEZ was sequenced and compared with the GenBank sequence of NCBI. Out of 30 AEZs 26 samples gave characteristic bands and were finally considered as M. graminicola based on the sequence homology of Genbank accessions (Supplementary table 4). Due to improper DNA concentration, 4 samples did not give the sequence. The utilization of ITS region for the correct identification of nematodes was reported by several researchers (Jabbar et al., 2021).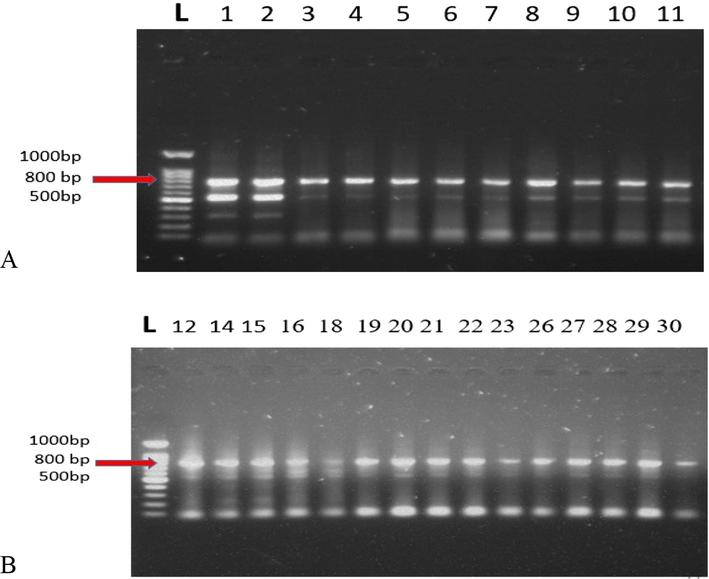
Amplification of genomic DNA using universal primers(18SF/26SR) for Meloidogynegraminicola. L:100 bp DNA ladder 1–30. Nematode samples from different AEZs of Bangladesh (A, B). An amplicon of 800 bp is visible for all nematode samples.
3.3 Phylogenetic analyses of the M. graminicola populations
For analyzing genetic distances and phylogenetic relationship of the populations of M. graminicola of different AEZs of Bangladesh based on the sequenced ITS region using neighbour joining method phylogenetic tree was constructed as given in Fig. 2. The phylogenetic tree showed that M. graminicola populations formed distinct groups and showed the highest homology with M. graminicola populations mostly from India, China, and the Philippines. The M. graminicola populations from different agro-ecological zones of Bangladesh formed different phylogenetic clade and also the length of the node is different meaning that a considerable level of genetic diversity exists among the populations of M. graminicola. The node length of the populations of MG16, MG12, MG20, MG22, MG30, MGO5, MG02, MG06, MG10, MG07, MG19, MG18, MG21, MG03, MG28, MG15, MG04 and MG29 longer compared to Indian and Philippines populations indicated that M. graminicola populations of Bangladesh are distantly related with the Indian and Philippines populations, while another group of nematode(MG01, MG08, MG11, MG26, MG23, MG14, MG09 and MG27) showed the highest homology with Chinese population but with the highest genetic distances.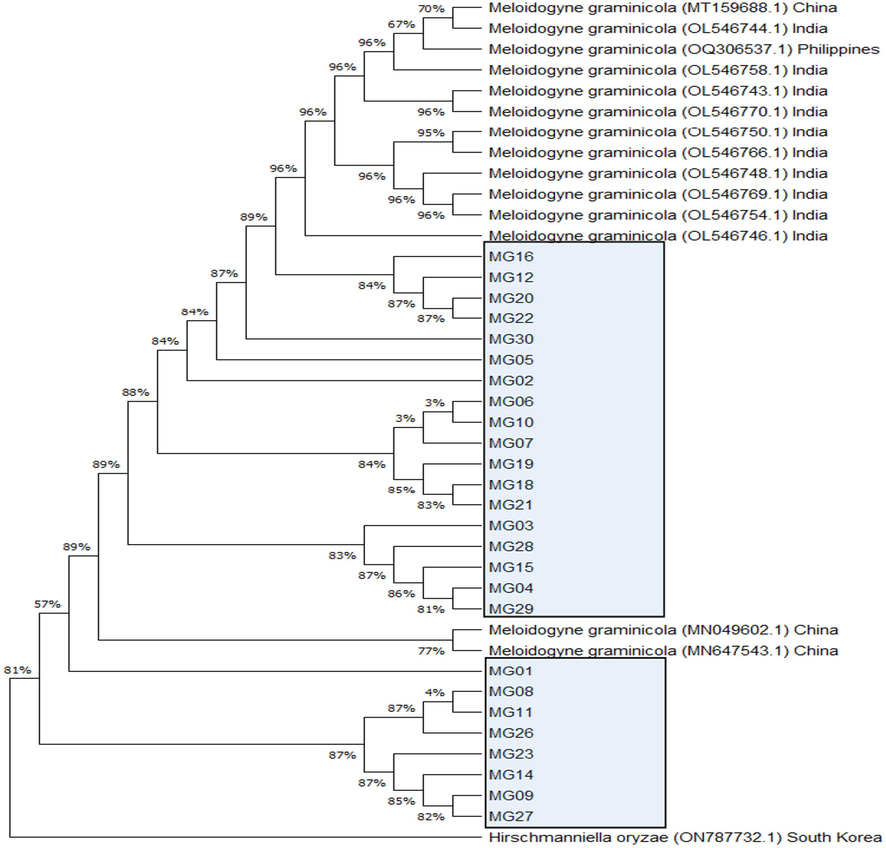
Phylogenetic tree of partial 18SF and 26SR universal sequence using Neighbor-Joining method describing the evolutionary relationship of the populations of M. graminicola with the closest neighbor of Genbank accessions of NCBI. The evolutionary distances were measured using the Maximum Composite Likelihood method and are in the units of the number of base substitutions per site. Taxon names contain the name of root-knot nematode populations, accession number of GenBank with their location, accessions of best matches in GenBank based on BLASTN against the sequences of one RKN species recovered from this study and the accession of an out-group (Hirschmennielia oryzae).
To understand the intra-generic variation among the M. graminicola population, a distance-based tree was also constructed (Fig. 3). Nematode populations from 26 AEZs of Bangladesh belong to six phylogenetic clades where MG26, MG11 and MG08 showed the highest genetic distances compared to other populations. Both phylogenetic analyses indicate that M. graminicola present in all rice fields (BRRI Dhan28) in all agro-ecological zones of Bangladesh having considerable genetic variation. Singh et al., (2023) and Fanelli et al., (2017) reported the phylogenetic variations among the populations of M. graminicola using ITS-based sequence.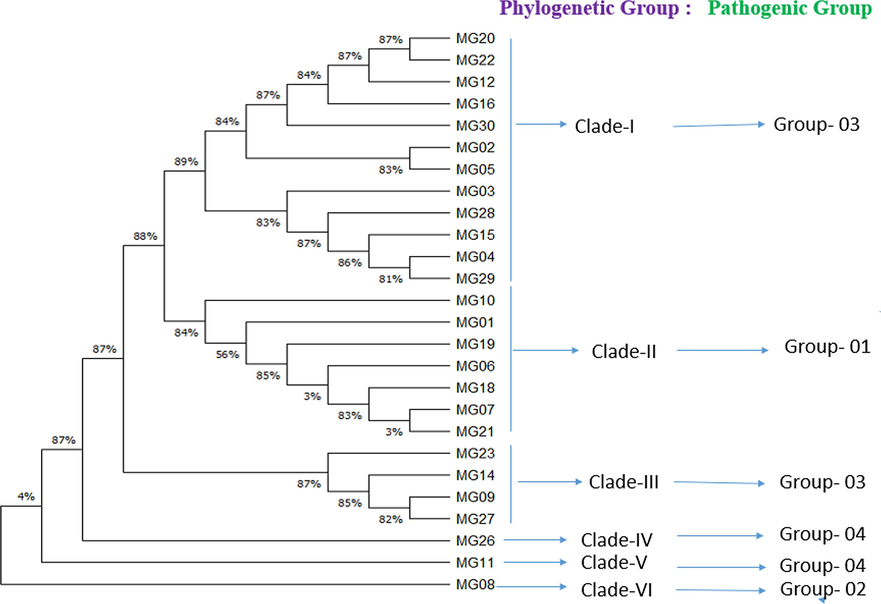
Phylogenetic tree of partial 18SF and 26SR universal sequence using a distance-based tree methoddescribing the evolutionary relationship among the populations of M.graminicolawith pathogenic groupcollected from different agro-ecological zones of Bangladesh.
3.4 Pathogenic variability
Whether genetic variability of the nematode populations is related to pathogenic variability, experiments were conducted to observe gall formation, and variation in agronomic and yield contributing traits were examined. Freshly hatched second-stage juveniles were inoculated in a susceptible rice cultivar (BRRI Dhan28) and the galling ability of nematode from each phylogenetic clade was evaluated at 95 days after inoculation (Fig. 4). Total number of galls/root system and galling index both varied significantly among the phylogenetic clades. Total number of galls /root system (178 ± 1.67) and galling index (5 ± 0) was highest in clade-II. Statistically different but higher no. of gall/root system (123 ± 3.53) and galling index (5 ± 0) was found in clade −VI. Nematode population of clade-II produced the highest number of galls indicating that these nematode populations are more aggressive. Because root galls can limit the uptake of water and nutrients by the roots. A great severity of root galling can thus cause considerable damage affecting plant growth and reducing yield (Table 1, Fig. 5). The severity of root galling can affect yield is reported (De Waele et al., 2013, Zhou et al., 2000; Anowar and McKenry, 2007; Fanelli et al., 2017). Values are the means of the six populations of six phylogenetic groups followed by the same letter are not significantly different among the six M. graminicola populations according to Tukey’s HSD test (P<0.05). (n = 3).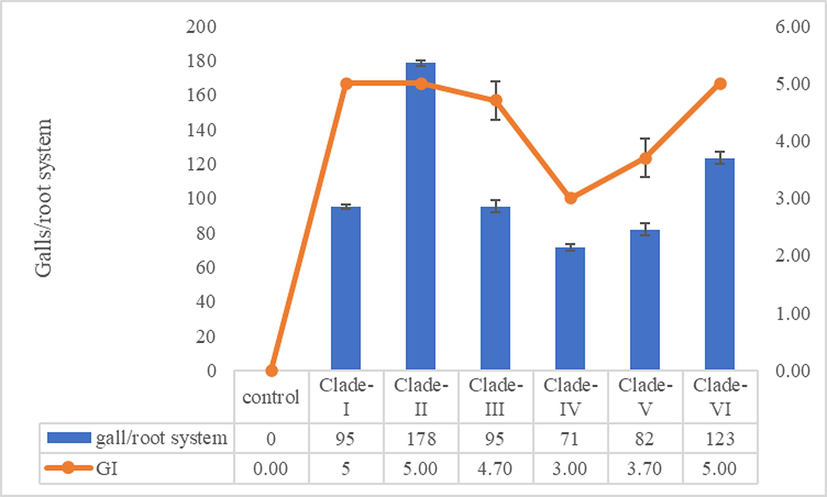
Pathogenic variability of Meloidogyne graminicola populations in BRRI Dhan28 in net-house experiment under artificial inoculation with nematodes of different phylogenetic clades.
Phylogenetic clade
Root length(cm)
Plant height(cm)
Fresh root weight(g)
Fresh shoot weight(g)
Clade-I
34.33B
105AB
99.54A
92.67B
Clade-II
26.6C
90C
73.33B
75.67C
Clade-III
34.33B
107.83A
103A
103B
Clade-IV
34.67B
112.83A
105A
128A
Clade-V
33.67B
109A
103.67A
125.67A
Clade-VI
27.67C
95BC
75B
76.67C
Control
41.67A
115A
110.67A
130A
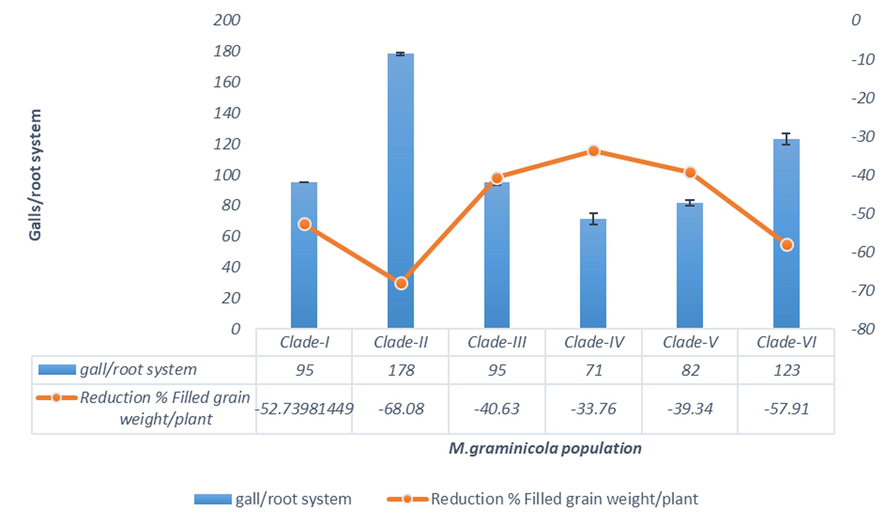
Pathogenic variability of Meloidogyne graminicola populations in reduction % filled grain weight/ plant of BRRIDhan28 in net-house experiment under artificial inoculation with nematodes of different phylogenetic clades.
3.4.1 Variability in agronomic and yield contributing traits
Variations among the phylogenetic groups were further assessed to understand whether the genetic variation is related to pathogenic variation. Different agronomic traits were observed after inoculation with nematodes of different phylogenetic clades (Table 1). All the agronomic traits showed significant variation in different clades. The lowest root length was recorded in clade −II which is 36.0 % lower compared to untreated control. The lowest plant height (90.0 cm), fresh root weight (73.3 g) and fresh shoot weight (75.6 g) was also observed in clade-II, compared to control, and 21.7 %, 33.7 % and 41.79 % reductions were observed respectively.
Several yields contributing traits showed significant differences (Table 2). The lowest no. of tillers/ plant (13.0), no. of spikelets/panicle (61.7), panicle length(19.2), no. of panicle/ plant (11.7), % filled grains/panicle(68.3), weight of 100 grains(1.84) and filled grains weight(9.08) was recorded in clade-II which was 40.9, 37.29,22.17,31.37,15.6,12.22 and 68.9 % lower compared to untreated control. Values are the means of the same populations of six phylogenetic groups followed by the same uppercase letter are not significantly different among the six M. graminicola populations according to Tukey’s HSD test (P<0.05). (n = 3).
Phylogenetic clade
Number of tillers/plants
Number of panicles/plants
Panicle length
Number of spikelets /panicles
Total number of spikelets/plants
% of filled grains/panicle
Weight of 100 grain(g)
Filled grain weight/plant(g)
Total Filled grain/plant(n)
Total unfilled grain/plant(n)
% of unfilled grains/panicle
Clade-I
18.7AB
12.7AB
21.3A
75.0C
948.67 BC
74.3C
1.9ABC
13.4BC
705 BCE
243.67A
25.667B
Clade-II
13C
11.7B
19.2A
61.67E
719.33C
68.3D
1.84C
9.08C
492C
227.33A
31.667A
Clade-III
19AB
13.7AB
20.7A
79.3B
1084.3 BC
76.3BC
2.03ABC
16.85B
827.33B
257.33A
23.667 BCE
Clade-IV
19.7A
14.3AB
21.0A
81.0B
1159B
78.0AB
2.07AB
18.75B
902.67B
256.33A
22CD
Clade-V
19.3AB
13.7AB
22.3A
79.3B
1084.3 BC
76.0BC
2.09A
17.24B
824.67B
259.67A
24 BC
Clade-VI
14BC
13AB
20.7A
72.0D
935.67 BC
68.6D
1.86BC
12.01BC
642.67 BC
293A
31.333A
Control
22A
17A
24.7A
98.33A
1671.3A
81.0A
2.1A
28.4A
1354A
317.33A
19 D
In case of both agronomic- and yield-contributing traits, the nematode populations from clade-II significantly reduced all parameters compared to other nematode groups, and untreated control considerable and statistically similar reduction also occurred in clade-VI (Table 3). These results reveal that the genetic diversity of M. graminicola significantly impacts on pathogenicity. The nematodes population of clade-VI can also cause severe damage to rice plants (BRRI Dhan28).Whether these two phylogenetic groups can cause similar damage to other varieties of rice can be an aspect of future research. In the present study, 36.0 %, 21.7 %, 34 %, and 41.9 % reductions were observed in root length, plant height, fresh root weight, and fresh shoot weight respectively compared to control treatment. Experiments conducted by Cabasan et al., 2017 where also reported 13.3 %, 27.3 %, 25.8 % reduction in plant height, fresh root weight, and fresh shoot weight in rice due to M. graminicola infestation which validates our research findings.
Phylogenetic clade
Root length (cm)
Fresh root weight(g)
Fresh shoot weight(g)
Plant height at the maturity (cm)
Number of tillers/plant
Number of panicles/plants
Panicle length(cm)
Number of spikelets/panicles
Total number of spikelets/plants
% of filled grains/panicle
Weight of 100 grain (g)
Filled grain weight/plant(g)
Total Filled grain(n)
Total unfilled grain(n)
% of unfilled grains/panicle
Clade-I
−17.61
−10.05
−28.72
−8.70
−15.15
−25.49
−13.53
–23.73
−44.23
−8.23
−9.52
−52.74
−47.85
–23.23
35.09
Clade-II
−36.01
–33.74
−41.79
−21.74
−40.91
−31.37
–22.17
−37.29
−58.36
−15.64
−12.22
−68.08
−63.69
−28.27
66.67
Clade-III
−17.61
−6.93
−20.77
−6.23
−13.64
−19.61
−16.23
−19.32
−36.00
−5.76
−3.02
−40.63
−38.88
−19.21
24.56
Clade-IV
−16.81
−5.12
−1.54
−1.88
−10.61
−15.69
−14.88
−17.62
−31.30
−3.70
−1.11
–33.76
–33.12
−19.58
15.79
Clade-V
−19.21
−6.33
−3.33
−5.22
−12.12
−19.61
−9.47
−19.32
−36.00
−6.17
−0.48
−39.34
−39.14
−18.07
26.32
Clade-VI
–33.61
–32.23
−41.03
−17.39
−36.36
–23.53
−16.23
−26.78
−45.11
−15.23
−11.43
−57.91
−52.55
−7.70
64.91
Control
−0.00
0.00
0.00
0.00
0.00
0.00
−0.00
0.00
0.00
0.00
−0.00
0.00
0.00
0.00
0.00
3.4.2 Variability in hatching ability of M. graminicola
Whether the genetic variability of nematodes has any impact on their hatching, an experiment was conducted in tap water. No significant variation among the phylogenetic groups was observed in hatching up to 5 weeks. The majority of the eggs were hatched within 5 weeks of incubation and gradually decreased afterward. In this study, eggs deposited in the seedlings did not show any remarkable variation in hatching. This experimentation indicates that the genetic variation of nematode is not directly related to the hatching ability of M. graminicola (Table 4). Different hatching pattern of Meloidgyne sp. exist that depend on the types of hosts and hosts age (Khokon et al., 2009). In the future study, it would be interesting to examine any kind of variation of hatching in senescing galls of M. graminicola. Values are log transformed. Each hatching value represents the percent of three replicates.
Phylogenetic clade
Percent hatched M. graminicola at different weeks after incubation (WAI)
1st WAI
2nd WAI
3rd WAI
4th WAI
5th WAI
Clade-I
1.1431 ± 0.0361
1.2299 ± 0.0148
1.2036 ± 0.0157
1.1754 ± 0.0167
0.9524 ± 0.0280
Clade-II
1.1754 ± 0.0167
1.2539 ± 0.0249
1.1735 ± 0.0337
1.1454 ± 0.0179
0.9985 ± 0.0252
Clade-III
1.1405 ± 0.0502
1.1999 ± 0.0436
1.1735 ± 0.0337
1.1754 ± 0.0167
0.9008 ± 0.0315
Clade-IV
1.0782 ± 0.0209
1.1741 ± 0.0301
1.1440 ± 0.0301
1.1735 ± 0.0337
0.9524 ± 0.0280
Clade-V
1.1440 ± 0.0301
1.1438 ± 0.0323
1.1115 ± 0.0323
1.1131 ± 0.0193
0.9008 ± 0.0315
Clade-VI
1.1454 ± 0.0301
1.1438 ± 0.0323
1.1440 ± 0.0301
1.1754 ± 0.0167
0.9985 ± 0.0252
3.5 Correlation matrices of agronomic and yield-contributing traits
The correlation study between agronomic and yield contributing traits with gall numbers per root system showed significant and negative relations except total number of unfilled grains (Supplementary Fig. 1). These results indicate that galling incidence varied in different phylogenetic clades which are inversely related to all agronomic and yield-contributing traits of rice.
Molecular identification of rice root-knot nematode has not been reported in Bangladesh so far (Supplementary table 5). Therefore, this is the first report of its kind in Bangladesh. This research also indicates that nematode populations are prevalent in all geographical regions. The future research should focus on the availability of resistant varieties of rice, the inclusion of effective biological agents that can be fitted in the existing IDM package for the management of root-knot disease of rice.
Funding
The research is fully funded by Bangabandhu Science and Technology Fellowship Trust, Ministry of Science and Technology.
CRediT authorship contribution statement
Nargis Akhter: Writing – original draft, Validation, Methodology, Investigation. Mohammad Tofazzal Hossain Howlader: Resources, Investigation, Data curation. Md. Atiqur Rahman Khokon: Writing – review & editing, Supervision, Project administration, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Molecular diagnostic key for identification of single juveniles of seven common and economically important species of root-knot nematode (Meloidogyne spp.) Plant Pathol.. 2007;56(1):190-197.
- [CrossRef] [Google Scholar]
- Ali, M.P., Nessa., Khatun, M.T., Salam, M.U., Kabir, M.S., 2021. A Way Forward to Combat Insect Pest in Rice. Bangladesh Rice J. 25 (1),1-22. doi.org/10.3329/brj.v25i1.55176.
- Bangladesh Bureau of Statistics, 2024. Yearbook of Agricultural Statistics 2023. Statistics and Information Division (SID). Ministry of Planning. Government of People’s Republic of Bangladesh, Dhaka, Bangladesh. www.bbs.gov.bd. 2024-06-13-05-41-8d348db80ecf814b6f1876432643639e.p.
- Intraspecific variability of the facultative meiotic parthenogenetic root-knot nematode (Meloidogyne graminicola) from rice fields in Vietnam. C. R. Biol.. 2015;338:471-483.
- [CrossRef] [Google Scholar]
- Cabasan, M. T. N., Kumar, A., Bellafiore, S., Waele, D.D. 2017. “Reproductive, pathogenic and genotypic characterisation of five Meloidogyne graminicola populations from the Philippines on susceptible and resistant rice varieties.” Nematology 20, no. 4,2018., 299-318. doi: 10.1163/15685411-00003142.
- Das S., Wadud, M.A., Heuer H., Khokon, M.A.R., 2021. Genetic variation of Meloidogyne spp. of brinjal reveals their difference in pathogenicity and hatching, Archives of Phytopathology and Plant Protection, 54:19-20, 2375-2396. doi: 10.1080/03235408.2021.1983383.
- Host response of rice genotypes to the rice root-knot nematode (Meloidogyne graminicola) under aerobic soil conditions. Arch. Phytopathol. Plant Protect.. 2013;46:670-681.
- [CrossRef] [Google Scholar]
- Detection and molecular characterization of the rice root-knot nematode Meloidogyne graminicola in Italy. Eur. J. Plant Pathol.. 2017;149:467-476.
- [CrossRef] [Google Scholar]
- Identification of quantitative trait loci underlying resistance and tolerance to the rice root-knot nematode, Meloidogyne graminicola, in Asian rice (Oryza sativa) Mol. Breed.. 2020;40(7):63.
- [CrossRef] [Google Scholar]
- Meloidogyne graminicola (Heteroderidae), a new species of root-knot nematode from grass. Proc. Helminthol. Soc. Wash.. 1965;32(2):228-231.
- [Google Scholar]
- Hesar, A., M., Mogadam, E., M., Maafi, Z., T., 2011. Morphometrical and genetic diversity of Meloidogyne javanica isolates from the north east of Iran. J. Nematode Morphol. Systemat. 14(1), 1–11. https://www.researchgate.net/publication/269785063.
- The development and molecular characterization of a rapid detection method for rice root-knot nematode (Meloidogyne graminicola) Eur. J. Plant Pathol.. 2016;146:281-291.
- [Google Scholar]
- Hussey, H., Janssen, G., J., W. 2002. Root-knot nematode: Meloidogyne species. In: Starr, J.L., Cook, R., Bridge, J. (Eds). Plant resistance to parasitic nematodes. Wallingford, UK, CAB International, pp. 43-70. doi: 10.1079/9780851994666.0043.
- Jabbar, A., Javed, N., Munir, A., Abbas, H., Khan, S. A., Moosa, A., Ali, M., A., 2021. Occurrence and molecular characterization of on rice in Central Punjab, Pakistan. Journal of Nematology, 52(1), 1-17. DOI: 10.21307/jofnem-2020-123.
- Quantitative analysis of the effects of diffusates from plant roots on the hatching of Meloidogyne chitwoodi from young and senescing host plants. Biosci. Biotechnol. Biochem.. 2009;73(10):2345-2347.
- [CrossRef] [Google Scholar]
- Kumar A.Khilari K., Yadav G.K., Kumar A., and Yada P., 2022. Evaluation of different basmati varieties of rice against root-knotnematode (Meloidogyne graminicola).Journal of Entomological Research 46, no. 3 (2022): 541-545. DOI :10.5958/0974-4576.2022.00094.9.
- Meloidogyne graminicola—A threat to rice production: Review update on distribution, biology, identification, and management. Biology. 2021;10:1163.
- [CrossRef] [Google Scholar]
- Meloidogyne graminicola population structure in China suggests a south-to-north expansion. Plant Dis.. 2023;107(7):2070-2080.
- [CrossRef] [Google Scholar]
- Incidence of the rice root-knot nematode, Meloidogyne graminicola, in Guangxi, China. Plant Pathol. J.. 2020;36(3):297.
- [CrossRef] [Google Scholar]
- Root-not nematodes in golf course greens of Western United States. Plant Dis.. 2012;96:635-647.
- [CrossRef] [Google Scholar]
- Understanding the dynamics of Meloidogyne incognita infestation in pomegranate orchards of Himachal Pradesh, India (year 2018, 2019 and 2021) and its management strategies. Heliyon. 2024;10(2024):e34752.
- [Google Scholar]
- Narasimhamurthy H.B., Sehgal M., and Naik R.G., 2023. Rice Root-Knot Nematode (Meloidogyne graminicola): A Major Menace in Rice Production. Sustainable Rice Production – Challenges, Strategies and Opportunities. Intech Open Journals Doi: 10.5772/intechopen.107752.
- Identification of Heterodera glycines using PCR with sequence characterised amplified region (SCAR) primers. Nematology. 2008;10(3):397-403.
- [CrossRef] [Google Scholar]
- Variability and the recognition of two races in Meloidogyne graminicola. Australas. Plant Pathol.. 2010;39:326-333.
- [CrossRef] [Google Scholar]
- Potential distribution and biosecurity risks from three economically important plant-parasitic nematodes. Ann. Appl. Biol.. 2022;180(3):371-382.
- [CrossRef] [Google Scholar]
- Detection of Meloidogyne graminicola (RRKN) in Kumaon region of the Indian Himalayas. India J. Agric. Sci.. 2023;93(7):710-714.
- [CrossRef] [Google Scholar]
- Morphological and molecular characterization of the rice root-knot nematode, Meloidogyne graminicola, Golden and Birchfeild, 1965 occurring in Zhejiang, China. J. Integr. Agric.. 2018;17(12):2724-2733.
- [Google Scholar]
- Intraspecific rDNA restriction fragment length polymorphism in the Xiphinema americanum group. Fundam. Appl. Nematol.. 1992;15:563-573.
- [CrossRef] [Google Scholar]
- Phylogenetic relationships among Bursaphelenchus species (Nematoda: Parasitaphelenchidae) inferred from nuclear ribosomal and mitochondrial DNA sequence data. Mol. Phylogenet. Evol.. 2007;43:1185-1197.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103472.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







