Translate this page into:
Oxidative stress mediated cytotoxicity of tellurite in MDA-MB-231 breast cancer cells
⁎Corresponding author at: Department of Microbiology & Molecular Genetics, University of the Punjab, New Campus, Lahore 54590, Pakistan. rehman.mmg@pu.edu.pk (Abdul Rehman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Among different metallic compounds, tellurium (Te) was investigated as having anticancer properties. In the current investigation, MDA-MB-231 breast cancer cell line was cultured with or without tellurite to measure live cells by crystal violet staining. TrxR systems can be acted as a leader for anticancer therapy by eliminating cancerous cells through inhibition of TrxR and glutathione reductase (GR). Oxidative stress, generated in higher concentration in tellurite exposed cells, leads to lower the cell reduced environment such as reduced glutathione GSH) and non-protein thiol. Tellurite exposed cells underwent both type of cell death via necrosis and apoptosis. The lateral was slowed as tellurite concentration increased which was measured by caspase-3 activity, thus this finding would be very useful for determination of type and extent of cell death occurred by tellurite via biochemical and morphological ways. By elucidating the role of tellurite in inducing cell death in carcinoma cells by increased production of reactive oxygen species, this method could be employed for the treatment of carcinoma cells.
Keywords
Tellurite
Breast cancer
Thioredoxin reductases
Oxidative stress
Caspase activity
1 Introduction
In all over the world, deaths due to cancer gain a lead overall and especially in underdeveloped countries (Jemal et al., 2011; Parrilha et al., 2014). Before 1960, the methods used for cancer treatments were surgery and radiotherapy, and later on discoveries of chemical agents against cancer treatment along with combinations were more useful (De Vita and Chu, 2008; Parrilha et al., 2014). Some metals (platinum, gold and bismuth), metalloid (antimony) and metal based molecules (cisplatin) have anticancer and antitumor activities against multiple cancers (Bratsos et al., 2007; Nobili et al., 2010; Lessa et al., 2012). They mostly affect the protein instead of DNA (Gabbiani et al., 2011). Bindoli et al. (2009) reported that inhibition of Thioredoxin reductase (TrxR) is the main cause of cytotoxicity and plays a significant role as anticancer agent (Parrilha et al., 2014; Cai et al., 2012; Soares et al., 2012; de Oliveira et al., 2013).
Among highly conserved systems present in the body, the thioredoxin system is involved in various pathways for the survival of cell, embryogenesis, RNA and DNA synthesis and repair, angiogenesis, and death signalling via oxidation reduction processes. The thioredoxin system is composed of three major components; thioredoxin (Trx), thioredoxin reductase (TrxR), and NADPH (Holmgren and Lu, 2010; Cai et al., 2012; Go and Jones, 2013). Thioredoxin reductase has two isoforms, TrxR1 and TrxR2, the former is abundantly present in cytosol and nucleus while the later is present within the mitochondria, and both isoforms are same in structure and catalysis (Rigobello and Bindoli, 2010; Lu and Holmgren, 2012; Liu et al., 2014).
Selenocysteine (Sec) is a major component, with evenly distributed Gly-Cys-Sec-Gly sequence on C-terminal, in mammalian TrxR and makes it more active enzyme for many substrates according to its specificity (Arner, 2009; Cai et al., 2012). Trx in its reduced form acts as an antioxidant enzyme and become substrate for another enzyme called ribonucleotide reductase used in DNA synthesis (Laurent et al., 1964; Gonzalez Porque et al., 1970; Brot et al., 1981; Gladyshev et al., 1990; Zhong et al., 2000; Arner, 2009; Liu et al., 2014). TrxR is involved in cell growth and helps in tumor proliferation, thus there is need to develop an agent to stop its activity and supports anticancer field (Millet et al., 2005; Cai et al., 2012; Sun et al., 2013; Oehninger et al., 2013; Klossowski et al., 2012; Liu et al., 2014). Te has a strong affinity to bind with thiols and selenol of TrxR, forming a complex which has the potential to induce oxidized environment within the cell (Liu et al., 2014).
This study reports tellurite as an anticancer agent, retarding TrxR activity and tellurite induced cytotoxicity in human breast cancer cells (MDA-MB-231), by stopping TrxR enzyme involved in maintenance of cellular homeostasis and induction of ROS production. The cellular reduced environment gets converted into oxidized (lowering thiol level) and activated the cell death process via apoptosis and necrosis (Liu et al., 2014). The main objective of this work was to inhibit the cancer cells growth by determining the extent of TrxR decreased through tellurite induced ROS production.
2 Materials and methods
2.1 MDA-MB-231 cell culture maintenance
From ATCC human breast cancer cell line MDA-MB-231 was cultured in Dulbecco’s modified Eagle medium (DMEM), supplemented with 10% fetal calf bovine serum, 2 mM L-glutamine (Gibco), penicillin (100 U/ml) and streptomycin (100 µg/ml) (Sigma), at 37 °C in 5% CO2 humidified incubator. The culture density was maintained at 5 × 104 cells/cm2 and split ratio was 1:3.
2.2 Metal as anti-proliferating agent
Potassium tellurite (100 mM) in PBS was used at 0.1 to 1 mM range.
2.3 Crystal violet staining
Tellurite treated and untreated cells were cultured, fixed with absolute ethanol for 20 s, stained with 0.4% crystal violet (Sigma) for 5–10 min, later on washed with PBS until clear and images were taken by using inverted microscope. For cell counting, 51% of Triton X 100 was added and absorbance was measured at 470 nm by ELISA reader (Humareader plus, HUMAN) (Ahmad et al., 2014).
2.4 RNA isolation, purification, quantification, cDNA synthesis and quantitative real time PCR
Total RNA from MDA-MB-231 treated and untreated (controlled) cells was extracted by growing cells in culture plates (100 mm diameter) up to 80% confluency after that medium was aspirate, washed twice with Phosphate Buffered Saline (PBS) and loosen the cells with cell scraper. The cell suspension was dissolved in 1 ml of TRIzol®, incubated for 5 min at room temperature and 0.2 ml chloroform was added to 1 ml TRIzol content. The mixture was vortexed for 30 s, incubated for 2–3 min at room temperature and again centrifuged at 15,000g for 15 min at 4 °C. The clear top layer was aspirated and mixed well with equal volume of isopropanol, incubated at 30 °C for 10 min and centrifuged at 12,000g for 10 min at 4 °C to pellet down the RNA. The pellet was washed with 75% ethanol (75 ml of ethanol and 25 ml of DEPC-treated water), centrifuged for 5 min at 4 °C and dried the pellet. The RNA pellet was dissolved in DEPC-treated water and stored at −80 °C until further use.
DNase1, RNase-free (Fermentas) kit was used to purify the RNA as described in given protocol; the reaction mixture contained 20 µl of raw RNA, 3 µl of 10X reaction buffer with MgCl2, 3 µl of DNase1, nuclease free water 4 µl and incubated at 37 °C for 30 min. Then, 3 µl of 50 mM EDTA was added, incubated for 10 min at 65 °C and placed on ice for 5 min. Finally, 4ul of each purified RNA used for RNA quantification as direct or diluting sample (to gain 500 ng/µl or 5ug RNA) and OD was measured at 260/280 nm on Picodrops 200. cDNA was synthesized from purified RNA by using random hexamer primer and revertaid enzyme for 1.5 h in thermocycler according to the procedure supplied with Thermo Scientific Revert Aid first strand cDNA synthesis kit.
TrxR1 and TrxR2 mRNA expression level of 0, 0.01, 0.1 and 1 mM tellurite treated was measured by qRT-PCR according to procedure described in Thermo scientific Maxima Sybr green ROX qPCR master mix (2x) and primers used were for TrxR1 primers, 5′-GCCCTGCAAGACTCTCGAAATTA-3′ and 5′-GCCCATAAGCATTCTCATAGACGA-3′ and TrxR2 5′-TCAGAAGATCCTGGTGGACTCC-3′ and 5-′TCGTGGGAACATTGTCGTAGTC-3′ (Rigobello et al., 2009) at a final concentration of 5 µM each. qPCR was performed on a Bio-Rad MYiQ™2 two color real-time PCR detection system (Bio-Rad) as previously reported by Rundlöf et al. (2007). For an endogenous control, Glucouranidase-β was used and 32 cycles Ct-value cut-off was setted and 90 ± 5% set as primers efficiency.
2.5 Measurement of ROS formation
Intracellular ROS generation was assessed with slight modifications according to Verrax and Calderon (2009). Cells were cultured under standard culture conditions up to 80% confluency and different tellurite concentration were added for 1 h in stressed labelled cells and then incubated at 37 °C with 25 µM DCFH-DA (Sigma) for 30 min and sonicated for 15 s twice on ice. The fluorescence intensity was measured at 485 excitation and 530 emission by fluorimeter as fluorescence units. It was normalized it to relative to control as oxidative stress index and fluorescence microscopic was used to capture fluorescence microscope images of tellurite treated cells.
2.6 Assessment of GSH content
The cells were grown with different concentration of tellurite and without tellurite in standard conditions. The cell culture was harvested and sonicated thrice on ice for 15 s in 5% sulfo-salicyclic acid. Reaction buffered (0.1 M phosphate buffer-pH 7 and 0.5 mM EDTA) and 3 mM 5′dithio-bis-(2-nitrobenzoic acid) was added into aliquot, and incubated for 5 min. The absorbance was measured at 412 nm for reduced glutathione (GSH), added 0.4 mM NADPH (Sigma) and glutathione reductase (GR) (Sigma), incubated for 20 min. The optical density was taken at 412 nm for total glutathione content.
In the second set of eppendorfs containing 100 µl of above sample mixture, reaction buffer (0.1 M phosphate buffer of pH 7 and 0.5 mM EDTA) and 1 mM 5′ dithio-bis-(2-nitrobenzoic acid) were also added. A blank without extract was also prepared and incubated for 10 min and optical density was taken at 412 nm (Rehman et al., 2010).
2.7 Caspase-3 activity
Caspase-3 activity was determined by Casp Glow Fluorescein Active Caspase-3 Staining Kit (BioVision), according to the procedure outline in the instructions and relative AFC (7-amino-4-trifluoromethyl coumarin) was measured.
2.8 Vitality staining
For the measurement of live and dead cells, tellurite treated and untreated cells were cultured and stained (FDA: 5 mg/ml, PI: 2 mg/ml) (Sigma) with freshly prepared solution, and incubated for 5 min and then all staining solution was removed, and washed with PBS. Finally, PBS was added and observed under fluorescence microscope.
2.9 Statistical analysis
All experiments were performed in triplet set of independent experiments, and results were represented as mean ± SD. Student’s t-test was measured between two-sample assuming equal variances and one-way ANOVA among more than two groups and P-values < 0.05 as statistically significant.
3 Results
3.1 MDA-MB-231 cell culturing
MDA-MB-231 cells were cultured without and with tellurite ranging from 0.01 to 1 mM. The percentage (%) of live cells was measured by crystal violet to access biomass of cells (Fig. 1) and morphologically characterized (Fig. S1). For crystal violet staining IC50 was 0.1 mM.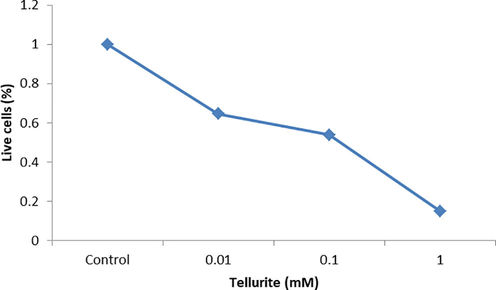
Crystal violet staining for the detection of live MDA-MB-231 cells.
3.2 Te effect on mRNA expression of TrxRs in MDA-MB-231 cells
The mRNA expression of was evaluated by RT-PCR and mRNA level was vary in MDA-MB-231 cell line in the presence of tellurite and level of its expression within mitochondria was low as compared to the cytosolic thioredoxin reductase in case of controlled cells (untreated) and treated with tellurite (Fig. 2a). In case of TrxR1 the level of its expression decreased with increasing tellurite stress while the pattern of TrxR2 expression vary as level of this enzyme decreased with increased tellurite concentration at except 0.1 mM tellurite conc. It was increased (Fig. 2b). Tellurite induced increased oxidative stress within the cell and the level of TrxR1 expression cannot maintain homeostasis of the cell and abundance of ROS disturbed the normal clock of cell and forced cell to cell death either by apoptosis and necrosis (Fig. 2c).
qRT–PCR expression of gene TrxR1 and TrxR2 (left to right) with tellurite treatment of breast cancer cells MDA-MB-231 on 1% agarose gel electrophoresis.
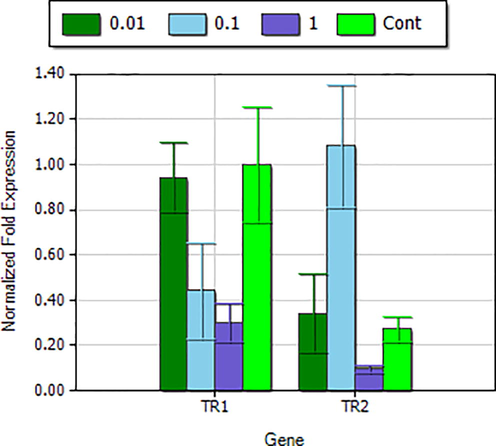
Relative deduction or quantification of gene TrxR1 and TrxR2 expression at different tellurite conc. and between cytosolic and mitochondrial thioredoxin reductase isoforms.
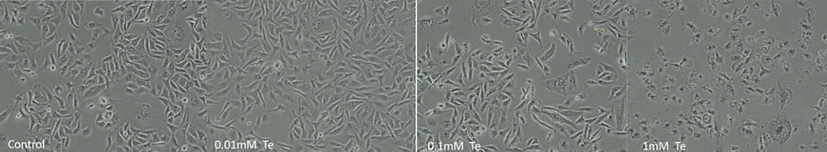
Morphological changes on 80% confluency of cells with different concentration of tellurite observed under inverted microscope.
3.3 Oxidative stress
In MDA-MB-231 cells, ROS formation induced by tellurite (Fig. 3a) was measured and oxidative stress was increased with increasing concentration of tellurite. The images were observed under florescence microscope (Fig. 3b).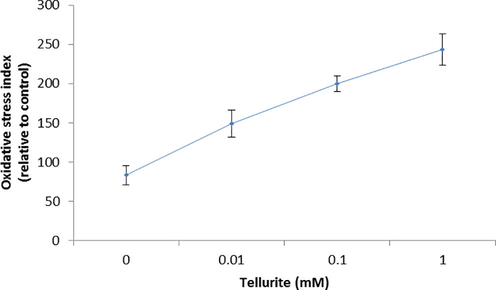
Measurement of ROS formation induced by tellurite. *(P < 0.05) represents statistically significant difference as compared to the control conditions and single factor ANOVA (represents all groups like as (different metal concentrations) are significantly different from each other as it represents F value greater then F critical (p-value < 0.05).
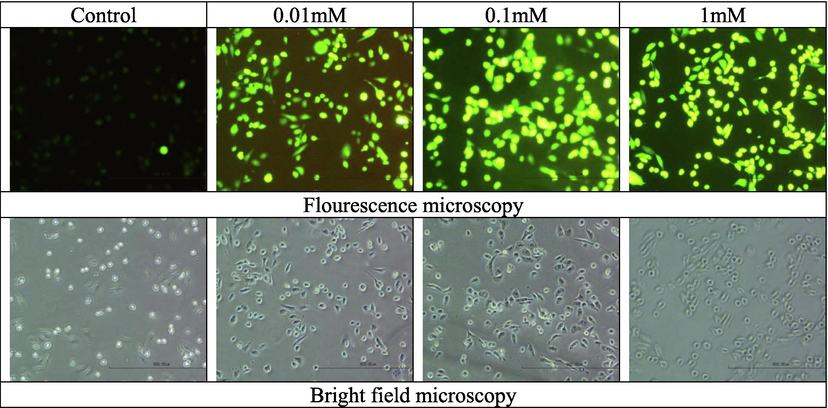
Fluorescence and bright field microscopy for ROS determination in MDA-MB-231 cells with and without tellurite.
3.4 GSH and Non-protein thiol measurement in Te stress
The level of non-protein thiols within cell was lowered with increasing concentration of tellurite as 0.1 mM decreased it to 8.8% (Fig. 3c). The oxidative stress induced by tellurite leads to enhance the oxidative stress and decreased the GSH concentration less than 50% at 0.01 mM tellurite treated cells. Increase tellurite concentration also decreased the GSH 18% while in control and NAC retain almost 100% GSH level that lowers the oxidative stress due to having antioxidant property and helps in cell proliferation (Fig. 3d).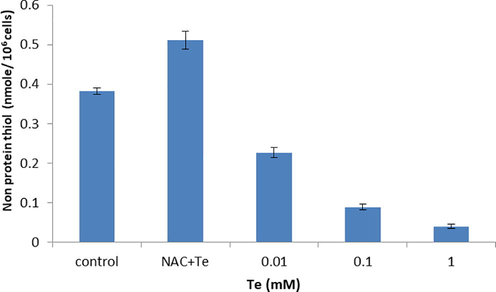
Non-protein thiol contents in the presence of tellurite stress. P < 0.05 represents statistically significant difference as compared to the control conditions and single factor ANOVA represents all groups like as (different metal concentrations) are significantly different from each other as it F value greater then F critical (p-value < 0.05).
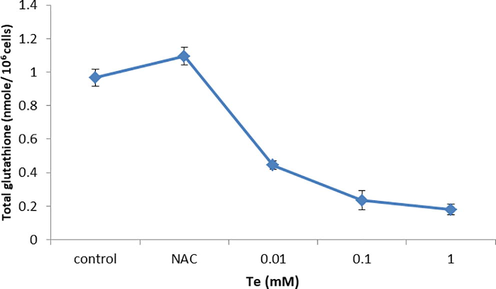
Level of GSH measurement in MDA-MB-231 cells with and without tellurite. *(P ≤ 0.05) represents statistically significant difference as compared to the control conditions.
3.5 Caspase-3 activity
The effect of tellurite on the activity of caspase-3 apoptosis-related protein was graphically represented in Fig. 4a and tellurite treated cells with 0.01 mM concentration were observed with more caspases-3 activity (Fig. S2). Fig. 4b represents the number of viable cells decreased with increasing concentration of tellurite (Fig. 4b) while dead cells increased in increasing tellurite concentration (Fig. 4c).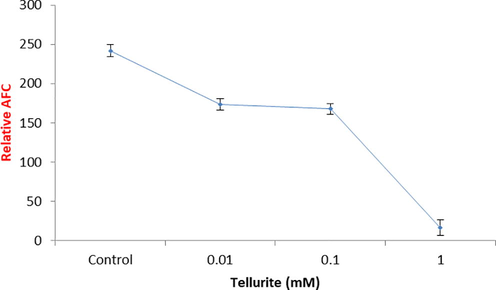
Caspase-3 activity in tellurite treated and untreated cells.
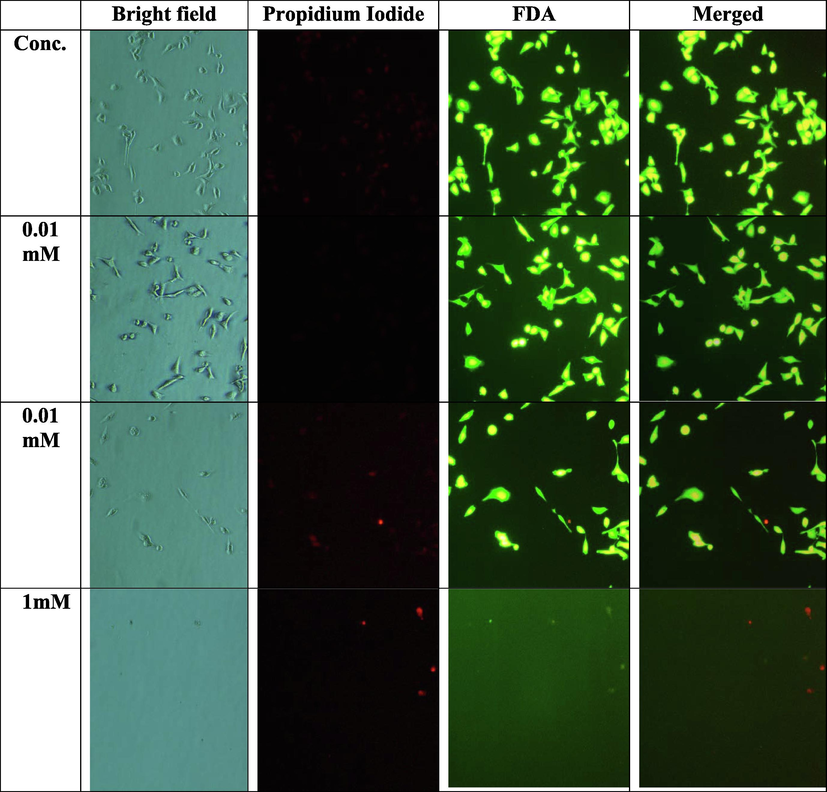
Fluorescence microscopy of live and dead cells by FDA and PI staining of MDA-MB 231 in the presence and absence of tellurite.
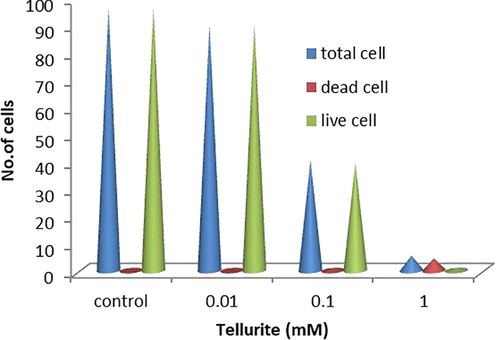
Measurement of live and dead cells by FDA and PI staining.
4 Discussion
In the present study, tellurite inhibited the human breast cancer cell line‘s (MDA-MB-231) growth in dose-dependent manner with 0–1 mM tellurite in increasing order. Tellurite at low concentration of 0.01 mM retained 64% live cells and as the concentration increased, more significant results were obtained at 0.1 mM with 53% cell viability (Fig. 1).
Current study reveals that tellurite has potential to inhibit TrxRs expression thus induced high ROS production which alter cellular reduced environment into oxidized as the level of GSH decreased as reported by Duan et al. (2014) shikonin retarded the function of TrxR in human promyelocytic leukaemia HL-60 cells. All these results are in good agreement with our findings. TrxR is a type of protein in the form of enzyme inhibited by Auranofin and retarded the Trx by using NADPH and stopped many cellular processes (Becker et al., 2000; Ott, 2009) and phenylarsenic oxide derivatives 2-(4-Aminophenyl)-1,3,2-dithiarsinane (PAO-PDT, 4) attacked on TrxR large protein‘s C-terminal and produced ROS by NADPH oxidase (Cai et al., 2012; Zhang et al., 2014; Duan et al., 2014; Liu et al., 2014). Tellurite can be used as anticancer agent because it has ability to decrease the expression of TrxR enzymes expression which are playing critical role in maintaining the cellular reduced environment (Fig. 2b) (Schuh et al., 2012; Wang et al., 2012; Duan et al., 2014). Lu et al. (2007) reported that arsenic trioxide (ATO) inhibited the expression of TrxR and can be used in cancer treatment (Liu et al., 2014).
For a healthy growth, damages removed by killing cells and this phenomena is known as apoptosis and in unhealthy cells these damages go unchecked and continued the proliferation, pause the apoptosis process and fight against anticancer agents introduced within the cells (Frankenet al., 2006; Gonçalves et al., 2012). To evaluate the mechanism of cell death induced by tellurite is either apoptotic or necrotic; present study performed the FDA/PI staining and observed under bright field and fluorescence microscopy i.e., nucleus with condense, shrunk and fragmented pieces, cell detachment, blebbing and shrinkage of cell membrane (Fig. 4b) as reported by Moongkarndi et al. (2004).
Duan et al. (2014) reported that shikonin induced the cell death via apoptotic mechanism activation at low concentration. Double staining with FDA/PI of MDA-MB-231 cells after treatment with tellurite and increasing apoptosis with increased time and concentration was observed. Our results are in good agreement with Duan et al. (2014). Duan et al. (2014) revealed that shikonin induced cell death by activation of other mechanisms rather than apoptosis with higher concentration of shikonin and our results with tellurite are exactly of same pattern.
Thus, one of the methods used against cancer is the apoptosis as reported by Ethiraj et al. (2014). For the determination of apoptosis, caspase activity was measured and significant decrease in caspase 3 activity was observed with the increasing concentration of tellurite stress as compared to the control (without tellurite) (Fig. 4a). Budihardjo et al. (1999) reported that caspase-3 is a major component of apoptotic machinery and was observed in cells treated with lower concentration as compared to the higher one, and Duan et al. (2014) also reported the similar results. The caspace 3 activity indicated that cells undergo both type of phenomena of apoptosis and necrosis but as the concentration of tellurite increases former are less occurred then lateral as reported by Xu et al. (2013).
Te induces ROS generation within the cells, as Te alters the membrane‘s potential which release cytochrome c that promotes caspase machinery resultant apoptosis. As in our case, with increase in tellurite concentration ROS generation increases as compared to the untreated cells (Fig. 3a). These increasing ROS induce apoptosis but their release was high that it causes abrupt necrosis in high concentrations. In the present study, tellurite increases ROS production more significantly in MDA-MB-231 cells in dose dependent manner and rendered the cell growth and proliferation ultimately lead to death phase i.e. apoptosis (Fig. 3b).
5 Conclusion
To sum up all findings, its suggested that Te can be used as one of the therapeutic agents as the level of TrxR suppressed with the increasing tellurite concentration in MDA-MB-231 cells and cause the major hindrance in proliferation of cells by disturbing normal cells homeostasis.
Acknowledgement
This work was supported by the research grant no. PU/Envr (59) from University of the Punjab, Lahore, Pakistan which is gratefully acknowledged.
Conflict of interest
The authors have declared that no competing interests exist.
References
- Anti-proliferative effect of arsenic, cadmium and lead on human placental chorion cells, Pakistan. J. Zool.. 2014;46:561-566.
- [Google Scholar]
- Focus on mammalian thioredoxin reductases important selenoproteins with versatile functions. Biochim. Biophys. Acta. 2009;1790:495-526.
- [Google Scholar]
- Thioredoxin reductase as a pathophysiological factor and drug target. Eur. J. Biochem.. 2000;267:6118-6125.
- [Google Scholar]
- Thioredoxin reductase: a target for gold compounds acting as potential anticancer drugs. Coord. Chem. Rev.. 2009;253:1692-1707.
- [Google Scholar]
- Ruthenium anticancer compounds: challenges and expectations. Chimia. 2007;61:692-697.
- [Google Scholar]
- Enzymatic reduction of protein bound methionine sulfoxide. Proc. Natl. Acad. Sci. U.S.A.. 1981;78:2155-2158.
- [Google Scholar]
- Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol.. 1999;15:269-290.
- [Google Scholar]
- Curcumin targeting the thioredoxin system elevates oxidative stress in HeLa cells. Toxicol. Appl. Pharmacol.. 2012;262:341-348.
- [Google Scholar]
- Butyltin(IV) benzoates: inhibition of thioredoxin reductase, tumor cell growth inhibition, and interactions with proteins. Chem. Med. Chem.. 2013;8:256-264.
- [Google Scholar]
- Shikonin targets cytosolic thioredoxin reductase to induce ROS-mediated apoptosis in human promyelocytic leukemia HL-60 cells. Free Radic. Biol. Med.. 2014;70:182-193.
- [Google Scholar]
- Synergistic anti-carcinogenic effect of interferon-β with cisplatin on human breast adenocarcinoma MDA MB231 cells. Int. Immunopharmacol.. 2014;23:222-228.
- [Google Scholar]
- Thioredoxin reductase, an emerging target for anticancer metallodrugs. Enzyme inhibition by cytotoxic gold(III) compounds studied with combined mass spectrometry and biochemical assays. Med. Chem. Commun.. 2011;2:50-54.
- [Google Scholar]
- Selenocysteine, identified as the penultimate C-terminal residue in human T- cell thioredoxin reductase, corresponds to TGA in the human placental gene. Proc. Natl. Acad. Sci. U.S.A.. 1990;93:6146-6151.
- [Google Scholar]
- Thiol/disulfide redox states in signalling and sensing. Crit. Rev. Biochem. Mol. Biol.. 2013;48:173-181.
- [Google Scholar]
- Cytotoxic effects of cadmium in mammary epithelial cells: protective role of the macrocycle [15] pyN5. Food Chem. Toxicol.. 2012;50:2180-2187.
- [Google Scholar]
- The involvement of the thioredoxin system in the reduction of methionine sulfoxide and sulfate. J. Biol. Chem.. 1970;245:2371-2374.
- [Google Scholar]
- Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochem. Biophys. Res. Commun.. 2010;396:120-124.
- [Google Scholar]
- Studies toward novel peptidomimetic inhibitors of thioredoxin-thioredoxin reductase system. J. Med. Chem.. 2012;55:55-67.
- [Google Scholar]
- Enzymatic synthesis of deoxy ribonucleotides. IV. Isolation and characterization of thioredoxin, the hydrogen donor from Escherichia coli B. J. Biol. Chem.. 1964;239:3436-3444.
- [Google Scholar]
- Gallium complexes as new promising metallodrug candidates. Inorg. Chim. Acta. 2012;393:53-63.
- [Google Scholar]
- Dithiaarsanes induce oxidative stress-mediated apoptosis in HL-60 cells by selectively targeting thioredoxin reductase. J. Med. Chem.. 2014;57:5203-5211.
- [Google Scholar]
- Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc. Natl. Acad. Sci. U.S.A.. 2007;104:12288-12293.
- [Google Scholar]
- Thioredoxin system in cell death progression. Antioxid. Redox. Signal.. 2012;17:1738-1747.
- [Google Scholar]
- Synthesis of 5-nitro-2- furan carbohydra zides and their cis-diammine dichloro platinum complexes as bitopic and irreversible human thioredoxin reductase inhibitors. J. Med. Chem.. 2005;48:7024-7039.
- [Google Scholar]
- Antiproliferation, antioxidation and induction of apoptosis by Garcinia mangostana (mangosteen) on SKBR3 human breast cancer cell line. J. Ethnopharmacol.. 2004;90:161-166.
- [Google Scholar]
- Gold compounds as anticancer agents: chemistry, cellular pharmacology, and preclinical studies. Med. Res. Rev.. 2010;30:550-580.
- [Google Scholar]
- A chemical-biological evaluation of rhodium(I) Nheterocyclic carbene complexes as prospective anticancer drugs. Chem. Eur. J.. 2013;19:17871-17880.
- [Google Scholar]
- On the medicinal chemistry of gold complexes as anticancer drugs. Coord. Chem. Rev.. 2009;253:1670-1681.
- [Google Scholar]
- Metal complexes with 2-acetylpyridine-N(4)- ortho chloro phenyl thio semicarbazone: cytotoxicity and effect on the enzymatic activity of thioredoxin reductase and glutathione reductase. Eur. J. Med. Chem.. 2014;84:537-544.
- [Google Scholar]
- Isolation and characterization of arsenite oxidizing Pseudomonas lubricans and its potential use in bioremediation of waste water. Afr. J. Biotechnol.. 2010;9:1493-1498.
- [Google Scholar]
- Mitochondrial thioredoxin reductase purification, inhibitor studies, and role in cell signaling. Meth. Enzymol.. 2010;474:109-122.
- [Google Scholar]
- Treatment of human cancer cells with selenite or tellurite in combination with auranofin enhances cell death due to redox shift. Free Radic. Biol. Med.. 2009;47:710-721.
- [Google Scholar]
- Quantification of alternative mRNA species and identification of thioredoxin reductase 1 isoforms in human tumor cells. Differentiation. 2007;75:123-132.
- [Google Scholar]
- Gold(I) carbine complexes causing thioredoxin1 and thioredoxin 2 oxidation as potential anticancer agents. J. Med. Chem.. 2012;55:5518-5528.
- [Google Scholar]
- N4-Phenyl-substituted 2-acetylpyridine thiosemicarbazones: cytotoxicity against human tumor cells, structure activity relationship studies and investigation on the mechanism of action. Bio. Org. Med. Chem.. 2012;20:3396-3409.
- [Google Scholar]
- A dinuclear cyclometalated gold(III)− phosphine complex targeting thioredoxin reductase inhibits hepatocellular carcinoma in vivo. Chem. Sci.. 2013;4:1979-1988.
- [Google Scholar]
- Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects, Free Radic. Biol. Med.. 2009;47:32-40.
- [Google Scholar]
- Ethaselen: a potent mammalian Thioredoxin reductase 1 inhibitor and novel organoselenium anticancer agent. Free Radic. Biol. Med.. 2012;52:898-908.
- [Google Scholar]
- Synergetic effect of functional cadmium–tellurium quantum dots conjugated with gambogic acid for HepG2 cell-labeling and proliferation inhibition. Int. J. Nanomed.. 2013;8:3729-3736.
- [Google Scholar]
- Highly selective off-on fluorescent probe for imaging thioredoxin reductase in living cells. J. Am. Chem. Soc.. 2014;136:226-233.
- [Google Scholar]
- Structure and mechanism of mammalian thioredoxin reductase: the actives site is a redox-active selenol thiol/selenenyl sulfide formed from the conserved cysteine selenocysteine sequence. Proc. Natl. Acad. Sci. U.S.A.. 2000;97:5854-5859.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2019.03.001.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







