Translate this page into:
Overexpression, purification and characterization of a heat-stable peroxisomal glutathione reductase from Oryza sativa involved in multifactorial abiotic stress tolerance
⁎Corresponding authors. gkchowdhary@kiitbiotech.ac.in (Gopal Chowdhary)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abiotic stress is the major bottleneck in obtaining the full potential of a standing crop. Various strategies have been investigated to impart abiotic stress tolerance. In the agricultural field, the stress conditions do not act in isolation rather they act synergistically in combinations against the wellbeing of the crop. The combined impact of abiotic stress conditions has been referred to as stress matrix or stress combination. Here, we have proposed a probable candidate that could be instrumental in imparting tolerance against stress matrix or stress combination. The investigation was made on a peroxisome localized glutathione reductase (GR) enzyme from the economically significant crop Oryza sativa. The protein was expressed in the E. coli system, purified and characterized. The enzyme was found to be active even at an elevated temperature of 55℃, demonstrating its abiotic stress tolerance properties. Further, the gene expression studies showed it to be upregulated under salinity, dehydration, low and high temperature. The expression was also investigated under various stress combinations and it was observed that the level of GR mRNA was found to be higher in the case of combined stress treatment as compared to individual stress conditions suggesting an additive effect phenomenon. The results obtained here put this peroxisomal GR in the spotlight for imparting abiotic stress combinations studies.
Keywords
Peroxisome
Glutathione reductase
Stress combination
Heat stable
- UNSDG
-
United Nations Sustainable Developmental Goals
- ROS
-
Reactive Oxygen Species
- GR
-
Glutathione reductase
- GSSG
-
Glutathione Disulfide
- GSH
-
Sulfhydryl form glutathione
- NADPH
-
Nicotinamide Adenine Dinucleotide Phosphate Hydrogen
- kDa
-
kilo Dalton
- APX
-
Ascorbate Peroxidase
- MDAR
-
Monodehydroascorbate Reductase
- DAR
-
Dehydroascorbate Reductase
- H2O2
-
Hydrogen peroxide
- PEG
-
polyethylene glycol
- RNA
-
Ribonucleic Acid
- cDNA
-
Complementary Deoxyribonucleic Acid
- PCR
-
Polymerase Chain Reaction
- CT
-
Critical Threshold
- Os
-
Oryza sativa
- OD
-
Optical Density
- IPTG
-
Isopropyl, β, D, thiogalactopyranoside
- NaCl
-
Sodium Chloride
- PMSF
-
Phenylmethanesulfonyl Fluoride
- rpm
-
rotation per minute
- Ni
-
NTA, Nickel, Nitrilotriacetic acid
- SDS
-
PAGE, SDS, Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis
- LC MS
-
Liquid Chromatography Mass Spectrometry
- g
-
gram
- DTT
-
Dithiothreitol
- ELISA
-
enzyme, linked immunosorbent assay
- PVDF
-
Polyvinylidene Fluoride
- PBS
-
Phosphate Buffer Saline
- HRP
-
Horseradish Peroxidase
- EDTA
-
Ethylenediaminetetraacetic Acid
- Km
-
Michaelis–Menten constant
- EYFP
-
Enhanced Yellow Fluorescent Protein
- CaMV
-
Cauliflower Mosaic Virus
- FAD
-
Flavin Adenine Dinucleotide
- NAD
-
Nicotinamide Adenine Dinucleotide
- PTS
-
Peroxisome Targeting Signal
- pI
-
Isoelectric Point
- mRNA
-
messenger RNA
Abbreviations
1 Introduction
The current scenario of climate change and its allied phenomena has led to serious deleterious effects on agriculture. The untimely onset of unfavourable environmental conditions leads to the loss of crops worth millions, on a global scale (Genders, 2023; Roy and Chowdhary, 2023). This is a serious bottleneck in achieving the UNSDG (United Nations Sustainable Developmental Goals) of a 70–100 % increase in crop productivity by 2050, leading to food security and zero hunger (Genders, 2023). This problem is further complexed by the increasing global population, which is expected to reach 10 billion by 2050, of which 60–70 % will be living in developing and underdeveloped nations including India (Etesami and Maheshwari, 2018). Feeding an ever-increasing population would require more agricultural land and protecting the standing crops from environmental damage. The primary abiotic stress conditions that affect crops worldwide are salinity, dehydration, low and high temperature. These are elements that affect plant’s morphological, physiological, biochemical, and molecular processes, as well as their development and growth (Zhang et al., 2022).
In natural field conditions, the environmental stresses do not act in isolation, rather they act in combinations. Agriculturally important stresses acting together have been termed “stress matrix” (Mittler, 2006) or “stress combinations” (Zandalinas et al., 2020). Currently, there are no means to protect the standing crop from combinations of environmental stresses. In this scenario, it becomes imperative to find alternate ways for sustainable agriculture practices, to protect the standing crop from combinations of environmental stresses. It has been observed that all abiotic stress conditions lead to an increase in reactive oxygen species (ROS) concentration in the cell. If this ROS surge is left unchecked, it could lead to cell damage and ultimately cell death. ROS is not only an after-effect of stress conditions but also acts as a signalling molecule (Bhat et al., 2023; Mansoor et al., 2022), therefore a critical balance in the scavenging and production of ROS must be carefully maintained.
Plants have developed complex enzymatic and non-enzymatic mechanisms of ROS homeostasis (Dorion et al., 2021). Glutathione reductase (GR) is a protein involved in enzymatic ROS homeostasis by reducing oxidative stress via the conversion of glutathione disulfide (GSSG) to its reduced form (GSH) (Hajiboland, 2014). GR, a member of the NADPH-dependent oxidoreductase family, has been purified from multiple plant species and exists as a homodimer having a molecular weight between 100 and 120 kDa, with subunit sizes ranging from 53 to 59 kDa (Wingsle, 1989; Anderson et al., 1990; Madamanchi et al., 1992). In the absence of thiols, GR shows a tendency to form larger forms including tetramers. Even though these bigger forms exhibit catalytic activity, under standard physiological conditions, the enzyme is maintained in its dimeric form. In the cells, GR maintains the GSH/GSSG ratio in favour of GSH. GR in addition to three additional enzymes — ascorbate peroxidase (APX), monodehydroascorbate reductase (MDAR), and dehydroascorbate reductase (DAR) — as well as GSH and ascorbate, plays a significant role in eliminating hydrogen peroxide (H2O2) from cell (Noctor and Foyer, 1998).
In plant cells, the majority of GR activity is present in chloroplasts followed by mitochondria. Cytosol also contains trace amounts of enzyme isoforms (Edwards et al., 1990; Jimenez et al., 1997). Recently, its presence in peroxisome was also demonstrated in Arabidopsis thaliana (Kataya and Reumann, 2010) and Oryza sativa (Roy and Chowdhary, 2023). While a lesser amount of enzyme activity has also been observed in root plastids, the majority of GR activity has been documented in leaf chloroplasts (Foyer and Halliwell, 1976). It has also been observed that distinct GR isoforms can be induced by a range of environmental stimuli and can perform a specific role in the adaptation of plant under various abiotic stress conditions (Stevens et al., 1997). Furthermore, while determining a plant's ability to withstand different abiotic stressors, GR and GSH, both are very important factors. GR is essential for maintaining the GSH pool, which is necessary for active protein function. Furthermore, millimolar (mM) quantities of GSH function as a crucial redox buffer, creating a virtual wall that keeps ROS and cysteine amino acids of target protein groups apart (Sies and Jones, 2020).
Peroxisomes in addition to chloroplast and mitochondria play a central role in cellular ROS homeostasis (Dietz et al., 2016), hence a peroxisome localized GR could be of greater significance in terms of ROS homeostasis and ultimately in imparting abiotic stress tolerance. Hence, in the present work, we characterized a GR enzyme, which was previously demonstrated to be localized in peroxisome by our group (Roy and Chowdhary, 2023) and validated its involvement in multiple abiotic stress conditions.
2 Material and method
2.1 Plant material and stress treatment
For all the experiments O. sativa IR 64 (Indica variety) rice was used. The seeds were acquired from the National Rice Research Institute, Cuttack, India. The seed sterilization, germination and plant growth were performed as per Roy and Chowdhary (2023). 13-day-old seedlings were used for all experimentation. The abiotic stresses were induced by incubating the seedlings in 200 mM sodium chloride, 15 % PEG, 4 ± 2 °C and 45 ± 2 °C for saline, dehydration, low and high-temperature stress respectively for 1, 2, 4, 8, 16 and 24 h. For stress combination studies, dehydration and low temperature (15 % PEG at 4 ± 2 °C) stress, dehydration and high temperature (15 % PEG at 45 ± 2 °C) stress and salinity and high temperature (200 mM sodium chloride at 45 ± 2 °C) stress were used for 4, 8, 16, 24 and 48 h. To determine the relative transcript level of OsGR the untreated seedlings were used.
2.2 RNA extraction, cDNA synthesis and expression analysis of OsGR
RNA isolation, cDNA synthesis and expression analysis were performed as per Roy and Chowdhary (2023). SYBR green chemistry was employed for expression analysis using real time PCR method. The fold change (relative change in the expression of OsGR under various stress treatments) was calculated by the ΔΔCT method. The primers used for OsGR expression studies were forward primer – CGACCTTTGACAGCACTGTTGG, reverse primer – TGGTCAAGGTCCGCATTGTCAC, OsActin forward primer – CAGCCACACTGTCCCCATCTA, reverse primer – AGCAAGGTCGAGACGAAGGA.
2.3 Subcloning of OsGR into pET21b
The OsGR (Accession number LOC_Os02g56850.1) was PCR-amplified from the OsGR-pCAT-EYFP (Roy and Chowdhary, 2023) using primers: forward GCAATTACATATGGCTAGGAAGATGCTCAAGG and reverse GCTACTCGAGCAAGTTTGTCTTTGGCTTGG using HiFidelity polymerase (Qiagen), with PCR cycling conditions of initial denaturation at 94 °C for 3 min, denaturation at 94 °C for 30 sec, annealing at 55 °C for 30 sec, extension at 72 °C for 90 sec, with a final extension of 10 min at the end of 30 cycles. The amplified PCR product was purified (PCR Cleanup Kit, Advanced Microdevices), digested using NdeI and XhoI restriction enzymes, ligated into the pET21b expression vector, and transformed into NEB5α competent cells. The positive colonies selected using ampicillin were used for the recombinant plasmid extraction. The presence of the insert was confirmed by restriction digestion analysis and automated DNA sequencing. The recombinant plasmid was then used for transforming the bacterial C41(DE3) competent cells which were generated using the calcium chloride method.
2.4 Overexpression and purification of OsGR
To overexpress OsGR protein, the recombinant pET21b plasmid was transformed to E. coli C41(DE3) cells which were grown at 37 °C in Luria-Bertani broth. Upon reaching an optical density (OD) of 0.5–0.6 at 600 nm (OD600), 0.4 mM IPTG was added for induction followed by growing at 37 °C for 4 h. The bacterial cultures were spun at 4000 g for 10 min at room temperature. The resulting bacterial pellet was resuspended in 10 volumes (w/v) of ice-cold lysis buffer (25 mM Tris- pH 7.5, 200 mM NaCl, 2 % glycerol, 20 mM imidazole and 1 mM PMSF), incubated on ice for 30 min, followed by sonication (amplitude 45 %, on-10 sec, off-10 sec) to lyse the cells. The lysed cells were then spun at 13000 rpm for 60 min, and the supernatant was passed through the Ni-NTA column. Post loading the Ni-NTA column was washed with 30 column volumes (CV) of wash buffer (25 mM Tris- pH 7.5, 200 mM NaCl, 2 % glycerol and 20 mM imidazole), and finally eluted using 10 CV of elution buffer (25 mM Tris- pH 7.5, 200 mM NaCl, 2 % glycerol and 250 mM imidazole). The Ni-NTA chromatography purified protein was further purified using size-exclusion chromatography (Sephacryl S-200). The purity of the recombinant protein was evaluated by SDS-PAGE. The identity of the recombinant protein was confirmed using tandem mass fingerprinting (LC-MS/MS at the protein analytical facility, KIIT-Technology Business Incubator, Bhubaneswar − 751024, Odisha, India).
2.5 Extraction of O. sativa total protein
For total protein extraction, 0.5 g of 13-day-old rice seedlings were crushed in liquid nitrogen and to the powder, protein extraction buffer (50 mM Tris- pH 7.0, 10 mM NaCl, 1 % SDS, 2 % β-Mercaptoethanol, 0.1 mM PMSF, 0.1 mM DTT) was added, followed by vortexing. It was centrifuged for 15 min, 4 °C at 10,000 rpm. The supernatant was used as the crude protein extract for further processing.
2.6 Antibody generation for OsGR
To generate antibodies, we employed the recombinant GR protein. Animal immunization and serum extraction were conducted as described previously (Sahu et al., 2024). Briefly, rats were intraperitoneally injected with 50 µg of the recombinant proteins. Initially, complete Freund’s adjuvant was used for emulsification of protein, which were administered on day 0. Two booster doses were applied on days 21 and 42 with incomplete Freund’s adjuvant. On day 60, terminal blood samples (500–750 µl/rat) were collected via retro-orbital puncture, after applying a topical anesthetic (0.5 % proparacaine). Antibody specificity was assessed using immunoblotting and ELISA.
2.7 Western blotting
Protein samples were resolved on 12 % SDS-PAGE followed by immobilization on a polyvinylidene fluoride (PVDF) membrane. The immune blotting was performed as per Sahu et al., (2024). The immobilized proteins were probed with rat α-GR antibodies (1:500 dilution). For signal development chemiluminescence method was used and it was captured using a ChemiDoc imaging system (GE Healthcare LifeSciences).
2.8 Glutathione reductase enzyme assay
For the enzyme assay, the assay buffer containing 100 mM potassium phosphate buffer, pH 7.5 with 1 mM EDTA and 2 mM oxidized glutathione solution was equilibrated at 25 °C for 10 min. 500 µl of 2 mM oxidized glutathione, 450 µl assay buffer, 10 µl sample and 50 µl of 2 mM NADPH was mixed by inversion and placed the cuvette in the spectrophotometer followed by reading the absorbance at 340 nm every 10 sec for 2 min. The concentration of enzyme in the sample was calculated using:
6.22 = εmM for NADPH
The amount of enzyme that will reduce 1 µmol GSSG per min per mL is considered as one unit of GR activity. The enzyme assay was repeated at varied temperatures of 5, 15, 25, 35, 45, 55 °C and pH 4, 5, 6, 6.5, 7, 7.5, 8. For the determination of the Km of OsGR, the assay was carried out by varying the concentration of GSSG and NADPH followed by plotting the curve of substate concentration-1 (GSSG/NADHP) vs reaction velocity-1.
3 Results
3.1 Recombinant expression of OsGR in E. coli system and purification
In the current investigation, OsGR having accession number LOC_Os02g56850.1 is used. In our previous studies (Roy and Chowdhary, 2023) we have demonstrated the cloning and peroxisomal localization of OsGR using biolistic bombardment followed by fluorescent microscopy. (Roy and Chowdhary, 2023). The peroxisomal localization was dependent on peroxisome targeting signal (PTS) type 1, primarily denoted by the three terminal amino acids present at the C-terminus (Reumann et al., 2016). In the case of OsGR the PTS type 1 is represented by TNL> (“>” denotes the end of the polypeptide chain). The theoretical pI and predicted molecular weight of OsGR were found to be 6.24 and 53.5 kDa respectively (https://www.expasy.org/). The recombinant protein construct was generated encompassing the entire OsGR protein (Fig. 1A). This recombinant protein construct was expressed in E. coli under the T7 lac promoter system with a histidine tag for purification. The recombinant protein was purified using metal affinity chromatography (Ni-NTA) followed by size exclusion chromatography. On SDS-PAGE, the purified protein ran an approximate molecular weight of 55 kDa (Fig. 1B). The identity of the protein construct was confirmed by tandem mass fingerprinting. The presence of GR in the crude protein was verified using western blot analysis (Fig. 1C).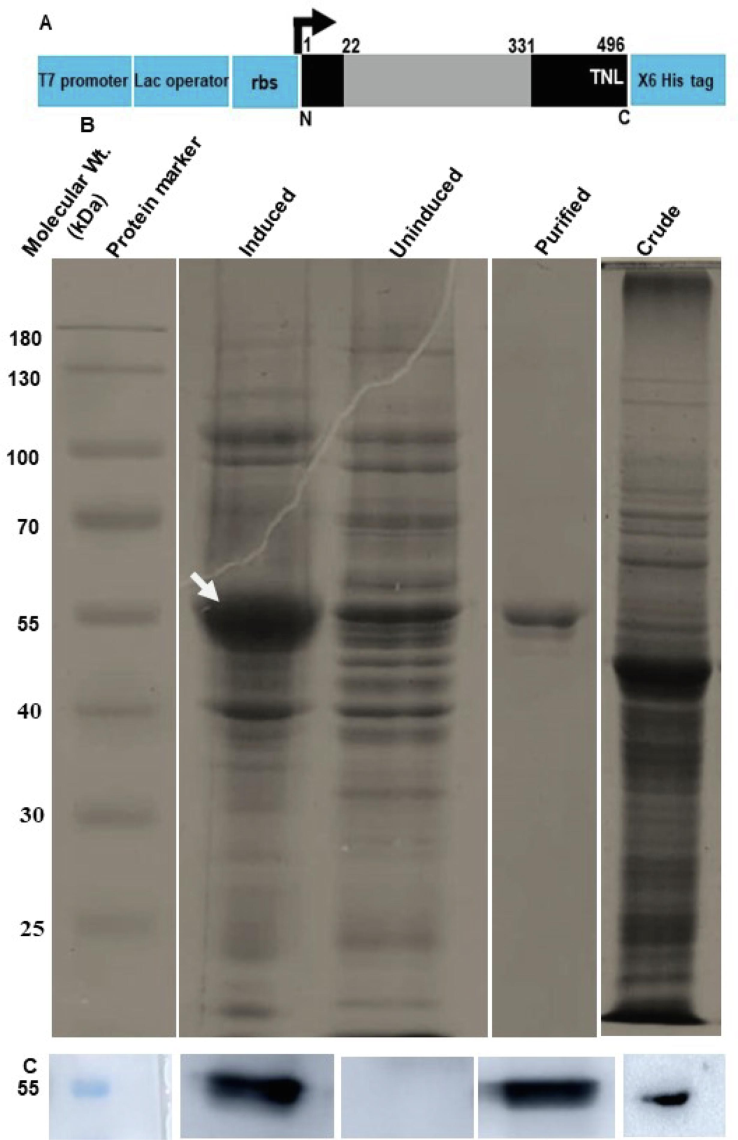
The overexpression, purification and western blot analysis of OsGR: The OsGR was subcloned in pET21b under a T7 lac promoter. (A) shows the diagrammatic representation of pET21b subcloning. The black and grey color bars represent the OsGR full-length protein and FAD/NAD(P) domain respectively. The blue color bars represent the parts of the vector. The numbers at the top indicate the position of amino acids. The “TNL” represents the peroxisome targeting signal type 1 present in OsGR. N – N terminus, C – C terminus, rbs – ribosome binding site. The subcloning was followed by induction in an IPTG-dependent manner (B). The bacterial extract of induced and uninduced samples was collected, sonicated and resolved in a 12 % SDS-PAGE. The induced sample was further processed via Ni-NTA column for purification based on 6X His affinity chromatography. The molecular weight was determined using the protein molecular weight marker (ThermoFisher Scientific). The digits at left indicate the molecular weight in kDa and the texts at the top of each lane describe the samples loaded on the corresponding lane. The white-colored arrow indicated the protein of interest. The lowest panel indicates the western blot of proteins using an anti-OsGR antibody. The leftmost lane indicates the protein molecular weight marker (55 kDa,), followed by induced, uninduced, purified and crude plant protein extracts respectively, probed with antibody (C).
3.2 Biochemical characterization of OsGR
3.2.1 Glutathione reductase assay at varied pH and temperature
GR assay was carried out as explained in materials and methods. This method measures the NADPH oxidation which is linked with GSSG reduction by the enzyme. The enzyme activity was measured as µmol of GSSG reduced per min per mL. In the case of pH, the best activity was observed at a pH of 7.5 with 11.3 enzyme units of activity. Upon decreasing pH either towards neutral (7.0) or increasing towards alkaline (8.0) conditions the enzyme activity dropped to 9.9 and 9.5 respectively. Further, reducing the pH to 6.5 a 26.6 % drop in the enzyme activity was observed (Fig. 2A). In the case of temperature, the best enzyme activity was observed at 35℃, having 11.4 units of activity. Further, increasing the temperature to 45℃ the enzyme activity reduced to 9.2 units. However, at 55℃ it showed a 32.4 % reduction in the activity. Similarly, upon reducing the temperature to 25℃ 30.34 % reduction in the enzyme activity was observed (Fig. 2B). Further, the Km for the OsGR was found to be 5.5 and 16.67 µM for NADPH and GSSG respectively.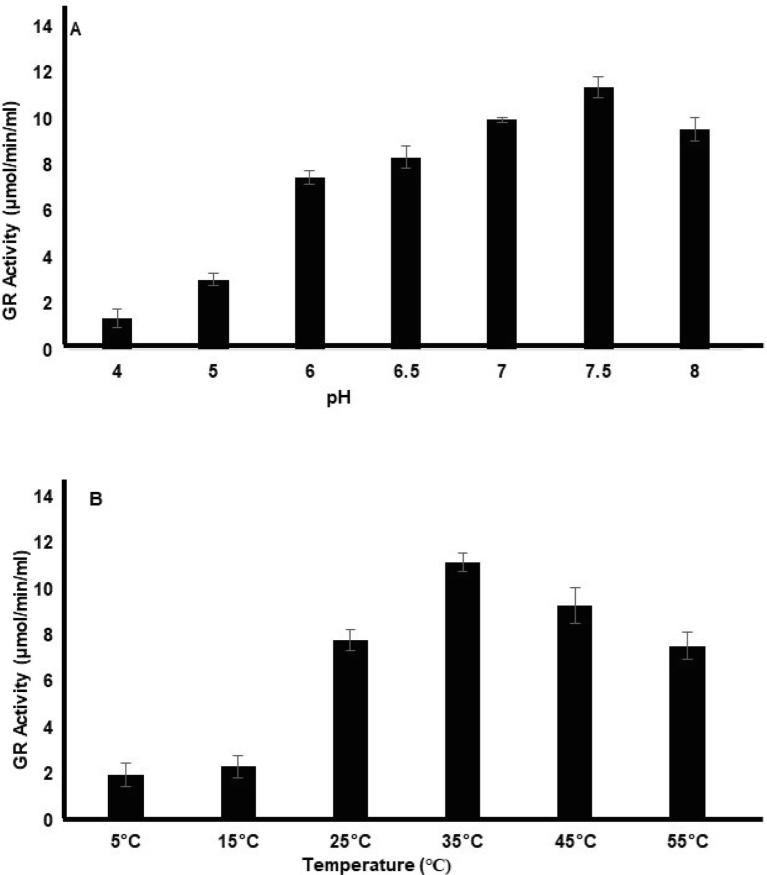
The peroxisomal isoform of glutathione reductase cloned from O. sativa, overexpressed and purified from the E. coli system. The purified protein was used for enzyme assay. The enzyme activity was measured as µmol of GSSG reduced per min per mL by the enzyme. This was measured by the change in the absorbance of NADHP at 340 nm. The activity was measured against various pH (A) and temperature (B). The X-axis shows the pH and temperature in the case of A and B respectively while the Y-axis represents the enzyme activity.
3.3 OsGR expression pattern investigation
The expression studies of OsGR were carried out under various abiotic stress conditions namely salinity, cold, heat and dehydration stress. The rice seedlings were treated with various abiotic stress conditions for 1, 2, 4, 8, 16 and 24 h, followed by freezing the seedlings in liquid nitrogen, which were later used for total RNA isolation, followed by cDNA synthesis. The RNA extracted from untreated seedlings was used as a control. The details of abiotic stress treatment and further procedures are described in the materials and method section. The expression data was depicted as “fold change” or “relative transcript accumulation” as calculated by the ΔΔCT method. The fold change essentially describes the change in the expression of OsGR under a treatment with respect to untreated conditions.
3.3.1 OsGR expression pattern under individual abiotic stress treatment
The expression data shows that under abiotic stress conditions, a basal level of OsGR expression at approximately 2 – 4-fold is maintained. The peak of OsGR mRNA expression in the case of cold and high temperature reached at 4 h of stress treatment which was found to be 10.2 and 9.3-fold respectively. A similar expression level was maintained till 8 h of stress treatment with a slight dip in expression at 16 h followed by reaching the basal of expression at 24 h of cold and high-temperature treatment. In the case of saline stress treatment, the peak of OsGR mRNA expression was found at 2 h of treatment. Elevated levels of OsGR mRNA were maintained under saline stress treatment till 16 h, which fell to the basal level at 24 h of stress treatment. However, in the case of dehydration stress the peak of OsGR mRNA expression reached at 16 h of stress treatment and elevated level was maintained till 24 h of dehydration stress treatment (Fig. 3A).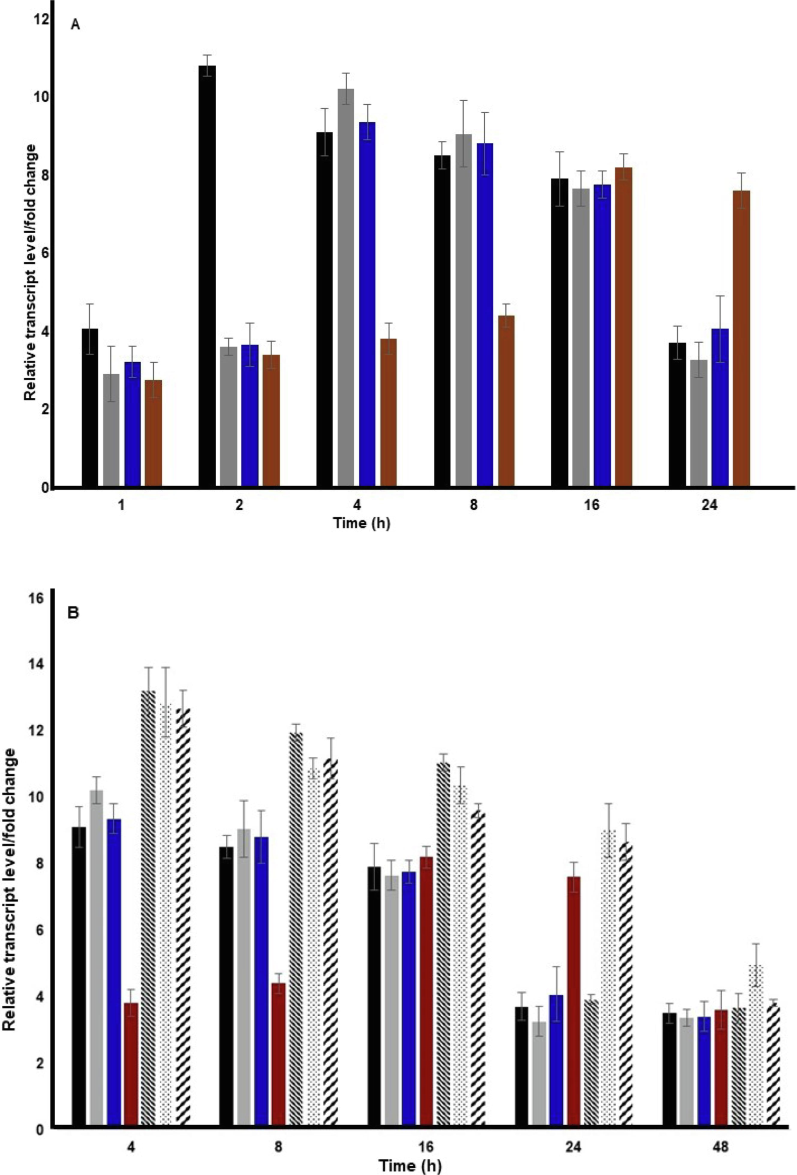
The abiotic stress treatment was given as explained in the materials and methods for the various time points. Upon the stress treatment, the samples were frozen in liquid nitrogen and RNA extraction was performed, followed by performing the real time PCR expression analysis. As endogenous control, OsActin was used. The assays were performed for a minimum of three times, ΔΔCT values were determined and fold change/relative transcript level was calculated. The X-axis shows the time points for treatment while the Y-axis shows the relative transcript level/fold change. A and B show the relative transcript level in the case of single stress treatment and multiple stress treatment respectively. The data provided is from three biological replicates and error bars (standard deviation) have been drawn. Black – salinity stress, grey – low-temperature stress, blue – high-temperature stress, maroon – dehydration stress, − “obtuse angled dashed line” − salinity + heat stress, “dotted line” − low-temperature + dehydration stress and “acute angled dashed line”- high – temperature + dehydration stress.
3.3.2 OsGR expression pattern under multifactorial abiotic stress treatment
Since, the OsGR expression was found to be upregulated by multiple abiotic stress conditions such as salinity, cold, high temperature and dehydration, its expression pattern was also analysed under the combination of abiotic stress conditions. The following stress combinations were chosen – high temperature and salinity, low temperature and dehydration and high temperature and dehydration. Upon the stress treatment, the expression analysis of OsGR was performed as explained above. Since the highest level of OsGR mRNA expression was found to be at 4 h or later point except for salinity stress, hence the expression analysis for multiple stress combinations was studied at 4 h and later time points. However, in the case of salinity stress the elevated level of OsGR mRNA was found to be till 8 h. The expression of OsGR mRNA in the case of combined stress treatment was comparatively higher than single stress treatment, suggesting an additive effect of stress conditions. In the case of high temperature and saline stress combination, the peak of expression was observed at 4 h with 13.2-fold. An elevated level of expression of about 11-fold was maintained till 24, which fell to 3.9-fold at 48 h of combined stress treatment. Similarly in the case of low temperature and dehydration stress the peak OsGR mRNA expression of 12.8-fold was observed at 4 h, maintaining an elevated level of about 9 – 10-fold till 24 h, which dropped to 4.9-fold at 48 h of combined stress treatment. In the case of combined high temperature and dehydration stress treatment, the peak of expression of 12.6-fold was observed at 4 h and an elevated level was maintained till 24 h followed by a reduction to 3.8-fold at 48 h of combined stress treatment (Fig. 3B).
4 Discussion
Being sessile in habit, crop plants cannot escape from unfavourable environmental conditions and hence, either they have to develop mechanisms to withstand the conditions or perish. In natural agricultural conditions, the problems get further worse as the stress factors do not act in isolation, rather multiple stressors act in combination to affect the growth and development of plants. These conditions have been termed “stress matrix” (Mittler, 2006) or “stress combinations” (Zandalinas et al., 2020). To combat unfavourable environmental conditions plants have developed various tolerance or resistant mechanisms, and homeostasis of ROS is one of them. The GR-mediated ascorbate–glutathione cycle is a major player in cellular ROS homeostasis. In the literature, enough information is available on the candidates and mechanisms imparting tolerance to individual abiotic stress conditions, but candidates involved in imparting tolerance to stress combinations are not well established and unexplored. Here, we have explored the potential of a peroxisomal GR from O. sativa in this regard (Fig. 4).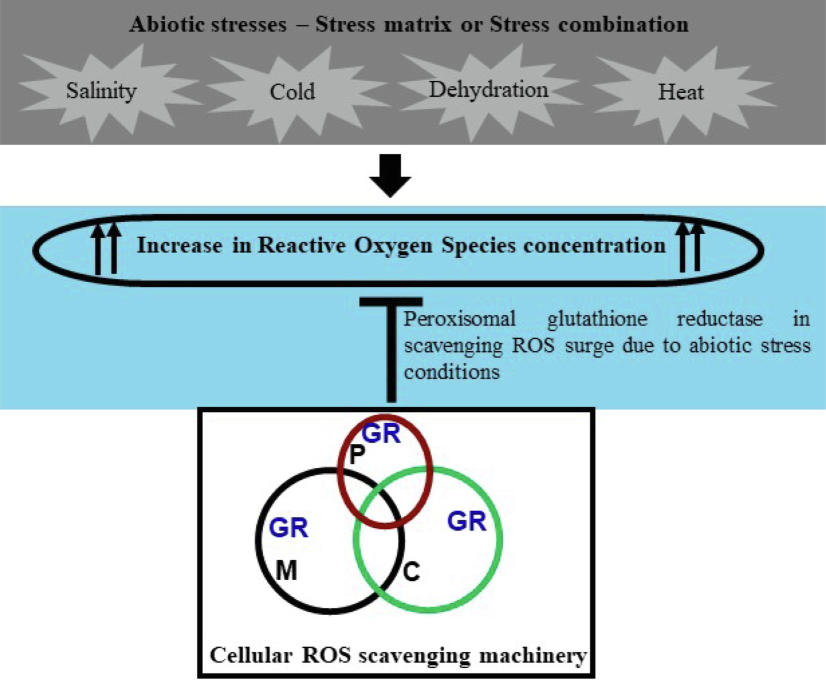
Reactive oxygen species (ROS) constitute a common aftereffect of all the abiotic stresses. The scavenging of ROS is primarily done by an interplay of three organelles; Mitochondria (M), Chloroplast (C), and Peroxisome (P). The involvement of peroxisomal glutathione reductase (GR) has been demonstrated in scavenging the cellular ROS surge.
Our laboratory has previously reported the peroxisomal localization of GR in O. sativa. The peroxisomal localization of OsGR was based on the peroxisome targeting signal (PTS) type 1, which is primarily represented by the last three amino acids present at the C-terminus. In the case of OsGR it is represented by TNL > . The study also reported the upregulation of OsGR mRNA in response to salinity, low and high temperature, specifically at 2 h of treatment (Roy and Chowdhary, 2023). Here, we have further performed a time course study beginning at 1 h to spanning till 24 h of stress treatment. The time course expression analysis revealed that the peroxisomal OsGR mRNA accumulation begins in the early time points of stress treatment and reaches its peak of expression somewhere between 2 – 4 h and a basal level of elevated expression is maintained till the stress factor is present. The upregulation of OsGR mRNA in the initial time points of stress treatment suggests its probable defensive role in the early stages of stress acclimation and tolerance. The maintenance of elevated levels of OsGR mRNA till the stressor is present suggests its crucial role in imparting stress tolerance over an extended period and establishing a systemic response. This would be the first-ever detailed expression analysis report for any peroxisomal localized GR.
Beyond peroxisomal GR, the expression analysis of GR with various abiotic stress conditions has been reported in multiple plant species, suggesting its significant involvement in abiotic stress responses. Enhanced expression of GR under chilling stress, was reported in O. sativa seedlings. GR activity has been positively correlated with low temperature-induced photoinhibition in O. sativa (Guo et al., 2006), Cucumis melo (Fogelman et al., 2011), Cucumis sativus (Dai et al., 2012) and Glycine max (Sun et al., 2011). High temperature has been found to enhance the GR activity in certain plant species such as Triticum aestivum (Hasanuzzaman et al., 2012), Nicotiana tabacum (Tan et al., 2011), Zea mays (Kumar et al., 2012), Phaseolus aureus (Kumar et al., 2011) and C. sativus (Dai et al., 2012). The involvement of GR in plant acclimation during low temperature and freezing conditions has been well documented (Kocsy et al., 2001). An increased GR activity has been reported in the roots and leaf tissues of Cicer arietinum, A. thaliana, T. aestivum, Brassica juncea, Vigna mungo and Capsicum annuum under saline stress (Yousuf et al., 2012).
Further, the expression pattern of OsGR was also studied in the presence of combinations of abiotic stress conditions in an attempt to simulate the natural field scenario inflicted on plants. The combinations studied were − dehydration and low-temperature stress, dehydration and high-temperature stress and salinity and high-temperature stress. The combinations were chosen based on their natural occurrences (Li et al., 2024; Swentowsky, 2023; Mittler, 2006; Wang et al., 2003). The OsGR mRNA accumulated to a higher level when the combination of stresses was used compared to individual stress conditions, suggesting an additive effect of stress factors. Also, the elevated level of OsGR mRNA was maintained in long-term stress acclimation as well, suggesting a systemic role of OsGR in abiotic stress tolerance. The studies related to stress combination in literature are limited. The synergistic negative impact of high temperature and dehydration has been demonstrated in O. sativa, resulting in a ROS surge (Yang et al., 2022). The higher expression of GR here could be important in curtailing the ROS surge and bringing about abiotic stress tolerance.
The current work also describes the heterologous expression of OsGR into E. coli followed by purification using affinity chromatography. The purified protein was further used for characterization and antibody generation. The protein was found to be of 55 kDa with all the known domains intact. The GR protein purified from Spinacia oleracea and Pisum sativum was found to be of 72 and 60 kDa respectively (Halliwell and Foyer, 1978; Connell and Mullet, 1986), however, none of these were peroxisomal isoforms. Another isoform from P. sativum having a subunit molecular mass of 55 kDa was also reported (Madamanchi et al., 1992). Two GR isoforms were reported from Pinus strobus having molecular masses of 54 and 57 kDa (Anderson et al., 1990), while another GR from Pinus sylvestris showed a molecular mass of 59 kDa (Wingsle, 1989). Normally, GR has been reported to exist as a homodimer having a combined molecular weight range of 100 – 120 kDa and subunit sizes ranging from 53 – 59 kDa (Wingsle, 1989; Anderson et al., 1990; Edwards et al., 1990; Madamanchi et al., 1992; Romero-Puertas et al., 2006). Jimenez et al. (1997) earlier reported the presence of GR activity in peroxisomes derived from P. sativum leaves, which was further confirmed by immunogold electron microscopy. The peroxisomal GR from P. sativum had a molecular mass of 56 kDa. (Romero-Puertas et al., 2006). The different isoforms of GR have been predicted to exhibit differential activities depending upon the differential environmental cues (Anderson et al., 1990). Further, the OsGR enzyme was also found to remain active over a wide range of pH from 6 to 8. Plant peroxisomes normally have a slightly alkaline pH (Shen et al., 2013), hence the functionality of OsGR at alkaline pH further supports its localization. Since, we also reported the upregulation of OsGR mRNA at various abiotic stress conditions, hence the enzyme stability at low and elevated temperatures was also verified and was found to be active even at 55 ℃, while the optimum temperature was found to be 35 ℃. The Km value of OsGR was found to be 16.67 and 5.55 µM for GSSG and NADHP respectively. Various purified GRs have been demonstrated to have a Km in the range of 9.1 to 196 µM and 1.0 to 8.8 µM for GSSG and NADPH respectively (Table 1). The relatively lower Km value of OsGR reflects its comparatively higher substrate affinity.
Source
Molecular mass (kDa)
Optimum pH
Km NADPH
Km GSSG
Reference
Pinus sylvestris
59
−
1 µM
28 µM
Wingsle, 1989
Pinus strobus
53–54
7.25–7.75
3.7 µM
15.3 µM
Anderson et al., 1990
Pinus strobus
57
7.25
8.8 µM
39.8 µM
Anderson et al., 1990
Spinacia oleracea
72
8.5–––9
2.8 µM
196 µM
Halliwell and Foyer, 1978
Pisum sativum
55
7.8
4.8 µM
56 µM
Madamanchi et al., 1992
Pisum sativum
60
−
3 µM
62 µM
Connell and Mullet, 1986
O. sativa
55
7.5
5.55 µM
16.67 µM
This study
Triticul aestivum
60
4.4–4.5
3.7 µM
9.1 µM
De Lamotte et al., 2000
Pennisetum glaucum
53.5
8.0
144.93 µM
153.85 µM
Achary et al., 2015
5 Conclusion
The current work reports the heterologous expression of a peroxisomal GR from O. sativa into E. coli, followed by its purification via Ni-NTA chromatography. The expression profile of OsGR was also analyzed in the presence of the individual as well as the combination of abiotic stress conditions. The expression pattern revealed its role in early stages as well as long-term stress acclimation. The stability of the enzyme under elevated temperatures and alkaline pH further supported the OsGR candidature for imparting abiotic stress acclimation. The data shows that it could be a suitable candidate for designing futuristic crop varieties able to withstand combinations of abiotic stress conditions in agricultural field conditions.
Consent to participate
All authors consent to participate in the manuscript publication.
Consent for publication
All authors approved the manuscript to be published.
Author Contributions
Conceptualization, GC and PCR, methodology, PCR, WS, DKO. visualization and supervision, GC and SR; writing, GC, SR, PCR; data analysis and review and editing, GC, SR PCR and MKAS. All authors have read and agreed to the published version of the manuscript.
Funding.
This research received no external funding.
CRediT authorship contribution statement
Pamela Chanda Roy: Writing – original draft, Methodology, Data curation. Deepak Kumar Ojha: Data curation. Welka Sahu: Data curation. Mohammad Khalid Al-Sadoon: Writing – review & editing, Data curation. K. Sony Reddy: Writing – original draft, Methodology. Gopal Chowdhary: Writing – review & editing, Writing – original draft, Project administration, Investigation, Conceptualization.
Acknowledgments
The authors would like to extend their sincere appreciation to KIIT University, SERB, Govt. of India and Department of Biotechnology, Govt. of India BUILDER support. The authors would also like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2024R410), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Glutathione reductase a unique enzyme: molecular cloning, expression and biochemical characterization from the stress adapted C4 plant, Pennisetum glaucum (L.) R. Br. mol. biol. rep.. 2015;42:947-962.
- [CrossRef] [Google Scholar]
- Purification, characterization, and immunological properties of two isoforms of glutathione reductase from Eastern white pine needles. Plant Physiol.. 1990;94:1402-1409.
- [CrossRef] [Google Scholar]
- Gibberellic acid mitigates nickel stress in soybean by cell wall fixation and regulating oxidative stress metabolism and glyoxalase system. Plant Physiol. Biochem.. 2023;198:107678
- [CrossRef] [Google Scholar]
- Pea chloroplast glutathione reductase: purification and characterization. Plant Physiol.. 1986;82(2):351-356.
- [CrossRef] [Google Scholar]
- Cinnamic acid pre-treatment enhances heat tolerance of cucumber leaves through modulating antioxidant enzyme activity. Environ. Exp. Bot.. 2012;79 1e10
- [CrossRef] [Google Scholar]
- Glutathione reductase in wheat grain. 1. Isolation and characterization. J. Agric. Food Chem.. 2000;48(10):4978-4983.
- [CrossRef] [Google Scholar]
- Redox-and reactive oxygen species-dependent signaling into and out of the photosynthesizing chloroplast. Plant Physiol.. 2016;171(3):1541-1550.
- [CrossRef] [Google Scholar]
- Glutathione metabolism in plants under stress: beyond reactive oxygen species detoxification. Metabolites. 2021;11(9):641.
- [CrossRef] [Google Scholar]
- Subcellular distribution of multiple forms of glutathione reductase in leaves of pea (Pisum sativum L.) Planta. 1990;180:278-284.
- [CrossRef] [Google Scholar]
- Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: action mechanisms and future prospects. Ecotoxicol. Environ. Saf.. 2018;156:225-246.
- [CrossRef] [Google Scholar]
- Antioxidative activity associated with chilling injury tolerance of muskmelon (Cucumis melo L.) rind. Sci. Hortic.. 2011;128:267-273.
- [CrossRef] [Google Scholar]
- The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21-25.
- [CrossRef] [Google Scholar]
- Sustainable Development Goals (UN Global Goals) In: Idowu S.O., Schmidpeter R., Capaldi N., Zu L., Del Baldo M., Abreu R., eds. Encyclopedia of Sustainable Management. Cham: Springer; 2023.
- [Google Scholar]
- Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Physiol. Biochem.. 2006;44:828-836.
- [CrossRef] [Google Scholar]
- Reactive oxygen species and photosynthesis. Oxidative Damage to Plants. Academic Press. 2014:1-63.
- [Google Scholar]
- Properties and physiological function of a glutathione reductase purified from spinach leaves by affinity chromatography. Planta. 1978;139:9-17.
- [CrossRef] [Google Scholar]
- Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat (Triticum aestivum) seedlings by modulating the antioxidant defense and glyoxalase system. Aust. J. Crop Sci.. 2012;6:1314-1323.
- [Google Scholar]
- Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol.. 1997;114(1):275-284.
- [CrossRef] [Google Scholar]
- Arabidopsis glutathione reductase 1 is dually targeted to peroxisomes and the cytosol. Plant Signal. Behav.. 2010;5(2):171-175.
- [CrossRef] [Google Scholar]
- Role of glutathione in adaptation and signalling during chilling and cold acclimation in plants. Physiol. Plant.. 2001;113:158.
- [CrossRef] [Google Scholar]
- Heat-stress induced inhibition in growth and chlorosis in mung bean (Phaseolus aureus Roxb.) is partly mitigated by ascorbic acid application and is related to reduction in oxidative stress. Acta Physiol. Plant.. 2011;33:2091-2101.
- [CrossRef] [Google Scholar]
- Comparative response of maize and rice genotypes to heat stress: status of oxidative stress and antioxidants. Acta Physiol. Plant.. 2012;34:75-86.
- [CrossRef] [Google Scholar]
- Hydrogen sulfide signaling in plant response to temperature stress. Front. Plant Sci.. 2024;15:1337250.
- [CrossRef] [Google Scholar]
- Purification of multiple forms of glutathione reductase from pea (Pisum sativum L.) seedlings and enzyme levels in ozone-fumigated pea leaves. Plant Physiol.. 1992;100:138-145.
- [CrossRef] [Google Scholar]
- Reactive oxygen species in plants: From source to sink. Antioxidants. 2022;11:225.
- [CrossRef] [Google Scholar]
- Abiotic stress, the field environment and stress combination. Trends Plant Sci.. 2006;11(1):15-19.
- [CrossRef] [Google Scholar]
- Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Biol.. 1998;49(1):249-279.
- [CrossRef] [Google Scholar]
- Characterization, prediction and evolution of plant peroxisomal targeting signals type 1 (PTS1s) Biochim. Biophys. Acta Mol. Cell Res.. 2016;1863(5):790-803.
- [CrossRef] [Google Scholar]
- Glutathione reductase from pea leaves: response to abiotic stress and characterization of the peroxisomal isozyme. New Phytol.. 2006;170(1):43-52.
- [CrossRef] [Google Scholar]
- Molecular cloning of glutathione reductase from Oryza sativa, demonstrating its peroxisomal localization and upregulation by abiotic stresses. Acta biochim. Pol.. 2023;70(1):175-181.
- [CrossRef] [Google Scholar]
- Plasmodium falciparum J‐dot localized J domain protein A8iJp modulates the chaperone activity of human HSPA8. FEBS Lett.. 2024;598(7):818-836.
- [CrossRef] [Google Scholar]
- Organelle pH in the Arabidopsis endomembrane system. Mol. Plant.. 2013;6(5):1419-1437.
- [CrossRef] [Google Scholar]
- Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol.. 2020;21:363-383.
- [CrossRef] [Google Scholar]
- Cloning and characterisation of a cytosolic glutathione reductase cDNA from pea (Pisum sativum L.) and its expression in response to stress. Plant Mol. Biol.. 1997;35:641-654.
- [CrossRef] [Google Scholar]
- Ascorbate-glutathione cycle of mitochondria in osmoprimed soybean cotyledons in response to imbibitional chilling injury. J. Plant Physiol.. 2011;168:226-232.
- [CrossRef] [Google Scholar]
- Multifactor agitation: several minor stresses severely compromise crop growth when combined. Plant Physiol.. 2023;194(3):1248-1249.
- [CrossRef] [Google Scholar]
- Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. J. Plant Physiol.. 2011;168:2063-2071.
- [CrossRef] [Google Scholar]
- Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 2003;218:1-14.
- [CrossRef] [Google Scholar]
- Purification and characterization of glutathione reductase from Scots pine needles. Physiol. Plant.. 1989;76:24-30.
- [CrossRef] [Google Scholar]
- Enhancement of heat and drought stress tolerance in rice by genetic manipulation: a systematic review. Rice (n y). 2022;15(1)
- [CrossRef] [Google Scholar]
- Role of Glutathione Reductase in Plant Abiotic Stress. In: Ahmad P., Prasad M., eds. Abiotic Stress Responses in Plants. New York: Springer; 2012.
- [Google Scholar]
- Signal transduction networks during stress combination. J. Exp. Bot.. 2020;71:1734-1741.
- [CrossRef] [Google Scholar]
- Abiotic stress responses in plants. Nat. Rev. Genet.. 2022;23(2):104-119.
- [CrossRef] [Google Scholar]







