Translate this page into:
Origanum majorana harvested from Al-Soda, Saudi Arabia promotes mitotic arrest and apoptosis in colon cancer cells
⁎Corresponding author. essamebrahim@hotmail.com (Essam H. Ibrahim)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Colorectal cancer is reported as the third major incentive of cancer doom. The present work is meant to examine the anticancer potential of Origanum majorana leaf acetone extract (OMAE) to fight HT-29 human colon cancer cells. Biomolecules in OMAE were examined using Fourier Transform Infrared (FT-IR) spectroscopy. Reactive oxygen species (ROS) in OMAE was determined using an immunosobant assay. The cytotoxic effect of OMAE was tested by MTT assay. p53 gene expression level of OMAE-tread cells was measured using quantitative real-time PCR (qPCR). Apoptotic and cell cycle arrest effects of OMAE on HT-29 were analyzed by flow cytometry. Results revealed the presence of many functional groups and considerable amount of ROS in the extract. The extract could raise p53 expression level five folds over control. OMAE arrested the HT-29 at G2/M phase. OMAE has an apoptotic effect rather than necrotic effects Our discoveries give solid proof that O. majorana acetone extract has a capacity to stop colon cancer activity, at least, through the enlistment of cell division arrest as well as apoptosis. These findings can suggest the use of OMAE as a natural therapeutic candidate against the colon cancer.
Keywords
Origanum majorana
Anticancer
P53
Cell cycle
ROS
1 Introduction
Approximately of 1.9 million cases are reported to have cancer, of them about one-third is accounted for colorectal cancer in both women and men (WHO, 2018, n.d.). Throughout history, plants have been used as a medicine. Now, it is a magnificent source for creating new medications, of them, anticancer drugs. Identification and advancement of unprecedented effective materials derived from plants to be used as cancer therapy have gained significant recognition and made scientists give more experimental work in cancer research. A large number of anticancer drugs are derived from plant or artificially-modified plant derivatives and phytochemicals (Amin et al., 2009; Cragg and Pezzuto, 2016; Iqbal et al., 2017). Plant-derived anticancer medications were shown to be more efficient and less harmful compared to synthetic ones. In the last few years, some plant-derived anticancer drugs are under clinical trial evaluation such as the Taxus brevifolia Nutt. and Camptotheca acuminate (the DNA topoisomerase I inhibitor) (Desai et al., 2008; Thoppil and Bishayee, 2011; Nabih et al., 2012; Greenwell and Rahman, 2015).
The mechanisms by which phytochemicals exert their anticancer effects include interference of gene expression leading to cell proliferation inhibition, induction of apoptosis, genotoxicity inhibition, increasing the anti-inflammatory and antioxidants activity, and cellular signaling pathways modulation (Greenwell and Rahman, 2015).
It is notable that cancer is a disease situation and related to evidently directed apoptosis. Recent researches proved that some plant extracts and phytochemicals could modulate the course of carcinogenesis through several ways including apoptosis. Apoptosis was shown to be one of the effective mechanisms in chemoprevention and chemotherapy studies in human trials and in animal and in vitro carcinogenesis models (Kuno et al., 2012; Pan and Ho, 2008; Thoppil and Bishayee, 2011).
Origanum majorana L. (O. majorana), a member of the Lamiaceae (mint family) is local to southern Europe, North Africa and Asia Minor. This plant is called Wazab in Saudi Arabia and has many uses with several health benefits and is utilized as an in house solution for gastrointestinal disorders, cramps, headaches, gloom, migraine, as diuretic, coughs and dizziness (Ouedrhiri et al., 2016; Vági et al., 2005). Origanum majorana has been reported to have a potential antimicrobial activity (Qureshi et al., 1994). Many investigations revealed that Origanum majorana oil (essential) and extracts (ethanolic/aqueous) have liver and kidney protective capacity against damage and genotoxicity (Leeja and Thoppil, 2007). These extracts contained many metabolites such as flavonoids, phenolic terpenoids, phenolic glycosides and sitosterol (Assaf et al., 1987; Hajlaoui et al., 2016). The O. majorana essential oil has a hepatoprotective, antioxidant and antimutagenic capacity (Mossa and Nawwar, 2011). O. majorana has been shown to exert a low anticancer activity on many hepatoma cell lines (Lin et al., 2002). Also, O. majorana extracts minimized the side effects of using the anticancer drug, cyclophosphamide, without affecting its cytotoxic activity (Qureshi et al., 1994).
In the current work, we examined the impact of OMAE on colon cancer cell lines regarding cytotoxicity, apoptosis, cell cycle and the level of p53 protein.
2 Materials and methods
2.1 Plant collection
In April 2021, O. majorana leaves were gathered from the Al Soda, Aeer, Saudi Arabia and identified. The plant was identified by an expert taxonomist at the Biology Department, King Abdul-Aziz University, Saudi Arabia.
2.2 Extract preparation
The air-dried leaves of O. majorana (200 g) were turned into a coarse powder utilizing an electrical processor and then soaked in acetone for 24 h with consistent mixing at room temperature. The mixture was filtered twice via filter paper (Whatman No.1). About 1.8 g were obtained after drying the filtrate. A stock solution (1%) was prepared by dissolving the obtained solid material in an appropriate amount of acetone (180 mL) (stock solution). The stock solution was sterilized (0.45 µm syringe filter, Millipore®) and kept at −40 °C.
2.3 Functional groups analysis
Active groups present in the prepared extract were discovered utilizing Fourier Transform Infrared (FT-IR) spectroscopy according to Ibrahim et al., (Ibrahim et al., 2021b).
2.4 Estimation of reactive oxygen species
Reactive oxygen species (ROS) was estimated utilizing the same immunosorbent assay kit, method and instrument as described by Ibrahim et al., (Ibrahim et al., 2021a).
2.5 Cell line maintenance
The human colon cancer HT-29 cell line (Merk) was seeded and maintained as described previously (Ghramh et al., 2020).
2.6 Study of extract cytotoxicity against HT-29 cancer cell line
The plant extract was added to HT-29 cancer cells and percent cell viability was assessed using MTT method to calculate the IC50 (concentration that produces half-maximal inhibition) as described previously (Ibrahim et al., 2021a, 2019).
2.7 Quantification of p53 gene expression
Quantification of p53 gene expression in extract-treated HT-29 cancer cells at IC50 concentration was done the same way as described by Ibrahim et al., (Ibrahim et al., 2021a).
2.8 Analysis of cell cycle using flow cytometer
The cell cycle of HT-29 cancer cells was tested utilizing the Propidium Iodide Flow Cytometry Kit (Abkam) for Cell Cycle Analysis. To each well of a 6-well tissue culture plate, HT-29 cancer cells (5X105) were added and incubated for 24 h before the addition of the extract at IC50 concentration for 48 h. All subsequent steps were done the same way as shown by Ibrahim et al., (Ibrahim et al., 2021a). Untreated cells were used as control cultures.
2.9 Apoptotic analysis with Annexin V staining
The cell cycle of HT-29 cancer cells was tested utilizing Annexin V-FITC Apoptosis Detection Kit (abkam, Cat#: ab14085) following the procedure shown by Ibrahim et al., (Ibrahim et al., 2021a).
3 Statistical Analysis
Acquired data were expressed as means 5 results ± SEM of the number of tests. A Student’s t-test was operated utilizing GraphPad Prism (Version 7.0) for paired or unpaired and a statistically significant p value of 0.05 was considered.
4 Results
4.1 Functional groups
A powerful broad beak emerged at 3341.6 cm−1 attributed to stretching O–H because of phenols and alcohols (Fig. 1). Arisen strong bands (2924.1 and 2853.3 cm−1) are assigned to stretching C—H bond which are identical to alkane. Emerged weak band (2118 cm−1) might be a result of stretching CΞC bond of alkyne. An arisen weak band (1863.3 cm−1) might be because of bending C—H bond of aromatic formula. Generated weak bands (1686–1653.1–1602.8–1507.7–1457.4 cm−1) because of disubstituted alkenes stretching C⚌C bond. Other peaks (strong–weak) emanated (1269.2–1209.5–1157.3 cm−1) ascribed to C—O stretching resultant of alkyl aryl ether. Engendered strong broad peaks (1067.9–1032.5 cm−1) because of C—O assigned to primary alcohols. Arisen powerful peaks (777.1–713.8 cm−1) may be the results of stretching C—H bond of monosubstituted benzene. Powerful peaks emanated at 661.6–520 cm−1 may assign to stretching C-Br halo formula.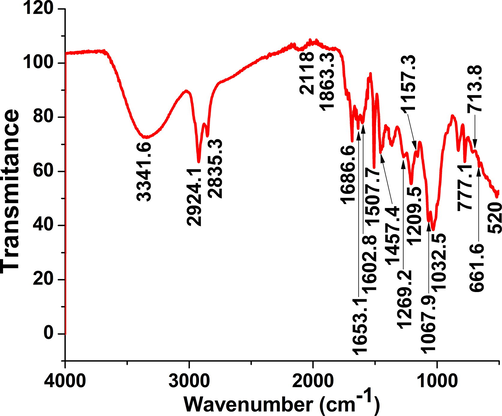
FT-IR spectra of O. majorana acetone extract.
4.2 Content of ROS in the plant extract
Results of quantifying the content of ROS in the O. majorana leaf acetone extract using ELISA revealed that the extract contains 164.2 pg/mL of ROS.
4.3 Extract cytotoxicity against HT-29 cancer cell line
Origanum majorana leaf extract showed a significant (p > 0.0001) inhibitory effects on HT-29 cell growth at the concentration 1000 μg/mL (11% versus 100% growth rate of untreated cells, Fig. 2) up to 62.5 μg/mL. These inhibitory effects continued nonsignificantly down to the concentration of 31.25 μg/mL. The concentrations 0, 0.49, 0.98, 1.95, 3.91, 7.81 and 15.63 μg/mL did not show any cell growth inhibition effect on the cultured cells. IC50 was shown to be at the concentration of 90 μg/mL.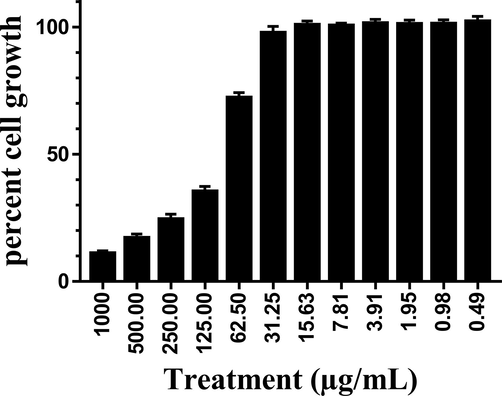
Cytotoxic effects of O. majorana acetone extract on human colon cancer HT-29 cell line.
4.4 Quantification of p53 gene expression
We used real-time RT-PCR to quantitatively analyze the expression of p53 after treatment of HT-29 cells with IC50 dose of O. majorana acetone extract in vitro. Results demonstrated that p53 mRNA expression increased 5.44 folds over control cells (Fig. 3).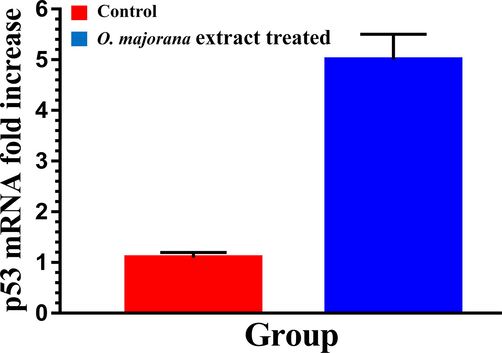
Apoptotic effects of O. majorana acetone extract on human colon cancer HT-29 cell line.
4.5 Cell cycle and DNA content
To investigate if the inhibitory effect of the extract on the cell line was due to cell cycle arrest induction, HT-29 cells were treated with O. majorana acetone extract at a concentration equal to IC50 for 48 h. Flow cytometry was used to test cell cycle progression. After the 48 h O. majorana acetone extract treatment, cell number in the G2/M population significantly (p > 0.001) increased from 18.36 % (control untreated) to 32.8%. As the G2/M phase cell population increased, the G1 phase cell population decreased from 51.88 % (control untreated) to 38.06% (extract treated) (Figs. 4 and 5).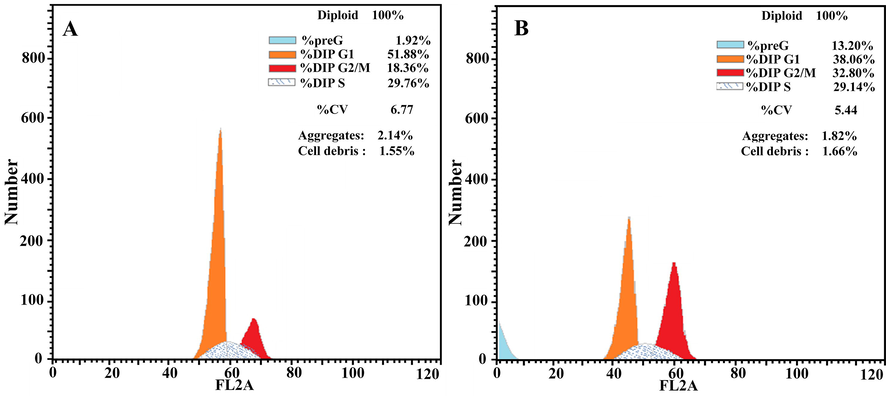
Analysis of cell cycle progression. Human colon cancer HT-29 cells were untreated (A) or treated with O. majorana acetone extract. The distribution and percentage of cells in preG-phase, G1, S and G2/M phase of the cell cycle are indicated.
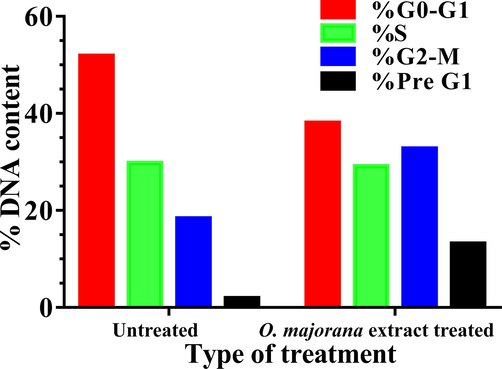
% DNA content during cell cycle progression. Human colon cancer HT-29 cells were untreated or treated with O. majorana acetone extract. The distribution of % DNA content at early and late apoptosis as well as necrosis of the cells are indicated.
4.6 Apoptotic effects of O. majorana acetone extract
The apoptotic effect of O. majorana acetone extract was evaluated with Annexin V staining (Figs. 6 and 7). The extract made the cells go through apoptosis, where apoptotic cell number significantly (p < 0.05) increased in treated cells (11.74 %) over the untreated cells (0.73%). This increase in the percent of the apoptotic cells (treated) was nearly equal at the early (5.92%) and late (5.82%) stages. The decrease in percent cell growth is referred to apoptosis rather than necrosis as necrotic cells were shown to be at a normal level in treated cells (1.46%) which is nearly similar (1.19%) to untreated cells.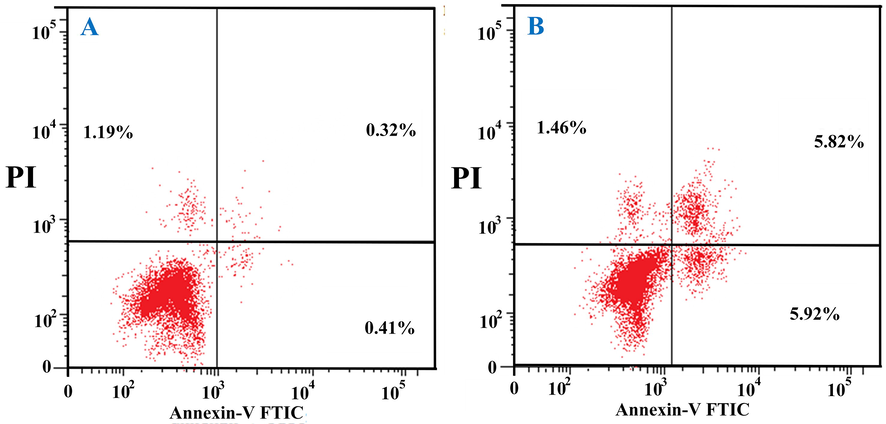
Human colon cancer HT-29 cells stained with anti-Annexin V and propidium iodide showing the percentage of apoptotic cells. A: Untreated cells; B: O. majorana acetone extract treated cells.
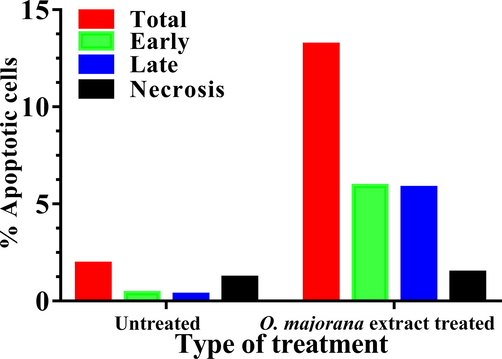
% Human colon cancer HT-29 apoptotic cells after treated with O. majorana acetone extract.
5 Discussion
To explore the anticancer capabilities of OMAE on HT-29 human colon cancer cells, we first tested the impact of different dilutions of OMAE on the proliferation of this cell line. Our outcomes indicated that the treatment of the HT-29 cells with OMAE lowered the viability of the HT-29 cells in a dose-dependent manner. The IC50 was around 90 µ/mL at 48 h.
FT-IR results in our study revealed the presence of phenolic groups in the OMAE. A previous study showed that O. majorana extract contains both phenolic and flavonoid components (Méabed et al., 2018). Among the phenolic compounds are the flavonoids, which have anticancer potentials by modulating the signal transduction pathways within cancer cells to inhibit cell division, metastasis and angiogenesis. Flavonoids can promote apoptosis (Ren et al., 2003).
Our results demonstrated the presence of ROS in the OMAE. In our work, OMAE was directly added to the HT-29 cells. Some workers used extracts of different sources and revealed that these extracts could trigger apoptosis in tested cells possibly through enhanced Bax expression, and Bcl-2 protein inhabitation. The increase in Bax/Bcl-2 ratio and the activation of both Caspase-3 and ROS are tangible fingers for such pathways (Murad et al., 2016; Sun et al., 2012). ROS is known to be involved in the stimulation of several cellular signaling pathways as well as transcription factors (e.g. phosphoinositide 3-kinase), mitogen-activated protein kinases, nuclear factor-κB and p53), which can stimulate cell fate (survival or death) (Bae et al., 2011; Covarrubias et al., 2008; Kaminskyy and Zhivotovsky, 2014; Zhang et al., 2016). ROS increase is usually observed in the apoptosis progress (Matés and Sánchez-Jiménez, 2000; Simon et al., 2000), and its generation or upregulation is considered as a sign of apoptosis induction. Many of the anticancer drugs are natural compounds (e.g. curcumin, garlic, quercetin, cisplatin, etc.) they induce the apoptosis in cancer cells through rising the amount of ROS (Berndtsson et al., 2007; Dirsch et al., 1998; Matés and Sánchez-Jiménez, 2000; Woo et al., 2003).
Mitotic arrest as well as apoptosis was noticed in HT-29 cells because of OMAE treatment. The capability of any anticancer agent to modulate cell cycle sequence is able to give clear data about its mechanism(s) of action to be cytotoxic. For this point of view, we test the impacts of OMAE on cell cycle apportionment utilizing flow cytometry. HT-29 cells were treated with the IC50 of OMAE for 48 h and its cell cycle was analyzed. This IC50 caused G/2M arrest on HT-29 cells. Actually, the population of G2/M has risen proving that OMAE could arrest HT-29 cells in the G2/M phase. This finding is in agreement with other workers and uses many different cell lines (Al Dhaheri et al., 2013b; Benhalilou et al., 2019).
Apoptosis has a valuable function in the demolition of the mutated and abnormal cells from the living organism as a protective mechanism to prevent the progression of cancer (Elmore, 2007; Igney and Krammer, 2002). The extract exerted apoptotic effects rather than cytotoxic effects on HT-29 cells as indicated in our results. The concentration of p53 increased more than five folds over the control. This finding is in agreement with other workers (Abdel-Massih et al., 2010). During the cell cycle, many checkpoints are being passed. These events are very important as control mechanisms. These include the G2/M checkpoint, which blocks the cycle to go through mitosis if the DNA is damaged (Taylor and Stark, 2001). The p53 protein regulates the G2/M transmission by controlling some regulatory protein complexes present in the cell (Bunz et al., 1998; Hermeking et al., 1997) or inducing the apoptosis process (Shaw et al., 1992; Yonish-Rouach et al., 1991). Normal p53 protein is essential in the p53-dependent pathway to control cell cycle arrest and apoptosis (Zhang et al., 2010).
It is settled that interference in contact among cancer cells in point of cancer progression is an essential stage required for the acquisition of invasive discriminatory and the decrease in that contact is always accompanied by a reduced expression of E-cadherin (Berx et al., 1995). E-cadherin expression can reduce cancer cells progression and invasion (Kowalski et al., 2003). Previous study (Al Dhaheri et al., 2013a) showed that O. majorana could induce overexpression of E-cadherin and homotypic cell–cell aggregation but inhibited cell migration in cancer cells. We presuppose that O. majorana in our study exerted an anti-migratory effect on HT-29 cells in the cell culture, to some extent, via the expression of E-cadherin gene reactivation. As a result, E-cadherin overexpression is the most possible mechanism that O. majorana can perform its anti-cancer effect on the HT-29 cells.
6 Conclusion
O. majorana acetone extract is a good anticancer as it could arrest tested cancer cell line at G2/M phase, induce cancer cell apoptosis and upregulate p53 expression.
Acknowledgements
Authors would like to acknowledge the support of the Research Center for Advanced Materials Science (RCAMS), King Khalid University, Abha Saudi Arabia for funding this work through research program RCAMS/KKU/G001/21.
Author’s contributions
The authors contributed equally in creating this manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The apoptotic and anti-proliferative activity of Origanum majorana extracts on human leukemic cell line. Leuk. Res.. 2010;34(8):1052-1056.
- [CrossRef] [Google Scholar]
- Anti-metastatic and anti-tumor growth effects of origanum majorana on highly metastatic human breast cancer cells: inhibition of NFκB signaling and reduction of nitric oxide production. PLoS ONE. 2013;8(7):e68808.
- [CrossRef] [Google Scholar]
- Mitotic arrest and apoptosis in breast cancer cells induced by Origanum majorana extract: upregulation of TNF-α and downregulation of survivin and mutant p53. PLoS ONE. 2013;8(2):e56649.
- [CrossRef] [Google Scholar]
- Overview of major classes of plant-derived anticancer drugs. Int. J. Biomed. Sci.. 2009;5:1-11.
- [Google Scholar]
- Preliminary study of phenolic glycosides from Origanum majorana; quantitative estimation of arbutin; cytotoxic activity of hydroquinone. Planta Med.. 1987;53(04):343-345.
- [CrossRef] [Google Scholar]
- Regulation of reactive oxygen species generation in cell signaling. Mol. Cells. 2011;32(6):491-509.
- [CrossRef] [Google Scholar]
- Origanum majorana ethanolic extract promotes colorectal cancer cell death by triggering abortive autophagy and activation of the extrinsic apoptotic pathway. Front. Oncol.. 2019;9:1-12.
- [CrossRef] [Google Scholar]
- Acute apoptosis by cisplatin requires induction of reactive oxygen species but is not associated with damage to nuclear DNA. Int. J. Cancer. 2007;120(1):175-180.
- [CrossRef] [Google Scholar]
- E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. EMBO J.. 1995;14(24):6107-6115.
- [CrossRef] [Google Scholar]
- Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science (80-.. 1998;282(5393):1497-1501.
- [Google Scholar]
- Function of reactive oxygen species during animal development: Passive or active? Dev. Biol.. 2008;320(1):1-11.
- [CrossRef] [Google Scholar]
- Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Principles Practice. 2016;25(2):41-59.
- [CrossRef] [Google Scholar]
- Medicinal plants and cancer chemoprevention. Curr. Drug Metab.. 2008;9:581-591.
- [CrossRef] [Google Scholar]
- Ajoene, a compound of garlic, induces apoptosis in human promyeloleukemic cells, accompanied by generation of reactive oxygen species and activation of nuclear factor κB. Mol. Pharmacol.. 1998;53(3):402-407.
- [CrossRef] [Google Scholar]
- Apoptosis: a review of programmed cell death. Toxicol. Pathol.. 2007;35(4):495-516.
- [CrossRef] [Google Scholar]
- Study of anticancer, antimicrobial, immunomodulatory, and silver nanoparticles production by Sidr honey from three different sources. Food Sci. Nutr.. 2020;8(1):445-455.
- [CrossRef] [Google Scholar]
- Medicinal plants: their use in anticancer treatment. Int. J. Pharm. Sci. Res.. 2015;6:4103-4112.
- [CrossRef] [Google Scholar]
- Chemical composition and in vitro evaluation of antioxidant, antimicrobial, cytotoxicity and anti-acetylcholinesterase properties of Tunisian Origanum majorana L. essential oil. Microb. Pathog.. 2016;95:86-94.
- [CrossRef] [Google Scholar]
- 14–3-3σ is a p53-regulated inhibitor of G2/M progression. Mol. Cell. 1997;1(1):3-11.
- [CrossRef] [Google Scholar]
- Rosemary extract and its biogenic silver nanoparticles induce apoptosis and arrest cell cycle in HT-29 colon cancer cells. Sci. Adv. Mater.. 2021;13(1):36-49.
- [CrossRef] [Google Scholar]
- Lepidium sativum and its biogenic silver nanoparticles activate immune cells and induce apoptosis and cell cycle arrest in HT-29 colon cancer cells. Biomater. Tissue Eng.. 2021;11:195-209.
- [CrossRef] [Google Scholar]
- Cellular proliferation/cytotoxicity and antimicrobial potentials of green synthesized silver nanoparticles (AgNPs) using Juniperus procera. Saudi J. Biol. Sci.. 2019;26:1689-1694.
- [CrossRef] [Google Scholar]
- Death and anti-death: tumour resistance to apoptosis. Nat. Rev. Cancer. 2002;2(4):277-288.
- [CrossRef] [Google Scholar]
- Plant-derived anticancer agents: a green anticancer approach. Asian Pac. J. Trop. Biomed.. 2017;7(12):1129-1150.
- [CrossRef] [Google Scholar]
- Free radicals in cross talk between autophagy and apoptosis. Antioxidants Redox Signal.. 2014;21(1):86-102.
- [CrossRef] [Google Scholar]
- E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res.. 2003;5:R217-R222.
- [CrossRef] [Google Scholar]
- Cancer chemoprevention through the induction of apoptosis by natural compounds. J. Biophys. Chem.. 2012;03(02):156-173.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of methanol extract of Origanum majorana L. (Sweet marjoram) J. Environ. Biol.. 2007;28:145-146.
- [Google Scholar]
- In vitro anti-hepatoma activity of fifteen natural medicines from Canada. Phyther. Res.. 2002;16(5):440-444.
- [CrossRef] [Google Scholar]
- Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int. J. Biochem. Cell Biol.. 2000;32(2):157-170.
- [CrossRef] [Google Scholar]
- Chemical analysis of aqueous extracts of Origanum majorana and Foeniculum vulgare and their efficacy on Blastocystis spp. cysts. Phytomedicine. 2018;43:158-163.
- [CrossRef] [Google Scholar]
- Free radical scavenging and antiacetylcholinesterase activities of Origanum majorana L. essential oil. Hum. Exp. Toxicol.. 2011;30(10):1501-1513.
- [CrossRef] [Google Scholar]
- Induction of G1-phase cell cycle arrest and apoptosis pathway in MDA-MB-231 human breast cancer cells by sulfated polysaccharide extracted from Laurencia papillosa. Cancer Cell Int.. 2016;16:39-50.
- [CrossRef] [Google Scholar]
- Potential anticancer activity of the medicinal herb, Rhazya stricta, against human breast cancer. African J. Biotechnol.. 2012;11(37):8960-8972.
- [Google Scholar]
- Mixture design of Origanum compactum, Origanum majorana and Thymus serpyllum essential oils: optimization of their antibacterial effect. Ind. Crops Prod.. 2016;89:1-9.
- [CrossRef] [Google Scholar]
- Chemopreventive effects of natural dietary compounds on cancer development. Chem. Soc. Rev.. 2008;37(11):2558.
- [CrossRef] [Google Scholar]
- Effect of alpinia galanga treatment on cytological and biochemical changes induced by cyclophosphamide in mice. Pharm. Biol.. 1994;32(2):171-177.
- [CrossRef] [Google Scholar]
- Flavonoids: Promising anticancer agents. Med. Res. Rev.. 2003;23(4):519-534.
- [CrossRef] [Google Scholar]
- Induction of apoptosis by wild-type p53 in a human colon tumor-derived cell line. Proc. Natl. Acad. Sci. U. S. A.. 1992;89(10):4495-4499.
- [CrossRef] [Google Scholar]
- Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415-418.
- [CrossRef] [Google Scholar]
- Cycle arrest and apoptosis in MDA-MB-231/Her2 cells induced by curcumin. Eur. J. Pharmacol.. 2012;690(1-3):22-30.
- [CrossRef] [Google Scholar]
- Regulation of the G2/M transition by p53. Oncogene. 2001;20(15):1803-1815.
- [CrossRef] [Google Scholar]
- Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. WJH. 2011;3(9):228.
- [CrossRef] [Google Scholar]
- Phenolic and triterpenoid antioxidants from Origanum majorana L. herb and extracts obtained with different solvents. J. Agric. Food Chem.. 2005;53(1):17-21.
- [CrossRef] [Google Scholar]
- WHO 2018, n.d. WHO Cancer Fact sheet (2018). Available online at: www.who.int/en/news-room/fact-sheets/detail/cancer.
- Molecular mechanisms of curcumin-induced cytotoxicity: Induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003;24(7):1199-1208.
- [CrossRef] [Google Scholar]
- Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991;352(6333):345-347.
- [CrossRef] [Google Scholar]
- ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev.. 2016;2016:1-18.
- [CrossRef] [Google Scholar]
- Huaier aqueous extract inhibits proliferation of breast cancer cells by inducing apoptosis. Cancer Sci.. 2010;101:2375-2383.
- [CrossRef] [Google Scholar]







