Organic farming innovations: Cultivation of wheatgrass microgreens in soil enriched with fruit peels and antimicrobial assessment
⁎Corresponding author. 20phfnf004@avinuty.ac.in (Krithika R),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
This study explores the viability of fruit peel-enriched soils as a sustainable growth medium, acting as a biofertilizer for wheatgrass microgreens. Additionally, the research seeks to evaluate the antimicrobial properties of fruit peels, typically considered agricultural waste, to determine their influence on plant growth parameters.
Methods
Fruit peels from pomegranate, orange, and sweet lime were collected and processed into powder, and diluted with water to create enriched soil. Wheatgrass microgreens were cultivated in conventional potting soil and soil enriched with fruit peel powder. Growth parameters, including germination rates, shoot and root length, and total yield of wheatgrass microgreens, were monitored over a 15-day growth cycle. Antimicrobial analysis was conducted on selected fruit peels, assessing their impact on Staphylococcus aureus and Escherichia coli compared to a control (Rifampicin).
Results
The results, analyzed through ANOVA and Tukey (post hoc) tests, indicate significant differences among fruit peel-enriched soils. Pomegranate peel emerged as a particularly effective enhancer of wheatgrass microgreen growth. Furthermore, the antimicrobial analysis revealed that pomegranate and sweet lime peels exhibited superior properties, with a notable zone of inhibition effects on Staphylococcus aureus and Escherichia coli compared to the control (Rifampicin).
Conclusion
This study demonstrates the potential of fruit peels as effective biofertilizers to promote wheatgrass microgreen growth in sustainable agricultural practices. The multifaceted benefits include enhanced crop development and the discovery of natural antimicrobial agents, particularly in pomegranate and sweet lime peels. These findings support the broader adoption of environmentally conscious approaches in agriculture, emphasizing the value of utilizing agricultural waste for plant growth promotion and the development of natural alternatives to synthetic antimicrobial agents.
Keywords
Environmental Challenges
Fruit peels
Global food security
Sustainable agriculture
and Wheatgrass
1 Introduction
Agricultural crop production is a cornerstone of global food security and economic sustainability (Arora, 2018). It is an intricate process that involves cultivating various crops to meet the ever-growing demands of a burgeoning global population (Nair, 2019). To increase soil fertility and maximize yield, adding nutrients is frequently necessary for the development of crops (Alasa et al., 2021).Table 2.
Due to the increased usage of chemical fertilizers, high crop output in the previous century was farmers' trend and habit of practice (Timsina, 2018). However, through time, they understood that chemical fertilizers alter soil fertility and eradicate the bacteria that facilitate crop growth (Srivastava et al., 2020). The main problem they encountered when employing chemical fertilizers was that they affected the agricultural land and the human population who consumed those agricultural products (Devi & Sumathy, 2017).
Biofertilizers emerged as a solution for farmers' issues when depending on chemical fertilizers. As the name suggests, biofertilizer is environmentally friendly and farmer-friendly (Dumitrescu et al., 2009). Biological waste and fertilizer do not contain harmful substances and improve the soil; they are utilized instead of chemical fertilizers (Laditi et al., 2012). Utilizing natural goods like biofertilizers in crop cultivation can help preserve the quality of crop products as well as the health of the soil (Singh et al., 2013).
According to (Sharma et al., 2023), food waste (FW) is a global issue that does not appear to improve. It causes problems for society, the economy, and the environment. According to the United Nations Environment Program, Food Waste Index Report 2021 (Lahiri et al., 2023), food waste production in India alone reached approximately 68.7 million metric tons in 2021 (Srivastava & Mishra, 2022). The global biofertilizer market, valued at USD 2.6 billion in 2022, is expected to grow at a compound annual growth rate (CAGR) of over 12.5 % from 2023 to 2028. Biofertilizers boost soil health, improve crop yields, and reduce chemical use, supporting sustainability and rising organic food demand. Using waste materials also cuts landfill waste, lowers emissions, and recycles nutrients for more sustainable farming (Markets and Markets, 2023).
Fruit waste is one of the biggest environmental concerns among all the recent elements that have affected the environment. For example, depending on the region and harvesting method, the number of wasted materials in most fruit processing industries tends to be very high (mango 30–50 %, banana 20 %, pomegranate 40–50 %, and citrus 30–50 %) (Parfitt et al., 2010).
Fruit peels are a great way to get calcium, iron, zinc, and potassium minerals. It serves as a kind of organic fertilizer (Nossier, 2021). Fertilizer comes in two primary varieties: organic and inorganic. Fertilizers are nutrients that are added to soil to support plant growth. Carbonaceous materials and other antioxidants are present in organic or natural fertilizers (Fatima et al., 2022). Commercial or inorganic fertilizers, such as ammonia sulfate, are typically manufactured, or they can be processed from quarries, which are a cheap and safe source of ingredients for plant growth. Fruit peels are used in the soil as fertilizer, to balance the pH of the soil, and to provide micronutrients like zinc, calcium, and iron (Qader, 2019).
In contemporary agriculture, fruit peels have emerged as potent biofertilizers, offering a sustainable solution to pressing environmental concerns (Yadav et al., 2024). This article explores the global state of fruit peel biofertilizer adoption, spanning diverse agricultural landscapes. Against soil degradation and chemical reliance, fruit peel biofertilizers present a compelling alternative rooted in ecological balance (Sharma et al., 2024). By harnessing the nutrient-rich properties of discarded peels, farmers worldwide stand to enhance soil fertility and crop yields while reducing environmental harm. This trend reflects a shift towards eco-conscious farming practices and sustainable agriculture on a global scale. Join us as we delve into the transformative potential of fruit peel biofertilizers in shaping the future of farming and environmental stewardship (Al-Tawaha et al., 2023).
(Gunjal et al., 2024) review explores the cultivation, bioactive potential, and health benefits of microgreens, based on data from extensive literature research. It also highlights their growing role in the culinary industry and potential as value-added food products. Microgreens grown in soil and cocopeat showed differences in growth, nutritional content, and antioxidant activity. Cocopeat was the most effective medium, especially for improving yield and bioactive properties (Gunjal et al., 2024).
Microgreens, rich in phytonutrients, are considered a great option for preventing malnutrition and improving health due to their nutrient-dense properties and short growth cycle. Challenges like fungal growth arise without proper practices despite their ease of cultivation. The global microgreens market, valued at $1.7 billion in 2022, is projected to grow to $2.61 billion by 2029, driven by their popularity in specialty foods, fast growth, and high economic potential (Singh et al., 2024).
(Gunjal et al., 2024) explores the nutritional composition, bioactive compounds, and health benefits of wheatgrass, including its cultivation, preservation, and utilization in food products.
2 Materials and methods
2.1 Collection of fruit peels
The study focused on fruit peels from pomegranate, orange, and sweet lime, gathered from a local fruit juice shop in Sai Baba Colony, Coimbatore, during July and August 2022. These peels were chosen because they exhibited the highest wastage among the selected fruit juice shops. Pomegranate peel is abundant in bioactive compounds, including phytochemicals such as flavonoids, phenolic acids, and lignin. It also possesses antibacterial and antimicrobial properties (Mo et al., 2022). Citrus peels, such as oranges and sweet limes, contain vital compounds like sugars and acids that influence soil acidity and play a role in photosynthesis, ultimately affecting plant growth (Qader, 2019). The collected fruit peels were cleaned and washed under tape water to remove the dirt in them and dried under the shade drying method for 10 days. After sun drying, it was made into fine powdered through siever with mesh no of 4.75 mm. Fruit peels dried under sunlight preserve nutrients, extend shelf life, and inhibit microbial growth, providing a natural and economical preservation technique.
2.2 Antimicrobial properties for selected fruit peel Powders
4 grams of the fruit peel powder was diluted with 100 ml of the distilled water to obtain aqueous extracts. These extracts are tested with one Gram-positive bacteria, Staphylococcus aureus, and one Gram-negative bacteria, Escherichia coli, for antimicrobial study. The antimicrobial properties of the selected fruit peel extracts were analyzed using disk diffusion or agar well diffusion method. This test was done to identify the sensitivity to bacteria. The disk-diffusion method tests the effectiveness of antibiotics on a specific microorganism. An agar plate is first spread with selected gram-positive and gram-negative bacteria. The disks were made by pouring 20 µl of extracts of selected fruit peels. The paper disk of antibiotic (Rifampicin) was used as the standard control disk. The bacteria were allowed to grow on the agar media by incubating overnight at 37 °C. The next day, the amount of space around each disk was noted using a measuring scale. The space around each disk indicates the lethality of the bacteria, i.e., bacteria that have not grown in this space. This is called the zone of inhibition and was measured and recorded as shown in Table 1(Saleem and Saeed, 2020).
| Sample | Zone of Inhibition (mm) | |
|---|---|---|
| Staphylococcus aureus | Escherichia coli | |
| Control (Rifampicin) | 37 mm | 20 mm |
| Pomegranate peel | 17 mm | 21 mm |
| Orange peel | 20 mm | 18 mm |
| Sweet lime peel | 24 mm | 21 mm |
| Parameters | Soil Incorporated with Selected Fruit Peels | |||
|---|---|---|---|---|
| Control | Pomegranate Peel (PP) |
Orange Peel (OP) |
Sweet lime Peel (SP) |
|
| Electrical Conductivity (dSm-1) | 0.65 (Good) |
1.14 (Medium) |
0.89 (Good) |
1.29 (Medium) |
| pH | 7.8 (Normal) |
6.2 (Normal) |
7.2 (Normal) |
7 (Normal) |
| Calcium Carbonate | Absent | Absent | Absent | Absent |
| Organic Carbon % | 0.48 | 0.51 | 0.54 | 0.48 |
| Phosphorous Kg/ac | 7.7 | 7.8 | 7.8 | 7.5 |
| Potassium Kg/ac | 285 | 269 | 273 | 244 |
| Iron (below critical limits) ppm | 3.9 | 4.3 | 4.7 | 6.2 |
| Manganese (above critical limits) ppm | 2.6 | 2.2 | 1.9 | 2.1 |
| Zinc (below critical limits) ppm | 0.9 | 0.6 | 0.7 | 0.9 |
| Copper (below critical limits) ppm | 0.7 | 0.9 | 0.7 | 0.8 |
| Borax (Boron) ppm | 1.5 | 2 | 1.5 | 1.5 |
| Nitrogen Kg/acre | 60 | 60 | 60 | 60 |
| Phosphorus pentoxide Kg/acre | 40 | 50 | 20 | 20 |
| Potassium oxide Kg/acre | 15 | 90 | 30 | 23 |
2.3 Comparison of soil nutrients incorporated with selected fruit peels
The respective variations of soil incorporated with selected fruit peels were analyzed for nutrient content such as electrical conductivity, pH, calcium carbonate, organic carbon, phosphorous, potassium, Iron, Manganese, zinc, copper, nitrogen, phosphorous pentoxide, potassium oxide, borax, Zinc sulfate, copper sulfate, zinc sulfate, manganese sulfate, and iron sulfate were measured using a standard procedure (National Mission for Sustainable Agriculture, 2020).
-
Electrical Conductivity (EC): EC was determined to assess the soil's ability to conduct electrical current, which provides insights into the overall ion concentration in the soil, Saturated solution of calcium sulphate and 0.01 N KC1 solution (0.7456 gm/lit) reagent is used (El-Naggar et al., 2021).
-
pH: Soil pH was measured to gauge its acidity or alkalinity, as this can significantly influence plant nutrient availability. The standard buffer solution of potassium hydrogen phthalate, toluene, sodium di hydrogen, and sodium tetra borate reagent is used and determined by the potentiometric method. (Msimbira & Smith, 2020).
-
Calcium Carbonate: The presence of calcium carbonate was determined using titration method, as it can impact soil alkalinity and nutrient availability. (Yu et al., 2023).
-
Organic Carbon: The organic carbon content was measured to assess the organic matter content of the soil, which is essential for microbial activity and soil fertility. 1 N standard potassium dichromate solution, ferrous ammonium sulphate solution, diphenylamine, ferroin indicator, and conc. Sulphuric acid is used and determined by titration method (Gerke, 2022).
-
Nutrient Elements: Essential nutrient elements, including phosphorus (Olesen’s method), potassium (Reagent: Ammonium acetate and standard potassium is used and determined by flame photometry method), iron, manganese, zinc, and copper, were quantified using Atomic Absorption Spectrophotometer (AAS) method. These elements are vital for plant growth and development (Bhatla et al., 2018).
-
Nitrogen: Nitrogen content was measured to assess the soil's nitrogen status, a key nutrient required for plant protein synthesis. 0.32 % potassium permanganate, 2.5 % sodium hydroxide, 2.0 % boric acid, BCG double indicator, potassium hydrogen phthalate, NaOH, and H2SO4 and determined by alkaline permanganate method (Hurisso et al., 2018).
-
Manganese Sulphate, Iron Sulphate, Copper Sulphate, Zinc Sulphate: The presence of these sulfate compounds was analyzed using the Atomic Absorption Spectrophotometer (AAS) method to assess the availability of these micronutrients in the soil (Kumar et al., 2020).
-
Borax: Borax content was measured to evaluate the presence of boron, a micronutrient critical for plant growth and development. The ammonium acetate, EDTA, glacial acetic acid, azomethine-H reagent, and standard boron solution were used and determined by the spectrophotometric method (Arunkumar et al., 2018).
These analyses were carried out using established and standardized procedures of NMSA to ensure accuracy and reliability. The data obtained from these assessments provide valuable insights into how incorporating different fruit peels affects the soil's nutrient profile, which is crucial for understanding the potential benefits of using fruit peels as biofertilizers. This comprehensive analysis enables us to sketch meaningful conclusions regarding the effects of fruit peel incorporation on soil health and its potential implications for agriculture and environmental sustainability.
2.4 Analysis of growth parameters and yield of wheatgrass microgreens
The growth parameters and yield of the wheatgrass microgreens were recorded. 25 g of wheat grains were sown in the four different trays. Variation I is pomegranate peel powder, Variation II entails using orange peel powder, and Variation III includes sweet lime peel powder incorporated into the soil. This incorporation is done by adding 1 g of fruit peel powder mixed with 100 ml of water, which is poured into the trays daily, and a controlled environment is maintained with consistent sunlight. The growth of wheatgrass remained unaffected by light at a temperature of 30 °C, even though the microgreens were exposed to daylight during the day. This cultivation occurred outdoors with an average relative humidity of 70 %. Growth parameters, including germination height, root, shoot height, and total yield, are measured on the 15th day of the cultivation period. A duration of 15 days in cultivation is adequate for wheatgrass microgreens to complete their growth cycle, from germination to maturity, ensuring optimal development (Kumar et al., 2017).
2.5 Statistical analysis
The obtained results underwent statistical analysis through one-way ANOVA. The significance of the results was assessed using a post-hoc test, specifically the Tukey test, with a probability level set at p < 0.05. This analysis used the Statistical Package for the Social Sciences (SPSS) version 13.0.
3 Results and discussion
3.1 Antimicrobial properties for selected fruit peels powder
Depicts the antimicrobial properties of selected fruit peels: Pomegranate peel (PP), orange peel (OP), and sweet lime peel (SP) (Fig. 1) represent the zone of inhibition by selected fruit peels. The antimicrobial properties of the selected fruit peels and antibiotic (Rifampicin) were identified using the two bacterial strains of Staphylococcus aureus and Escherichia coli using a standard procedure (Farahmandfar et al., 2020). Staphylococcus aureus and Escherichia coli in agricultural soil pose a contamination risk, indicating potential soil degradation and ecosystem harm, leading to reduced crop yields and environmental damage.
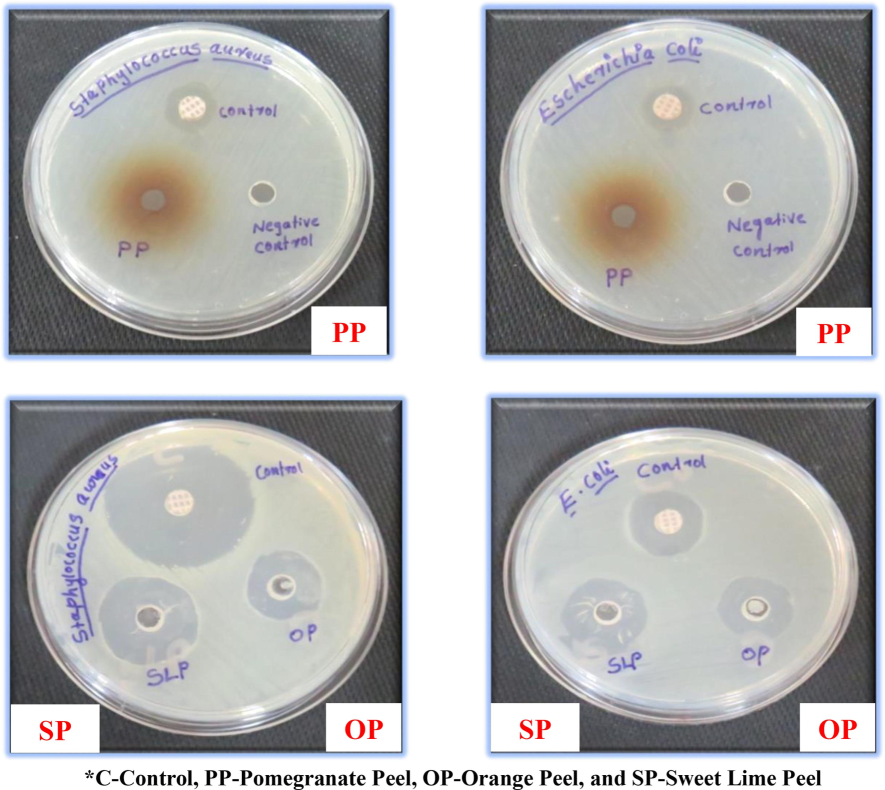
- Zone of Inhibition by Selected Fruit Peels Against Staphylococcus aureus and Escherichia coli.
The zone of inhibition of pomegranate peel was 17 mm against Staphylococcus aureus and 21 mm against Escherichia coli, orange peel was 20 mm Staphylococcus aureus and 18 mm against Escherichia coli, and sweet lime peel was 24 mm against Staphylococcus aureus and 21 mm against Escherichia coli. The control Staphylococcus aureus shows the maximum zone of inhibition compared to all three selected fruit peels. In the control group, Escherichia coli shows 20 mm as the minimum inhibition zone compared to pomegranate and sweet lime peels. Still, orange peel shows a very low zone of inhibition, about 18 mm. In comparing all three fruit peels, sweet lime peel shows the maximum zone of inhibition in Staphylococcus aureus and pomegranate peel, and sweet lime peel shows the maximum zone of inhibition in Escherichia coli. The antimicrobial properties of selected fruit peels against Staphylococcus aureus and Escherichia coli, the sweet lime peel was found to have good antimicrobial properties compared to the pomegranate and orange peels. In the research conducted by (Saleem & Tariq Saeed, 2020), it was observed that orange peel exhibited a zone of inhibition measuring 14 mm against Staphylococcus aureus and 19 mm against E. coli. However, the present study reveals an even greater zone of inhibition for orange peel.
3.2 Evaluation of selected fruit peel incorporated in soil
The soil analysis was done using the standard procedure mentioned in the Soil Health Card Scheme training manual, Government of Tamil Nadu, Department of Agriculture. The electrical conductivity of the soil is influenced by the concentration of dissolved salts and ions in the soil. The normal electrical conductivity rating in the soil is s0-1.0 dSm-1, which is good, and 1.0–3.0 dSm-1 indicates medium ratings for electrical conductivity. In pomegranate peel (PP) and sweet lime peel (SP), 1.14 dSm-1 and 1.29 dSm-1 electrical conductivity ratings showed a medium level in soil, control (C) and orange peel (OP) 0.65 dSm-1 and 0.89 dSm-1 electrical conductivity ratings showed good levels in soil. The electrical conductivity results inferred that variations of pomegranate peel (PP) and sweet lime peel (SP) have the maximum range of soil salinity and nutrient content. PH is used to measure the acidity or alkalinity of soil. All the variations of pomegranate peel (PP), orange peel (OP), orange peel (SP), and control (C) show a normal range of pH. The organic carbon in soil is important for nutrient, moisture retention, and microbial activity. The ratings of organic carbon % (<0.5 %= Low, 0.51–0.75 % = medium and > 0.75 %= High. In (C) and (SP), 0.48 % was found, which indicates a low level of organic carbon. In (PP), it is 0.51 %; in (OP), it is 0.54 %, indicating a medium level of organic carbon in the soil. The rating of phosphorous in the soil is < 4.5 Kg/ac is low, 4.5––9 Kg/ac is medium, and > 9.0 Kg/ac. In (PP) and (OP) both showed 7.8 Kg/ac, and (C) and (SP) showed 7.7 Kg/ac and 7.5 Kg/ac showed medium level of phosphorous in the soil. The potassium rating in the soil is 0–48 Kg/ac, which is low, 48–113 Kg/ac is medium, and > 113 Kg/ac is high. In (C) −285 Kg/ac, (PP) − 269 Kg/ac, (OP) − 273 Kg/ac, and (SP) has 244 Kg/ac, and all the variations showed high values indicating the presence of potassium in the soil. The ratings of micronutrients such as Zinc < 1.20 below the critical level (in ppm) and 1.21–5.0 above the critical level (in ppm). All the variations showed below the critical level in (in ppm), that is, (PP), (OP), (SP), and (C) have zinc content of 0.6 ppm, 0.7 ppm, 0.9 ppm, and 0.9 ppm. The normal value of copper < 1.20 below the critical level (in ppm) and 1.21–5.0 is above the critical level (in ppm). All the variations showed below the critical level in (in ppm), that is, (PP), (OP), (SP), and (C) have a copper content of 0.9 ppm, 0.7 ppm, 0.8 ppm, and 0.7 ppm. The normal value of manganese < 2.0 below the critical level (in ppm) and 2.01–12.0 is above the critical level (in ppm). In all the variations, manganese values are above the critical level that (PP), (OP), (SP), and (C) have a manganese content of 2.5 ppm, 15 ppm, 4 ppm, and 2.5 ppm. The normal value of iron < 3.7 below the critical level (in ppm) and 4.5–24.0 is above the critical level (in ppm). The variation of (PP) and the control group has below critical limits of iron content in the soil that is 3.9 and 4.3 ppm, and (OP) and (SP) have above critical limits of iron content that is 4.7 and 6.2 ppm. The normal value of boron is < 0.5 ppm is low, 0.5–1.0 ppm is medium, and > 1.0 ppm is high in levels in soil. All the variations showed higher values of boron present in the soil, that is, (PP), (OP), (SP), and (C) have boron content of 2 ppm, 1.5 ppm, 1.5 ppm, and 1.5 ppm. The normal nitrogen value is < 280 kg/acre is low, 280–560 kg/acre is medium, and > 560 kg/acre is high. (PP), (OP), (SP), and (C) have a low level of nitrogen, with 60 kg/acre of nitrogen content present in the soil. The normal phosphorous pentoxide value is < 22.5 kg/acre is low, 22.5–55 kg/acre is medium, and > 55 kg/acre is a high level of phosphorous pentoxide in soil, (PP) and (C) has a medium level of phosphorous pentoxide that is 50 kg/acre and 40 kg/acre present in the soil. In (OP) and (SP), 20 kg/acre is found, with a low level of phosphorous pentoxide in the soil. The normal potassium oxide value is < 144 kg/acre is low, 144–336 kg/acre is medium, and > 336 kg/acre is low. (PP), (OP), (SP), and (C) have a low level of potassium oxide at 90 kg/acre,30 kg/acre, 23 kg/acre, and 15 kg/acre. The variation (PP) has the maximum content of potassium oxide compared to (OP), (SP), and (C). The Control group (C) has the minimum potassium oxide content compared to other variations.
3.3 Evaluation of growth Characteristics of wheatgrass microgreens
Wheatgrass offers therapeutic qualities and may help manage conditions like diabetes, atherosclerosis, and cancer, making consumer awareness essential for its market potential (Gunjal M. et al.,2024). The growth parameters of wheatgrass microgreens were monitored under controlled environmental conditions. Several growth parameters were monitored, including germination height, root length, and shoot length. The experiment was conducted over a period of 15 days.
i Germination rate
The germination rate of wheatgrass microgreens was found to be rapid in the variation of PP, with nearly 95 % of the seeds germinating within the first 4 days of the experiment in all the variations (PP), (OP), (SP) and (C). A measuring scale was used to determine the height of the germination seeds. The maximum height of the germination of seeds observed in (PP) is 2.2 ± 0.149 cm, whereas (OP) and (SP) had 0.65 ± 0.108 cm and 1.15 ± 0.108 cm, and the (C) control group had 1.08 ± 0.131 cm. The variation of (OP) shows the minimum height of the germination rate of seeds as shown in Fig. 2
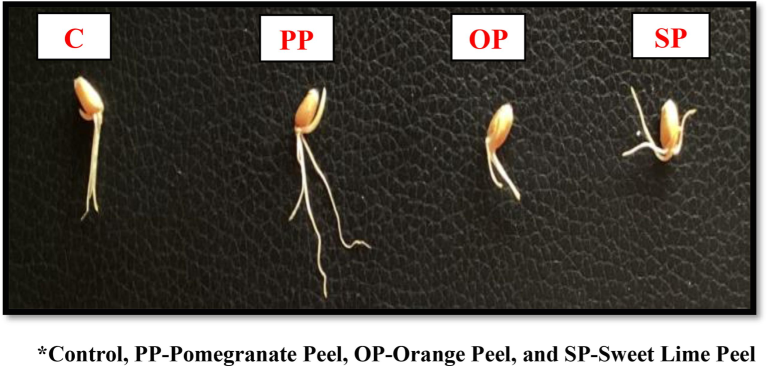
- Effect of Selected Fruit Peels on Wheat Grain Germination.
The ANOVA (Analysis of Variance) Table 3 shows that there is a high statistical significance difference between all the groups with a 95 % level of significance p-value < 0.05 (i.e., the p-value is 0.000). The (PP) group has a higher mean value when compared with all other groups; hence, we conclude that the (PP) group has more growth in the germination of wheatgrass grains. The Tukey honestly significant test is used to determine where the difference occurs. The results show that the (PP) group has more differences than the other groups when compared to the control, (OP), and (SP) groups. The Mean difference for the Control with the (PP) group is 1.120 cm with a p-value of 0.000 with a 95 % level of significance, for the (PP) with (OP) and (SP) groups, the mean difference is 1.550 cm and 1.050 cm where the p-value for both the groups is highly significance with the p-value of 0.000 (i.e., p-value < 0.05).
| Groups | Mean ± S. D (cm) |
Range (Minimum-Maximum) | ANOVA p-value Between Groups |
Tukey (Post Hoc Test) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Group |
Mean Difference |
Std-Error | 95 % Confidence Interval | p-value | |||||
|
Lower Bound |
Upper Bound | ||||||||
| C | 1.08 ± 0.132 | 0.9–––1.3 | 0.000* | PP | −1.120 | 0.056 | −1.271 | −0.969 | 0.000* |
| OP | 0.430 | 0.056 | 0.279 | 0.581 | 0.000* | ||||
| SP | −0.070 | 0.056 | −0.221 | 0.081 | 0.601 | ||||
| PP | 2.2 ± 0.149 | 2.0–––2.4 | 0.000* | C | 1.120 | 0.056 | 0.969 | 1.271 | 0.000* |
| OP | 1.550 | 0.056 | 1.399 | 1.701 | 0.000* | ||||
| SP | 1.050 | 0.056 | 0.899 | 1.201 | 0.000* | ||||
| OP | 0.65 ± 0.108 | 0.5–––0.8 | 0.000* | C | −0.430 | 0.056 | −0.581 | −0.279 | 0.000* |
| PP | −1.550 | 0.056 | −1.701 | −1.399 | 0.000* | ||||
| SP | −0.500 | 0.056 | −0.651 | −0.349 | 0.000* | ||||
| SP | 1.15 ± 0.108 | 1.0–––1.3 | 0.000* | C | 0.070 | 0.056 | −0.081 | 0.221 | 0.601 |
| PP | −1.050 | 0.056 | −1.201 | −0.899 | 0.000* | ||||
| OP | 0.500 | 0.056 | 0.349 | 0.651 | 0.000* | ||||
* The mean difference is significant at the 0.05 level.
ii Height of the microgreens
The measurement of shoot length revealed a consistent increase in height over the 15th day. On average, the shoot length of (OP) is 15.91 ± 0.732 cm and has the maximum height of the shoot length compared to the other variations (PP), (SP), and (C). The minimum height of the shoot length recorded in the control group was about 7.76 ± 0.134 cm. (PP) variations have a 15.3 ± 1.272 cm height of shoot length, and (SP) has a 12.38 ± 0.846 cm height of shoot length seen in wheatgrass microgreens. The root development in wheatgrass microgreens also showed consistent progression throughout the study. The root lengths were measured with maximum (PP) and minimum (C). The mean and standard deviation of root length seen in (PP), (OP), (SP), and (C) are 8.31 ± 0.412 cm, 7.18 ± 0.404 cm, 6.9 ± 0.483 cm, and 4.09 ± 0.412 cm respectively. The overall full height of the wheatgrass microgreens seen in the (PP) variation is about 24.47 ± 0.283 cm, whereas (OP), (SP), and (C) are 24.43 ± 0.176 cm, 19.79 ± 0.993 cm, and 12.18 ± 0.335 cm respectively as shown in Fig. 3.
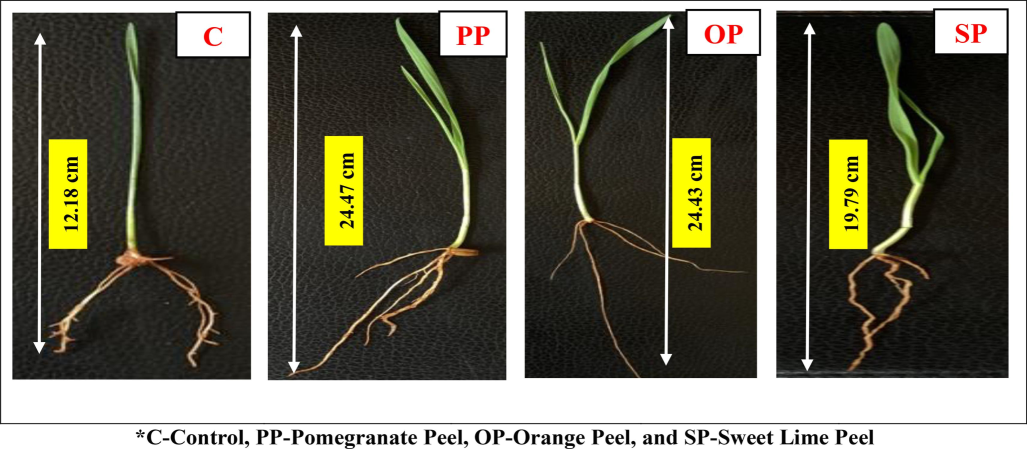
- Effect of Selected Fruit Peels on Growth of Wheatgrass Microgreens.
The ANOVA (Analysis of Variance) Table 4 shows that there is a high statistical significance difference between all the groups with a 95 % level of significance p-value < 0.05 (i.e., the p-value is 0.000). The (PP) group has a higher mean value when compared with all other groups; hence, we conclude that the (PP) group has more growth. The Tukey honestly significant test is used to determine where the difference occurs. The results show that the (PP) group has more differences than the other groups when compared to the control, (OP), and (SP) groups. The Mean difference for the Control with the (PP) group is 12.290 cm with a p-value of 0.000 with a 95 % level of significance, for the (PP) with (SP) groups, the mean difference is 4.680 cm where the p-value for both the groups is highly significant with the p-value of 0.000 (i.e. p-value < 0.05) for the (OP) group the mean difference is 0.040 cm with the p-value of 0.998 which is not statistical significance (i.e., p value > 0.05) with the 95 % level of significance.
| Groups | Mean ± S. D (cm) |
Range (Minimum-Maximum) | ANOVA p-value Between Groups |
Tukey (Post Hoc Test) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Group |
Mean Difference |
Std-Error | 95 % Confidence Interval | p-value | |||||
|
Lower Bound |
Upper Bound | ||||||||
| C | 12.18 ± 0.336 |
11.5 – 12.5 | 0.000* | PP | −12.290 | 0.246 | −12.953 | −11.627 | 0.000* |
| OP | −12.250 | 0.246 | −12.913 | −11.587 | 0.000* | ||||
| SP | −7.610 | 0.246 | −8.273 | −6.947 | 0.000* | ||||
| PP | 24.47 ± 0.283 | 24 – 24.8 | 0.000* | C | 12.290 | 0.246 | 11.627 | 12.953 | 0.000* |
| OP | 0.040 | 0.246 | −0.623 | 0.703 | 0.998 | ||||
| SP | 4.680 | 0.246 | 4.017 | 5.343 | 0.000* | ||||
| OP | 24.43 ± 0.177 | 24.1 – 24.8 | 0.000* | C | 12.250 | 0.246 | 11.587 | 12.913 | 0.000* |
| PP | −0.040 | 0.246 | −0.703 | 0.623 | 0.998 | ||||
| SP | 4.640 | 0.246 | 3.977 | 5.303 | 0.000* | ||||
| SP | 19.79 ± 0.994 |
18.3 – 21.1 | 0.000* | C | 7.610 | 0.246 | 6.947 | 8.273 | 0.000* |
| PP | −4.680 | 0.246 | −5.343 | −4.017 | 0.000* | ||||
| OP | −4.640 | 0.246 | −5.303 | −3.977 | 0.000* | ||||
* The mean difference is significant at the 0.05 level.
iii Total yield of wheatgrass microgreens
This experiment aimed to investigate the total yield of the wheatgrass microgreens on the 15th day of the cultivation period, focusing on the impact of different selected fruit peels as biofertilizers. Four variations were tested, and the results revealed significant differences in total yield.
The maximum yield of the wheatgrass microgreens was recorded in the variation (PP) about the mean ± S.D of 32.4 ± 2.05 g of wheatgrass microgreens yield out of 25 g of seeds sown in the tray whereas, (C) had 14 ± 2.44 g, (OP) and (SP) where 17.7 ± 2.05 g and 22.3 ± 2.05 g respectively as shown in Fig. 4. This study concluded that pomegranate peel-incorporated soil has increased nutrient availability in the soil through plants and also influenced the height and yield of the wheatgrass microgreens. Pomegranate peels greatly act as biofertilizers for plants to improve their overall height and yield, as shown in Table 3
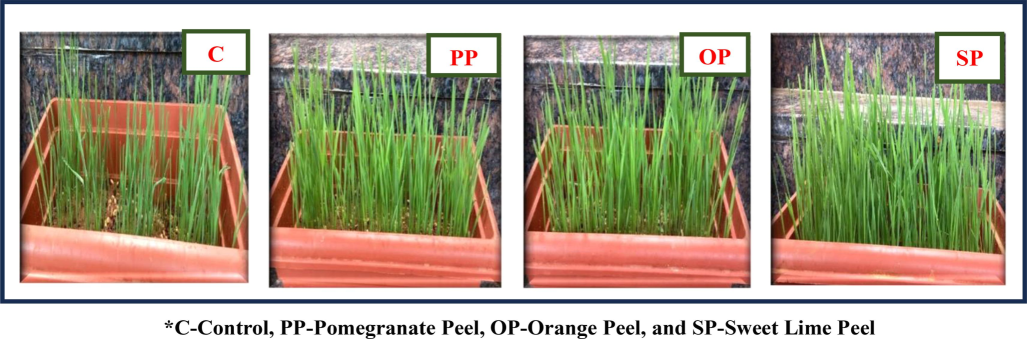
- Effect of Selected Fruit Peels on Yield of Wheatgrass Microgreens.
The ANOVA (Analysis of Variance) Table 5 shows that there is a highly statistically significant difference between all the groups with a 95 % level of significance p-value < 0.05 (i.e., the p-value is 0.000). The Tukey honestly significant test is used to determine where the difference occurs. The (PP) group has a higher mean value when compared with all other groups; hence, we conclude that the (PP) group has more growth. The results show that the (PP) group has more differences than the other groups when compared to the control, (OP), and (SP) groups. The Mean difference for the Control with the (PP) group is 18.333 cm with a p-value of 0.000 with a 95 % level of significance, for the (PP) with (OP) groups, the mean difference is 14.667 cm where the p-value for the groups is significant with the p-value of 0.001 (i.e., p-value < 0.05) and the (PP) group with the (SP) group the mean difference is 10 cm with the statistical significance with the p-value of 0.007 (i.e., p- value < 0.05) with the 95 % level of significance. This study analyzed the height and yield of wheatgrass microgreens over the first 15 days of cultivation, focusing on microgreens. Future research could investigate mature plants, assess the bioactive compounds in wheatgrass, and explore its health benefits and potential for value-added product development.
| Groups | Mean ± S. D (g) |
Range (Minimum-Maximum) | ANOVA p-value Between Groups |
Tukey (Post Hoc Test) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Group |
Mean Difference |
Std-Error | 95 % Confidence Interval | p-value | |||||
|
Lower Bound |
Upper Bound | ||||||||
| C | 14 ± 3.000 | 11 – 17 | 0.000* | PP | −18.333 | 2.160 | −25.250 | −11.420 | 0.000* |
| OP | −3.667 | 2.160 | −10.580 | 3.250 | 0.384 | ||||
| SP | −8.333 | 2.160 | −15.250 | −1.420 | 0.020* | ||||
| PP | 32.33 ± 2.517 | 30 – 35 | 0.000* | C | 18.333 | 2.160 | 11.420 | 25.250 | 0.000* |
| OP | 14.667 | 2.160 | 7.750 | 21.580 | 0.001* | ||||
| SP | 10.000 | 2.160 | 3.080 | 16.920 | 0.007* | ||||
| OP | 17.67 ± 2.517 | 15 – 20 | 0.000* | C | 3.667 | 2.160 | −3.250 | 10.580 | 0.384 |
| PP | −14.667 | 2.160 | −21.580 | −7.750 | 0.001* | ||||
| SP | −4.667 | 2.160 | −11.580 | 2.250 | 0.214 | ||||
| SP | 22.33 ± 2.517 | 20 – 25 | 0.000* | C | 8.333 | 2.160 | 1.420 | 15.250 | 0.020* |
| PP | −10.000 | 2.160 | −16.920 | −3.080 | 0.007* | ||||
| OP | 4.667 | 2.160 | −2.250 | 11.580 | 0.214 | ||||
* The mean difference is significant at the 0.05 level.
4 Conclusion
This study investigates fruit peel-enriched soils as a sustainable growth medium and biofertilizer for wheatgrass microgreens, focusing on repurposing agricultural waste. Pomegranate peels significantly enhanced growth and yield while exhibiting antimicrobial properties against pathogens like Staphylococcus aureus and Escherichia coli. Sweet lime peel showed stronger antimicrobial properties than pomegranate and orange peels, with orange peel exhibiting greater inhibition zones against the same pathogens, particularly in this study. The pomegranate peel (PP) group showed the most notable improvements in wheatgrass germination height, full height, and yield when compared to the control, orange peel (OP), and sweet lime peel (SP) groups.
The results highlight fruit peels as an eco-friendly, sustainable alternative in agriculture, promoting higher yields and reducing environmental impact. Future research will focus on wheatgrass's nutritional and phytochemical properties in peel-enriched soils. These findings are promising for global food security and environmental sustainability, demonstrating how agricultural waste can be transformed into valuable biofertilizers. The study underscores the importance of integrating sustainable practices into farming systems, offering a more resilient and environmentally responsible path for agriculture that aligns with the planet's needs.
Acknowledgement
We would like to express our sincere thanks to Avinashilingam Institute for Home Science and Higher Education for Women, Coimbatore, India for their valuable support and laboratory facilities, which are crucial to the success of our research.
Authors contributions.
Krithika. R contributed to drafting the original manuscript, while Raajeswari Paramasivam reviewed and made corrections. All authors have reviewed and consented to the publication of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Experimental study on the use of banana and pineapple peel waste as biofertilizers, tested on Hibiscus sabdariffa plant: Promoting sustainable agriculture and environmental sanitation. Arid Zone Journal of Engineering, Technology and Environment. 2021;17(4):547-554.
- [Google Scholar]
- Al-Tawaha, A. R. M. S., & Ondrasek, G. (Eds.). (2023). Integrated nutrients management: An approach for sustainable crop production and food security in changing climates. Frontiers Media SA.
- Agricultural sustainability and food security. Environmental Sustainability. 2018;1(3):217-219.
- [Google Scholar]
- Boron: A critical micronutrient for crop growth and productivity. Journal of Pharmacognosy and Phytochemistry. 2018;7(2):2738-2741.
- [Google Scholar]
- Bhatla, S. C., A. Lal, M., Kathpalia, R., & Bhatla, S. C. (2018). Plant mineral nutrition. Plant physiology, development and metabolism, 37-81.
- Production of biofertilizer from fruit waste. European Journal of Pharmaceutical and Medical Research. 2017;4(9):436-443.
- [Google Scholar]
- Obtaining biofertilizer by composting vegetable waste, sewage sludge and sawdust. Bulletin of the Transilvania University of Brasov. Engineering Sciences. Series I. 2009;2:117.
- [Google Scholar]
- Imaging the electrical conductivity of the soil profile and its relationships to soil water patterns and drainage characteristics. Precis. Agric.. 2021;22(4):1045-1066.
- [Google Scholar]
- Comparison of different drying methods on bitter orange (Citrus aurantium L.) peel waste: Changes in physical (density and color) and essential oil (yield, composition, antioxidant and antibacterial) properties of powders. J. Food Meas. Charact.. 2020;14:862-875.
- [Google Scholar]
- Accompanying effects of sewage sludge and pine needle biochar with selected organic additives on the soil and plant variables. Waste Manag.. 2022;153:197-208.
- [Google Scholar]
- The central role of soil organic matter in soil fertility and carbon storage. Soil Systems. 2022;6(2):33.
- [Google Scholar]
- Microgreens: cultivation practices, bioactive potential, health benefits, and opportunities for its utilization as value-added food. Food Biosci.. 2024;105133
- [Google Scholar]
- Comparative analysis of morphological, nutritional, and bioactive properties of selected microgreens in alternative growing medium. S. Afr. J. Bot.. 2024;165:188-201.
- [Google Scholar]
- Nutritional Significance of Wheatgrass: Cultivation Practices and Opportunities for its Processing and Preservation. Recent Advances in Food. Nutrition & Agriculture; 2024.
- Soil protein as a rapid soil health indicator of potentially available organic nitrogen. Agric. Environ. Lett.. 2018;3(1):180006
- [Google Scholar]
- Growth parameters and productivity of wheat as influenced by crop establishment methods and different seed rate. International Journal of Pure and Applied Bioscience. 2017;5(4):2134-2140.
- [Google Scholar]
- Evaluation of microbial inoculants as biofertilizers for the improvement of growth and yield of soybean and maize crops in savanna soils. Afr. J. Agric. Res. 2012
- [Google Scholar]
- A critical review on food waste management for the production of materials and biofuel. Journal of Hazardous Materials Advances. 2023;100266
- [Google Scholar]
- Markets and Markets. (2023). Biofertilizers Market - Global Forecast to 2028.
- Pomegranate peel as a source of bioactive compounds: A mini review on their physiological functions. Front. Nutr.. 2022;9:887113
- [Google Scholar]
- The roles of plant growth promoting microbes in enhancing plant tolerance to acidity and alkalinity stresses. Front. Sustainable Food Syst.. 2020;4:106.
- [Google Scholar]
- Utilizing crop wild relatives to combat global warming. Adv. Agron.. 2019;153:175-258.
- [Google Scholar]
- Orientation Training on Soil Analysis for Agricultural Officers. Soil Health Card Scheme. Training Manual. 2020.
- Impact of organic fertilizers derived from banana and orange peels on tomato plant quality. Arab Universities Journal of Agricultural Sciences. 2021;29(1):459-469.
- [Google Scholar]
- Food waste within food supply chains: quantification and potential for change to 2050. Philos. Trans. r. Soc., B. 2010;365(1554):3065-3081.
- [Google Scholar]
- Influence combination of Fruits Peel and Fertilizer Methods on growth and yield of Chickpea (Cicer areitinum) L. Plants. ZANCO. J. Pure Appl. Sci.. 2019;31(3):45-51.
- [Google Scholar]
- Potential application of waste fruit peels (orange, yellow 2lemon and banana) as wide range natural antimicrobial agent. Journal of King Saud University-Science. 2020;32(1):805-810.
- [Google Scholar]
- Food waste digestate as biofertilizer and their direct applications in agriculture. Bioresour. Technol. Rep.. 2023;101515
- [Google Scholar]
- Microbial Biofertilizers for Soil Health. In: Advancements in Microbial Biotechnology for Soil Health. Springer Nature Singapore: Singapore; 2024. p. :119-147.
- [Google Scholar]
- Production of Biofertilizer from agro-waste by using Thermotolerant Phosphate Solubilising Bacteria. International Journal of Bioinformatics and Biological Science. 2013;1(2):227-244.
- [Google Scholar]
- Emergence of microgreens as a valuable food, current understanding of their market and consumer perception: A review. Food Chem.. 2024;X:101527
- [Google Scholar]
- Food waste valorization for handling environmental problems: a review. Environmental Sustainability. 2022;5(4):401-421.
- [Google Scholar]
- Can organic sources of nutrients increase crop yields to meet global food demand? Agronomy. 2018;8(10):214.
- [Google Scholar]
- Valorisation of Agri-Food Waste for Bioactive Compounds: Recent Trends and Future Sustainable Challenges. Molecules. 2024;29(9):2055.
- [Google Scholar]
- Ameliorating soil acidity with calcium carbonate and calcium hydroxide: effects on carbon, nitrogen, and phosphorus dynamics. J. Soil Sci. Plant Nutr.. 2023;23(4):5270-5278.
- [Google Scholar]
Further Reading
- Management of micronutrients in soil for the nutritional security. Nutrient Dynamics for Sustainable Crop Production 2020:103-134.
- [Google Scholar]







