Translate this page into:
Optimization of treatment blocking the gustatory sense and feeding ethogram of red imported fire ant, Solenopsis invicta Buren (Hymenoptera: Formicidae) to sugar
⁎Corresponding authors. waqar4me@yahoo.com (Waqar Jaleel), waqarjaleel@gdppri.com (Waqar Jaleel), lhlu@gdppri.com (Lihua LYU)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The red imported fire ant is one of the notorious species of ants all over the world. The most effective control of red imported fire ant is the broadcast application of baits, i.e., fatty oil. However, to improve the sugar bait efficacy against fire ants, one of the most important ways is that understand the sugar taste mechanism of fire ant workers via feeding behavior/ethogram. Therefore, we first time described the feeding ethogram of minor fire ant workers to sucrose solution. As well as, we first time explained that, how we can block or deactivate the sensitivity of gustatory sensilla on gustatory appendages of fire ant workers (minor size, head width 0.5–0.8 mm). We used different techniques (ablation, 2–silicon components and HCL solution) for blocking the sensitivity of antennal and tarsal sensilla in in the fire ant workers. Feeding ethogram confirmed that fore legs tarsi of fire ant workers are main gustatory organ to sucrose solution. In antennal treatments, the survival % of workers without a flagellum (ablated of flagellum) was maximum as compared to silicon treated workers. In tarsal treatments, the survival % of HCL treated workers were maximum as compared to other treated workers (ablation of foreleg tarsi and silicon treated foreleg tarsi). The results implied that ablation for antennae flagellum and HCL solution for foreleg tarsi were more suitable for study the gustatory behavior of fire ant workers on the sucrose solution. This study would be more useful for the study regarding the gustatory sense of fire ant through antennal and tarsal sensilla.
Keywords
Solenopsis invicta
Gustatory sensilla
Ablation
Silicon
HCL solutions
1 Introduction
The red imported fire ant, Solenopsis invicta Buren is also known as the fire ant or RIFA, and the dangerous invasive species of ants worldwide especially in China (Wang et al., 2019). The fire ant’s invasion created serious issues, e.g., public safety, human health, agricultural, and veterinary places all over the world especially in China (Vinson, 1997; Wang et al., 2020). The most effective control of fire ant is a broadcast application of sugar or oily baits (Luo et al., 2006; Wang et al., 2020). However, it is necessary to understand the gustatory mechanisms of fire ant workers to sugar baits for their better management.
Antennae, mouthparts, legs, ovipositors, and wings of insects have gustatory sensilla to sugar and bitter compounds (Agnihotri et al., 2016; Araya and Padilla, 2020; Liman et al., 2014; Stocker, 1994; Yosano et al., 2020). Antennae and foreleg tarsi of flies, bees, and moths have gustatory sensilla for sweet, and bitter compounds (Chun and Schoonhoven, 1973; Solari et al., 2016; Yosano et al., 2020; Zhang et al., 2011). Tarsus sensilla of fruit flies, honey bee and beetles have the gustatory sense to sugar compounds especially sucrose (de Brito Sanchez et al., 2014; Thoma et al., 2016; Yosano et al., 2020). Sensilla basiconic and trichoid on antennae of Drosophila, ants and H. armigera play the main role in chemoreceptors (main olfactory and partially gustatory sense) (Ning et al., 2019). Sensilla chaetic have a gustatory response to sugar and bitter compounds and usually found on the tarsus and antennae of beetle and most of the Hymenopteran insects (de Brito Sanchez et al., 2014; Yosano et al., 2020).
In insect, ablation is the most useful technique for blocking the sensitivity of sensilla (Chui Ting et al., 2018; Kunert and Weisser, 2005; Pontes et al., 2014; Potting et al., 2005). In honey bees, to block the sensitivity of antennae, two–silicon compounds were used to cover the antennae (Letzkus et al., 2006). HCl solution is the best acid to deactivate sensilla of antennae and legs in insects (Ramaswamy et al., 1987). And Ramaswamy et al. (1987) concluded that the HCL solution blocked the sensitivity of sensilla on antennae and leg tarsi of Heliothis virescens. However, no records were published on fire ants.
A sucrose solution is often considered as a phagostimulant for ants, meanwhile important ingredient of sugars baits (Deby et al., 1999, Madsen et al., 2017; Skinner, 1980; O’brien and Hooper-bùi, 2005; Howard et al., 1980). Therefore, we selected the sucrose in our study. This study mainly hypothesized that the foreleg tarsi and antennae flagellum may play an important role in sugar taste and both foreleg tarsi and antennae flagellum contain similar types of gustatory sensilla. We report our findings on the optimization of a suitable treatment for blocking gustatory sensilla in the fire ant workers; meanwhile, we observed their gustatory behavior on sucrose solution.
2 Material and methods
2.1 Fire ant colonies
Colonies of fire ants were collected with soil from Nansha (22.602149 N, 113.584083 E) and Baiyun (23.394736 N, 113.737307 E) Districts, Guangzhou city, Guangdong Province, the Peoples’ Republic of China in 2019. Fire ant colonies with soil were shoveled into a bucket (20–litre capacity) and then transferred to the laboratory in the Plant Protection Research Institute, Guangdong Academy Agricultural Sciences, located in No. 7 Jinying Rd., Tianhe District 510640, Guangzhou city, Guangdong Province, China.
Colonies of fire ants were extracted from the bucket by slowly dripping water (Lei et al., 2019). An iron sieve (9 cm) was used to swab the floated ant raft into the plastic container (45 × 38 × 15 cm) lined with Fluon F4–1 to prevent the ants from escaping. Each nest container was fitted with the artificial nest that made in a Petri dish (15 cm diameter × 1.5 cm high) with a layer of 1.25 cm plaster. The lid of the nest was coated with black color. Water was provided in a glass test tube (2.5 × 19.5 cm), which was half–full of water and closed with cotton. A Petri dish (9 × 1.5 cm) containing minced mealworms, Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae), was placed in the plastic container and replaced twice a week. Fire ant colonies consisted of 10,000 workers, more than 10 queens, and more broods. The controlled environment chambers were set at a photoperiod of 14:10 (L:D) h, at 25 ± 2 °C and 60–70% relative humidity. All colonies were acclimatized for 20 d in the laboratory before the experiments (Lei et al., 2021). There is big variation in fire ant workers as minima, minor, media, and major (Greenberg et al., 1985; Hedges, 1998; Wood and Tschinkel, 1981). We selected/screened minor workers having head widths from (0.5 to 0.8 mm) for each experiment.
2.2 Feeding ethogram of minor fire ant workers to sucrose solution
The purpose of this experiment is to find out the feeding pattern of minor fire ant workers (0.5–1 mm) on sucrose solution. The 20% sucrose solution was prepared in distilled water. An individual worker was transferred into the Petri dish (3 × 1.5) by fine and soft forceps. The worker allows to acclimatized for at least 48 h in the Petri–dish, and then the Petri dish was placed for 2 h in the dark black condition (de Brito Sanchez et al., 2014). A droplet (10 μL) of 20% sucrose solution (n = 20) was dispensed by a micropipette (1–20 μL) into the centre of Petri dish. Its foraging behaviour was observed until touching a droplet up to 25 min. If a worker did not access to the droplet, then it was discarded. Observations were done for 3 hr under a Keyence VHX–5000 digital microscope (Jaleel et al. 2018; Pozuelo et al. 2015). The controlled conditions were similar, as described above. Twenty workers were tested from each colony. A total of 4 different colonies were used for categorizing ethogram of workers (olfaction and gustation).
2.3 Optimization of treatment for blocking gustatory sensilla on antennae and foreleg tarsi.
The gustatory sense of fire ant was evaluated on antennae and foreleg tarsi after blocking the sensitivity of sensilla by ablation, 2–silicon components or HCL solution according to the modified methodologies from some previous studies (Letzkus et al., 2006; Pontes et al., 2014; Ramaswamy et al., 1987).
2.3.1 Ablation
Minor of workers were collected and shifted into the plastic containers as described in the section of feeding ethogram. The workers were deprived of any carbohydrate source and water for 48 h. A total of 4 colonies were used for the experiment, 30 workers from each colony. After starvation, 30 workers were separated into a glass vial (10 ml capacity with Fluon F4–1 to prevent the ants from escaping) from the sub colony. The glass vial containing workers were immobilized on the icebox. Under dissecting microscope (Carl Zeiss, Germany), a worker was fixed inside the small plastic centrifuge tube (0.2 ml) (the tip of centrifuge tube was removed) by extruding the antennae and forelegs tarsi. A sterile fine scissor (8.5 cm in length, the tip of scissor 8 mm) was used to cut antennae and forelegs tarsi.
2.3.2 2–silicon compounds
Workers were collected and prepared for the experiment as the same described in the section of ablation. Then 2–silicon components were mixed by ratio 1:1 and coated thinly on antennae flagellum. Similarly, forelegs tarsi were coated by silicon compounds (Huge Kouqiang Cailiao Ltd. Shandong, China).
2.3.3 HCL treatment
The HCL solution is more suitable for blocking the sensitivity of sensilla. Workers were collected and prepared in the experiment as the same described above. Five different concentrations of HCL solution (10%, 20%, 30%, 40%, and 50%) were prepared and applied on the worker forelegs tarsi for the 20 s using pasture pipette under dissecting microscope in a preliminary test, in which mortality of the workers was <20% in the treatment of 20% HCL solution.
Two main experiments were designed as following: first antennal treatment as with ablation of the flagellum (Flagellum-); with 2–silicon compounds (Flagellum Silicon), and without any measures (Intact). Second foreleg tarsal treatments as with ablation (Tarsi -), with 2–silicon compounds covered (Tarsi Silicon), with HCL solution (Tarsi HCL), and without any measures (Intact) as control.
2.4 Survival of fire ant workers
The survival rate is an important parameter for study the behavior of insects (Potting et al., 2005). Minor of workers were collected and prepared for the experiment as the same described in the above sections. Thirty workers were shifted into a Petri dish (9 cm). Survival of each type/treated workers (ablated, silicon, and HCL) was observed for 10 days. Survival % were calculated after 10 days.
2.5 Feeding latency of fire ant workers
Sucrose solutions is often considered as a phagostimulant, especially for ants (Deby et al., 1999; Madsen et al., 2017; Skinner, 1980). The 20% sucrose solution in comparison to simple sugar might be less viscous and more energized to ants (Detrain and Prieur, 2014). Based on the literatures, we selected 20% sucrose solution to find out the feeding latency of minor fire ant workers.
The treated workers were prepared as described above and designed in antennal and tarsal treatments. A treated worker was shifted into a Petri dish (3 × 1.5 cm). The Petri dish was placed for 2 h in a dark back condition before the experiment. A droplet (10 μL) of 20% sucrose solution was dispensed using a micropipette in the centre of the dish. The foraging was observed until to reach a droplet up to 20 min. If a worker would not be accessed to the droplet, then it was discarded. Observations were done for 3 h using a Keyence VHX–5000 digital microscope. The feeding latency, define as “the time spent between the first searching and fulfil its feeding on the sucrose droplet” were calculated. This experiment was replicated with 120 times/treated worker, the 30 workers from one colony. A total of 4 colonies were used for this experiment.
2.6 Statistical analysis
All data in the bar graph were presented as mean ± SEM. Statistical tests were conducted using SPSS version 22.0 (International Business Machines Corp., Armonk, NY, USA). The data (Mean ± SEM) of feeding latency (min) among antennal treated workers, e.g., with ablation of the flagellum (Flagellum-); with 2–silicon compounds (Flagellum Silicon), and without any measures (Intact) were analyzed in SPSS using statistical significance (0.05) using one–way ANOVA and followed by post hoc Tukey. A similar analysis was done for tarsal treatments, e.g., foreleg tarsal treatments as with ablation (Tarsi -), with 2–silicon compounds covered (Tarsi Silicon), with HCL solution (Tarsi HCL), and without any measures (Intact) as control.
3 Results
3.1 Feeding ethogram of small fire ant workers to sucrose solution
Ethogram of feeding behavior in minor fire ant was categorized into two main steps, first olfactory sense and second gustatory sense. Gustatory sense was further categorized into three steps.
First type used their antennae to touch sucrose droplet
Second used their foreleg tarsi
Third used their palps
Our ethogram result showed that, more workers of fire ant used their foreleg tarsi to taste the sucrose droplet as compared to antennae flagellum and palps (Fig. 1).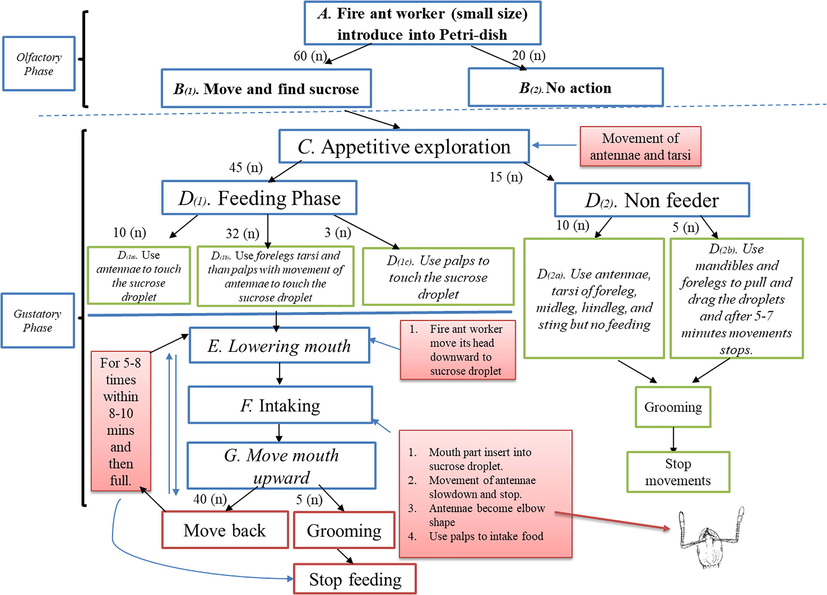
Feeding ethogram of fire ant worker (minor having head width 0.5–0.8 mm) to sucrose solution (n: representing number of fire ant workers).
3.2 Survival of fire ant workers
The significant difference was observed in the survival % among the antennae treated workers, e.g., flagellum ablation, flagellum covered with silicon and untreated (F2,357 = 6242, P < 0.0001). The survival % of flagellum ablated workers were maximum as compared workers having flagellum covered with silicon, while survival % of both treated workers was less than control/untreated workers (Fig. 2A). The results implied that workers without antennae flagellum were less effected in comparison to silicon treated workers.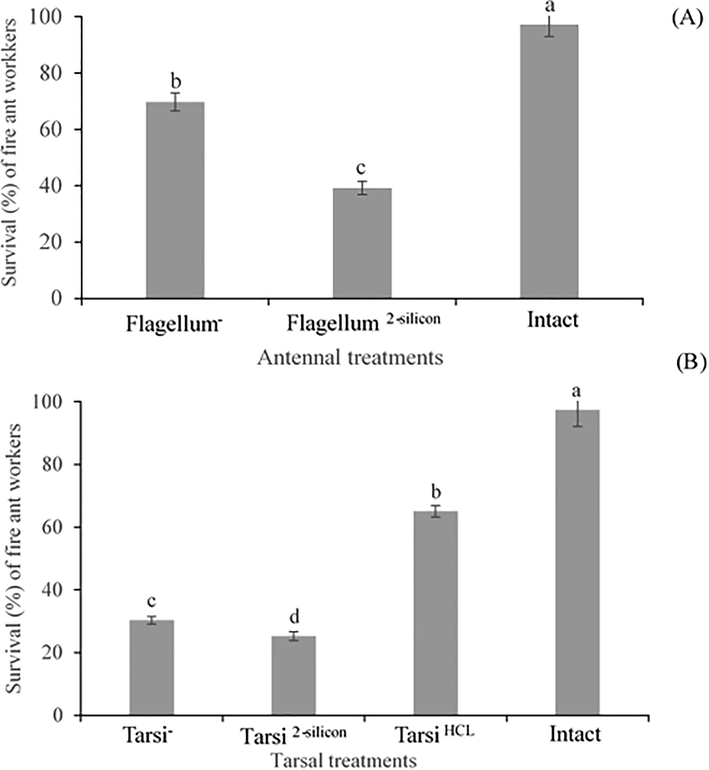
Survival (%) of fire ant workers with antennal (A) and tarsal (B) treatments. Within the different treated workers, the means with different letters are significantly different (One-way ANOVA, at P < 0.05) (n = 120 per treatment). The fire ant workers’ antennae flagellum was ablated (Flagellum-), Antennae flagellum covered with 2-silicon components (Flagellum2-silicon). Foreleg tarsi was ablated (Tarsi-), Foreleg tarsi covered with 2-silicon components (Tarsi 2-silicon), Foreleg tarsi treated with HCL solution (Tarsi HCL), and Untreated workers (Intact).
The significant difference was observed in the survival % among the tarsi treated workers, e.g., with ablation (Tarsi -), with 2–silicon compounds covered (Tarsi Silicon), with HCL solution (Tarsi HCL), and without any measures (Intact) as control (F3,476 = 6159, P < 0.0001). In tarsal treatments, the survival % of HCL treated workers were maximum as compared to other treated workers (ablation of foreleg tarsi and silicon treated foreleg tarsi). While survival % of all tarsi treated workers was less than control/untreated workers (Fig. 2B). The results implied that workers treated with HCL solution were less injured as compared to ablated and silicon treated fire ant workers.
3.3 Feeding latency
There were significant differences among the fire ant workers with antennal treatments as without antennae flagellum, flagellum covered with 2–silicon compounds and untreated in terms of feeding latency (F2,357 = 7038, P < 0.0001, Fig. 3A). Similar results were found in the tarsus treatments of fire ant workers for feeding latency (F2,357 = 26326, P < 0.0001, Fig. 3B) when assayed on sucrose droplet.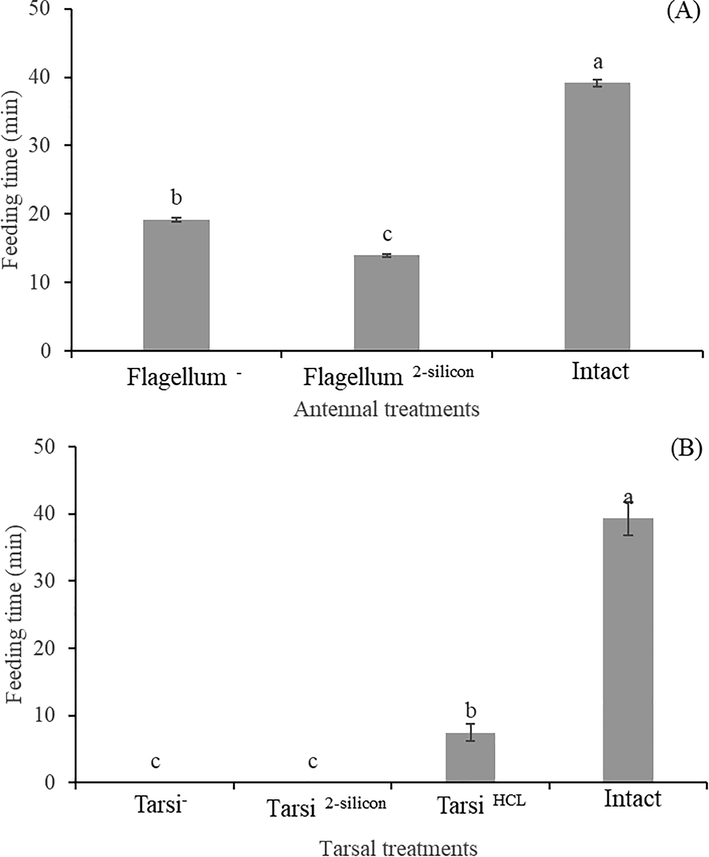
Feeding time of fire ant workers with antennal (A) and tarsal (B) treatments on sucrose solution. Within the different treated workers, the means with different letters are significantly different (One-way ANOVA, at P < 0.05) (n = 120 per treatment). The fire ant workers’ antennae flagellum was ablated (Flagellum-), Antennae flagellum covered with 2-silicon components (Flagellum2-silicon). Foreleg tarsi was ablated (Tarsi-), Foreleg tarsi covered with 2-silicon components (Tarsi 2-silicon), Foreleg tarsi treated with HCL solution (Tarsi HCL), andUntreated workers (Intact).
Antennal treated fire ant workers, e.g., without antennae flagellum and flagellum covered by 2–silicon compounds spent significantly less time on feeding the droplet in comparison to untreated workers. In antennal treatments, the workers without flagellum took a long time for feeding on the droplet as compared to silicon treated workers. In comparison, both types of antennal treated workers spent less time on feeding the droplet in comparison to untreated workers (Fig. 3A).
In tarsal treatments, ablated and silicon treated fire ant workers were unable to feed on the sucrose droplet except HCL treated fire ant workers. And HCL treated fire ant workers spent significantly minimum time on feeding the droplet as compared to untreated workers (Fig. 3B).
The results implied that ablation for antennae flagellum and HCL solution for foreleg tarsi were more suitable for study the gustatory behavior of fire ant worker on sucrose solution.
4 Discussion
In Hymenoptera, most of the insects use their tarsal sensilla for tasting the sugars (de Brito Sanchez et al., 2014). However, in the case of fire ants, no works were reported on gustatory body parts.
A sucrose solution is often considered as a phagostimulant for insects, especially ants (Madsen et al., 2017; McCalla et al., 2020; Vander Meer et al., 1995). But there is no records available on the feeding ethogram of minor RIFA workers. Feeding ethogram is an important behaviour and plays a essential role in the taste recognition of insects. The feeding ethogram of fruit fly have been explained by Thoma et al. (2017). Feeding ethogram of insects can be summarized into 3 principles first making appetitive exploration with body appendages, second contact/touch the food items with feeding, and third stop the movement of body appendage (Joseph and Carlson, 2015). Based on the results of this study, the fore legs tarsi and antennae of minor fire ant workers have major gustatory sensilla to sucrose solution.
Ablation or removal of antennae and legs were found the suitable technique in R. prolixus and cockroaches for the study of gustatory behavior on sweet and bitter compounds (Lockey and Willis, 2015; Pontes et al., 2014). The sensitivity of antennal sensilla has been blocked successfully by two silicon compounds in honey bees (Letzkus et al., 2006). HCL solution was found a good source to deactivate the sensitivity of tarsal and antennal sensilla in H. virescens (Ramaswamy et al., 1987) and cockroaches. In our study, the ablation for antennae flagellum and HCL solution for foreleg tarsi were suitable techniques for blocking the sensitivity of sensilla in fire ant workers. The ablated cockroaches were unable to approach to food in comparison to normal cockroaches (Lockey and Willis, 2015). In our findings, the feeding ability of flagellum ablated and HCL treated fire ant workers were significantly decreased on sucrose solution as compared to normal fire ant workers.
5 Conclusion
Based on behavioral bioassay, we infer that in fire ant workers, the foreleg tarsi and antennae flagellum might be kept major potential porous gustatory sensilla for sucrose. We concluded that ablation and HCL solution were more suitable techniques for blocking the sensitivity of sensilla on flagellum and foreleg tarsi respectively in fire ant workers. Future works will be done on the confirmation of gustatory sense to sucrose via single sensillum recording (SSR). Our observations might be a good basis for the study regarding sugar taste recognition via antennal and tarsal sensilla and also for sugar bait improvement in the fire ant.
Acknowledgement
This research work was supported by the “President Foundation of the Guangdong Academy of Agricultural Sciences (201817B) and National Natural Science Foundation of China (31801805).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could influence the work reported in this paper.
References
- Gustatory receptors in Lepidoptera: chemosensation and beyond. Insect Mol. Biol.. 2016;25(5):519-529.
- [Google Scholar]
- Araya, M.R., Padilla, S.G., 2020. Feeding behavior pattern and glycosylated hemoglobin in people with type 2 diabetes at the beginning and end of an educational intervention. Endocrinología Diabetes Y. Nutr. 67, 155–163.
- Involvement of the Antennal and Maxillary Palp Structures in Detection and Response to Methyl Eugenol by Male Bactrocera dorsalis (Diptera: Tephritidae) J. Insect Sci.. 2018;18:19-26.
- [Google Scholar]
- Tarsal contact chemosensory hairs of the large white butterfly Pieris brassicae and their possible role in oviposition behaviour. Entomol. Exp. Appl.. 1973;16:343-357.
- [Google Scholar]
- The tarsal taste of honey bees: behavioral and electrophysiological analyses. Front. Behav. Neurosci.. 2014;8:25-32.
- [Google Scholar]
- Regulation of Diet in the Fire Ant, Solenopsis invicta. J. Insect Behav.. 1999;12:307-328.
- [Google Scholar]
- Sensitivity and feeding efficiency of the black garden ant Lasius niger to sugar resources. J. Insect Physiol.. 2014;64:74-80.
- [Google Scholar]
- Differences in worker size and mound distribution in monogynous and polygynous colonies of the fire ant Solenopsis invicta Buren. J. Kansa. Entomol. Soc.. 1985;58:9-18.
- [Google Scholar]
- Hedges, S.A., 1998. Field Guide for the Management of Structure Infesting Ants, 2nd Ed.(Moreland D, editor) pp. 202–216. G.I.E. Publishers, Cleveland, Ohio.
- Howard, D.F., Tschinkel, W.R., Sociobiology. 1980. The effect of colony size and starvation on food flow in the fire ant, Solenopsis invicta (Hymenoptera: Formicidae). Behavioral Ecol. 7:293-300.
- Using two–sex life tables to determine fitness parameters of four Bactrocera species (Diptera: Tephritidae) reared on a semi–artificial diet. Bull. Entomol. Res.. 2018;108(6):707-714.
- [Google Scholar]
- Drosophila chemoreceptors: a molecular interface between the chemical world and the brain. Trends Genet.. 2015;31(12):683-695.
- [CrossRef] [Google Scholar]
- The importance of antennae for pea aphid wing induction in presence of natural enemies. Bull. Entomol. Res.. 2005;95:125-131.
- [Google Scholar]
- Sugar and amino acid preference in the black garden ant Lasius niger (L.) J. Insect Physiol.. 2017;100:140-145.
- [Google Scholar]
- McCalla, K.A., Tay, J.W., Mulchandani, A., Choe, D.H. and Hoddle, M.S., 2020. Biodegradable alginate hydrogel bait delivery system effectively controls high–density populations of Argentine ant in commercial citrus. J. Pest Sci. 93:1031–1042.
- Lei, Y., Zhou, Y., Lü, L., He, Y., 2019. Rhythms in foraging behavior and expression patterns of the foraging gene in Solenopsis invicta (Hymenoptera: Formicidae) in relation to photoperiod. J. Econ. Entomol. 112, 2923–2930.
- Lei, Y., Jaleel, W., Shahzad, M.F., Ali, S., Azad, R., Ikram, R.M., Ali, H., Ghramh, H.A., Khan, K.A., Qiu, X. and He, Y., 2021. Effect of constant and fluctuating temperature on the circadian foraging rhythm of the red imported fire ant, Solenopsis invicta Buren (Hymenoptera: Formicidae). Saudi J. Biol. Sci. 28(1),.64–72.
- Letzkus, P., Ribi, W.A., Wood, J.T., Zhu, H., Zhang, S.–W., Srinivasan, M., 2006. Lateralization of olfaction in the honeybee Apis mellifera. Curr. Biol. 16, 1471–1476.
- Liman, E.R., Zhang, Y.V., Montell, C., 2014. Peripheral Coding of Taste. Neuron 81, 984–1000.
- One antenna, two antennae, big antennae, small: total antennae length, not bilateral symmetry, predicts odor–tracking performance in the American cockroach Periplaneta americana. J. Exp. Biol.. 2015;218:2156-2165.
- [Google Scholar]
- Controlling the fire ant, Solenopsis invicta by N-BPS bait in Southern China. Plant Prot.. 2006;32:22-27.
- [Google Scholar]
- Ning, D., Yang, F., Xiao, Q., Ran, H., Xu, Y., 2019. A simple and efficient method for preventing ant escape (Hymenoptera: Formicidae). Myrmecol. News 29.
- Hunger in red imported fire ants and their behavioral response to two liquid bait products. J. Eco. Entomol.. 2005;98(6):2153-2159.
- [Google Scholar]
- Effect of diamondoids on the microstructure and mechanical behavior of nanostructured Mg–matrix nanocomposites. Mater. Sci. Eng. C MAT. 2015;633:200-208.
- [Google Scholar]
- Pontes, G., Minoli, S., Insaurralde, I.O., De, B.S., M. G., Barrozo, 2014. Bitter stimuli modulate the feeding decision of a blood–sucking insect via two sensory inputs. J. Exp. Biol. 217, 3708–3717.
- Insect behavioural ecology and other factors affecting the control efficacy of agro–ecosystem diversification strategies. Ecol. Model.. 2005;182(2):199-216.
- [Google Scholar]
- Sensory cues and receptors for oviposition by Heliothis virescens. Entomol. Exp. Appl.. 1987;43:159-168.
- [Google Scholar]
- Skinner, G., 1980. The feeding habits of the wood–ant, Formica rufa (Hymenoptera: Formicidae), in limestone woodland in north–west England. J. Anim. Ecol. 49, 417–433.
- Solari, P., Crnjar, R., Masala, C., Loy, F., Isola, M., 2016. Morphological and electrophysiological analysis of tarsal sensilla in the medfly Ceratitis capitata (Wiedemann, 1824) (Diptera: Tephritidae). Ital. J. Zool. 83, 456–468.
- The organization of the chemosensory system in Drosophila melanogaster: a rewiew. Cell Tissue Res.. 1994;275(1):3-26.
- [Google Scholar]
- Functional dissociation in sweet taste receptor neurons between and within taste organs of Drosophila. Nat. Commun.. 2016;7:10678.
- [Google Scholar]
- Thoma, V., Kobayashi, K., Tanimoto, H., 2017. The Role of the Gustatory System in the Coordination of Feeding. Eneuro 4:0324.
- Vander Meer, R.K., Lofgren, C.S. and Seawright, J.A., 1995. Specificity of the red imported fire ant (Hymenoptera: Formicidae) phagostimulant response to carbohydrates. Fla Entomol. 78: 144–154.
- Invasion of the red imported fire ant (Hymenoptera: Formicidae) spread, biology, and impact. Am. Entomol.. 1997;43:23-39.
- [Google Scholar]
- Wang, L., YiJuan, X.U., Zeng, L., YongYue, L.U., 2019. Impact of the red imported fire ant Solenopsis invicta Buren on biodiversity in South China: A review. J. Integr. Agric. 18, 788–796.
- Prevalence and management of Solenopsis invicta in China. Neobiota. 2020;54:89-124.
- [Google Scholar]
- Quantification and modification of worker size variation in the fire ant Solenopsis invictaQuantifizeirung und Veränderung der Grösse der Arbeiter in der Ameisenspezies Solenopsis invicta. Insectes Soc.. 1981;28(2):117-128.
- [CrossRef] [Google Scholar]
- Yosano, S., Kutsuwada, Y., Akatsu, M., Masuta, S., Hori, M., 2020. Taste recognition through tarsal gustatory sensilla potentially important for host selection in leaf beetles (Coleoptera: Chrysomelidae). Sci. Rep. 10, 4931.x
- Tarsal taste neurons of Helicoverpa assulta (Guenee) respond to sugars and amino acids, suggesting a role in feeding and oviposition. J. Insect Physiol.. 2011;57:1332-1340.
- [Google Scholar]







