Translate this page into:
Optimization of process parameters in mixed sulfide oxidation bacterial culture using response surface methodology as a tool
⁎Corresponding author at: Faculty of Chemical and Natural Resources Engineering, Universiti Malaysia Pahang (UMP), Lebuhraya Tun Razak, 26300 Gambang, Kuantan, Pahang, Malaysia. mmahmadu@kustwudil.edu.ng (Mani Malam Ahmad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The integrated bacterial mixed culture consortium with disproportionate oxygen demand was discovered to mutually cooperate with one single biofilm in oxidizing sulfide at different concentration. The present work was carried out to verify the potential of bacterial mixed culture (BMC) in developing a predictive optimum condition for sulfide oxidation in a laboratory batch mode. A face centered central composite design (FCCCD) under response surface methodology (RSM) was employed to predict the synergistic effects of initial hydrogen sulfide concentration (100–500 ppm), temperature (30–40 °C) and aeration rates (50–250 vvm) on BMC sulfide oxidation. A total number of 20 experimental runs with 6 centre points were carried out. The obtained results were analyzed using design expert and statistical validation indices to check the adequacy of the obtained quadratic models. The analysis of variance showed that more than 99% of the variation was explained by the models. There was a good agreement between experimental and predicted data. The optimum sulfide removal of 448.75 ppm was achieved at the temperature of 32.4 °C, initial hydrogen sulfide of 500 ppm and aeration rates of 110.06 vvm in 8 h. Therefore, the finding depicts the adequacy of the obtained model in enhancing BMC sulfide oxidation conditions. The model is further affirmed through SEM-EDXS analysis, revealing oxidized sulfide product aggregate of the micrographs coupled with elemental identification and quantitative composition.
Keywords
Optimization
Mixed-culture
Sulfide oxidation
RSM
Batch mode
1 Introduction
The menace due to metabolism of sulfate reducing bacteria (SRB) has remained the major debilitating effect associated with sewer system wastewaters. The toxicity, corrosive nature, unpleasant malodor and aggressive oxygen affinity enhance its emission potential, thus necessitating its mitigation from the surrounding Mani et al. (2016) and Zytoon et al. (2014). Apparently, sulfide accumulations in industrial set-up cause several damages and loss of efficiency to the systems like corrosion of concrete systems and steel pipelines. The characteristic pungent “rotten eggs” smell of sulfide is detectable in a dilution as small as 20 ppb of concentration and 20–30 ppm at a higher concentration that can deaden the olfactory sense of the brain Buisman et al. (1990). Although, the classical physicochemical approach to sulfide removal was successful but suffered some drawbacks, including huge capital investment for handling, maintenance and production of secondary pollutants Montalvo (2015), and Zytoon et al. (2014). However, biological sulfide oxidation (BSO) comparatively has the potential in providing a perfect different option for the evacuation of sulfide at the different concentration, alongside the recuperation of sulfur Liang et al. (2015).
There are many different approaches to biological sulfide oxidation based on immobilized and suspended bioreactor types. However, such techniques at a conventional level have failed to explain the interactions effects of operational variables in the oxidation process, prediction output response to change in variables weight and inability to establish an optimum yield at optimum independent variables ranges Habeeb et al. (2016). Therefore, statistical design of experiment approach using face-centered central composite design (FCCCD) under response surface methodology (RSM) was applied to overcome the constraints. In addition, RSM shows the significant level of each process parameter. However, this option is not meant to substitute the classical approaches, but rather to serve as efficient and cost effective methods of sulfide oxidation with a minimum number of experimental runs.
The bacterial mixed culture (BMC) consortium, Pseudomonas putida (ATCC 49128)/S1 and Bacillus cereus (ATCC 14579)/S2 have been reportedly pointed in several biological treatments of recalcitrant wastewaters specifically petrochemical industries effluents Cerqueira et al. (2012), Das and Chandran (2011), Jacques et al. (2008), Vinothini et al. (2015) and Reshma (2014). The metabolic capabilities was found to be enhanced through complementary effect of these spore-forming and vegitative isolates with disproportionate morphological and physiological make-up (Patel et al., 2012). Although some few texts exist relate to their application for BSO as pure culture Ahmad et al. (2017), Liang et al. (2015) and Mani et al. (2016), however their integration as mixed culture is not much popular Mani et al. (2017). The parametric effect of aeration cycles, influent sulfide concentration and medium temperature had been reported to influence sulfide oxidation. The rate of aeration to reacting medium in the reactor and sulfide concentration determine the type and rate of product formed; either elemental sulfur or sulfate, while temperature determines oxidation rate of the process by activating and sustaining an enzymatic drive (Buisman et al., 1990; Duetz et al., 2000). Therefore, the aim of this study is to develop a model that can effectively predict the operating conditions in achieving maximum sulfide removal using a tolerant mesophilic BMC.
2 Materials and methods
2.1 Test organism and cultivation
Two BMC isolates, S1 and S2 were used for this study. The nutrient broth was purchased from Merck (Darmstadt, Germany) and prepared according to the manufacturer’s instruction. Typically, 8 g of nutrient broth (NB) was suspended in a litre of deionized water and agitated on a hot plate until it dissolved. Thereafter, it was sterilized in an autoclave (H + P Varioklav Steam Sterilizer ESCO, Japan) at 121 °C for 15 min, cooled in a water bath to 47 °C and later dispensed in 20 ml Eppendorf bottles. The stock cultures of BMC were maintained throughout the experimental process using a periodic sub-culture at least fortnightly on nutrient agar (NA) and refrigerated at 4 °C until use. To prepare the pre-culture, 1–3 loopful of cells from a 24 h actively growing culture on a nutrient agar plate was dispensed in bottles containing sterile nutrient broth (10% w/v) and incubated at 37 °C (Mummert-Germany/BE 600) for 24 h. The inoculation was aseptically performed inside a biosafety cabinet to avoid contamination, and the flask was sterilized by passing it over a bunsen flame before and after inoculation. To ensure proper bacterial growth, the inoculation was carried out in triplicates.
2.2 Media and synthetic wastewater
The media and additional composite exogenous carbon and nutrients sourced formulation were in accordance with the methods described by Fajardo et al. (2012) and Li et al. (2009) with little modifications. Concisely, the synthetic wastewater contained all the essential constituents for bacterial growths were used. The two solutions S1 and S2 containing DI water; 7.5 g sucrose, 7.5 g NaS.7H2O, 3.5 g NaHCO3, 3.6 g KH2PO4, 5.5 g NaNO3, 5.46 g KNO3, and 0.08 g MgSO4. The solutions were thoroughly mixed, top up with tap water to balance other micro elements required, the pH was adjusted and maintained at 8.5 using standard buffer solution. Sodium sulfide at a concentration range of 100–500 ppm was added at a specific time interval after start up to enable that the cultures acclimatized to the new environment.
2.3 Experimental set-up
The experiment was conducted in a 2 L laboratory-scale batch reactor type BIOTRON (LiFlus GX, Intran, Korea). Prior to start up, the fermenter was stocked with media (with exception of Na2S.9H2O), sterilized with the buffer solution and other accessories at 121 °C for 15 min. After cooling, the calibrated reactor was inoculated with 90 ml S1 and 60 ml S2 (10% v/v of the total reactor volume) at the ratio of 60:40. The variation in the strain volume was to create complementary impact of helper bacteria Mani et al. (2017). The efficiency of the process (aerobic) depends on influent sulfide concentration and aeration rate, thus need more of aerobic isolate (P. putida). While S2 being a spore-forming and facultative anaerobe was needed as a supportive isolate against the inhibitory effect of sulfide and toxic metabolites as reported in Garcia (2016) and Mani et al. (2017). The final working volume of the reactor was put at 1.5 L to avoid any splashing effect due to foaming. The operation was carried out batch-wise. The temperature of the medium was maintained at required range using a thermostat water jacket from water bath Moghanloo et al. (2010). Complete homogeneity was maintained inside the reactor with double Rushton mechanical turbine with one foam breaker operated at an agitation of 150 and 200 rpm, respectively. Aeration was achieved using an air compressor (HIBLOW HP-80, Japan) from the reactor base. Furthermore, dissolved oxygen (DO) was maintained within the range of 20 to 5 mg/l as the least value to the end of the experimental cycle. Thermostat control was used to maintain the temperature between 30 and 40 °C. Likewise, the BRT was operated at a retention time of 8 h and each experimental cycle with a four periodic sampling of analysis. The oxidation rate of the system was estimated using the Eq. (1).
where RE is the removal efficiency, ΔS is the difference in sulfide concentration gradient between the influent and at time t, So is the initial sulfide concentration.
2.4 Analysis methods
For sample analysis, 2.5 ml aliquots were withdrawn periodically at fixed intervals throughout the 8 h run. Analysis of the samples for the quantification of sulfide depletion was done spectrophotometrically using standard methyl blue method (Trüper and Schlegel, 1964) in Hach (2800DR) spectrophotometer. A standard sulfide reagent kit ((5 to 800 µg/L S2−) range) was used after a serial of dilution and the result multiplied by corresponding dilution factor (DF) to arrive at exact sample sulfide range.
2.5 Experimental design
The factors influencing sulfide oxidation was screened using the fractional factorial design of experiment (FFDOE) based on the preliminary experiment (Ahmad et al., 2017). Three process parameters, namely, influent sulfide concentration, temperature and aeration were optimized using FCCCD in design expert software (Stat-Ease, Inc., Version 10.0.5.0 Minneapolis, USA). A total number of 20 experimental at three-coded level (−1, 0 and +1) were performed (Tables 1 and 2). Regression analysis to correlate the influence of independent variables on sulfide oxidation was done using an empirical model of the second-order polynomial (Eq. (2)).
Terms
Parameters
Units
Coded level
−1
0
+1
A
Initial Sulfide Concentration
ppm
100
300
500
B
Temperature
°C
30
35
40
C
Aeration
vvm
50
150
250
Run
Independent variables
Experimental values
Predicted values
A
B
C
Sulfide Reduction (ppm)
Sulfide Reduction (ppm)
1
300
35
250
221
227
2
300
30
150
240
242
3
100
35
150
85
89
4
300
40
150
223
222
5
500
35
150
450
446
6
300
35
50
235
235
7
100
30
250
73
72
8
500
30
250
431
431
9
500
40
50
425
425
10
500
40
250
401
402
11
100
40
50
70
69
12
500
30
50
445
446
13
100
30
50
78
76
14
100
40
250
60
58
15
300
35
150
242
241
16
300
35
150
241
239
17
300
35
150
242
239
18
300
35
150
238
241
19
300
35
150
240
238
20
300
35
150
238
240
2.6 Validation of the model
The obtained model was validated by predicted with experimented values using bias factor (Bf) and accuracy factor (Af) validation indices. Bf measure how far the distance experimental values are relative to the reference point of equivalence, while Af measure on average the space between individual points relative to the point of equidistance, thus indicated how closely related experimental values are to predicted.
2.7 SEM-EDXS biofilm analysis
Samples for this analysis were withdrawn 8 h after start-up in 35 ml Eppendorf tube, and transferred to Karnovsky’s fixative solution for primary fixation as described Lohwacharin and Annachhatre (2010) and Patel et al. (2015, 2016) with some modification. Washing was achieved in thrice distilled water for 15 min with centrifugation at 5000 rpm for 5 min each. This was followed by dehydration in a graded ethanol solutions (30%, 50%, 70%, and 90%) for 10 min each and absolute ethanol (100%) for 15 min. The samples were pipetted to 1 cm diameter aluminum foil paper and air-dried at room temperature in an enclosed bio-safety cabinet overnight. The sample specimens were stuck onto a stub using double-sided colloidal silver for mounting. Samples images were analysed using SEM (Hitachi, TM3030Plus, Japan).
3 Results and discussion
3.1 Model fitting and optimization of BMC sulfide removal efficiency
Table 2 shows face-centered central composite design (FCCCD) matrix for experimental and predicted results for the biological sulfide oxidation by S1 and S2, BMC system. It has been reported that a multivariable system tends to be influenced by some of the linear effects and less significant interactions. From the regression analysis, the quadratic model was the most fitted for the BMC sulfide removal. In addition, the selected quadratic model presented an acceptable adjusted and predicted values, highest F-value and a highly significant model p-value (Table 3). Modified Gompertz function using a nonlinear regression model was adopted to evaluate sulfide removal at effective variables levels.
Source
Sum of Square
df
Mean Square
F-Value
P-Value
Model
3.23 × 105
9
35837.64
6066.93
<0.0001
A
3.19 × 105
1
3.19 × 105
53999.86
<0.0001
B
774.40
1
774.40
131.10
<0.0001
C
448.90
1
448.90
75.99
<0.0001
AB
105.13
1
105.13
17.80
0.0018
AC
66.13
1
66.13
11.19
0.0074
BC
28.13
1
28.13
4.76
0.0541
A2
2121.14
1
2121.14
359.09
<0.0001
B2
186.14
1
186.14
31.51
0.0002
C2
378.20
1
378.20
64.03
<0.0001
Residual
59.07
10
5.91
Lack of Fit
42.24
5
8.45
2.51
0.1678
Pure Error
16.83
5
3.37
Corr. Total
3.23 × 105
19
From the results in Table 2, the percentage sulfide removal was in the range of 70–89.2%. The highest depletion was recorded at the optimum temperature of 35 °C, 150 vvm of aeration and retention time of 8 h; with 89.2% (446 ppm) in 500 ppm, 80.7% (242 ppm) in 300 ppm and 85% (85 ppm) in 100 ppm, respectively. The maximum percentage sulfide removal had a high fitness with the curves indicated by significant R2 values (0.9976 to 0.9998) for all tested conditions. Analysis of variance (ANOVA) was used to assess the adequacy of the model (Table 3), while the sensitivity of the response to independent variables was evaluated on the basis of the quadratic polynomial equation below:
where A is the initial sulfide concentration (ppm), B is the temperature (°C) and C is the aeration (vvm).
The relative contribution of model terms to residual variance was evaluated using Fisher’s variance ratio index. A large ratio value indicates that more of the variance is explained by the model Garg et al. (2015). Moreover, lack of fit indicates a variation of the data around a fitted model, which is not significant in a fitted model, whereas it is significant if the model does not fit the data adequately Garg et al., (2015). The F value of this model (5601.16) indicates a high level of significance (p < .0001), and the non-significant lack-of-fit test (14.49%) showed the validity of the model. The measures indices used for testing goodness of fit of the model are R2 and adj. R2. An R value close to 1 indicates a high degree of correlation between the observed and predicted values (Zhou et al., 2010), which should not be less than 0.8 for biological processes Ölmez (2009). The R2 and adj. R2 values of this model were 0.9998 and 0.9996, respectively (Table 3). The R2 value of 0.9998 indicates that approximately only 0.02% of the total variation cannot be explained by the model thus spells a high significance of the model.
The predicted R2 of 0.9875 was also in agreement with adj R2 of 0.9996. Moreover, a low value (1.03%) of the coefficient of variation (CV) indicates an appreciable degree for experimental values adequacy (Table 3). Likewise, there was a significant relationship between predicted and experimental values for the BMC sulfide oxidation, indicating a well-fitted model (Fig. 1a). The closeness of the two values, which were depicted graphically by the distribution of the predicted values near to the straight-line reasonably agrees with the experimental data (R2 0.9998). Indeed, this further confirmed good prediction ability of the model. Furthermore, the model terms B, C, BC, B2, and C2 regardless of being significant, but have a negative effect and their effect on growth spell at the low range Peng et al. (2014). Results from the present study showed that significant sulfide reduction by 70–90% in 100, 300 and 500 ppm under optimized process variables. Generally, sulfide reduction increased with increase in influent sulfide concentration at low temperature and aeration rate with a plateaued reduction at optimum aeration of 150 rpm and temperature of 35 °C in 100 ppm, 300 ppm, and 500 ppm, respectively. It has been reported that biological sulfide oxidation is faster with high sulfide concentration and low oxygen dosing with elemental sulfur as the main product, while low sulfide and high aeration tend to favour sulfate production Diaz et al. (2011), Krayzelova et al. (2014) and Wang et al. (2016).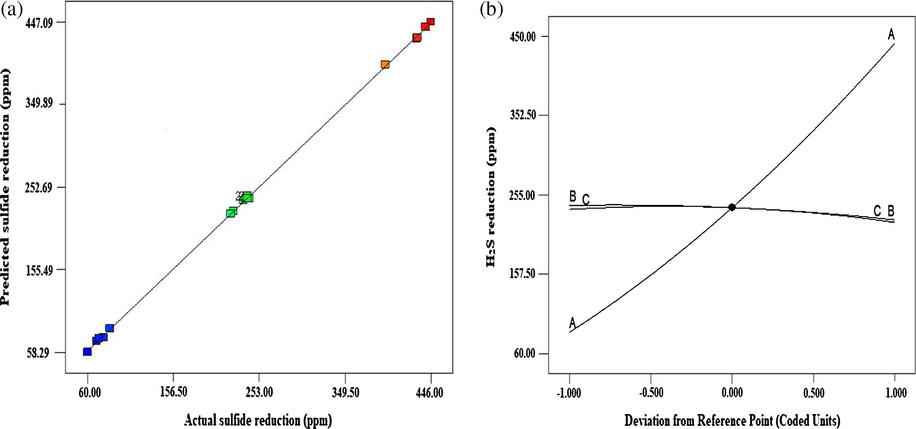
(a) Predicted versus experimental values and (b) Perturbation plot for P. putida biological sulfide oxidation.
3.2 Parametric effect on sulfide removal efficiency
The linear impact of influent sulfide concentration, temperature and aeration rate on sulfide reduction is shown in Fig. 1b. Perturbation plot does not show interactions effect, thus it reflected a one-variable-at-a-time pattern. However, it can be utilized in comparing the effects of factors by default in their corresponding center levels in the design space. It is plotted through varying a factor over its range while the other factors remain fixed. It can be clearly seen that only initial sulfide concentration shows positive linear effect, while the other variables indicated negative linear effect, with factors highly significant (p < .0001), although no much difference between positive and negative level in terms of temperature and aeration linear effects. Moreover, interactions effect of the three variables on BMC sulfide oxidation were all significant (p < .0001).
Fig. 2a shows a response surface plot for influent sulfide concentration and temperature on BMC sulfide oxidation. This plot indicated that sulfide reduction rate was sensitive to both the interacting variables. Increased in reduction trend was observed with increase in H2S and temperature, although the response to temperature was a little bit insensitive at a higher level. In addition, the sensitivity of sulfide on the response was found to be optimum at a moderate range, with concentration of 300 ppm as the effective, probably due to sulfide inhibitory effect on BMC growth and removal Mani et al. (2017). Such a moderate concentration ensures effective utilization and minimum metabolites generation. However, due to complementary helper bacteria effect Garcia, (2016), BMC system is reported to withstand toxic and recalcitrant pollutants, hence the highest removal rate was observed in 500 ppm sulfide concentration. Findings from this study were by far more than what was reported elsewhere in which a concentration of 5 to 30 ppm inhibit certain bacterial strain Buisman et al. (1991) and Janssen et al. (1995). This effect may be due to the fact that one of the bacterial strain in the consortium, B. cereus is a spore-forming mesophilic bacterium with reported tolerant to the oxidative stress Periago et al. (2002). This was found to activate gene expression sigma factors involved in various biochemical and physiological mechanisms which may excite enzymatic activities and subsequent metabolism Ynte et al. (2004). It was also reported that, in such a complementary helper effect, one of the strain may posseses toxic substance enzyme degrading system, while the other provides essential growth substances needed by the other isolate Garcia (2016) and Kimura and Ito (2001). Biological sulfide oxidation was shown to proceed faster at higher concentration and low aeration rate, with elemental sulfur as a favored product over sulfate Diaz et al. (2011), Krayzelova et al. (2014) and Wang et al. (2016), although not all sulfide is converted to sulfur Janssen et al. (1995). Similarly, the interaction effect of H2S and aeration rate (Fig. 2b) on BMC sulfide oxidation appears to follow the same trend with the plot in Fig. 2a. It is quite clear that removal pattern was sensitive at an aeration rate of between 50–150 vvm. In both plots, optimum removal of 88% was achieved at an optimum temperature and aeration of 35 °C and 150 vvm within a residence time of 8 h. The results agree with some findings reported by Alcántara et al. (2004) under steady dilution and aeration. The interactions effect of temperature and aeration show that the response is not affected much by the increase or decrease in these factors as both depend on sulfide concentration to cast their effect. However, highest response was observed at the two lower ranges (Fig. 2c).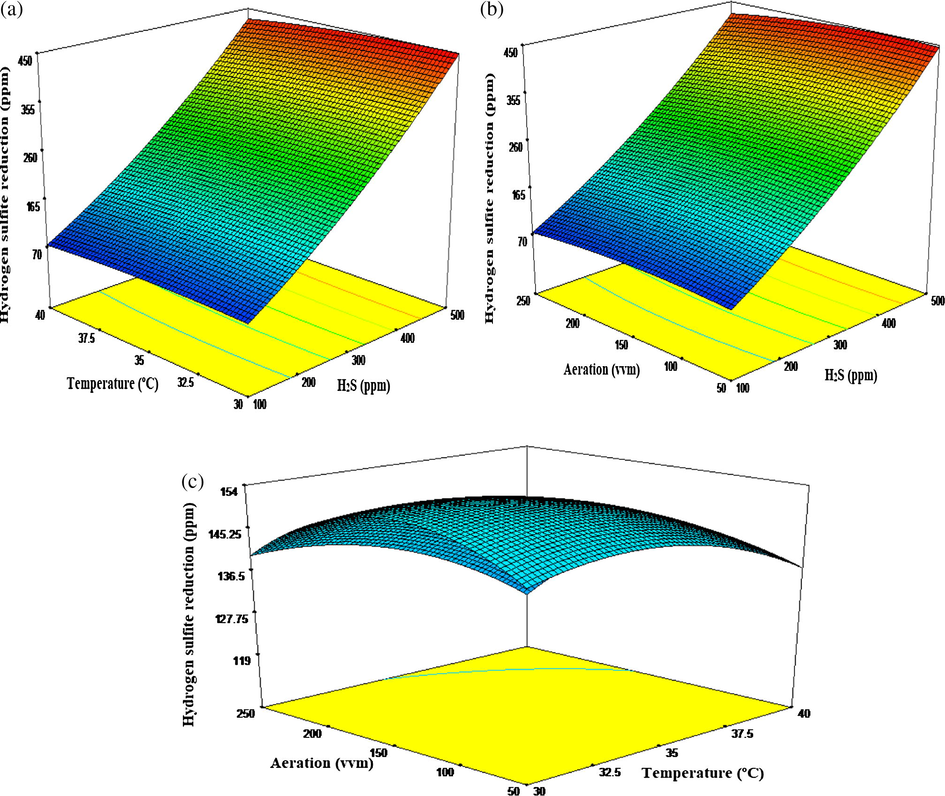
Three-dimensional (3D) response surface plots showing P. putida sulfide oxidation to interactions of process parameters H2S and temperature (a) H2S and aeration (b); temperature and aeration (c).
3.3 Model validation
The reliability of this model was evaluated using Bias and Accuracy factor index (Eqs. (4) and (5)). Table 4, shows the four different optimal conditions from the predicted matrix validation experiment. The closeness between predicted and observed values (y = x) line depict a satisfactory performance of the model. The Bf indicated that, on average, the predicted values were either above or below the line of equivalence. Ross (1996) proposed that a Bf in the range 0.90 to 1.05 should be considered good. Also, a Bf of either 0.70 to 0.90 or 1.06 to 1.15 should be considered acceptable, while that of 0.7 or 1.5 should be considered unacceptable (bad). The Bf of this model was 1.01, which is within the range considered good and acceptable. However, Bf does not provide an indication of the average accuracy of estimates, so Af was necessary. Furthermore, the larger the Af the less accurate is the average estimate te Giffel and Zwietering (1999), with Af of 1.0 indicating a good and accurate estimate. The average Af for this study was 1.00, indicating a high degree of accuracy. Therefore this model can be recommended as a reference point for optimum BMC sulfide oxidation under optimized process conditions of small-scale BRT. Based on the established model, the maximum BMC sulfide removal was 446 ppm at an optimum temperature and aeration of 35 °C and 150 rpm.
Run
Parameters
Response (Sulfide depletion ppm)
A
B
C
Experimental
Predicted
1
500.00
30.00
50.00
446.90
447.09
2
499.93
32.40
110.06
448.75
448.91
3
499.54
32.60
145.00
443.20
446.34
4
500.00
35.09
125.41
446.21
443.53
3.4 SEM-EDXS biofilm micrographs
Fig. 3 showed morphology of BMC (P. putida and B. cereus) biofilm from oxidized sulfide samples with an overwhelming sulfur deposition, while the EDXS analytes show a proportionate elemental product percentage. A sample from the dispersed active sludge of the batch reactor where sulfur sediment was presumably retained was withdrawn through the sampling port. From this actively growing BMC, two individual samples from the surface and bottom layer of the sampling bottle were taken. High influent sulfide concentration and low aeration rate have been shown to favour elemental sulfur formation; thus initial sulfide concentration and aeration rate of 50 vvm and temperature of 35 °C were used for this phase of biological sulfide oxidation. The micrographic imaging and elemental analysis for treated sample were compared against the blank (Fig. 3a, b and c).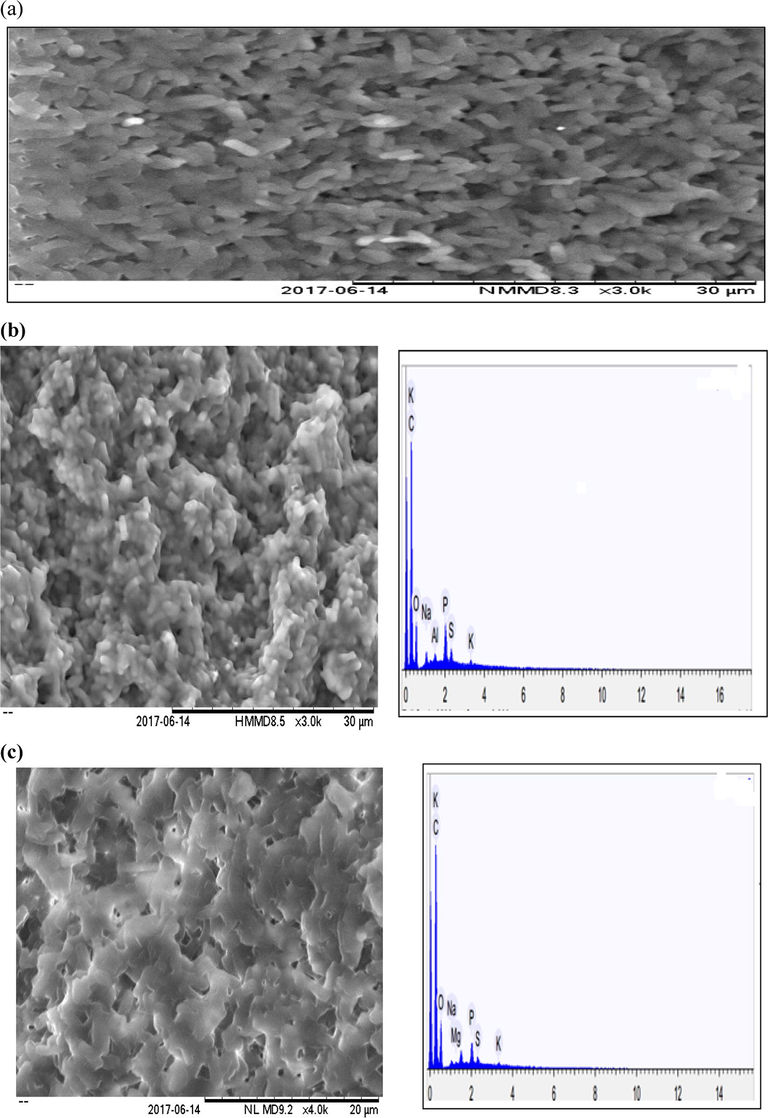
Micrographs of SEM-EDXS of blank sample containing growth medium only (a); treated sample from the surface layer of the port (b); treated sample from the bottom layer of the port (c).
The SEM-EDXS analysis of the two gram-negative and positive rod-shaped chemolithotrophic bacterial mixed cultures was highlighted for the oxidized sulfide samples. Fig. 3b shows a high sulfur deposition proportion for the sample collected from the lower layer. The SEM image shows the aggregation of the BMC with less visible outer sulfur excretes with dominant B. cereus species that shade less sulfur extracellularly compared to the other complimentary isolate (P. putida). However, the micrographs in Fig. 3c from surface layer indicates a more visible extracellularly sulfur deposition due to the active metabolic sulfide oxidation Moreover, EDXS analysis from bottom layer (Fig. 3b) revealed the highest sulfur content (0.953% wt) as against 0.483% wt from the surface layer (Fig. 3c), further affirming the oxidation potential of the BMC. The astonishing outer sulfur accumulation micrograph from the surface layer was probably due to aerobic nature of sulfur oxidizing bacteria, P. putida. This finding agrees well with Lohwacharin and Annachhatre (2010), on the potential of rod-shaped chemolithotrophic bacteria on sulfide oxidation to elemental sulfur. In addition, the EDXS analysis shows the disproportionate elemental composition of the culture, with high carbon content as an indication of a high cell biomass production (cell growth).
4 Conclusion
In this study, the potential of BMC for sulfide oxidation was successfully established and modelled at optimized conditions. This model approach using RSM was adequately applied for optimization of sulfide reduction. The validity of the model was proven by fitting the values of the variables to the model equation and by carrying out experiments using these values. The optimization of the analyzed response demonstrated that the best results for BMC percentage sulfide oxidation (448.75 ppm) were obtained with 499.93 ppm of initial sulfide concentration, 32.4 °C temperature, and 110.06 vvm aeration rates. Moreover, removal rate was also tied to the interactions effect of influent sulfide concentration, with other independent variables of temperature, and aeration at specific optimum level. The validation of a model for adequacy was tested based on the available indices which revealed almost 100% correlation between observed and predicted values, signaling a passable reliability of the revised model. The potential of this BMC to oxidize hydrogen sulfide to sulfur or sulfate is further strengthened with SEM-EDXS analysis, which revealed a proportionate sulfur aggregate and overwhelming elemental identification and quantitative composition information.
Acknowledgement
The authors acknowledge the financial support from Universiti Malaysia Pahang through Postgraduate Research Scheme (PGRS) and Doctorate Scholarship Scheme (DSS). Likewise, we appreciate the technical support of Faculty of Chemical and Natural Resources Engineering.
Conflict of interest
The authors declare no conflicts of interest.
References
- Biological sulfide oxidation and its impact on cell biomass synthesis by mesophilic bacterium. J. Chem. Eng. Ind. Biotechnol.. 2017;1:83-96.
- [Google Scholar]
- Hydrogen sulfide oxidation by a microbial consortium in a recirculation reactor system: sulfur formation under oxygen limitation and removal of phenols. Environ. Sci. Technol.. 2004;38:918-923. Doi 10.1021/Es034527y
- [Google Scholar]
- Optimization of sulfur production in a biotechnological sulfide-removing reactor. Biotechnol. Bioeng.. 1990;35:50-56.
- [Google Scholar]
- Kinetic parameters of a mixed culture oxidizing sulfide and sulfur with oxygen. Biotechnol. Bioeng.. 1991;38:813-820.
- [CrossRef] [Google Scholar]
- Cerqueira, V.S., Hollenbach, E.B., Maboni, F., Camargo, F.A.O., Peralba, M., do C.R., Bento, F.M., 2012. Bioprospection and selection of bacteria isolated from environments contaminated with petrochemical residues for application in bioremediation. World J. Microbiol. Biotechnol. 28, 1203–1222 doi:10.1007/s11274-011-0923-z.
- Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnol. Res. Int.. 2011;2011:941810.
- [CrossRef] [Google Scholar]
- Effect of oxygen dosing point and mixing on the microaerobic removal of hydrogen sulphide in sludge digesters. Bioresour. Technol.. 2011;102:3768-3775.
- [CrossRef] [Google Scholar]
- Methods for intense aeration, growth, storage, and replication of bacterial strains in microtiter plates. Appl. Environ. Microbiol.. 2000;66:2641-2646.
- [CrossRef] [Google Scholar]
- Cross effect of temperature, pH and free ammonia on autotrophic denitrification process with sulphide as electron donor. Chemosphere. 2014;97:10-15.
- [CrossRef] [Google Scholar]
- Modeling and Optimization for H 2 S Adsorption from Wastewater Using Coconut Shell Based Activated Carbon. Aust. J. Basic Appl. Sci.. 2016;10:136-147.
- [Google Scholar]
- Mixed cultures as model communities: Hunting for ubiquitous microorganisms, their partners, and interactions. Aquat. Microb. Ecol.. 2016;77:79-85.
- [CrossRef] [Google Scholar]
- Response surface methodology for optimization of process variable for reactive orange 4 dye discoloration by Pseudomonas putida SKG-1 strain and bioreactor trial for its possible use in large-scale bioremediation. Desalin. Water Treat.. 2015;54:3122-3133.
- [CrossRef] [Google Scholar]
- Microbial consortium bioaugmentation of a polycyclic aromatic hydrocarbons contaminated soil. Bioresour. Technol.. 2008;99:2637-2643.
- [CrossRef] [Google Scholar]
- Biological sulphide oxidation in a fed-batch reactor. Biotechnol. Bioeng.. 1995;47:327-333.
- [CrossRef] [Google Scholar]
- Two bacterial mixed culture systems suitable for degrading terephthalate in wastewater. J. Biosci. Bioeng.. 2001;91:416-418.
- [CrossRef] [Google Scholar]
- Microaeration for hydrogen sulfide removal in UASB reactor. Bioresour. Technol.. 2014;172:297-302.
- [CrossRef] [Google Scholar]
- Sulfide removal by simultaneous autotrophic and heterotrophic desulfurization-denitrification process. J. Hazard. Mater.. 2009;162:848-853.
- [CrossRef] [Google Scholar]
- Aerobic biodegradation of odorous dimethyl disulfide in aqueous medium by isolated Bacillus cereus GIGAN2 and identification of transformation intermediates. Bioresour. Technol.. 2015;175:563-568.
- [CrossRef] [Google Scholar]
- Biological sulfide oxidation in an airlift bioreactor. Bioresour. Technol.. 2010;101:2114-2120.
- [CrossRef] [Google Scholar]
- Mani Malam Ahamd, Abd. Aziz Mohd Azoddein, Mior Ahmad Khushairi Mohd Zahari, M.N., bin A.S., M.S.J., 2017. Microbial interactions in response to sulfide effect on mesophilic bacterial mixed culture (BMC) growth. J. Adv. Res. Mater. Sci. 1, 10–20.
- Mani Malam Ahamd, Abd.Aziz Mohd Azoddein, Mior Ahmad Khushairi bin Mohd Zahari, M.N., bin A.S., F.A.B.M.A., 2016. Assessment of sulfide concentration effects in a micro- oxygenated condition on the growth and removal. J. Adv. Res. Mater. Sci. 1, 1–10.
- Biological oxidation of hydrogen sulfide in mineral media using a biofilm airlift suspension reactor. Bioresource Technol.. 2010;101(21):8330-8335.
- [CrossRef] [Google Scholar]
- The optimization of Cr(VI) reduction and removal by electrocoagulation using response surface methodology. J. Hazard. Mater.. 2009;162:1371-1378.
- [CrossRef] [Google Scholar]
- Enhancing biological hydrogen production through complementary microbial metabolisms. Int. J. Hydrogen Energy. 2012;37:10590-10603.
- [CrossRef] [Google Scholar]
- Integrative approach to produce hydrogen and polyhydroxybutyrate from biowaste using defined bacterial cultures. Bioresour. Technol.. 2015;176:136-141.
- [CrossRef] [Google Scholar]
- Enhancement of methanol production from synthetic gas mixture by Methylosinus sporium through covalent immobilization. Appl. Energy. 2016;171:383-391.
- [CrossRef] [Google Scholar]
- Screening and optimization of low-cost medium for Pseudomonas putida Rs-198 culture using RSM. Brazilian J. Microbiol.. 2014;45:1229-1237.
- [Google Scholar]
- Identification of Proteins Involved in the Heat Stress Response of Bacillus cereus ATCC 14579. Appl. Environ. Microbiol.. 2002;68:3486-3495.
- [CrossRef] [Google Scholar]
- Indices for performance evaluation of predictive models in food microbiology. J. Appl. Bacteriol.. 1996;81(5):501-508.
- [Google Scholar]
- Sulphur metabolism in Thiorhodaceae I. Quantitative measurements on growing cells of Chromatium okenii. Antonie Van Leeuwenhoek. 1964;30:225-238.
- [CrossRef] [Google Scholar]
- Biodegradation of petroleum and crude oil by Pseudomonas putida and Bacillus cereus. Int. J. Curr. Microbiol. Appl. Sci.. 2015;4:318-329.
- [Google Scholar]
- Effect of dissolved oxygen on elemental sulfur generation in sulfide and nitrate removal process: characterization, pathway, and microbial community analysis. Appl. Microbiol. Biotechnol.. 2016;100:1-11.
- [CrossRef] [Google Scholar]
- Growth and Sporulation of Bacillus cereus ATCC 14579 under defined conditions: temporal expression of genes for key sigma factors. Appl. Environ. Microbiol.. 2004;70:220-230.
- [CrossRef] [Google Scholar]
- Optimization of media constituents through response surface methodology for improved production of alkaline proteases by Serratia rubidaea. J. Chem. Technol. Biotechnol.. 2007;82:1115-1121.
- [CrossRef] [Google Scholar]
- Optimization of fermentation conditions for production of anti-TMV extracellular ribonuclease by Bacillus cereus using response surface methodology. Bioprocess Biosyst. Eng.. 2010;33:657-663.
- [CrossRef] [Google Scholar]
- Bioconversion of high concentrations of hydrogen sulfide to elemental sulfur in airlift bioreactor. Hindawi Publishing Corporation. Sci. World J.. 2014;2014:675673.
- [CrossRef] [Google Scholar]







