Optimization and characterization of exopolysaccharide produced by Bacillus aerophilus rk1 and its in vitro antioxidant activities
⁎Corresponding author. rkthampu@kakatiya.ac.in (Raja Komuraiah Thampu) g.ravi320@gmail.com (Raja Komuraiah Thampu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
In this study, the isolation and identification of Exopolysaccharide (EPS) producing bacteria from polluted soil samples and its in vitro antioxidant activities were investigated.
Methods
Isolation and identification of bacteria was investigating using 16 s rDNA genome sequencing method. Further, optimization (pH, time, and temperature), purification and characterization of EPS were performed using different (UV, FT-IR, C13-NMR and H1-NMR) methods. In addition, 2, 2-diphenyl-1-picrylhydrazyl (DPPH) and hydrogen peroxide (H2O2) scavenging activities of EPS were performed.
Results
The molecular characterization of isolated strain was confirmed as Bacillus aerophilus by 16S rDNA sequencing. The sequence was submitted to NCBI (Genbank accession number MH553072). Characterization of EPS by UV, FT-IR, C13-NMR and H1-NMR revealed the presence of hydroxyl, carbonyl, and carboxyl functional groups. SEM analysis of EPS showed a solid surface with an irregular shape. The optimum conditions for EPS production were pH 7.0, cultivation time 72 h (3.73 ± 0.211 g/L) and temperature 30 °C respectively. The yeast and sucrose extract showed higher EPS production. In addition, in vitro DPPH, H2O2 studies showed 56.6 and 67.5% of scavenging activity at 4 mg/mL of concentration.
Conclusions
From this study, the novel EPS producing Bacillus aerophilus rk1 was identified. Further, the purified EPS showed good antioxidant activity.
Keywords
Bacillus aerophilus
Exopolysaccharides
Optimization
Purification
DPPH and H2O2 activities
1 Introduction
EPS are the outer cellular macromolecules excreted by microorganisms, which play a significant role against the many cellular functions such as cell eating, phage defense, osmatic stress aggregation of bacterial cells, and surface adherence (Angelin and Kavitha, 2020). The EPS contains structural elements of biofilm and involved in the water preservation to reduce cell desiccation, processing of exogenous organic compounds and inorganic ions (Tian, 2008; Wingender et al., 1999). The production of EPS was highly influenced by different factors such as concentration; pH, yeast and sucrose extract source (Krishnamurthy et al., 2019). Therefore, the yield of EPS production can be enhanced by optimizing the different parameters. Previously, different researchers were reported the improvement of EPS production after optimization. Si et al. (2017) studied the EPS production form Lactobacillus plantarum YM-2 which showed 24 mg/L before and 257 mg/L after optimization.
EPS used for numerous medical and industrial applications such as drug delivery system, medical coating devises, scaffolds, surgical sealants (Mohd Nadzir et al., 2021), and antitumor (Farag et al., 2020) activity. Similarly, the EPS has been acting as good anti-oxidant agent due to the bioavailability in nature. In current years, rising interest in identifying EPS due to its high range of biological applications (Andrew and Jayaraman, 2020). The microorganisms from extreme habitats that produced antibiotics and enzymes were very promising in biotechnological applications (Sarika et al., 2021; Govindarajan et al., 2019). Different studies were focused on the development of bacterial EPS because of which contain larger quantities of nucleic acids, proteins, and polysaccharides (Costa et al., 2018).
The search for new EPS producing bacteria is most essential part as only less number of bacterial strains is known for the high level of EPS production. In addition, gram positive Bacillus species have been confirmed as EPS producing bacteria, and which are now consider as significant antioxidant agent (Pei et al., 2020). However, to the best of our knowledge, there are no reported studies on isolation, identification and EPS production from bacteria collected from sugarcane-polluted soil area in Telangana, India. In this study, we investigated the optimization and characterization of EPS produced from Bacillus aerophilus rk1. Further, in vitro antioxidant and free radical scavenging activities were performed.
2 Material and methods
2.1 EPS producing bacteria isolation and screening
2.1.1 Bacterial isolation
The soil sample was obtained from Nizam sugar factory (18°39′44.7″N 77°54′34.4″E; 18°39′48.1″N 77°54′36.5″E), Nizamabad district, Telangana, India. The sugar industry effluent was collected aseptically at a depth of 6 cm and transported to the microbiology laboratory, after the soil was dried in an oven at 30 °C, after the dry soil sample was used for further isolation process.
The soil sample was diluted 10-fold on NA HiVegTM Agar plates and incubated at 30 °C for 24 h; the colonies was picked and used for further research. The pure specific colony was collected and used for further bacterial growth (Tallgren et al., 1999). The viscosity tests were performed in the basal broth medium for secondary sampling analysis of the isolates. The bacterial glycerol stocks were preserved in broth medium and stored at −20 °C.
2.1.2 Isolation and identification of Bacillus aerophilus rk1
Isolated pure colonies of bacterial culture were identified by 16S rDNA gene sequencing as described by Ruimy et al. (1994). The DNA was isolated using PCR genomic DNA isolation kit (Thermo Fisher Scientific GeneJET). The test was carried out at 95 °C for 4 min for a total of 30 cycles as: 94 °C for 1 min, 55 °C for 1 min, 72 °C for 2 min and 10 min at 72 °C respectively. The ideal temperature for PCR was 55 °C. PCR product was further taken to the subject using agarose gel electrophoresis to remove unwanted nucleotides and amplified by PCR through 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-TACGGTTACCTTGTTACGACTT-3′) primers using. The genome sequencing was performed at Eurofins Genomics, India Pvt Ltd, (Bangalore, India). The acquired sequences were compared with BLAST program with GenBank sequences (Altschul et al., 1997) and were submitted to NCBI Genbank and retrieved accession number. Further, the phylogenetic tree was constructed by neighbour-joining method using MEGA7 software to identify the similarity genome relationship (Kumar et al., 2016). In addition, the structural morphology was observed using colony morphology, gram staining and SEM analysis.
2.1.3 Bacterial growth condition and EPS production
The isolated strain was cultured in basal medium (g L-1) (yeast extract (5 g), sodium acetate (12 g), K2HPO4 (10 g), l-cysteine (0.5 g), sodium chloride (2.5 g), Peptone (1.0 g), MgSO4·7H2O (0.3 g), KH2PO4 10 (mg), sucrose (20 g) and Vitamin B1) as the additional supplement. After the pH 6.8 was adjusted, and the culture vials were allowed to grow at 30 °C overnight (Atlas and Parks, 1997).
EPS production was done by using basal medium in 250 mL Erlenmeyer flasks in batch culture. The medium inoculum having a cell count of ∼3 × 106 (cells/mL−1), after incubated in orbital shaker with 120 rpm. (Thermo scientific maxQ 6000) for 72 h at 30 °C.
2.1.4 Isolation and purification of EPS
Acetone precipitation method was used to isolate the EPS as followed (Sudhamani et al., 2004). Briefly, with modifications the bacterial culture cells were separated by centrifugation at 9000 rpm for 7 min, after the supernatant containing EPS was precipitated with double volumes of chilled acetone and incubated at 4 °C for overnight. The obtained pellets were suspended and centrifuged at 12000 rpm for 15 min at 4 °C, the EPS was collected. The milli-Q water was used for washing (three times), dialyzed 3500 kDa tubing cellulose membrane was used for filter, and then EPS was frost-dried (Freeze-dryer Model-CHRIST, ALPHA 1–2 LD plus, 118 Germany) at −80 °C for 24 h.
The collected crude EPS was further purified by Diethylaminoethyl cellulose (DEAE-C) anion exchange chromatography using Sephadex G-100 column and collected EPS was measured (Chang, 1987; Dorina et al., 2020). The phenol–sulfuric acid method used for EPS content (1 mg) was estimated (DuBois et al., 1956).
2.1.5 Optimization of EPS production
The effects of various carbon (glucose, sucrose, lactose and galactose), nitrogen (yeast extract, beef extract, peptone, tryptone, (NH4)2 SO4, NH4NO3, NH4Cl, tri-ammonium citrate, NaNO2 and KNO3) sources, incubation temperatures (20, 25, 30, 37 and 45 °C), time and initial pH values (6.0, 6.5, 7.0, 8.0) were screened. Further, the individual sucrose concentration on EPS production was investigated (Farag et al., 2020; Fourati-Ben Fguira et al., 2005).
2.2 Characterization of EPS
2.2.1 UV–Vis spectra and FT-IR analysis of purified EPS
The EPS was dissolved (5 mg in 2.5 mL) in distilled water used for ultra-violet visible spectroscopy analysis. The sample was analyzed with a spectrophotometer (Shimadzu) in the range between 200 nm and 600 nm range (Ye et al., 2012).
Fourier transform infrared spectroscopy (FT-IR) was used to identify the major functional groups of the EPS produced from bacteria. In present study, EPS sample 10 mg was homogenized with potassium bromide pellet at room temperature, compressed and analyzed using (Spectrum 100 Optica–PerkinElmer) with a frequency range of 4000–400 cm−1 (Mathivanan et al., 2021).
2.2.2 Characterization of EPS through nuclear magnetic resonance (NMR) spectroscopy
A 5 mm reversed probe Bruker AVANCE400 MHz spectrometer was used for the NMR analysis. The purified EPS was dissolved at concentrations of 10 mg/mL−1 in deuterium oxide (D2O) and evaluated the frequency of 400 MHz at the parameters of 1H spectrum and C13 NMR (Yildiz et al., 2014).
2.2.3 Analysis of scanning electron microscopy (SEM) and Energy-dispersive X-ray spectroscopy (EDX)
SEM analysis was used to observe the structural morphology of purified EPS and EDX (TESCAN, VEGA 3 LMU instrument, South Korea) was used for elemental analysis of EPS. Present study, the aluminum stubs dried with EPS (1 mg/mL−1) was used for SEM and EDX analysis. The sample was analyzed through an SC7620 sputter coater. This study was performed at National Institute of Technology-Warangal, Telangana, India.
2.3 EPS antioxidant studies
The different concentrations (0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4 mg/mL) of purified EPS sample were prepared for DPPH radical scavenging activity (Adesulu-Dahunsi et al., 2018). Briefly, 2 mL of deionized water and 2.0 mL DPPH solution (0.16 mM) were added to 1.0 mL of various concentrated EPS samples. The mixture was incubated at 37 °C in a dark room for 30 min, after the absorbance was measured at 517 nm.
AS = Absorbance of the EPS sample (s); Ab = Absorbance of blank; AC = Absorbance of control.
The hydroxyl radical scavenging activity of the EPS samples was measured with the Fenton reaction. Briefly, 1 mL of EPS sample with different concentrations were added to the reaction mixture containing 1.0 mL of brilliant green (0.435 mM), 2.0 mL of FeSO4 (0.5 mM), and 1.5 mL of H2O2 (3.0%,w/v) and incubated at 37 °C for 20 min. The Absorbance was measured at 520 nm (Balakrishnan et al., 2011; Adesulu-Dahunsi et al., 2018).
AS = Absorbance of the sample; A0 = Absorbance of the control; A = Absorbance of deionized water without the sample and Fenton reaction.
2.4 Statistical analysis
All the statistical studies were performed in triplicate. The experimental data were calculated by mean ± SD analysis using GraphPad Prism 5.0 for windows.
3 Results and discussion
3.1 Isolation and identification of bacteria
The isolated bacteria were white irregular shaped and showed positive results for gram staining. The SEM analysis clearly showed the presence of rod shaped bacteria (Fig. 1A). The phylogenetic tree of 16S rDNA gene sequence results suggested the isolated strain showed higher similarity to Bacillus aerophilus (Fig. 1B). The sequences were submitted to Genbank and retrieved accession number MH553072.
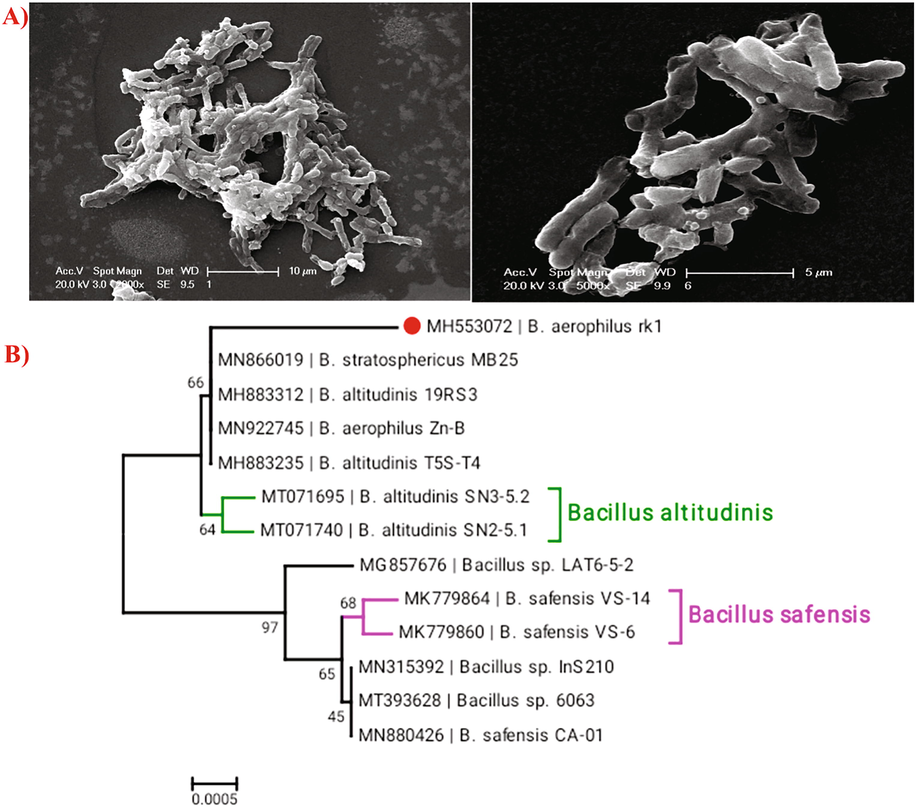
- A) Scanning electron microscopic images of rod shaped Bacillus aerophilus rk1B) Construction of sequence based evolutionary tree of our target isolates B. aerophilus rk1 (MH553072) and its closely related species. Present identified strain was highlighted with symbol.
3.2 Purification of exopolysaccharide
In the present study, sucrose used as a primary carbon source for EPS production, 10% sucrose was used in the fermentation process. The growth and EPS production were studied at 30 °C at 120 rpm. In this study, EPS yield (3.73g/L ± 0.211) was observed after 72 h. The EPS production influenced by the bacteria depends on the type of strain, culture medium, optimum conditions (pH, temperature, and time), carbon source, and inoculums size used (Adesulu-Dahunsi et al., 2018). The rk1 strain produced 3.71 g/L−1 of EPS which was higher than earlier reported studies of EPS produced from Bacillus tequilensis-GM and Bacillus mycoides (2.9, 2.78 g/L) respectively (Abid et al., 2019; Farag et al., 2020). However, lower than Bacillus megaterium PFY-147 EPS which produced 4.82 g/L (Pei et al., 2020).
3.3 Optimization of the EPS
A preliminary screening was performed to determine the best processing parameters for high yield EPS production. The different studies have shown that environmental conditions such as temperature, pH, and oxygen and food sources are majorly influenced the production of bio-products (Kekez et al., 2015). Our findings showed that the yield of EPS was increased with the incubation time, temperature, carbon and nitrogen sources. The highest rate of EPS production was observed at 30 °C (Fig. 2A), pH 7.0 (Fig. 2B), at 72 h of time (Fig. 2C). Ahmad et al. (2015) reported the Carbon supply was a significant factor for EPS production. In current study, we used different carbon source such as glucose, sucrose, lactose and galactose, among all the carbon sources, the sucrose had the highest EPS production (Fig. 3). In present study Bacillus aerophilus rk1 EPS production was higher, when compared to other strain of Bacillus subtilis AF17 of EPS production, which showed the highest yield (2.85 g/L 0.235) at 72 h (Bouallegue et al., 2020). Ale et al (2016) reported the EPS production from Lactobacillus fermentum, which showed 1 g/L at 30 °C for 72 h.
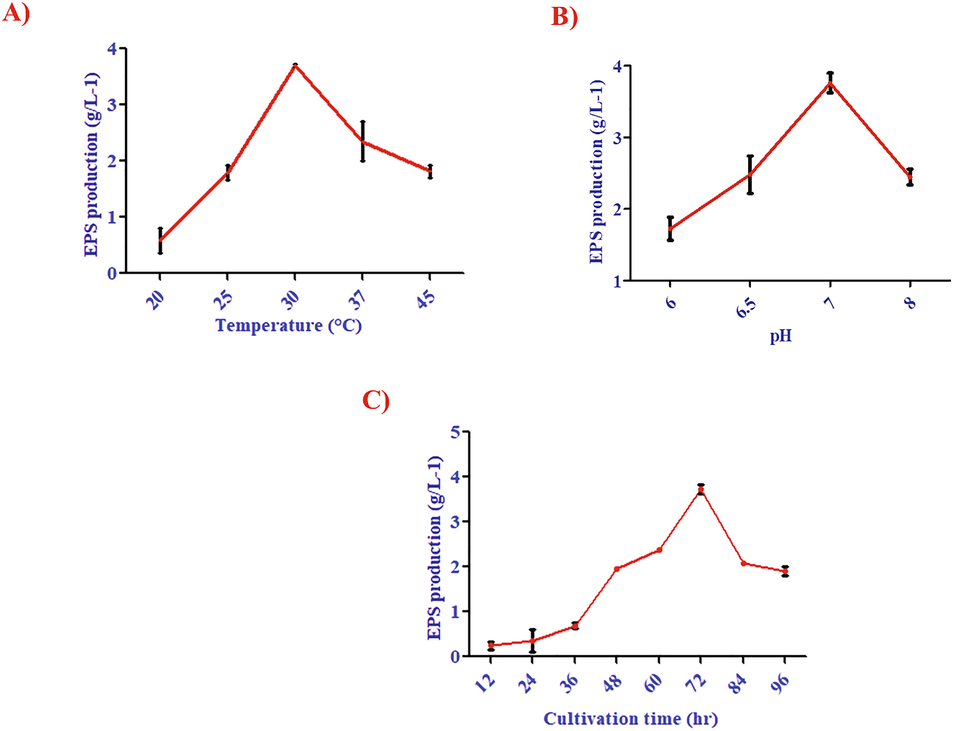
- Influence of A) temperature B) pH C) incubation time on EPS production by Bacillus aerophilus rk1.
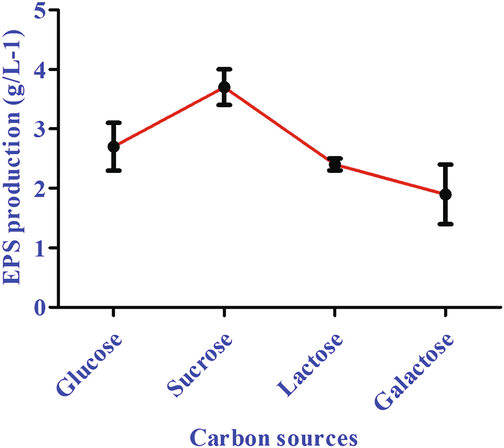
- Effect of different carbon sources on EPS production by Bacillus aerophilus rk1.
Yeast extract showed improved EPS production with an overall yield of 3.71 g/L−1, compared with inorganic nitrogen sources and beef extract (Fig. 4A). This study investigated the impact of various nitrogen (organic and inorganic) sources (Fig. 4B). The essential sugar was sucrose, an ideal carbon source for increased EPS production. The type of output EPS can be influenced by carbon source (Miqueleto et al., 2010). The present study was not affected by EPS production or cell growth from inorganic nitrogen sources (NH4)2SO4, NH4NO3, NH4Cl, Tri-Ammonium citrate NaNO2 or KNO3). The study showed that the effectiveness of organic nitrogen source and growth factors were enhanced the cell mass growth (Liangzhi et al., 2007). Present study a high amount of EPS production was observed for pH 7.0, at 30 °C, and Yeast extract acting as a best carbon sources.
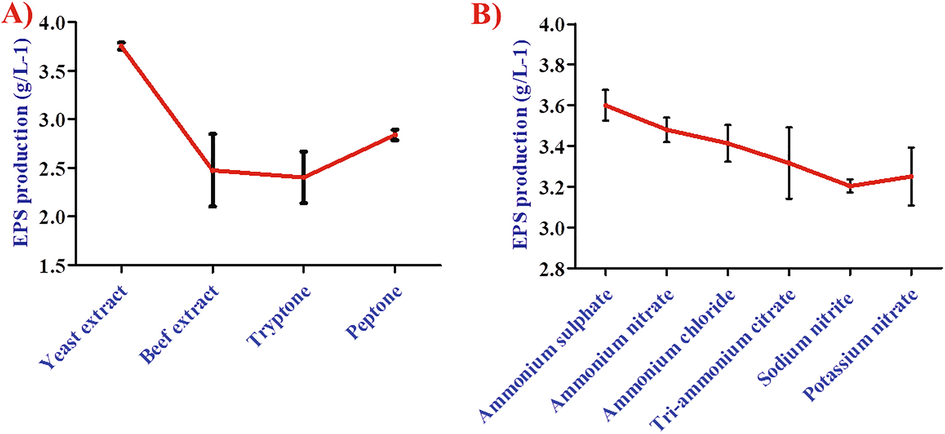
- Effect of different A) growth mediums and B) inorganic sources on EPS production by Bacillus aerophilus rk1.
3.4 Chemical characterization of EPS
3.4.1 UV and FT-IR spectral analysis
Using UV–vis spectroscopy, the absorption of electrical transitions of EPS was evaluated. The results showed, a strong peak occurred at around 250 nm. The physical property responsible for much of visible light absorption, between 250 and 280 nm, is the π-π* transformations of aromatic and polyaromatic compounds (Trabelsi et al., 2009). The absorption wavelength matches with OH, OCH3, CO2, and COOH functional groups bound to an aromatic ring in the molecule.
FT-IR is asses to identify structural properties such as macromolecular conformation and molecular interaction and spectrometer in the range 400–4000 cm−1 (Fig. 5). The results of the FT-IR spectrum showed a large stretch high peak at 3398 cm−1 characteristics of polysaccharide hydroxyl groups and a weaker C–H band at around 2926 cm−1 (Govindarajan et al., 2021; Asgher et al., 2020; Dumas and Miller, 2003). At about 1649 cm−1, the absorption peak reflects the carbonyl group’s presence (Parikh and Madamwar, 2006). The peak between 928 and 1229 cm−1, indicates the presence of the ether functional group. The bending vibrations in the 539 cm−1 array are seen. Asgher et al. (2020) and Kumar et al. (2007) reported the, presence of functional groups such as COOH and OH indicate the polysaccharides characteristics in the FT-IR spectrum.
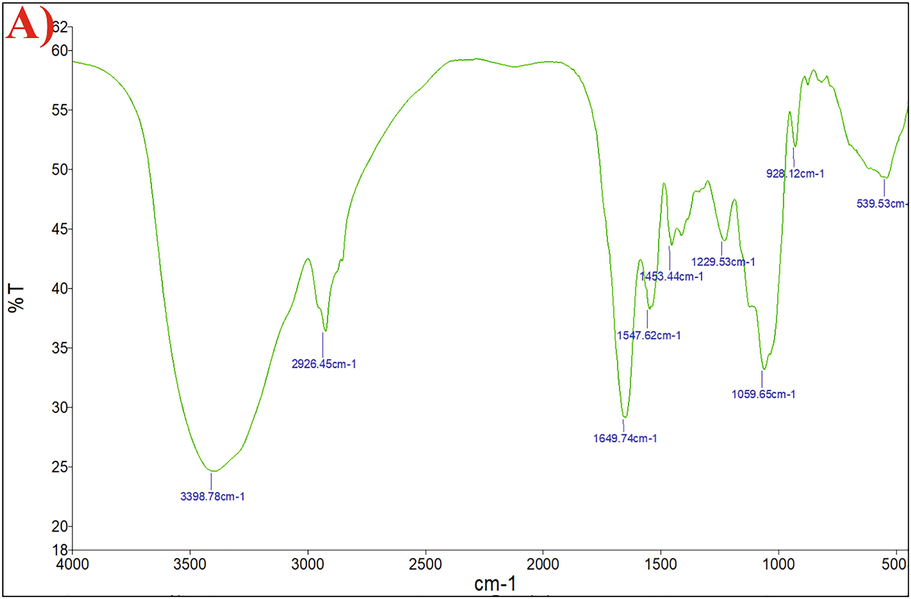
- Characterization of Bacillus aerophilus rk1 EPS A) FT-IR analysis.
3.5 1H, 13C NMR analysis
All proton signals are currently isolated (typically with 500 MHz or better NMR instruments) for unique mono polysaccharide molecules, and only a 1D NMR spectrum is available. However, large molecules, especially in the non-anomeric region, get a considerable proton signal overlap, while large molecules must have notable proton signal overlap, particularly in the non-anomeric region (3–4 ppm).
In present study, 1H NMR (400 MHz, D2O) δ 1H NMR (400 MHz, D2O) δ 5.38 (1H, s, anomeric), 4.14–4.10 (5H, m, non-anomeric) 3.99–4.03 (5H, m, non-anomeric), 3.87–3.81 (10H, m, non-anomeric), 3.74–3.68) (8H, m, non-anomeric), 3.60–3.57 (6H, m, non-anomeric), 3.49–3.46 (H, m, non-anomeric).13C NMR (101 MHz, CDCl3) δ 106.73, 106.19, 101.76, 94.68, 83.88, 82.82, 78.80, 77.71, 76.49, 74.91, 73.58, 71.72, 65.92, 63.84, 62.41. : IR Data: ʋ in cm−1: 3398 (–OH), 2926 (C–H Stretch), 1649 (C–C bond), 1059 (C-O) (Fig. 6A) signals were observed.
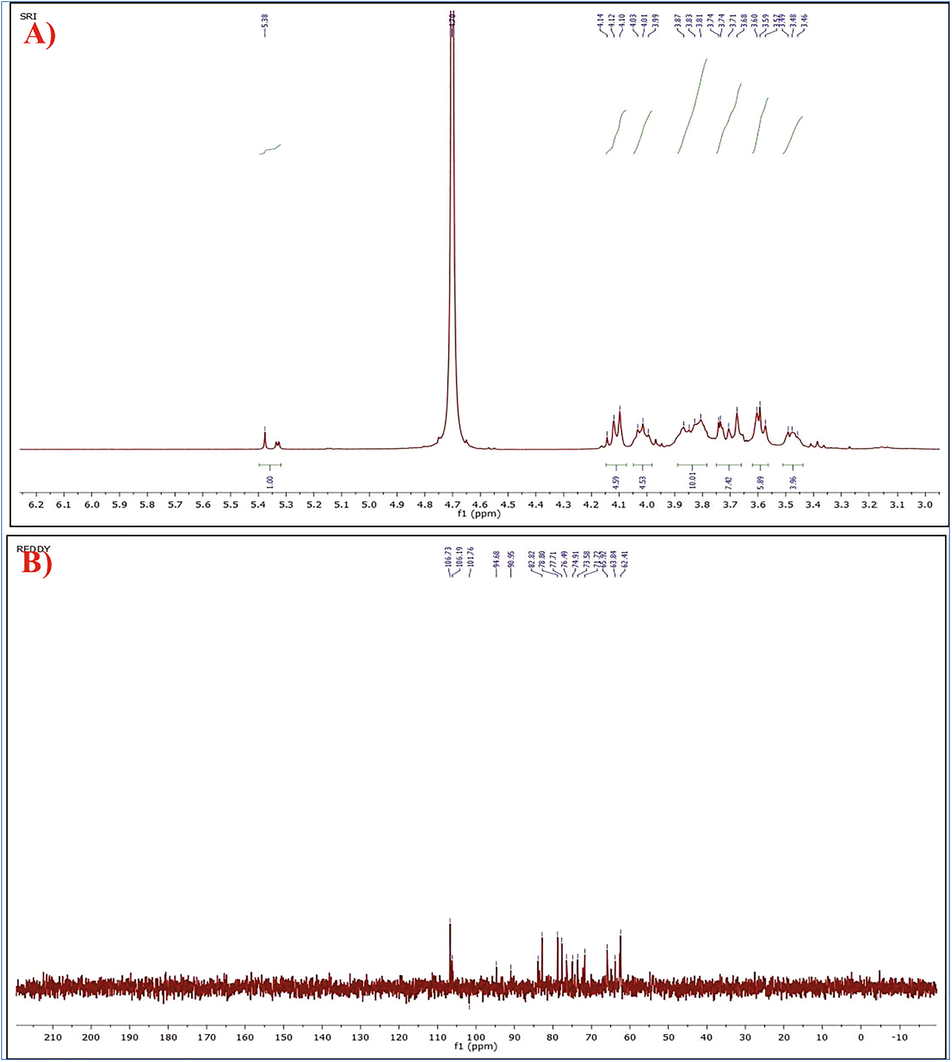
- A) 1H-NMR B) 13C-NMR profile of EPS production by Bacillus aerophilus rk1.
Main large signals and a strong overlap with low intensity between 4.1 and 3.1 ppm are shown by the 1H NMR, as predicted for a high molecular weight complex polysaccharide. one signal in the anomeric region at 5.38 ppm. Multiplet signals were found between 4.10 and 4.14 ppm, 3.99–4.03 ppm, 3.81–3.87 ppm, 3.68–3.74 ppm, 3.57–3.60 ppm 3.46–3.49 ppm, which represents the presence of sugars in the cyclic structure of the non-aromatic region. The 1H spectra agree well with the data obtained in the polysaccharide composition. In 13C NMR data, all the carbons appear between 60 and 110 ppm, which indicates all the carbon atoms in the anomeric region, 106.73 ppm, 106.19 ppm, 101.76 ppm anomeric carbons. Hydroxy-bearing carbons of the sugar ring: 68–77 ppm, sugar carbon in open- form with a 71–75 ppm hydroxy function. Collectively, these data were confirmed through the FT-IR results (Fig. 6B).
3.6 SEM-EDAX analysis
The SEM image of purified EPS showed solid surface with an irregular shape (Fig. 7A). Similar irregular and uneven surface shaped EPS produced by Bacillus cereus KMS3 (Krishnamurthy et al., 2019). The EDAX analysis confirms the presence of different element composition C and O atoms (Fig. 7B). Kanamarlapudi and Muddada (2017) explained that different physio-chemical compositions might decide the shape and structure of EPS.
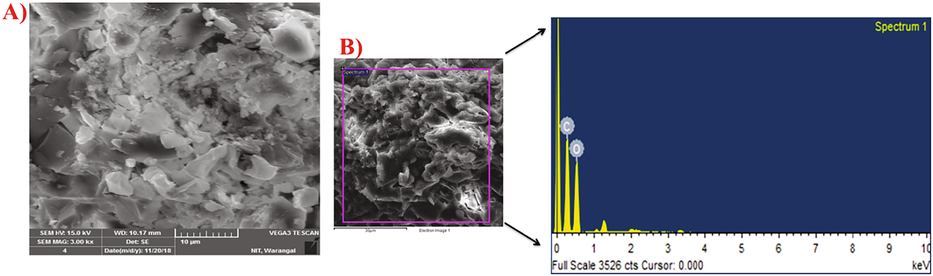
- A) SEM images showing the shape and surface morphology and B) EDAX analysis of EPS produced by Bacillus aerophilus rk1.
3.7 Determination of antioxidant properties
3.7.1 DPPH free radical scavenging activity
The DPPH scavenging activity was observed in EPS. The ascorbic acid's scavenging activity was significantly higher than the EPS, which showed the 57.6% of radical scavenging activity respectively (Fig. 8A). This result showed higher activity, when compared to the other EPS isolated from Lactobacillus plantarum, which showed 52.23% of DPPH radical scavenging activity at 4.0 mg/mL of concentration (Zhang et al., 2013). These results showed that EPS produced from Bacillus aerophilus rk1 have a significant DPPH activity.
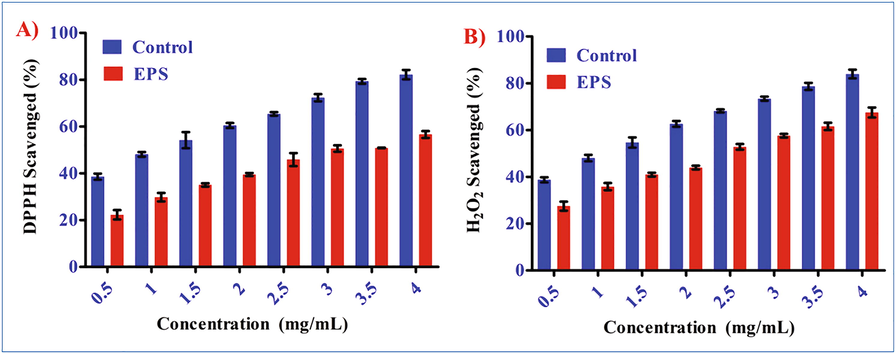
- Antioxidant activities of Bacillus aerophilus rk1 EPS in In vitro assays. A) DPPH scavenging activity B) H2O2 activity. (All the results are expressed in triplicate as Mean ± SD).
3.7.2 Hydroxyl radical scavenging activity
The EPS sample exhibited a moderate scavenging effect against hydroxyl radicals. However, ascorbic acid showed higher hydroxyl radical scavenging activity and was significantly different from the EPS sample. At a 4 mg/mL concentration, the scavenging activity for EPS rk1 and ascorbic acid was 67.5% and 81.8% respectively (Fig. 8B). In present study, by increasing the EPS concentrations the increased hydroxyl activity was observed. In comparison to the EPS rk1 sample, ascorbic acid has shown higher antioxidant activity. The result is higher to 78.6% at 10 mg/mL of EPS produced from Lactobacillus delbureckii-EPSMLD reported earlier (Adebayo-Tayo and Fashogbon 2020). Sahu and Gray (1997) explained the mechanism of super oxide activity, according to his findings Fe 2+ reacts with H2O2 results in Fenton reaction which leads into a oxidation of biomolecules as a results hydroxyl radicals are forming.
4 Conclusion
In this study, the novel strain Bacillus aerophilus rk1 was identified and characterized. In addition, the production, optimization, and antioxidant properties of EPS produced by Bacillus aerophilus rk1 were investigated. The optimization studies showed the EPS yield was higher at pH 7, 30 °C, and 72 h of incubation in Yeast and sucrose extract. The characterization studies confirmed that, EPS is a polysaccharide in nature and which is composed of hydroxyl, carbonyl, and carboxyl functional groups. The SEM analysis of EPS showed solid in nature with irregular in shape. Overall, results suggested that the EPS of Bacillus aerophilus rk1 could be a good antioxidant agent. Further, this could be used for industrial and biomedical applications.
CRediT authorship contribution statement
Gangalla Ravi: Conceptualization, Methodology, Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Visualization, Supervision. Gattu Sampath: Conceptualization, Methodology, Formal analysis, Writing - original draft, Writing - review & editing, Visualization, Supervision. Beduru Srinivas: Investigation. Kasarla Sarika: Investigation, Visualization. Rasiravathanahalli Kaveriyappan Govindarajan: Investigation, Visualization, Supervision. Raja Komuraiah Thampu: Conceptualization, Methodology, Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Visualization, Supervision.
Acknowledgments
This research was funded by the Deanship of Scientific Research at princess nourah bint abdulrahman university through the Fast track Research Funding Program.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Potential biotechnological properties of an exopolysaccharide produced by newly isolated Bacillus tequilensis-GM from spontaneously fermented goat milk. LWT - Food Sci. Tech.. 2019;105:135-141.
- [Google Scholar]
- In vitro antioxidant, antibacterial, in vivo immunomodulatory, antitumor and hematological potential of exopolysaccharide produced by wild type and mutant Lactobacillus delbureckii subsp. bulgaricus. Heliyon. 2020;6(2):e03268.
- [CrossRef] [Google Scholar]
- Production of exopolysaccharide by strains of Lactobacillus plantarumYO175 and OF101 isolated from traditional fermented cereal beverage. Peer J.. 2018;6:5326.
- [Google Scholar]
- Microbial polysaccharides and their modification approaches: a review. Int. J. Food Prop.. 2015;18(2):332-347.
- [Google Scholar]
- Exopolysaccharide from Lactobacillus fermentum Lf2 and its functional characterization as a yogurt additive. J. Dairy Res.. 2016;83(4):487-492.
- [Google Scholar]
- Gapped BLAST and PSIBLAST: a newgeneration of protein database search programs. Nucleic Acids Res.. 1997;25:3389-3402.
- [Google Scholar]
- Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr. Res.. 2020;487:107881.
- [CrossRef] [Google Scholar]
- Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biolmacromol.. 2020;162:853-865.
- [Google Scholar]
- Improved exopolysaccharide production from Bacillus licheniformis MS3: optimization and structural/functional characterization. Int. J. Biol. Macromol.. 2020;151:984-992.
- [Google Scholar]
- Principles of Microbiology. Toronto: Wm. C. Brown Publishers; 1997.
- In-vitro antioxidant and antibacterial properties of hydrolyzed proteins of delimed tannery fleshings: Comparison of acid hydrolysis and fermentation methods. Biodegradation. 2011;22:287-295.
- [Google Scholar]
- Levan from a new isolated Bacillus subtilis AF17: Purification, structural analysis and antioxidant activities. Int. J. Biol. Macromol.. 2020;144:316-324.
- [Google Scholar]
- Microbial extracellular polymeric substances: ecological function and impact on soil aggregation. Front. Microbiol.. 2018;9:1636.
- [Google Scholar]
- A newstrategy for a combined isolation of EPS and pigments from cyanobacteria. J. Appl. Phycol. 2020
- [CrossRef] [Google Scholar]
- Colorimetric method for determination of sugars and related substances. Anal. Chem.. 1956;28:350-356.
- [Google Scholar]
- The use of synchrotron infrared microspectroscopy in biological and biomedical investigations. Vib. Spectrosc.. 2003;32(1):3-21.
- [Google Scholar]
- Antitumor effect of exopolysaccharide produced by Bacillus mycoides. Microb. Pathog.. 2020;140:103947.
- [CrossRef] [Google Scholar]
- Purification and structureelucidation of antifungal and antibacterial activities of newly isolated Streptomyces sp.strain US80. Res. Microbiol.. 2005;156(3):341-347.
- [Google Scholar]
- Tannin acyl-hydrolaseproduction by Bacillus subtilis KMS2-2: purification, characterization, and cytotoxicity studies. J. King Saud Univ. Sci.. 2021;33(3):101359.
- [CrossRef] [Google Scholar]
- Purification, structural characterization and biotechnological potential of tannase 388 enzyme produced by Enterobacter cloacae strain 41. Process Biochem.. 2019;77:37-47.
- [Google Scholar]
- Characterization of exopolysaccharide produced by Streptococcus thermophilus CC30. Biomed. Res. Int. 2017
- [CrossRef] [Google Scholar]
- High levan production by Bacillus licheniformis NS032 using ammonium chloride as the sole nitrogen source. Appl. Biochem. Biotechnol.. 2015;175:3068-3083.
- [Google Scholar]
- Optimization, compositional analysis, and characterization of exopolysaccharides produced by multi-metal resistant Bacillus cereus KMS3-1. Carbohydr. Polym.. 2019;227:115369.
- [CrossRef] [Google Scholar]
- Evaluation of biosurfactant/bioemulsifier production by a marine bacterium. Bull Environ. Contam. Toxicol.. 2007;79:617-621.
- [Google Scholar]
- MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol.. 2016;33(7):1870-1874.
- [Google Scholar]
- Nitrogen sources affect streptolydigin production and related secondary metabolites distribution of Streptomyces lydicus AS 4.2501. Chin. J. Chem. Eng.. 2007;15:403-410.
- [Google Scholar]
- Characterization and biotechnological functional activities of exopolysaccharides produced by Lysinibacillus fusiformis KMNTT-10. J. Polym. Environ. 2021:1-10.
- [Google Scholar]
- Influence of carbon sources and C/N ratio on EPS production in anaerobic sequencing batch biofilm reactors for wastewater treatment. Bioresour. Technol.. 2010;101(4):1324-1330.
- [Google Scholar]
- Biomedical applications of bacterial exopolysaccharides: A review. Polymers. 2021;13(4):530.
- [Google Scholar]
- Partial characterization of extracellular polysaccharides from cyanobacteria. Bioresour. Technol.. 2006;97(15):1822-1827.
- [Google Scholar]
- Purification and structural characterization and antioxidant activity of levan from Bacillus megaterium PFY-147. Int. J. Biol. Macromol.. 2020;161:1181-1188.
- [CrossRef] [Google Scholar]
- Phylogenetic analysis and assessment of the genera Vibrio, Photobacterium, Aeromonas, and Plesiomonas deduced from small-subunit rRNA sequences. Int. J. Syst Evol. Microbiol.. 1994;44:416-426.
- [Google Scholar]
- Lipid peroxidation and DNA damage induced by morin and naringenin in isolated rat liver nuclei. Food. Chem. Toxicol.. 1997;35:443-447.
- [Google Scholar]
- Antimicrobial and antifungal activity of soil actinomycetes isolated from coal mine sites. Saudi J. Biol. Sci. 2021
- [CrossRef] [Google Scholar]
- Optimization of biosynthesis conditions for the production of exopolysaccharides by Lactobacillus plantarum YM-2. Food Sci. 2017;38:24-30.
- [Google Scholar]
- Isolation and characterization of an extracellular polysaccharide from Pseudomonas caryophylli CFR 1705. Carbohydr. Polym.. 2004;56:423-427.
- [CrossRef] [Google Scholar]
- Exopolysaccharide-producing bacteria from sugar beets. Appl. Environ. Microbiol.. 1999;65(2):862-864.
- [Google Scholar]
- Behaviour of bacterial extracellular polymeric substances from activated sludge: A review. Int. J. Environ. Pollut.. 2008;32(1):78.
- [CrossRef] [Google Scholar]
- Partial characterization of extracellular polysaccharides produced by Cyanobacterium Arthrospira platensis Biotechnol. Bioprocess. Eng.. 2009;14(1):27-31.
- [Google Scholar]
- Microbial Extracellular Polymeric Substances. Berlin, Heidelberg: Springer Berlin Heidelberg; 1999.
- Antioxidant activities of an exopolysaccharide isolated and purified from marine Pseudomonas PF-6. Carbohydr. Polym.. 2012;87(1):764-770.
- [Google Scholar]
- Brevibacillus themoruber: a promising microbial cell factory for exopolysaccharide production. J. Appl. Microbiol.. 2014;116:314-324.
- [Google Scholar]
- Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int. J. Biol. Macromol.. 2013;54:270-275.
- [Google Scholar]







