Translate this page into:
Omics-based approach in characterising mechanisms of entomopathogenic fungi pathogenicity: A case example of Beauveria bassiana
⁎Corresponding author. maizom@ukm.edu.my (Maizom Hassan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Biopesticides are gaining interests as an alternative to chemical-based pesticides for arthropod pest management. Among the widely-used biopesticides is the entomopathogenic fungi Beauveria bassiana due to its efficacy and broad range of arthropod hosts. Although the general mechanisms of infection by B. bassiana are known, the underlying complexity of molecular mechanisms at each infection stage is largely not well-understood. Characterising the mechanisms of pathogenicity allows for a more effective pest control by synergising between multiple pesticides or biopesticides without overlapping modes of action and by characterising novel toxic molecules that can expand the biopesticide arsenal. Systems biology refers to a large scale, high-throughput analysis of biological molecules at the systemic level. It incorporates the ‘omics’ methods, allowing identification of genes (genomics), along with their RNA (transcriptomics), proteins (proteomics) and metabolites (metabolomics) expression levels. The high-throughput research approach accelerates the process of characterising pathogenicity. The use of omics is a powerful tool to drive the discovery of the complex process of B. bassiana infections. This review categorises infection processes into distinct steps, and presents the overview of the genes, proteins and metabolite expressions relevant for the B. bassiana pathogenicity.
Keywords
Biological control
Fungi
Pathogenesis
Proteomics
Transcriptomics
Metabolomics
1 Introduction
Arthropod pests have been a significant threat to the agricultural productivity and human health worldwide. Different arthropod pests attack different types of crops, causing serious damages to crop yield globally. Rice stem borers, being the most significant pest to rice crops, inflicted between 5 and 10% yield loss to all rice-producing regions worldwide (Savary et al., 2019). In Southeast Asia, 54, 388 ha of paddy lands were decimated by arthropod pest colonisation (AFSIS, 2018). Other insects like mosquitoes act as disease vectors, transmitting diseases including lethal ones such as malaria and dengue. Malaria had the most severe mortality rate, with 435,000 deaths reported worldwide in 2017 (WHO, 2018). Meanwhile, dengue rapidly evolved and became widespread over a few decades, with four serotypes increasing to ten serotypes in 1990 and 2015, respectively (Guo et al., 2017), and with approximately 390 million dengue infections annually (Bhatt et al., 2013).
The conventional pest management strategy using broad-range chemical pesticides looms with concerns. Most notably, the use of excessive broad-range chemical pesticides to control brown planthopper, Nilaparvata lugens in rice led to the decimation of its natural enemies, consequently causing an even greater surge of outbreaks following the use of the pesticides (Way and Heong, 1994). Furthermore, certain chemical pesticides also pose dangerous risks to the ecosystem by disrupting food chains or being hazardous to human health. An example is dichlorodiphenyltrichloroethane (DDT). It persists for a long time in the environment, accumulating in the food chain and tissues of living organisms, and it has been found later to be carcinogenic (Turusov et al., 2002).
Thus, with the rising concerns over the use of chemical-based pesticides, the pest management research is diverting towards a more sustainable and safer approach, such as biopesticides. Biopesticides are biologically-based pesticides that can be either toxic molecules harvested from entomopathogenic microbe cultures or live entomopathogenic microbes (EPMs) included in the pesticide formulations as mycoinsecticides (Senthin-Nathan, 2015). Various biopesticides have been developed from various EPMs with varying degree of pathogenicity for targeted arthropod pests (Li et al., 2010).
Beauveria bassiana is a well-established biological control agent with a broad range of efficacy (Faria and Wraight, 2007). Furthermore, B. bassiana can colonise the soil or plants as a saprophyte or an endophyte, respectively (Boomsma et al., 2014). Consequently, B. bassiana is capable of long-term protection with minimal applications, effectively reducing insecticide application costs, and benefitting both farmers and consumers. However, to assess whether B. bassiana can be an effective and sustainable option for managing arthropod pests, it is crucial to understand its mechanisms of pathogenicity at the molecular level.
The advancement of molecular biology has led to a new approach termed “systems biology” has been developed, with a promising capacity to holistically understand an EPM’s mechanisms of pathogenicity compared to the conventional reductionist approach. Systems biology employs omics technologies at the level of genes (genomics), RNA transcripts (transcriptomics), proteins (proteomics), and metabolites (metabolomics), as well as bioinformatics to produce a snapshot of the total expression of the molecules from the samples and infer how these molecules interact with one another to produce a phenotype (Tini et al., 2017). Through omics, new models can be produced from the wealth of data and thus, new hypotheses can be tested. This review aims to discuss how the systems biology approach using omics techniques can accelerate the characterisation of the mechanisms of pathogenicity of EPMs. With B. bassiana as an example, this review highlights how the key genes, proteins and metabolites relevant to pathogenesis could be identified through high-throughput analysis.
2 Infection process of B. bassiana
B. bassiana shares common mechanisms of pathogenicity with other entomopathogenic fungi (EPF). The infection process of B. bassiana can be broadly divided into three stages: (1) host arthropod adhesion; (2) penetration of arthropod cuticle; and (3) arthropod haemocoel colonisation (Fig. 1) (Hajek and St. Leger, 1994; Wojda et al., 2009). At each stage of infection, B. bassiana adapts by changing its structure to efficiently overcome the host’s defences (Hajek and St. Leger, 1994). The overview of relevant genes, proteins and metabolites for each stage is summarised in Table 1.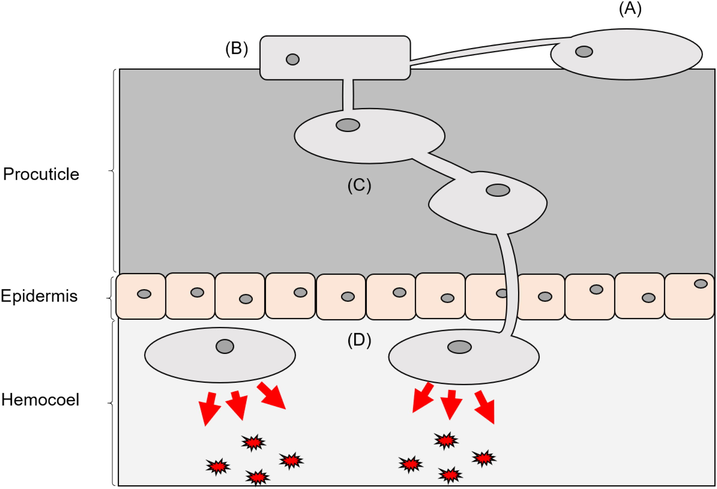
Overview of the mechanism of Beauveria bassiana pathogenicity. (A) B. bassiana attaches itself to insect host via hydrophobic interactions. (B) B. bassiana modifies its structure for forming appresorium that secretes of chitin degrading enzymes and exerting mechanical pressure to breach the cuticle. (C) Host cuticle penetration and germination of B. bassiana inside insect procuticle. (D) Formation of blastospores, invasion of haemocoel and secretion of toxic molecules.
Gene/protein/metabolite
Role
Reported In
References
CFEM domain-containing protein
Cuticle adhesion
Transcriptomics
Chen et al. (2018), Wang et al. (2017)
Fasciclin domain-containing protein
Cuticle adhesion
Proteomics
Santi et al. (2018)
Hydrophobin
Cuticle adhesion
Transcriptomics
Chu et al. (2016), Wang et al. (2017)
Mammalian-like perilipin 1 (MPL1)
Cuticle adhesion
Transcriptomics
Lai et al. (2017)
Metarhizium anisopliae adhesin-like protein (MAD1)
Cuticle adhesion
Transcriptomics
(Chen et al., 2018), Chu et al. (2016), Lai et al. (2017), Wang et al. (2017)
Metarhizium anisopliae adhesin-like protein (MAD2)
Cuticle adhesion
Transcriptomics
Wang et al. (2017)
Cell wall proteins
Cell wall remodelling
Transcriptomics
Chu et al. (2016), Lai et al. (2017), Wang et al. (2017)
Chitin synthase
Cell wall remodelling
Transcriptomics
Lai et al. (2017)
β-1,3-glucanase
Cell wall remodelling
Transcriptomics
Lai et al. (2017)
Metarhizium anisopliae HOG 1 (MaHOG1)
Signalling
Transcriptomics
(Chen et al., 2018)
Osmosensor protein MOS1 (Mos1)
Signalling
Transcriptomics
(Chen et al., 2018), Lai et al. (2017)
Oxylipin
Signalling; Stress tolerance
Metabolomics
(Zhang et al., 2016)
Protein Kinase A
Signalling
Transcriptomics
(Chen et al., 2018), Lai et al. (2017)
Carboxypeptidase
Cuticle penetration
Transcriptomics
Lai et al. (2017)
Cytochrome P450 (CYP)
Cuticle penetration; Host immune defence
Transcriptomics; Proteomics
Lai et al. (2017)
GH18 Chitinase
Cuticle penetration
Transcriptomics; Proteomics
Chu et al. (2016), (Chen et al., 2018), Dionisio et al. (2016), Lai et al. (2017), Santi et al. (2018),
Subtilisin-like Pr1A
Cuticle penetration
Transcriptomics; Proteomics
Chu et al. (2016), Dionisio et al. (2016), (Zhou et al., 2018)
Subtilisin-like Pr1B
Cuticle penetration
Transcriptomics; Proteomics
Chu et al. (2016), Dionisio et al. (2016), (Zhou et al., 2018)
Subtilisin-like Spm1
Cuticle penetration
Proteomics
Dionisio et al. (2016)
Catalase
Antioxidative enzymes; Stress tolerance
Transcriptomics; Proteomics
(Chen et al., 2018), Chu et al. (2016), Santi et al. (2018)
Flavin adenine dinucleotide-dependent oxidoreductase (FOXRED1)
Antioxidative enzymes; Stress tolerance
Proteomics
Santi et al. (2018)
Glutathione S-transferase (GST)
Antioxidative enzymes; Stress tolerance
Transcriptomics
Lai et al. (2017)
Peroxidases (POX)
Antioxidative enzymes; Stress tolerance
Proteomics
Dionisio et al. (2016)
Superoxide dismutase (SOD)
Antioxidative enzymes; Stress tolerance
Transcriptomics
Lai et al. (2017)
Thioredoxin
Antioxidative enzymes; Stress tolerance
Transcriptomics
(Chen et al., 2018), Lai et al. (2017)
Thioredoxin reductase
Antioxidative enzymes; Stress tolerance
Proteomics
Dionisio et al. (2016)
Heat shock protein 30 (HSP30)
Stress tolerance
Transcriptomics
Chu et al. (2016)
Heat shock protein 40 (HSP40)
Stress tolerance
Transcriptomics
Chu et al. (2016)
Heat shock protein 70 (HSP70)
Stress tolerance
Transcriptomics; Proteomics
Chu et al. (2016)
ATP binding casette (ABC) transporter
Host immune defence
Transcriptomics
Lai et al. (2017)
Polyketide synthase (PKS)
Host immune defence
Transcriptomics
Lai et al. (2017)
Small secreted cysteine-rich proteins with LysM binding domain (SSCP-LysM)
Host immune defence
Transcriptomics; Proteomics
Dionisio et al. (2016), Lai et al. (2017)
Beauvericin
Toxin
Metabolomics
de Bekker et al. (2013), (Zhang et al., 2016)
Beauverolide
Toxin
Metabolomics
de Bekker et al. (2013), (Zhang et al., 2016)
Several literature reviews had discussed B. bassiana pathogenicity; however, much of the knowledge of the genes were acquired from gene-knockout studies and enzymatic assays performed with low-throughput analyses (Butt et al., 2016; Valero-Jiménez et al., 2016). The more recent studies that applied omics methods found similar genes reported in the gene-knockout studies. Moreover, these studies have reported an involvement of additional genes, proteins, and metabolites prevalent at a particular stage of infection which were not previously reported (Fig. 2).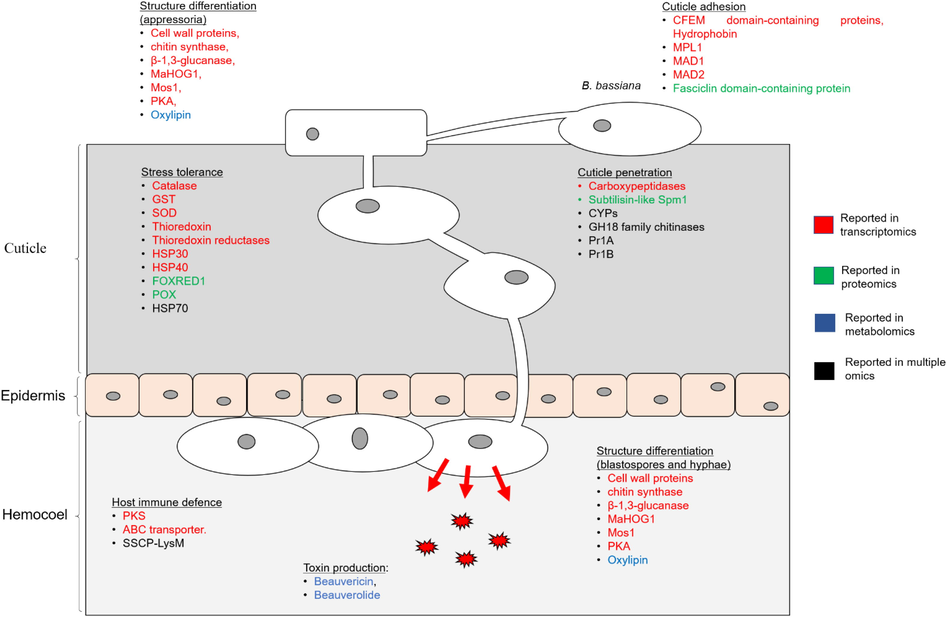
Mechanism of B. bassiana pathogenicity as elucidated from transcriptomics, proteomics and metabolomic studies. ABC, ATP-binding cassette; CYP, cytochrome P450; DLD, dihyrolipoly dehydrogenase; FOXRED1, flavin adenine dinucleotide-dependent oxidoreductase; GST, glutathione S-transferase; HSP, heat shock protein; MAD1, Metarhizium anisopliae adhesin-like protein 1; MAD2, Metarhizium anisopliae adhesin-like protein 2; MaHOG1, Metarhizium anisopliae HOG1; MOS1, Metarhizium anisopliae osmosensor; MPL1, mammalian-like perilipin; PKA, protein kinase A; Pr1A, pathogenesis-related protein Pr1A; Pr1B, pathogenesis-related protein Pr1B; SSCP-LysM, small secreted cysteine-rich proteins.
3 Host adhesion
The aerial conidia of B. bassiana facilitate adhesion to insect cuticles through hydrophobic interactions (Boucias et al., 1988). To facilitate adhesion, the aerial conidia are coated with hydrophobins which form a hydrophobic coating (Holder and Keyhani, 2005). B. bassiana expresses several genes that play a role in lipid homeostasis which influences the hydrophobicity of its conidia.
Transcriptomics analyses of B. bassiana showed an up-regulation of gene expressions for Metarhizium adhesin-like protein 1, 2 (MAD1, MAD2), and hydrophobins (Chen et al., 2018; Lai et al., 2017; Zhou et al., 2018). Adhesins and hydrophobins are vital for B. bassiana to attach itself to the insect’s cuticle via hydrophobic interactions (Holder and Keyhani, 2005; Wang and St. Leger, 2007a). Furthermore, the studies have also reported an over-expression of mammalian-like perilipin 1 (MPL1) and CFEM-domain containing genes (Chen et al., 2018; Wang et al., 2017). MPL1 facilitates lipid transport and storage in the conidia while maintaining the lipid homeostasis of fungus. The deletion of the MPL1 gene leads to reduced appressoria turgor pressure which impairs the adhesiveness of B. bassiana on hydrophobic surfaces (Wang and St. Leger, 2007b). CFEM-domain genes are unique to fungi, sharing a commonly conserved eight-cysteine residue (Kulkarni et al., 2003). CFEM domain-containing proteins have roles in the surface sensing and signalling for fungal biological processes associated with pathogenesis, including conidial germination and appressorium formation (Sabnam and Roy Barman, 2017). However, further characterisation of each of the CFEM domain-containing genes is necessary to elucidate the specific roles of each genes with regards to host adhesion.
Proteomic studies have reported secretion of proteins related to insect host adhesion. B. bassiana secretes sphingomyelin phosphodiesterase and fasciclin domain-containing proteins upon contact with insect’s cuticles (Dionisio et al., 2016; Santi et al., 2018). Sphingomyelin phosphodiesterase is a lipase that has a role in sphingolipid metabolism on the cell membranes (Feng et al., 2011). In fungi, sphingomyelin phosphodiesterase shares a homology with acid sphingomyelinases which can affect the composition of sphingolipids on biological membranes (de Bekker et al., 2015). The extracellular secretion of sphingomyelin phosphodiesterase by B. bassiana suggests its role in disrupting the biological membrane of the insect host. Fasciclin domain-containing proteins are present across different species, mediating cellular adhesion (Miyazaki et al., 2007). In phytopathogenic fungi, Magnaporthe oryzae, fasciclin plays a role in the conidial adhesion to a plant’s cell wall (Liu et al., 2009). The over-expression of this protein in B. bassiana suggests a similar role of adhesion onto insect hosts.
4 Germination and cell body differentiation
Upon adhesion, the conidia of B. bassiana begin to germinate and develop appressoria for cuticular penetration (Hajek and St. Leger, 1994). The appressorium structure enables mechanical pressure and enzymatic digestion to function synergistically on a much smaller surface area, thus increasing penetration efficiency (Chandler, 2017; Singh et al., 2017). As the penetration progresses, B. bassiana germinates its hyphae through the cracks inside the insect’s exoskeleton and produces secondary hyphal bodies inside the cuticular layer of the insect host. In the haemocoel, B. bassiana is exposed to a hyperosmotic environment. Therefore, it switches from hyphae to blastospores which are more hydrophilic, motile and better adapted for host immune evasion (Holder et al., 2007; Ortiz-Urquiza and Keyhani, 2016).
Several genes related to cell wall remodelling and signalling were over-expressed throughout B. bassiana infection. Notably, several cell wall protein-conferring genes, chitin synthase and β-1,3-glucanase were up-regulated in previous transcriptomic studies (Chen et al., 2018; Chu et al., 2016; Lai et al., 2017). Cell wall protein-conferring genes are responsible for the building blocks of B. bassiana cell wall. Chitin synthases are involved in producing chitin which is a vital component of the cell wall (Tartar et al., 2005) whereas β-1,3-glucanases are involved in cell wall softening, hence allowing germination (Mouyna et al., 2013). The B. bassiana signalling-related genes, mitogen-activated-protein kinases (MAPKs) and osmosensor Mos1 are vital for the cell body differentiation in fungi (Chen et al., 2018; Lai et al., 2017; Zhou et al., 2018). Two MAPK genes vital for B. bassiana were identified from previous omics studies: protein kinase A (PKA) and Metarhizium anisopliae HOG1 (MaHOG1) genes. The expression of PKA, MaHOG1 and Mos1 have been found to be vital for appressorium and blastospore formation, and the disruption of these genes has been linked with the delayed cell body differentiation and reduced pathogenicity (Jin et al., 2012; Luo et al., 2012).
5 Cuticle penetration
Insect cuticles consist of non-polar hydrocarbons and numerous components of cuticular proteins that act as a physical barrier against microbial infection. The outermost layer, the epicuticle, is rich in lipids and the procuticle layer is rich in chitin and sclerotised proteins (Ortiz-Urquiza and Keyhani, 2013). An appressorium serves as the site at which B. bassiana unleashes its arsenal of hydrolytic enzymes to degrade and penetrate the insect’s cuticle (Samuels et al., 2016). Once the epicuticle layer is breached, B. bassiana germinates hyphae which penetrate through the cracks and into the procuticle layer (Hajek and St. Leger, 1994). The penetrating hyphae not only continue to secrete hydrolytic enzymes, but also begin to release defensive molecules against the insect host’s immune responses (Butt et al., 2016). The digested proteins and hydrocarbons from the cuticle serve as a nutrient source for further hyphal growth (Pedrini et al., 2013). Moreover, these hydrolytic enzymes are also necessary for detoxifying antimicrobial compounds from quinones, alkanes, lipids, and free fatty acids commonly present in the epicuticle layer (Wang and Wang, 2017). These antimicrobial compounds could inhibit spore germination and fungal growth, thus preventing successful infection.
Several proteases, chitinases, carboxypeptidases, and lipases have been reported from both transcriptomics and proteomics analyses pertinent to cuticle penetration. Among the more notable ones are the expression of subtilisin-like protease (Pr) isoform 1A (Pr1A) and 1B (Pr1B), GH18 family chitinases, and cytochrome P450s (CYPs) which have been consistently found in proteomic and transcriptomic studies. Pr1 family proteins are extracellular cuticle degrading enzymes that are vital to penetrate the insect’s exoskeleton (Wang and Wang, 2017). Expectedly, the over-expression of Pr1A and Pr1B genes were correlated with the increased insect killing ability of M. anisopliae and B. bassiana (Fang et al., 2009; St. Leger et al., 1996). GH18 chitinases serve to degrade and derive nutrient from chitin while also vital for facilitating the growth of an entomopathogenic fungi (Hartl et al., 2012; Mondal et al., 2016). The analysis of B. bassiana genome has shown high abundance of GH18 chitinase genes (Xiao et al., 2012) which correlate to the abundance of different types of GH18 chitinases in the transcriptomic and proteomic studies (Chen et al., 2018; Dionisio et al., 2016; Lai et al., 2017; Santi et al., 2018). CYPs are a superfamily of monooxygenases that hydroxylate xenobiotic compounds, including alkanes and fatty acids (Lin et al., 2011). Their expression has been found to be induced by the presence of insect lipids, such as the cuticles (Lin et al., 2011; Zhang et al., 2012) and demonstrated to have a role in detoxifying the insect host’s toxic molecules (Xing et al., 2017).
6 Stress response and immune evasion
Successful cuticle penetration allows B. bassiana an access to the host’s haemocoel. To prevent colonisation inside the haemolymph, the insect host responds to the breach in the haemocoel by activating melanisation, and releasing protease inhibitors, antimicrobial peptides (AMPs), and reactive oxygen species (ROS) (Butt et al., 2016; Ortiz-Urquiza and Keyhani, 2016; Valero-Jiménez et al., 2016).
B. bassiana overcomes the host insect defences by expressing genes related to stress management. These are produced as early as the cuticle penetration stage. Furthermore, genes related to immune evasion are also upregulated upon breaching into the host insect’s haemocoel. During the cuticle penetration and haemocoel colonisation stage, several anti-oxidative enzyme-conferring genes, including glutathione S-transferases (GSTs), superoxide dismutase (SODs), thioredoxins, catalases, oxidoreductases, and peroxidases are over-expressed (Chen et al., 2018; Chu et al., 2016; Lai et al., 2017; Santi et al., 2018). Additionally, the expression of several types of heat shock proteins (HSPs) are also significantly increased. The anti-oxidative enzymes protect the fungi against the oxidative stress from the increased ROS as a result of the insect host’s defence response (Ortiz-Urquiza and Keyhani, 2016). HSPs serve as protein chaperones that protect the integrity of the internal cellular structure against various forms of stress. Moreover, the over-expression of signalling genes from the aforementioned MaHOG1, PKA, and MOS1 is vital for the B. bassiana survivability inside the haemocoel. Besides regulating the formation of blastospores, the increased expression of these signalling genes is also linked with the increased tolerance to osmotic shock to survive under high osmotic pressure (Jin et al., 2012; Wang et al., 2008).
Furthermore, there is an up-regulation of polyketide synthase (PKS), small secreted cysteine rich proteins (SSCPs) with LysM domain, and ATP binding cassette (ABC) transporters at the cuticular and haemocoel stages (Lai et al., 2017). PKS synthesises oosporein that inhibits polyphenol oxidase (PPO) activity, which in turn, suppresses anti-fungal peptide expression (Feng et al., 2015). In addition to the defence against host immune response, PKS is involved in the synthesis of toxic metabolites secreted by B. bassiana (Chandler, 2017). SSCPs with LysM domain have yet reported any established role in the B. bassiana pathogenicity. However, the expression of these SSCPs has been found to be correlated with the suppression of chitin-triggered immunity by the host plants against phytopathogenic fungi (Mentlak et al., 2012). The ABC transporters are multidrug efflux pumps which protect the fungi against a range of toxic compounds (Morschhäuser, 2010). Thus, the increased expression of ABC transporter genes may serve to protect the fungi against harmful host’s molecules. The cell wall remodelling genes discussed previously also play important roles in host immune evasion. The increased activities of β-1,3-glucanase but lowered expression of chitin synthase genes inside the haemocoel (Lai et al., 2017) may be associated with removing all presence of antigenic compounds, such as galactomannan and chitin, thus allowing the evasion of host insect defences.
7 Toxin production
B. bassiana also produces toxic secondary metabolites, including beauverolides, bassianolide, beauvericin, oosporeins, tenellins, and isarolides which are responsible for insect cell cytotoxicity (Chandler, 2017). Each of these metabolites has different degrees of toxicity that depends on the insect host. For example, bassianolide contributes significantly to the pathogenicity of the greater wax moth (Galleria mellonella), beet armyworm (Spodoptera exigua), and corn earworm (Helicoverpa zea), but tenellin and beauvericin do not (Butt et al., 2016).
The over-expression of PKS gene in the haemocoel as mentioned previously is involved in oosporein biosynthesis (Lai et al., 2017). However, it was noted that the expression of genes related to beauvericin, bassianolide, and tenellin biosynthesis was not significantly increased in the transcriptomic studies although the genes were already characterised and annotated in the B. bassiana genome (Xiao et al., 2012). In contrast, the metabolomic studies on B. bassiana have confirmed the expression of toxic secondary metabolites. Interestingly, B. bassiana seems to be able to distinguish between living and dead hosts. It secretes a significantly higher amount of beauverolides in the presence of live insect tissues compared to dead tissues (de Bekker et al., 2013). Besides that, the presence of pupae extract yielded no significant difference for the beauvericin and beauverolide secretion compared to the minimal media control (Luo et al., 2015). The lack of toxins produced by B. bassiana in the presence of the cuticular extract is expected as these toxins are released by the fungus inside an insect’s haemocoel (Lacey et al., 2015; Singh et al., 2017).
8 Conclusion
The application of omics in B. bassiana illustrates the big picture of the plethora of biological processes at work during pathogenesis and the modulated expressions at each stage of the attack (Table 1). Overall, a single omics approach is insufficient to capture the complexity of B. bassiana’s mechanism of pathogenesis. An integrated omics approach is necessary to elucidate an in-depth and broad view of the complete expressions of genes, proteins, and secondary metabolites throughout each stage of infection.
It must be noted that some gene expressions do not correlate well with their corresponding protein and metabolite expressions. For example, the majority of the toxic secondary metabolite biosynthesis related genes were not significantly overexpressed (Chen et al., 2018; Lai et al., 2017; Zhou et al., 2018) but the corresponding metabolites were found in abundance in metabolomic studies (de Bekker et al., 2013; Luo et al., 2015). This contradiction can be attributed to the difference in samplings, and the host differences that could impact the expression patterns for genes, proteins, and metabolites reported in the omics studies. Therefore, to acquire a more precise, systemic overview of the modulation of the genes, proteins, and metabolites of B. bassiana during an infection process, it is imperative that the experimental designs to closely match each other studies in terms of the insect host species, the developmental stage during experiments, and the timing of sampling. Ideally, the transcriptomics, proteomics, metabolomics, and any additional omics of interests should be conducted on the same experimental sample. However, carrying out different omics studies on the same B. bassiana infection experiment is a monumental challenge for a research group. Therefore, different research groups that use different omics approaches need to collaborate in designing the omics experiments to investigate B. bassiana pathogenicity, subsequently running different omics studies from the same biological sample. Perhaps, we can expect more research consortiums being formed with each contributing omics method that cumulatively provides comprehensive systemic insights into the complexity of EPF pathogenesis.
Future research could be conducted based on the knowledge of genes, proteins and metabolites relevant to B. bassiana pathogenicity. They include a genetic manipulation to produce transgenic B. bassiana that yields greater insect-killing potency or to produce transgenic crops expressing B. bassiana pathogenicity-related genes. For the former, the research efforts have been ongoing. For example, transgenic strains of B. bassiana have been developed to produce heterologous toxins from scorpions (Lu et al., 2008) or insect host’s hormones that result in endocrinal imbalance, increasing the host’s susceptibility (Fan et al., 2012). Ultimately, these researches will collectively improve the potential of B. bassiana as an alternative to the conventional insecticides, augmenting the arsenal of the insect pest control.
Acknowledgement
The work is supported by Universiti Kebangsaan Malaysia (Grant number DIP-2016-012).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Report on Early Warning information. Bangkok: Asean Food Security Information System; 2018.
- Evolutionary interaction networks of insect pathogenic fungi. Annu. Rev. Entomol.. 2014;59:467-485.
- [CrossRef] [Google Scholar]
- Nonspecific factors involved in attachment of entomopathogenic deuteromycetes to host insect cuticle. Appl. Environ. Microbiol.. 1988;54:1795-1805.
- [Google Scholar]
- Entomopathogenic fungi: new insights into host-pathogen interactions. Adv. Genet.. 2016;94:307-364.
- [CrossRef] [Google Scholar]
- Basic and applied research on entomopathogenic fungi. In: Lacey L., ed. Microbial Control of Insect and Mite Pests: From Theory to Practice. Cambridge (MA), USA: Academic Press; 2017. p. :69-89.
- [CrossRef] [Google Scholar]
- Genes involved in Beauveria bassiana infection to Galleria mellonella. Arch. Microbiol.. 2018;200:541-552.
- [CrossRef] [Google Scholar]
- Genome-wide host-pathogen interaction unveiled by transcriptomic response of diamondback moth to fungal infection. PLoS One. 2016;11:1-15.
- [CrossRef] [Google Scholar]
- Gene expression during zombie ant biting behavior reflects the complexity underlying fungal parasitic behavioral manipulation. BMC Genomics. 2015;16:1-23.
- [CrossRef] [Google Scholar]
- Metabolomics reveals the heterogeneous secretome of two entomopathogenic fungi to Ex Vivo cultured insect tissues. PLoS One. 2013;8
- [CrossRef] [Google Scholar]
- Label-free differential proteomics and quantification of exoenzymes from isolates of the entomopathogenic fungus Beauveria bassiana. Insects. 2016;7
- [CrossRef] [Google Scholar]
- Exploiting host molecules to augment mycoinsecticide virulence. Nat. Biotechnol.. 2012;30(1):35-37.
- [CrossRef] [Google Scholar]
- Expressing a fusion protein with protease and chitinase activities increases the virulence of the insect pathogen Beauveria bassiana. J. Invertebr. Pathol.. 2009;102:155-159.
- [CrossRef] [Google Scholar]
- Mycoinsecticides and Mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control. 2007;43:237-256.
- [CrossRef] [Google Scholar]
- Molecular characterization of a Stagonospora nodorum lipase gene LIP1. Plant Pathol.. 2011;60:698-708.
- [CrossRef] [Google Scholar]
- Fungal biosynthesis of the bibenzoquinone oosporein to evade insect immunity. Proc. Natl. Acad. Sci. U.S.A.. 2015;112:11365-11370.
- [CrossRef] [Google Scholar]
- Global epidemiology of dengue outbreaks in 1990–2015: a systematic review and meta-analysis. Front. Cell. Infect. Microbiol.. 2017;7:1-11.
- [CrossRef] [Google Scholar]
- Interactions between fungal pathogens and insect hosts. Annu. Rev. Entomol.. 1994;39:293-322.
- [CrossRef] [Google Scholar]
- Fungal chitinases: diversity, mechanistic properties and biotechnological potential. Appl. Microbiol. Biotechnol.. 2012;93:533-543.
- [CrossRef] [Google Scholar]
- Adhesion of the entomopathogenic fungus Beauveria (Cordyceps) bassiana to substrata. Appl. Environ. Microbiol.. 2005;71:5260-5266.
- [CrossRef] [Google Scholar]
- Surface characteristics of the entomopathogenic fungus Beauveria (Cordyceps) bassiana. Microbiology. 2007;153:3448-3457.
- [CrossRef] [Google Scholar]
- MaHog1, a Hog1-type mitogen-activated protein kinase gene, contributes to stress tolerance and virulence of the entomopathogenic fungus Metarhizium acridum. Microbiology (United Kingdom). 2012;158:2987-2996.
- [CrossRef] [Google Scholar]
- An eight-cysteine-containing CFEM domain unique to a group of fungal membrane proteins. Trends Biochem. Sci.. 2003;28:116-118.
- [CrossRef] [Google Scholar]
- Insect pathogens as biological control agents: back to the future. J. Invertebr. Pathol.. 2015;132:1-41.
- [CrossRef] [Google Scholar]
- In vivo gene expression profiling of the entomopathogenic fungus Beauveria bassiana elucidates its infection stratagems in Anopheles mosquito. Sci. China Life Sci.. 2017;60:839-851.
- [CrossRef] [Google Scholar]
- Biological control of insects in Brazil and China: history, current programs and reasons for their successes using entomopathogenic fungi. Biocontrol Sci. Technol.. 2010;20:117-136.
- [CrossRef] [Google Scholar]
- The MrCYP52 cytochrome P450 monoxygenase gene of Metarhizium robertsii is important for utilizing insect epicuticular hydrocarbons. PLoS One. 2011;6
- [CrossRef] [Google Scholar]
- MoFLP1, encoding a novel fungal fasciclin-like protein, is involved in conidiation and pathogenicity in Magnaporthe oryzae. J. Zhejiang Univ. Sci. B. 2009;10:434-444.
- [CrossRef] [Google Scholar]
- Insecticidal evaluation of Beauveria bassiana engineered to express a scorpion neurotoxin and a cuticle degrading protease. Appl. Microbiol. Biotechnol.. 2008;81:515-522.
- [CrossRef] [Google Scholar]
- Differential metabolic responses of Beauveria bassiana cultured in pupae extracts, root exudates and its interactions with insect and plant. J. Invertebr. Pathol.. 2015;130:154-164.
- [CrossRef] [Google Scholar]
- The MAP kinase Bbslt2 controls growth, conidiation, cell wall integrity, and virulence in the insect pathogenic fungus Beauveria bassiana. Fungal Genet. Biol.. 2012;49:544-555.
- [CrossRef] [Google Scholar]
- Effector-mediated suppression of chitin-triggered immunity by Magnaporthe oryzae is necessary for rice blast disease. Plant Cell. 2012;24:322-335.
- [CrossRef] [Google Scholar]
- The fruiting-specific Le.flp1 gene, encoding a novel fungal fasciclin-like protein, of the basidiomycetous mushroom Lentinula edodes. Curr. Genet.. 2007;51:367-375.
- [CrossRef] [Google Scholar]
- Journey of enzymes in entomopathogenic fungi. Pac. Sci. Rev. A: Nat. Sci. Eng.. 2016;18:85-99.
- [CrossRef] [Google Scholar]
- Regulation of multidrug resistance in pathogenic fungi. Fungal Genet. Biol.. 2010;47:94-106.
- [CrossRef] [Google Scholar]
- β-1,3-glucan modifying enzymes in Aspergillus fumigatus. Front. Microbiol.. 2013;4:1-9.
- [CrossRef] [Google Scholar]
- Molecular genetics of Beauveria bassiana infection of insects. In: Advances in Genetics. Elsevier Ltd.; 2016.
- [CrossRef] [Google Scholar]
- Action on the surface: entomopathogenic fungi versus the insect cuticle. Insects. 2013;4:357-374.
- [CrossRef] [Google Scholar]
- Targeting of insect epicuticular lipids by the entomopathogenic fungus Beauveria bassiana: hydrocarbon oxidation within the context of a host-pathogen interaction. Front. Microbiol.. 2013;4:1-18.
- [CrossRef] [Google Scholar]
- WISH, a novel CFEM GPCR is indispensable for surface sensing, asexual and pathogenic differentiation in rice blast fungus. Fungal Genet. Biol.. 2017;105:37-51.
- [CrossRef] [Google Scholar]
- Entomopathogenic organisms: conceptual advances and real-world applications for mosquito biological control. Open Access Insect Physiol.. 2016;6:25.
- [CrossRef] [Google Scholar]
- Secretomic analysis of Beauveria bassiana related to cattle tick, Rhipicephalus microplus, infection. Folia Microbiol. (Praha). 2018;64:361-372.
- [CrossRef] [Google Scholar]
- The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol.. 2019;3:430-439.
- [CrossRef] [Google Scholar]
- A review of biopesticides and their mode of action against insect pests. In: Environmental Sustainability. Tamil Nadu: Springer India; 2015. p. :1-324.
- [CrossRef] [Google Scholar]
- Entomopathogenic fungi: an effective biocontrol agent for management of insect populations naturally. J. Pharm. Sci. Res.. 2017;9:830-839.
- [Google Scholar]
- Construction of an improved mycoinsecticide overexpressing a toxic protease. Proc. Natl. Acad. Sci. U.S.A.. 1996;93:6349-6354.
- [CrossRef] [Google Scholar]
- Differential expression of chitin synthase (CHS) and glucan synthase (FKS) genes correlates with the formation of a modified, thinner cell wall in in vivo-produced Beauveria bassiana cells. Mycopathologia. 2005;160:303-314.
- [CrossRef] [Google Scholar]
- Multi-omics integration—a comparison of unsupervised clustering methodologies. Brief. Bioinform. 2017:1-11.
- [CrossRef] [Google Scholar]
- Dichlorodiphenyltrichloroethane (DDT): ubiquity, persistence, and risks. Environ. Health Perspect.. 2002;110:125-128.
- [Google Scholar]
- Genes involved in virulence of the entomopathogenic fungus Beauveria bassiana. J. Invertebr. Pathol.. 2016;133:41-49.
- [CrossRef] [Google Scholar]
- MOS1 osmosensor of Metarhizium anisopliae is required for adaptation to insect host hemolymph. Eukaryot. Cell. 2008;7:302-309.
- [CrossRef] [Google Scholar]
- The MAD1 adhesin of Metarhizium anisopliae links adhesion with blastospore production and virulence to insects, and the MAD2 adhesin enables attachment to plants. Eukaryot. Cell. 2007;6:808-816.
- [CrossRef] [Google Scholar]
- The Metarhizium anisopliae perilipin homolog MPL1 regulates lipid metabolism, appressorial turgor pressure, and virulence. J. Biol. Chem.. 2007;282:21110-21115.
- [CrossRef] [Google Scholar]
- Insect pathogenic fungi: genomics, molecular interactions, and genetic improvements. Annu. Rev. Entomol.. 2017;62:73-90.
- [CrossRef] [Google Scholar]
- Transcriptomic analysis of two Beauveria bassiana strains grown on cuticle extracts of the silkworm uncovers their different metabolic response at early infection stage. J. Invertebr. Pathol.. 2017;145:45-54.
- [CrossRef] [Google Scholar]
- The role of biodiversity in the dynamics and management of insect pests of tropical irrigated rice—a review. Bull. Entomol. Res.. 1994;84:567-587.
- [CrossRef] [Google Scholar]
- WHO, 2018. World Malaria Report. World Health Organization 2018, Geneva.
- Humoral immune response of Galleria mellonella larvae after infection by Beauveria bassiana under optimal and heat-shock conditions. J. Insect Physiol.. 2009;55:525-531.
- [CrossRef] [Google Scholar]
- Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci. Rep.. 2012;2
- [CrossRef] [Google Scholar]
- RNA-seq analyses for two silkworm strains reveals insight into their susceptibility and resistance to Beauveria bassiana infection. Int. J. Mol. Sci.. 2017;18
- [CrossRef] [Google Scholar]
- Metabolic responses of Beauveria bassiana to hydrogen peroxide-induced oxidative stress using an LC-MS-based metabolomics approach. J. Invertebr. Pathol.. 2016;137:1-9.
- [CrossRef] [Google Scholar]
- CYP52X1, representing new cytochrome P450 subfamily, displays fatty acid hydroxylase activity and contributes to virulence and growth on insect cuticular substrates in entomopathogenic fungus Beauveria bassiana. J. Biol. Chem.. 2012;287:13477-13486.
- [CrossRef] [Google Scholar]
- In vivo transcriptomic analysis of Beauveria bassiana reveals differences in infection strategies in Galleria mellonella and Plutella xylostella. Pest Managee. Sci.. 2018;75:1443-1452.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.101332.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







