Translate this page into:
Novel drug delivery to the brain for neurodegenerative disorder treatment using carbon nanotubes
⁎Corresponding author. mmyalzahrani@imamu.edu.sa (Mohammed Al-zharani),
⁎⁎Corresponding author. saquib.hasnain@marwadieducation.edu.in (Md Saquib Hasnain) msaquibhasnain@gmail.com (Md Saquib Hasnain)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Neurodegenerative disorders pose a significant challenge in drug delivery because of the substantial obstacle presented by the blood–brain barrier (BBB). Conventional therapeutic agents frequently encounter constraints in efficiently penetrating the brain, thus requiring inventive delivery strategies. This review explores the potential aspects of the carbon nanotubes (CNTs) as an advanced drug delivery system for treating neurodegenerative disorders. CNTs, with their distinctive structural and physicochemical characteristics, present an intriguing framework for addressing challenges related to drug delivery across the BBB. The review emphasizes the functionalization of CNTs, utilizing diverse chemical modification techniques to improve their biocompatibility and effectiveness as drug carriers. The variations have a significant effect on crucial factors related to the growth of neurons.
Moreover, the review emphasizes the therapeutic ability of CNTs in treating neurodegenerative disorders. By introducing purified CNT substrates, an increase in the growth of dendrites and improved adhesion of hippocampal neurons was reported. This has led to significant progress in the processing of neuronal signals and the development of neural circuits. Finally, this review offers in-depth understanding of the innovative and enhanced nano-scaffolds provided by CNTs for transporting therapeutic substances to the brain. This offers potential for more efficient treatments for neurodegenerative disorders.
Keywords
Neurodegenerative disorders
Carbon nanotubes
Drug delivery
Blood–brain barrier
- AD
-
Alzheimer’s disease
- Aβ
-
amyloid-β-derived diffusible ligands
- AME
-
adsorptive mediated endocytosis
- ALS
-
Amyotrophic lateral sclerosis
- BBB
-
Blood Brain Barrier
- CNTs
-
carbon nanotubes
- DA
-
dopamine
- DDS
-
Drug delivery system
- EE2
-
Ethinylestradiol
- EPR
-
enhanced permeation and retention
- HD
-
Huntington’s disease
- nm
-
nanometers
- NSCs
-
neural foundational microorganisms
- NL
-
nanoliposomes
- ND
-
Neurodegenerative disease
- NDDS
-
nanotechnology-based drug delivery systems
- O-MWNTs
-
oxidized multi-walled carbon nanotubes
- RME
-
receptor mediated endocytosis
- ROS
-
reactive oxygen species
- SWNTs
-
single-walled carbon nanotubes
- MWNTs
-
multi-walled carbon nanotubes
- CNHs
-
carbon nanohorns
- NMR
-
nuclear magnetic resonance
- PD
-
Parkinson’s disease
- TJs
-
tight junctions
- AJs
-
adherents junctions
- NPs
-
nanoparticles
Abbreviations
1 Introduction
The brain serves as the central command center for the body and necessitates effective protection against potential threats such as xenobiotics, neurotoxic agents, and microbial pathogens (Alajangi et al., 2022). Ensuring the safety of the brain from external factors is paramount, and the blood–brain barrier (BBB) plays a pivotal role in this regard. It comprises of endothelial cells that form the lining of cerebral blood vessels, the BBB acts as a barrier, separating the extracellular fluid of the brain from the bloodstream (Alajangi et al., 2022). This selective and sensitive barrier poses challenges for the delivery of neurotherapeutics. Existing treatments are available in the market which faces limitations in effectively addressing neurovascular disorders, primarily due to challenges related to their successful transport and sustained delivery within the brain. Consequently, these hurdles have led to the adoption of invasive strategies for managing brain disorders.
Further, central nervous system (CNS) disorders, encompassing conditions like cerebrovascular diseases, brain tumors, and neurodegenerative disorders, pose significant health risks. Addressing these ailments has spurred the development of various nano-therapeutics, among which CNTs, alongside polymer nanoparticles, inorganic nanoparticles, liposomes, micelles, and fullerenes, have gained attention (Srikanth and Kessler, 2012). Neurodegenerative disorders, including Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS), are circumstancesmanifested by the progressive corrosion of neurons. CNT-based drug delivery systems are being investigated for these disorders because of their capacity to penetrate biological barriers, deliver drugs directly to affected cells, and provide controlled drug release (Guo et al., 2017).
Carbon nanotubes (CNTs) are cylindrical structures made of carbon atoms prearranged in a hexagonal pattern. CNTs have a unique length-to-diameter ratio that can reach up to 28,000,000:1, making them pseudo-one-dimensional. Their exceptional electrical conductivity, thermal stability, and mechanical strength, along with their large surface area, make them highly valuable in various applications. In biomedical fields, CNTs show promise for drug delivery owing to their capability to penetrate biological barriers and deliver therapeutic agents directly to target cells.Functionalizing CNTs improves their solubility and biocompatibility, further enhancing their potential for targeted therapeutic applications (Aslam et al., 2023).
Classification:
Carbon nanotubes are categorized based on their structure into two primary types: primary categories (Fig. 1):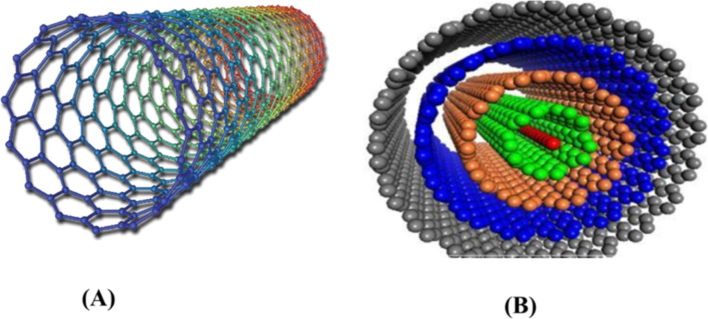
Molecular structure of carbon nanotube: (A) single walled carbon nanotube; and (B) multiwalled carbon nanotube.
Single-Walled Carbon Nanotubes (SWNTs): These nanotubes are comprised of a single sheet of graphene rolled into a cylindrical structure, approximately 1 nm in radius. They are closed at both endings, resembling a cap-like structure throughout synthesis (Foldvari and Bagonluri, 2008).
Multi-Walled Carbon Nanotubes (MWNTs): MWNTs are produced by several layers of graphene sheets, resulting in an external diameter of over 10 nm (Dresselhaus et al., 2004). They are categorized into two structural arrangements: the Russian-doll model, where graphitic sheets arrange in concentric layers, and the parchment model, where graphite single sheet rolls around itself (Goya et al., 2003). SWNTs differ structurally from MWNTs due to the distinct arrangement of carbon atoms, providing three configurations: (a) chiral or helical arrangement, (b) armchair arrangement characterized by perpendicular chairs to the tube axis, and (c) zigzag arrangement identified by a V-shape perpendicular to the tube axis (Foldvari and Bagonluri, 2008; Iijima et al., 1999).
Additionally, there are other CNT structures:
-
Carbon Nanohorns (CNHs)
-
Nanobuds
These variations possess unique modified structures that find applications in drug delivery due to their specific characteristics.
Double-Walled CNTs (DWNTs) consist of two concentric graphene cylinders, providing increased chemical and thermal stability. Moreover, CNTs are further classified based on:
-
Structural modifications
-
Preparation methods
CNTs possess distinctive physicochemical and electronic properties (Saito et al., 2009). Functionalized CNTs demonstrate enhanced translocation across plasma membranes, entering cells passively via direct translocation or actively through endocytosis. Various synthesis techniques (Fig. 2) for CNTs exist, including arc discharge, laser vaporization, and thermochemical vapor deposition. Upon entering cells, CNTs engage in cytoplasmic translocation, facilitating interaction with mammalian cells and enabling the delivery of diverse therapeutic agents (Cai et al., 2005; Gao et al., 2006; Kostarelos et al., 2007; Zheng et al., 2004).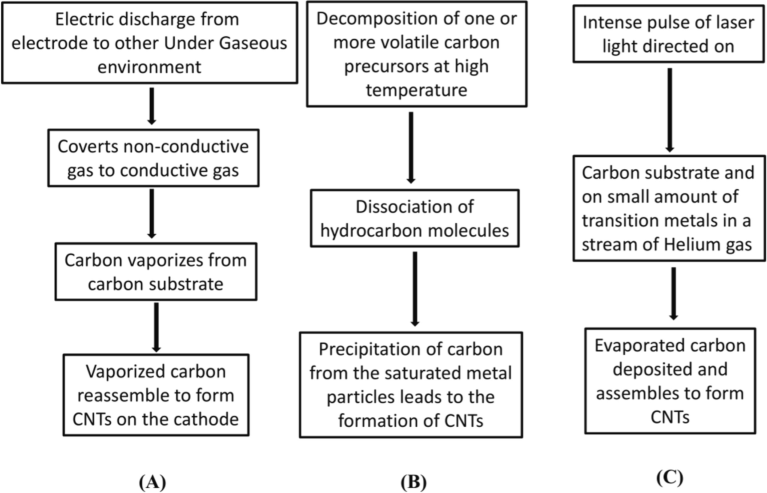
Synthesis of carbon nanotube by following methods/mechanisms: (A) Arc discharge method (B) chemical vapor deposition method (C) laser ablation method.
Structurally, Carbon Nanotubes (CNTs) feature cylindrical shapes, ranging from diameters of 1 to several dozen nanometers (nm), with lengths extending into microns. These tubes are formed by rolling graphite planes, as reported in multiple studies (Ebert, 1997; Saito et al., 1998). Their cylindrical configuration grants CNTs a higher surface area, robust tensile strength, excellent absorption, and conductivity abilities, thus positioning them as novel nanomaterials for drug delivery and biomedical applications. Moreover, they find diverse applications across multiple fields such as nano-conductors, nano-switches, and storage mediums for hydrogen and natural gasamong others, is represented below (Fig. 3): (Park et al., 1999).
Broad applications of Carbon nanotubes.
It's worth noting that inhalation of CNTs may induce toxicity, with potential harm more severe than carbon black or comparable to quartz (Cui et al., 2005). Research indicates toxicity concerns not only in cultured cells like T-lymphocytes, human keratinocytes, and kidney cells (Flahaut et al., 2003; Joselevich, 2004; Bottini et al., 2006), but also in animal studies (Heller et al., 2005).
Characterization of CNTs:
Characterization is crucial for understanding CNT properties pertinent to pharmaceutical and biological applications, including shape, size, solubility, purity, and more. Techniques such as: AFM, TEM, SEM, and various spectroscopic analyses (Infrared and Raman spectroscopy) are employed to examine CNTs, each offering specific insights into their structure and properties (Lacerda et al., 2006).
The Blood-Brain Barrier: Gateway to Neurological Disorders.
Comprising endothelial cells (ECs), astrocytes, pericytes and microglial cells, the BBB prevents the passage of numerous elements into the brain while maintaining an optimal extracellular fluid environment for CNS homeostasis. The BBB allows only specific molecules, like small lipophilic compounds and select nutrients, to pass through using various transport mechanisms, including carrier-mediated and receptor-mediated transport, among others (Abbott et al., 2006).
Physiology of the Brain and BBB Structure: Brain ECs form the cerebral microvascular endothelium, controlling the transportation of substances and maintaining brain homeostasis (Hawkins and Davis, 2005). This control involves tight junctions (TJs) and adherents’ junctions (AJs) that seal intercellular spaces, particularly through claudins 1 and 5 proteins. The barrier prevents polar molecules from freely crossing, directing molecular traffic mainly through transcellular routes across brain endothelium (Nagy et al., 1984).
Neurological Disorders and Nanotechnology: Numerous CNS disorders, including migraine, chronic pain, and psychiatric conditions, pose significant challenges in diagnosis and treatment due to their complex and often unknown pathological mechanisms (Srikanth and Kessler, 2012). Nanotechnology offers promising prospects for developing therapeutic medications with fewer side effects, particularly in neuroscience. It allows for effective drug delivery across the BBB (Saito et al., 2009).
This revised version provides a structured and concise overview of CNT characterization, the BBB, brain physiology, and the role of nanotechnology in addressing CNS disorders.
2 Usage of CNTs in various neurological disorders
2.1 Alzheimer’s disease
Alzheimer’s disease (AD) is a prevalent neurodegenerative condition marked by progressive cognitive decline and memory loss, impacting over 35 million individuals worldwide. The primary cause behind memory deficits and synaptic injury in AD is attributed to tinymasses/aggregations of amyloid-β (Aamyloid-β-derived diffusible ligands (Bisgaier and Newton, 2001).
AD diagnosis: Georganopoulou et al. employed gold nanoparticles, known for their effectiveness in studying Aβ peptide aggregation kinetics (Georganopoulou et al., 2005). Aβ-coupled iron oxide nanoparticles, whether monocrystalline or superparamagnetic, possess significant surface area, magnetic properties, and low toxicity. They're used for detecting amyloid deposition via μ MRI in AD transgenic mice. These nanoparticles can traverse the BBB and have FDA approval as MRI contrast agents for liver imaging (Yang et al., 2011). Additionally, in vitro techniques utilizing quantum dots (QD) conjugated with streptavidin have demonstrated high sensitivity in identifying APP, surpassing conventional fluoro-immunoassay methods (Feng et al., 2010).
Treatment: AD treatment through nanotechnologies involves nanoparticles targeting Aβ aggregation, reducing both blood and brain peptide levels (known as the ‘sink effect’). Gobbi et al. utilized phosphatidic acid or cardiolipin with nanoliposomes (NL) to create this effect, reducing toxicity in vitro and demonstrating high affinity for Aβ. Canovi et al. engineered an NL adorned with an anti-Aβ monoclonal antibody, exhibiting high affinity for Aβ both ex-vivo and in-vitro, even in post-mortem AD brain samples (Bereczki et al., 2011).
Parkinson’s disease:
Parkinson's disease (PD) is a prevalent progressive neurodegenerative condition, primarily affecting older individuals. It involves the dopaminergic neurons deterioration in the substantia nigra pars compacta, resulting in reduced dopamine levels in the striatum. It affects about 2–3 % of individuals above 65 years old. The symptoms of Parkinson's disease encompass motor issues such as rigidity, tremors, akinesia, bradykinesia, hypokinesia, and postural instability, along with non-motor symptoms like depression, cognitive decline, hallucinations, sleep disturbances, neuropsychiatric problems (such as fatigue, pain, anhedonia, apathy, anxiety, dementia), restless leg syndrome, panic attacks, psychosis, and sensory disturbances including numbness, paresthesia, and visual anomalies. Around 30–50 % of PD patients experience pain (Pfeiffer, 2016).
2.2 PD diagnosis
Baron and colleagues devised an in-vitro quantitative assay utilizing gold nanoparticles that leverage plasmon absorbance to detect neurotransmitters linked to Parkinson's disease pathology (Baron et al., 2005). Additionally, a high-sensitivity photoelectrochemical immunosensor was utilized for the recognition of α-synuclein, employing Au-doped TiO2 nanotube arrays for in vitro diagnosis (An et al., 2010).
PD therapy:
Nano-based technologies have been explored for dopamine release, showcasing promise in Parkinson’s disease treatment (Modi et al., 2010). Trapani et al. developed a chitosan nano preparation loaded with dopamine (DA), observing enhanced brain delivery to the striatum after intraperitoneal administration, demonstrating reduced cytotoxicity compared to standard DA (Trapani et al., 2011). These nanostructures also serve as gene therapy vehicles for PD, offering an alternative to viruses. Huang et al. demonstrated the efficacy of lactoferrin-modified nanoparticles encapsulating the human neurotrophic factor gene, leading to improved locomotor activity, increased dopamine levels in the PD rat brain, and reduced dopaminergic neuronal loss (Huang et al., 2010).
2.3 Neuronal tissue regeneration
The field of neuronal tissue regeneration has witnessed the extensive use of extracellular scaffolds, supported axonal growth and facilitated neuronal attachment. These electrically conducting nano-scaffolds, when used as scaffolds and delivery vehicles, have shown promise in enhancing neuronal proliferation and differentiation (Prabhakaran et al., 2011).
In brain glioma therapy, nanotechnology-based drug delivery systems (NDDS) play a crucial role in diagnosis and cancer treatment. These nanocarriers, loaded with anti-tumor drugs, effectively accumulate at the tumor site, enhancing chemotherapeutic effects and minimizing side effects through improved permeation and retention (EPR) effects or active targeting delivery (Agarwal et al., 2011; Koo et al., 2006; Yang, 2010). Nanocarrier strategies often target adsorptive mediated endocytosis (AME), carrier mediated transports (CMT) systems, and receptor-mediated endocytosis (RME) (Fig. 4) (Huang et al., 2007; Béduneau et al., 2007).
Illustration of(a) non-receptormediated endocytosis, (b) receptor mediated endocytosisand(c) endocytosis independent pathway of CNTs.
Ren and colleagues (2012) utilized a dual-targeting drug delivery method to treat brain glioma, employing oxidized MWNTs as carriers and angiopep-2 as a targeting ligand. This system, labeled with fluorescein Isothiocyanate (FITC) to determine intracellular distribution, demonstrated high biocompatibility and low toxicity while exhibiting superior anti-glioma effects compared to DOX alone (Ren et al., 2012).
Lu et al. (2012) developed a magnetic dual-targeted nanocarrier employing folate-conjugated magnetic multi-walled carbon nanotubes for cancer treatment. These nanotubes loaded with doxorubicin (DOX) showed increased cytotoxicity against U87 human glioblastoma cells compared to free DOX. The carriers facilitated intracellular release of DOX and its subsequent transport into the nucleus, suggesting promise for targeted DOX delivery in cancer treatment (Lu et al., 2012).
Functionalized CNTs have emerged as potential agents for treating infectious diseases like acute respiratory syndrome, leishmaniasis, tuberculosis, and flu due to their aptitude to conjugate with drugs such as amphotericin B, dapsone, etc. These conjugations enhance antimycotic efficiency and antimicrobial properties against bacteria like Escherichia coli, inducing oxidative stress that leads to cell death (Lim et al., 2003).
2.4 Huntington's disease (HD)
HD is an autosomal neuronal disorder distinguished by movement issues, behavioral disturbances, cognitive dysfunction, and psychiatric symptoms. It results from a mutation in the huntingtin gene located on chromosome 4p16. This mutation leads to CAG expansion within the gene, causing repeated glutamine development in the huntingtin protein.
The disorder primarily affects the striatum, globus pallidus, hypothalamus, amygdala, and nucleus accumbens, causing neuronal loss, particularly in the cortex and striatum, impacting neurons containing γ-aminobutyric acid and enkephalin (Arrasate et al., 2004; Rosas et al., 2003). Various neurotransmitters, including glutamate, dopamine, and γ-aminobutyric acid, are affected (Frank, 2014).
HD's pathogenesis involves multiple theories, such as oxidative damage, excessive glutamate activation of excitatory receptors leading to ROS production, mitochondrial dysfunction, toxic neuronal aggregates, excitotoxicity, transcriptional dysregulation, and dysfunction of synapticvesicles (Beal, 1996; Bence et al., 2001; Olney and Gubareff, 1978; Ravikumar et al., 2002).
The indications of Huntington's disease (HD) can appear anytime from 1 to 90 years of age, impacting both males and females equally. About 5–10 % of instances present with juvenile onset, with symptoms emerging before the age of 20 (Foroud et al., 1999; Ribaï et al., 2007). Symptoms like dystonia, epilepsy, speech changes, ataxia, bradykinesia, dysphagia, and upper motor neuron signs are more familiar in juvenile HD (Robertson et al., 2012).
Treatment strategies for Huntington's disease (HD) aim to alleviate symptoms, particularly chorea, and encompass medications such as dopamine antagonists, dopamine-depleting agents, dopamine agonists, benzodiazepines, glutamate antagonists, antiseizure prescriptions, lithium, cannabinoids, acetylcholinesterase inhibitors, deep brain stimulation, and fetal cell transplantation (Bagchi, 1983). These treatments aim to alleviate symptoms;however do not cure the fundamental cause of the disease.
2.5 Amyotrophic lateral sclerosis (ALS)
ALS, also acknowledged as Motor Neuron Disease, is an advancing and lethal neurodegenerative condition marked by the degeneration of upper and lower motor neurons within the brain and spinal cord (Brooks et al., 2000). This condition leads to weakness in limb and bulbar muscles, atrophy, weight loss, spasticity, and ultimately respiratory failure.
The global incidence of ALS is around 2 per 100,000 person-years, with an estimated 25,000 prevalent patients in North America alone (McGuire et al., 1996).
Regarding pharmacological therapy, Riluzole is the merely approved drug for the treatment of ALS in the United States, Australia, and many European countries (Miller et al., 2012). Riluzole is known to modestly extend survival by reducing the damage to motor neurons and is used as a standard medication for managing ALS symptoms. However, it doesn't completely arrest or overturn the progression of the disease.
2.6 Mechanism/pathways on fate of CNTs into brain
Safeguarding the structure and function of neurons, commonly referred to as neuroprotection, stands as a fundamental strategy in addressing a range of CNS disorders, including stroke, brain injuries and neurodegenerative diseases. The overarching objective is to avert or mitigate the loss of neurons. While the symptoms and presentations may vary, the commonality in the mechanisms underlying neurodegeneration is apparent across diverse CNS disorders (Seidl and Potashkin, 2011).
Neuroprotective drugs often target oxidative stress as well as excitotoxicity, which are linked to CNS disorders. The blend of these factors can cause neuronal damage. Hence, strategies to counteract these factors are crucial for neuroprotection. Neuroprotective drugs, such as glutamate antagonists and antioxidants, strive to mitigate excitotoxicity and oxidative stress, respectively (Zádori et al., 2012). Using these medications can shield neurons from degenerative conditions.
Specific medications, such as methylprednisolone, a steroid, have been shown to inhibit secondary impairment and decrease neuroinflammation following spinal cord injury. Moreover, Sygen® newer drug (Fidia SpA, San Mauro Torinese, Italy), alleviates the loss of nerve function (John et al., 2015).
Nanotechnology holds significant promise in treating both peripheral nervous system (PNS) and CNS disorders, offering broader treatment options for patients and clinicians. The progress in synthetic as well as characterization techniques across disciplines such as materials science and biology, combined with the evolution of nanoengineered applications in the nervous system, holds significant promise. Substantial research focusing on neurophysiology, neuropathology, and neurology will be pivotal in harnessing the benefits of nanotechnology.
The CNTs and graphene demonstratespotential various aspects of nanomedicine, particularly in neurology. Their applications in neuroregeneration and the restoration of neuronal cells are being extensively studied and hold potential for addressing neurological disorders.
2.7 CNTs in neuroregeneration
Neurological disorders pose a substantial challenge to human biological functions. While there has been considerable research and information regarding the therapeutic options available for the neurodegenerative disorders are still restricted, underscoring the need for continued investigation into the underlying mechanisms. Various conditions affecting the CNS, including AD, stroke, spinal cord injury, traumatic brain injury, and PD contribute to the dysfunction of spinal cord cells including brain (Nunes et al., 2012). The focalapproaches to facilitate self-repair of injured axonal connections include axonal regeneration and reconstruction of neuronal circuits. Effective regenerative engineering requires preserving neurons neuro-restoration (preserving neurons), creating a favorable environment for damaged neurogenesis (neuronal regrowth), and reconnecting neuronal circuits, while also enhancing the adaptability of neuronal tissue (neural plasticity) (Ellis-Behnke, 2007).
Research conducted by Zhang and colleagues showcased the electrical conductivity and strong mechanical characteristics of carbon nanotubes (CNTs), emphasizing the morphological resemblances between CNTs and neurons (Zhang and Webster, 2009). The similarity between CNTs and neuronal configurationsakin tocomponents of the neuronal cytoskeleton, signaling proteins, and ion channels may enhance molecular-level neuronal communication, potentially improving neuronal physiological function and information processing (Cellot et al., 2009). Studies conducted by Mattson and their team demonstrated the growth of hippocampal neurons in rats on 4-hydroxynonenal coated MWNTs, confirming the biocompatibility of CNTs as a potential candidate for neuroregeneration (Mattson et al., 2000).
The features and patterns of neuronal growth, encompassing elements for instance length, branching, and the abundance of growth cones, were impacted by distinct chemical alteration methods employed to functionalize CNTs. For instance, MWNTs coated with poly(ethylene glycol) diamine exhibited an augmentation in both the count of growth cones and neurite branches (Hu et al., 2005). Polyethylenimine (PEI)-functionalized SWNTs demonstrated an increased facilitation of neurite growth and branching when compared to the sole PEI substrate (Hu et al., 2005). Research conducted by Matsumoto and colleagues demonstrated that MWNTs, when coated by means of nerve growth factor or brain-derived neurotrophic factor, facilitated neuronal expansion on a CNT platform. Additionally, they exhibited influence over neuronal differentiation and viability (Matsumoto et al., 2007). Furthermore, investigations conducted by Lovat et al showcased improved dendrite elongation and increased adhesion of hippocampal neurons through the integration of purified multi-walled carbon nanotube (MWNT) substrates. This resulted in enhanced processing of neuronal signals (Lovat et al., 2005).
Further, similarly, research by Mazzatenta and colleagues proposed that purified SWNTs stimulate the hippocampal neuronal growth and support the neuronal circuit development. This enhanced electrical signal transmission within the neuronal network is pivotal for fostering significant growth in the neuronal circuit (Mazzatenta et al., 2007). Moreover, Jan and colleagues validated the suitability of CNT-substrates for NSCs (NSCs; neural stem cells), demonstrating favorable cell viability and neuronal process development comparable to that observed with the widely utilized poly-L-ornithine, agrowth substrate (Jan et al., 2009). The effectiveness of CNTs was evidenced by their ability to transport NSCs to affected regions of the CNS and facilitate their delineation into neurons.
Innovative and enhanced nano-scaffolds have been created/produced to mend or augment neural tissue function through the provision of mechanical support to neurons. The potential for neural tissue engineering is optimistic with CNTs owing to their advantageous electrical as well asmechanical properties, along with their compatibility with neuronal cells (Jan et al., 2009). Roman et al emphasized the usefulness of CNTs in addressing neurodegenerative diseases. In a rat model, PEG-modified single-walled nanotubes (SWNTs) demonstrated effectiveness for neuroregeneration. The CNT framework exhibited efficacy through a reduction in the damaged tissue and enhancement in neurofilament number near the injury site, and the restoration of functionality in the hind limb of rat. This report also highlights the potential of CNT-based substrates for fostering sustained restoration of impaired neural tissues and neurons, paving the way for new possibilities in the area of neuronal disorders and neuroregeneration (Roman et al., 2011).
2.8 CNTs in drug delivery
Nanodrug delivery holds promise for achieving neuroprotection in chronic neurological disorders by facilitating drug transport across the BBB. Nanotechnology applied in therapeutic approaches, known as nanomedicine, enables the delivery of drug to the brain. Recent investigations suggest that the amount of nanomedicine needed for neurodegenerative conditions is significantly lower than that required for cancer and infectious diseases (Sharma et al., 2013). Hence, the continuous advancement of nanotechnologies for creating delivery systems for neurotrophins holds potential for activating neurotrophin signaling, contributing to both neuro-regeneration and protection (Tan et al., 2012).
This approach represents a significant advancement in leveraging nanotechnology to target and support neuronal function and survival, offering potential avenues for neuroprotection and regeneration in neurological disorders.
Carbon nanotubes (CNTs) offer a promising avenue as delivery tools for treating CNS disorders owing to their exceptional properties, including improved solubility in physiological solvents, high surface area, amenability to modification with drug molecules, and compatibility with neural systems. In an application targeting Alzheimer's disease (AD), Zhang et al utilized SWNTs modified with acetylcholine for treatment. Through gastric gavage, doses of SWNTs up to 300 mg/kg facilitated drug delivery into neuronal lysosomes, signifying the effectiveness of this therapeutic approach (Yang et al., 2010).
The management of brain tumors poses ongoing challenges despite considerable progress in understanding carcinogenesis. The limited permeability of anti-tumor molecules of drug across the BBB has spurred investigations into strategies involving carbon nanotubes (CNTs). In a particular study, a drug delivery system employing CNTs was employed, markedly amplifying the impact of CpG oligodeoxynucleotide immunotherapy in treating gliomas (tumors originating from glial cells) and in tumor prevention (Zhao et al., 2011). This approach shows promise for addressing various neurodegenerative conditions.
Amine-functionalized SWNTs, created via amidation reaction, have shown potential in protecting neurons against ischemic damage without the need for therapeutic or drug molecules. This method enhances neuronal tolerance and facilitates the recovery of their functions. However, the precise mechanism by which amine-functionalized SWNTs protect neurons remains unclear. These resultsdraw attention to the potential of CNT-based therapies in protecting neurons and treating various neurological conditions.
Recent research has brought attention to the neurotoxic effects of environmental contaminants and pharmaceuticals on the nervous system (Ali-Boucetta and Kostarelos, 2013). Ethinylestradiol (EE2), a compound found in birth control pills, has been shown to disrupt nervous system expansion and swimming manners in zebrafish larvae, illustrating the impact of pharmaceutical pollutants on neural development (Nasri et al., 2021). Similarly, biopesticides and pesticides at nanomolar concentrations have demonstrated neurotoxicity in zebrafish, resulting in altered gene expression, neurochemical changes, and behavioral disturbances (Nasri et al., 2016). These findings highlight the need for advanced drug delivery systems like carbon nanotubes (CNTs), which offer the potential to minimize neurotoxicity by targeting specific brain regions and reducing off-target effects, thereby addressing the challenges in treating neurodegenerative disorders.
CNTs have demonstrated varying degrees of cytotoxicity depending on their functionalization, size, and concentration. Non-functionalized CNTs may trigger oxidative stress, inflammation, and cellular damage (Akturk, 2024). However, functionalized CNTs, which are modified to improve solubility, generally show better biocompatibility and reduced toxicity (Dubey et al., 2021). The distribution of CNTs within the body depends on their structural and chemical properties. Investigations have indicated that CNTs can accumulate in organs like the spleen lungs, and liver. To improve safety, surface modifications can enhance their clearance from the body through pathways such as renal excretion or biodegradation (Ali-Boucetta and Kostarelos, 2013). Prolonged exposure to CNTs raises concerns about chronic toxicity and immune responses. Research indicates that inhalation or high doses may cause fibrosis or granuloma formation, particularly in the lungs.
Therefore, more studies are needed to assess the long-term effects of CNTs in drug delivery applications (Ali-Boucetta and Kostarelos, 2013). The use of CNTs in clinical settings must adhere to regulatory safety standards. This includes thorough toxicity assessments to identify safe dosage levels and ensure minimal risks to patients.
3 Conclusion
In conclusion, carbon nanotubes (CNTs) offer significant translational potential as innovative DDS for the treatment of ND disorders. Their distinct physicochemical and structural features namely their large surface area, substantial mechanical strength, and BBB-crossing capability —position CNTs as impendingcandidates for targeted and efficient drug delivery to the brain. Furthermore, the functionalization of CNTs through diverse chemical modification techniques has enhanced their biocompatibility and drug-carrying capabilities, allowing them to triumph over a number of traditional challenges alliedby way of crossing the BBB.
Preclinical studies have demonstrated CNTs' potential to improve neuronal growth and survival in neurodegenerative disease models, highlighting their role not only as drug carriers but also as agents that can promote neural regeneration. However, quite a few challenges ought to be addressed prior to CNT-based systems can be widely adopted in clinical applications. These challenges include optimizing biocompatibility, minimizing toxicity, ensuring controlled biodistribution, and achieving consistent clearance from the body. Additionally, the regulatory hurdles associated with introducing new nanomaterials into therapeutic use must be overcome.
Future research should focus on refining functionalization strategies, conducting comprehensive long-term toxicity studies, and addressing the pharmacokinetics and biodistribution challenges that are critical for clinical translation. CNTs' versatility in promoting neural cell growth and supporting neuroregenerative processes underscores their potential to revolutionize drug delivery for neurodegenerative disorders, offering a promising approach to overcoming the barriers of traditional therapies and improving patient outcomes. By addressing these challenges, CNTs may offer aunsullied frontier in the management and treatment of neurodegenerative diseases.
Credit authorship contribution statement
Mohammed Al-zharani: Writing – original draft, Supervision, Resources, Methodology, Funding acquisition, Conceptualization. Md Saquib Hasnain: Writing – review & editing, Visualization, Validation, Supervision, Methodology, Conceptualization. Mohammed S. Al-Eissa: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Funding acquisition, Conceptualization. Reem A. Alqahtani: Writing – original draft, Visualization, Validation, Formal analysis, Data curation.
Acknowledgements
The authors extend their appreciation to the King Salman Center for Disability Research for funding this work through Research Group No. KSRG-2023-163.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006
- [CrossRef] [Google Scholar]
- Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev. Mol. Med. 2011
- [CrossRef] [Google Scholar]
- Akturk O., 2024. Biocompatibility, Toxicity, and Immunological Effects of Functionalized Carbon Nanostructures, in: Handbook of Functionalized Carbon Nanostructures. doi: 10.1007/978-3-031-14955-9_73-1.
- Blood–brain barrier: emerging trends on transport models and new-age strategies for therapeutics intervention against neurological disorders. Mol. Brain 2022
- [CrossRef] [Google Scholar]
- Pharmacology of carbon nanotubes: toxicokinetics, excretion and tissue accumulation. Adv. Drug Deliv. Rev. 2013
- [CrossRef] [Google Scholar]
- A photoelectrochemical immunosensor based on au-doped TiO2 nanotube arrays for the detection of α-synuclein. Chem. A Eur. J.. 2010;16
- [CrossRef] [Google Scholar]
- Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 2004
- [CrossRef] [Google Scholar]
- Functionalized carbon nanotubes for biomedical applications. Functionalized Carbon Nanotubes for Biomedical Applications 2023
- [CrossRef] [Google Scholar]
- Differential interactions of phencyclidine with tetrabenazine and reserpine affecting intraneuronal dopamine. BiochemPharmacol.. 1983;32
- [CrossRef] [Google Scholar]
- Dopamine-, L-DOPA-, adrenaline-, and noradrenaline-induced growth of Au nanoparticles: assays for the detection of neurotransmitters and of tyrosinase activity. Anal Chem.. 2005;77
- [CrossRef] [Google Scholar]
- Mitochondria, free radicals, and neurodegeneration. CurrOpinNeurobiol.. 1996;6
- [CrossRef] [Google Scholar]
- Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;1979:292.
- [CrossRef] [Google Scholar]
- Liposomes functionalized with acidic lipids rescue Aβ-induced toxicity in murine neuroblastoma cells. Nanomedicine. 2011;7
- [CrossRef] [Google Scholar]
- Bisgaier, C.L., Newton, R.S., 2001. Methods For Treating Alzheimer’s Disease. WO Patent WO/2001/056,579.
- Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol. Lett.. 2006;160
- [CrossRef] [Google Scholar]
- El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler.. 2000;1
- [CrossRef] [Google Scholar]
- Highly efficient molecular delivery into mammalian cells using carbon nanotube spearing. Nat. Methods. 2005;2
- [CrossRef] [Google Scholar]
- Carbon nanotubes might improve neuronal performance by favouring electrical shortcuts. Nat. Nanotechnol.. 2009;4
- [CrossRef] [Google Scholar]
- Effect of single wall carbon nanotubes on human HEK293 cells. Toxicol. Lett.. 2005;155
- [CrossRef] [Google Scholar]
- Electronic, thermal and mechanical properties of carbon nanotubes. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2004
- [CrossRef] [Google Scholar]
- Functionalized carbon nanotubes: synthesis, properties and applications in water purification, drug delivery, and material and biomedical sciences. Nanoscale. Adv. 2021
- [CrossRef] [Google Scholar]
- Ellis-Behnke, R., 2007. Nano Neurology and the Four P’s of Central Nervous System Regeneration: Preserve, Permit, Promote, Plasticity. Medical Clinics of North America. doi: 10.1016/j.mcna.2007.04.005.
- Feng, L., Li, S., Xiao, B., Chen, S., Liu, R., Zhang, Y., 2010. Fluorescence imaging of APP in Alzheimer’s disease with quantum dot or Cy3 : A comparative study. Journal of Central South University (Medical Sciences) 35.
- Gram-scale CCVD synthesis of double-walled carbon nanotubes. Chem. Commun.. 2003;3
- [CrossRef] [Google Scholar]
- Carbon nanotubes as functional excipients for nanomedicines: I. pharmaceutical properties. Nanomedicine 2008
- [CrossRef] [Google Scholar]
- Differences in duration of Huntington’s disease based on age at onset. J. Neurol. Neurosurg. Psychiatry. 1999;66
- [CrossRef] [Google Scholar]
- Carbon nanotube delivery of the GFP gene into mammalian cells. Chembiochem.. 2006;7
- [CrossRef] [Google Scholar]
- Nanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer’s disease. Proc. Natl. Acad Sci. U S A. 2005;102
- [CrossRef] [Google Scholar]
- Static and dynamic magnetic properties of spherical magnetite nanoparticles. J. Appl. Phys.. 2003;94
- [CrossRef] [Google Scholar]
- Carbon nanotubes-based drug delivery to cancer and brain. J. Huazhong University of Sci. Technol - Medical Sci.. 2017;37
- [CrossRef] [Google Scholar]
- The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005
- [CrossRef] [Google Scholar]
- Single-walled carbon nanotube spectroscopy in live cells: towards long-term labels and optical sensors. Adv. Mater.. 2005;17
- [CrossRef] [Google Scholar]
- Polyethyleneimine functionalized single-walled carbon nanotubes as a substrate for neuronal growth. J. Phys. Chem. B. 2005;109
- [CrossRef] [Google Scholar]
- Gene therapy using lactoferrin-modified nanoparticles in a rotenone-induced chronic Parkinson model. J. Neurol. Sci.. 2010;290
- [CrossRef] [Google Scholar]
- Efficient gene delivery targeted to the brain using a transferrin-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. FASEB J.. 2007;21
- [CrossRef] [Google Scholar]
- Nano-aggregates of single-walled graphitic carbon nano-horns. Chem. Phys. Lett.. 1999;309
- [CrossRef] [Google Scholar]
- Layered carbon nanotube-polyelectrolyte electrodes outperform traditional neural interface materials. Nano Lett.. 2009;9
- [CrossRef] [Google Scholar]
- Carbon nanotubes and graphene as emerging candidates in neuroregeneration and neurodrug delivery. Int. J. Nanomedicine 2015
- [CrossRef] [Google Scholar]
- Electronic structure and chemical reactivity of carbon nanotubes: a Chemist’s view. ChemPhysChem. 2004
- [CrossRef] [Google Scholar]
- Brain cancer diagnosis and therapy with nanoplatforms. Adv. Drug Deliv. Rev. 2006
- [CrossRef] [Google Scholar]
- Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat. Nanotechnol.. 2007;2
- [CrossRef] [Google Scholar]
- Carbon nanotubes as nanomedicines: from toxicology to pharmacology. Adv. Drug Deliv Rev. 2006
- [CrossRef] [Google Scholar]
- Selection of quantum dot wavelengths for biomedical assays and imaging. Mol. Imaging. 2003;2
- [CrossRef] [Google Scholar]
- Carbon nanotube substrates boost neuronal electrical signaling. Nano. Lett.. 2005;5
- [CrossRef] [Google Scholar]
- Dual targeted delivery of doxorubicin to cancer cells using folate-conjugated magnetic multi-walled carbon nanotubes. Colloids Surf B Biointerfaces. 2012;89
- [CrossRef] [Google Scholar]
- Neurite outgrowths of neurons with neurotrophin-coated carbon nanotubes. J. BiosciBioeng.. 2007;103
- [CrossRef] [Google Scholar]
- Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J. Mol. Neurosci.. 2000;14
- [CrossRef] [Google Scholar]
- Interfacing neurons with carbon nanotubes: electrical signal transfer and synaptic stimulation in cultured brain circuits. J. Neurosci.. 2007;27
- [CrossRef] [Google Scholar]
- Relationship of the Tufts Quantitative Neuromuscular Exam (TQNE) and the Sickness Impact Profile (SIP) in measuring progression of ALS. Neurology. 1996;46
- [CrossRef] [Google Scholar]
- Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Cochrane Database Syst. Rev. 2012
- [CrossRef] [Google Scholar]
- Advances in the treatment of neurodegenerative disorders employing nanotechnology. Ann. N Y Acad Sci. 2010
- [CrossRef] [Google Scholar]
- Fracture faces of cell junctions in cerebral endothelium during normal and hyperosmotic conditions. Lab. Invest.. 1984;50
- [Google Scholar]
- Neurotoxicity of a biopesticide analog on Zebrafish larvae at nanomolar concentrations. Int. J. Mol. Sci.. 2016;17
- [CrossRef] [Google Scholar]
- Ethinylestradiol (EE2) residues from birth control pills impair nervous system development and swimming behavior of zebrafish larvae. Sci. Total Environ.. 2021;770
- [CrossRef] [Google Scholar]
- Application of carbon nanotubes in neurology: Clinical perspectives and toxicological risks. Arch. Toxicol. 2012
- [CrossRef] [Google Scholar]
- Further studies of the interaction of hydrogen with graphite nanofibers. J. Phys. Chem. B. 1999;103
- [CrossRef] [Google Scholar]
- Electrospun composite nanofibers for tissue regeneration. J. NanosciNanotechnol. 2011
- [CrossRef] [Google Scholar]
- Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum. Mol. Genet. 2002;11
- [CrossRef] [Google Scholar]
- The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials. 2012;33
- [CrossRef] [Google Scholar]
- Psychiatric and cognitive difficulties as indicators of juvenile Huntington disease onset in 29 patients. Arch. Neuro.l. 2007;64
- [CrossRef] [Google Scholar]
- Current pharmacological management in Juvenile Huntington’s disease. PLoSCurr. 2012
- [CrossRef] [Google Scholar]
- Single-walled carbon nanotubes chemically functionalized with polyethylene glycol promote tissue repair in a rat model of spinal cord injury. J. Neurotrauma.. 2011;28
- [CrossRef] [Google Scholar]
- Evidence for more widespread cerebral pathology in early HD: an MRI-based morphometric analysis. Neurology. 2003;60
- [CrossRef] [Google Scholar]
- Physical properties of carbon nanotubes. Physical Properties of Carbon Nanotubes 1998
- [CrossRef] [Google Scholar]
- The promise of neuroprotective agents in Parkinson’s disease. Front Neurol. NOV 2011
- [CrossRef] [Google Scholar]
- Novel therapeutic strategies using nanodrug delivery, stem cells and combination therapy for CNS trauma and neurodegenerative disorders. Expert Rev. Neurother. 2013
- [CrossRef] [Google Scholar]
- Nanotechnology - novel therapeutics for CNS disorders. Nat. Rev. Neurol. 2012
- [CrossRef] [Google Scholar]
- Nanoporous peptide particles for encapsulating and releasing neurotrophic factors in an animal model of neurodegeneration. Adv. Mater.. 2012;24
- [CrossRef] [Google Scholar]
- Characterization and evaluation of chitosan nanoparticles for dopamine brain delivery. Int. J. Pharm.. 2011;419
- [CrossRef] [Google Scholar]
- Nanoparticle-mediated brain-specific drug delivery, imaging, and diagnosis. Pharm Res. 2010
- [CrossRef] [Google Scholar]
- Detection of amyloid plaques targeted by USPIO-Aβ1-42 in Alzheimer’s disease transgenic mice using magnetic resonance microimaging. Neuroimage. 2011;55
- [CrossRef] [Google Scholar]
- Pharmacological and toxicological target organelles and safe use of single-walled carbon nanotubes as drug carriers in treating Alzheimer disease. Nanomedicine. 2010;6
- [CrossRef] [Google Scholar]
- Mitochondrial disturbances, excitotoxicity, neuroinflammation and kynurenines: novel therapeutic strategies for neurodegenerative disorders. J. Neurol. Sci.. 2012;322
- [CrossRef] [Google Scholar]
- Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today 2009
- [CrossRef] [Google Scholar]
- Carbon nanotubes enhance CpG uptake and potentiate antiglioma immunity. Clin. Cancer Res.. 2011;17
- [CrossRef] [Google Scholar]







