Translate this page into:
Neuroprotective effect of huperzine-A against cadmium chloride-induced Huntington's disease in Drosophila melanogaster model
⁎Corresponding author. drsubaraja89@gmail.com (Mamangam Subaraja)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
Huntington's disease is an incurable neurodegenerative disease caused by a huntingtin gene defect; it causes changes in the brain, which affect movement and mental functions. The molecular mechanism still challenges the research to find the treatment of Huntington's disease. The present study aims to develop the neuroprotective potential of huperzine-A against Huntington's disease through in-silico, in-vivo, and in-vitro approaches.
Methods
The preliminary screening of the huperzine-A, linalool, norsanguinine, tumerone, ungeremine, tetrabenazine, and astragalin ligands that interact with neuropathological proteins was performed by Auto Dock. In further studies, the biochemical and m-RNA levels of huperzine-A against cadmium chloride-induced in-vitro and in-vivo models were carried out.
Results
The interaction of huperzine-A with huntingtin has shown a binding energy of −7.85 kcal/mol. The level of wnt3a and CAMK2A were significantly increased (*p ≥ 0.05), and superoxide dismutase and catalase activities were significantly reduced (*p ≥ 0.05) in cadmium chloride-treated SH-SY5Y cells when compared to that of the control group. The dopamine and serotonin levels were significantly increased (*p ≥ 0.05), and the neuronal behavior pattern was significantly altered in the cadmium chloride-induced group compared to control flies.
Conclusions
These results indicate the neuroprotective effect of huperzine-A against cadmium chloride-induced degeneration in Drosophila melanogaster and huperzine-A could minimize neurodegenerative symptoms like − Huntington's disease.
Keywords
Huntington's disease
Huperzine-A
Cadmium chloride
In-silico
SH-SY5Y cells and Drosophila melanogaster
- HD
-
Huntington's disease
- Cd
-
Cadmium
- HA
-
Huperzine-A
- ROS
-
Reactive oxygen species
- MAO-A
-
Monoamine oxidase A
- MAO-B
-
Monoamine oxidase B
Abbreviations
1 Introduction
Huntington's disease is a fatal neurodegenerative disorder characterized by motor incoordination, cognitive dysfunction, and neuropsychiatric problems that progress to dementia and mortality (Peavy et al., 2010). It is one among the group of neurodegenerative diseases caused by mutations of the huntingtin gene encoded in the polyglutamine protein due to an enlarged CAG trinucleotide repeat tract (Rawlins et al., 2016). In 2020, the estimated Huntington's disease incidence rate was 2.71 per 100,000 worldwide (Yohrling et al., 2020; Pringsheim et al., 2012). It is expected to affect 365 million in 2022 and 1517 million in 2030 (Bunting et al., 2022; Dorsey, 2012). Gamma-aminobutyric neurotransmitters are involved in motor and cognitive function. The imbalance between these neurotransmitters leads to Alzheimer’s disease, Parkinson’s disease, and Huntington's disease (André et al., 2010). Acetylcholine is a cholinergic neurotransmitter that regulates neuronal behaviors like cognitive and locomotion functions. Acetylcholine esterase is cholinergic at the postsynaptic neuromuscular junction. The accumulation of acetylcholine esterase in the brain could lead to Alzheimer’s disease, Parkinson's disease, and Huntington's disease (Prado et al., 2017).
The air and soil are major sources of cadmium contamination in the environment. Cadmium is a neurotoxin, and it can easily cross the blood–brain barrier (BBB), causing neuronal death and neurodegeneration (Kamitsuka et al., 2023). Additionally, cadmium inhibits the catalase, superoxide dismutase, and glutathione peroxidase activities, enhancing the levels of reactive Oxygen Species (ROS) and lipid peroxidation leads to neurodegeneration (Cuypers et al., 2010). Huntingtin is important in protein trafficking, vesicle transport, postsynaptic signaling, and transcriptional regulation. Accordingly, a deficiency of the huntingtin protein and the mutant huntingtin causes an interruption in intracellular pathways. Moreover, excitotoxicity, dopamine toxicity, metabolic impairment, mitochondrial dysfunction, and oxidative stress have been implicated in Huntington's disease (Caviston and Holzbaur, 2009; Cowan and Raymond,2006). Excessive formation of reactive oxygen species in cells can damage cellular proteins, lipids, and DNA, leading to neurodegeneration (Unsal et al., 2020).
Molecular docking is a“lock and key” hypothesis used to discover the best-fit orientation of ligands and proteins (Tripathi and Bankaitis, 2017; Guedes et al., 2014). Molecular docking studies have recently been used to predict potential inhibitors of neurodegenerative diseases like Parkinson's features (Subaraja et al., 2020). The fruit fly is an excellent model organism in neuroscience research. 75 % of fly genes are similar to humans (Pandey and Nichols, 2011; Bilen and Bonini, 2005). However, Huperzine-A is a sesquiterpene alkaloid that crosses the blood–brain barrier. Huntington's disease therapy has not yet been found for the treatment of patients (Friedli and Inestrosa, 2021). Thus, the objective study was to find out the targeted therapy of neurodegeneration like −Huntington's disease based on the analyses of In-silico, In-vitro, and In-vivo studies.
2 Material and methods
2.1 Preparation of proteins
The crystal structure of proteins were retrieved from the protein databank (PDB).
2.2 Preparation of ligand
The seven compounds, namely astragalin, linalool, tumerone, tetrabenazine, ungeremine, huperzine-A and norsanguinine structure data file (SDF) were retrieved from the PubChem database.
2.3 Molecular docking analysis
Further, the In-Silico study was analyzed by using Auto Dock version 4.2. The binding interaction and energy between proteins and ligands were obtained, and the docked complexes were analyzed.
2.4 In-vitro studies
2.4.1 Cells maintenance and differentiation
Human neuroblastoma SH-SY5Y cells were pouched from the National Centre for Cell Science, Pune, India. Cells were cultivated in 75 cm2 flasks with Dulbecco's modified eagle medium (DMEM) containing 25 mM glucose, 5 % of fetal bovine serum (FBS), 100 µg/ml of penicillin, 20 µg /ml of streptomycin, and 200 mM of glutamine and incubated for 5 % CO2 at 37 °C and then passed at 80 % confluence of SH-SY5Y cells. The culture media was changed every two days, and cells were sub-cultured by trypsin treatment.
2.4.2 Assessment of cytotoxicity assay
To study the toxicity of cadmium chloride and huperzine-A, cells in the medium were incubated with various concentrations of cadmium chloride (5, 10, 50, 100, and 200 nM) and huperzine-A (5, 10, 20, 50, 100, 200, 500 nM and 1 µM) for 24 hr. To examine the neuroprotective effect of huperzine-A, SH-SY5Y cell was pre-treated with different concentrations of huperzine-A (5, 10, 20, and 50 nM) for 24hr. Then the cells were incubated with 150 µl of (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) for 3hr. The absorbance was read at 570 nm. Data were expressed as a percentage compared to control.
2.4.3 Experimental design
SH-SY5Y neuroblastoma cells were divided into five experimental groups following:
Group1: Untreated control cell
Group2: Cells were incubated with 100 nM of cadmium chloride
Group3: Cells were treated to 100 nM of cadmium chloride with 50 nM of huperzine –A
Group4: Cells were treated with 50 nM of huperzine –A
Group 5: Cells were treated with tetrabenazine
Therefore, after experimental periods of 24 hr and biochemical plus mRNA were analyzed.
2.4.4 Lactate dehydrogenase (LDH) assay
After the treatment, the extracellular media was collected and centrifuged at 1789g at 37 °C for 5 min. Then 20 µl of supernatant, 20 µl of 2,4-dinitrophenylhydrazine and 250 µl of sodium hydroxide was mixed and incubated for15 min at 37 °C.The absorbance was read at 630 nm.
2.4.5 Reactive oxygen species (ROS) production
After the treatment, cells were washed with phosphate buffer saline (pH-7.4) and incubated with 10 µl of dichloro-dihydro-fluorescein diacetate for 30mins at 37°Cin the dark. The fluorescence emission intensity was read at 488 nm and expressed as the % of control cells.
2.4.6 Malondialdehyde, superoxide dismutase, catalase, and glutathione peroxidase
After the treatment, cells were digested with trypsin and centrifuged at 112g for 5 min at 37 °C. The cells were suspended in 500 µl of phosphate buffer saline and lysed by sonication in protease inhibitor, followed by centrifugation at 1789g for 15 mins at 37 °C, and the supernatant was collected for assays. The estimation of lipid peroxidation (Ayna, 2020), superoxide dismutase (Nebot et al., 1993), catalase (Beers and Sizer, 1952), and glutathione peroxidase (Paglia and Valentine, 1967) were done.
2.4.7 Extraction of RNA, reverse transcription and quantitative real-time PCR
After the treatments, total RNA was extracted from SH-SY5Y cells using the TRIzol® reagent method. The RNA to cDNA synthesized was performed (iScript™cDNA synthesis kit). The PCR reactions were as follows: The initial polymerase activation step at 95 °C for 2 min, denaturation of 40 cycles at 95 °C for 5 sec, annealing at 60 °C for 5 sec, and extension 60 °C for 30 sec.
2.5 In-vivo study
2.5.1 Maintenance and husbandry of drosophila melanogaster
Drosophila melanogaster (Oregon-R Stains) were maintained on a standard diet containing 100 g of wheat flour, 100 g of Jaggery, 100 of Agar agar, and 7.5 ml of Propionic acid and Yeast granules at 24 ± 2 °C and 60–80 % humidity, on a 12 h: 12 h light: dark cycle.
2.5.2 Cadmium chloride concentration induced Drosophila melanogaster
Drosophila were exposed to various concentrations of cadmium chloride (0, 2, 4, 6,8,10 mM) in the diet for 24 and 48 h to validate lethality. Flies were monitored for the incidence of movement dysfunction and mortality rates. Based on the data screening, an 8 mM concentration of cadmium chloride was chosen as suitable for induction. Flies were exposed to cadmium chloride on movement dysfunction and lethality for seven days. The effects were assessed by treating with 8 mM cadmium chloride of D. melanogaster, and lethality and movement dysfunction were recorded for 24 and 48 h(15 flies per group / three replicate).
2.5.3 Huperzine-A concentration for dietary supplement in flies
The fly diet was supplemented with 0.01, 0.04, 0.2, 0.4, 0.6, 0.8, and 1 mM of Huperzine-A, and the survival rates were chosen (0.2, 0.4, and 0.6 mM of huperzine-A) the survival rates of the compared between control and treated flies. The three concentrations of huperzine-A represent low and high concentrations to determine the neuroprotective effect of huperzine-A in the movement behavior of flies. The high concentration of huperzine-A (0.8 and 1 mM) shows the neuroprotection effects for 7 days. Hence, 0.6 (mM) was chosen as an ideal concentration of huperzine-A for 24 and 48 hr (15 flies per group / 3 replicate).
2.6 Experimental designs
For the experiments, flies were divided into five groups of twenty-five flies, and the experimental groups were as follows:
Group 1:Flies were served as the medium
Group 2: Flies were treated with cadmium chloride
Group 3: Flies were treated with cadmium chloride and huperzine-A
Group 4: Flies were treated with huperzine-A
Group 5: Flies were treated with tetrabenazine
All experiments were conducted for a period of five days; flies were assessed for behavior assays after which the brain was removed and subjected to biochemical assays.
2.6.1 Climbing assay
The locomotor ability of flies was assessed by their ability to climb up a vertical glass tube (2.2 cm diameter). Flies were gently tapped to the bottom of the tube. At the end of each trial, the number of flies that could climb 8 cm within 10 sec was recorded. Each trial was performed thrice, and flies were tested from each group (15 Flies).
2.6.2 Rough eye phenotype assessment
After the treatment, the external surface of the fly eye was examined, and localization was analyzed by light microscopy (15 flies /3 replicated). The image J software was used to measure the phenotypic score from the disordered ommatidia in the fly eye. The eye phenotype area score was calculated.
2.6.3 Feeding assay
After the treatment, flies were transferred to blue yeast paste (2.5 % w/v) and allowed to feed for 5hrs. After feeding, each group of flies was washed in water and dried. Flies were homogenized with phosphate buffer saline (pH-7.4), centrifuged at 1370g for 10 min at 37 °C, and absorbance was read at 625 nm. The labeled food on the fly was calculated from a standard curve.
2.6.4 Biochemical assay
After treatment, the flies' brains were removed after anesthetization, and the brains were pooled. Brain samples were homogenized with phosphate buffer solution (pH- 7.4) and centrifuged at 112g for 5 min at 4 °C, and the supernatant was used for Biochemical assay. Acetylcholine esterase activity was determined by the method of Ellman et al., 1961. The protein level was determined by the method (Lowry et al., 1951) using Bovine serum albumin (BSA) standard.
2.6.5 Neurotransmitters assays
After the experiment, the whole brain was dissected. Brain tissue was homogenized in 5 ml hydrochloric acid-butanol-heptane for 1 min and then centrifuged at 447g for 10 min. The method estimated the serotonin content (Schlumpf et al., 1974). The content of dopamine was determined by the method (Dilip Kumar Pal et al., 2009).
2.6.6 Extraction of RNA, reverse transcription and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated using the TRIzol® reagent method (Thermo Fisher Scientific). The qRTPCR reactions were performed using the relative standard curve method to analyze the SYBR® master mix with Rox dye. The following PCR conditions were set: Reverse Transcription of 1 cycle at 61 °C for 20 sec, denaturation of 1 cycle at 95 °C for 1 min, and annealing of 45 cycles at 95 °C for 5 sec, followed by extension at 72 °C for 20 sec.
2.6.7 Histopathological examination
Drosophila tissues were incubated in 8 % paraformaldehyde (w/v) for 8 hrs. After fixation, tissues were rinsed in PBS for 10 min at 37 °C. The tissue was dehydrated. Blocks were mounted and sectioned on a microtome. The sections were stained with Ehlich’s Haemotoxylin and Eosin for 2 min. Finally, the slides were observed under the light microscope.
2.7 Data analysis
Statistical analysis is reported as mean ± standard error of the mean (SEM) using GraphPad Prism software (Version 5.0) followed by one-way analysis of variance (ANOVA) with a Tukey's test of multiple comparisons to examine significance between groups. The significance was set as p < 0.05.
3 Results
3.1 In-silico studies
The active site of glutamate receptor bound to huperzine-A interacting with VAL227, ILE125, ILE149 and LEU143; bound to linalool interacting with ARG108, LEU102, THR103 SER158, THR159, GLU209, LEU154, and TYR73; bound to norsanguinine interacting with TYR73, LEU102, LEU154, LEU208, GLU209, and TYR206; bound to tetrabenazine interacting with LEU102, TYR73, GLU209, LEU208, TYR206, and LEU154; bound to tumerone interacting with ARG108, TYR73, THR103, and GLU209; bound to ungeremine interacting with HIS170, VAL201, PHE202, TRP65, TRP278, GLU251 and ILE276; and bound to astragalin by interacting with ARG108, TYR73, THR103, GLU209, and LEU154 with a binding energy of −7.20, −6.11, −7.30, −6.73, −5.82, −6.30, and −4.77, respectively (Fig. 1).The huntingtin protein bound to huperzine-A interacting with LYS2236, TYR2576, and HIS2574; that is bound to linalool interacting with HIS3077, VAL3078, ASP3089, and TRP 3056; bound to norsanguinine interacting with ASN2039, LEU2038, and MET2035; bound to tetrabenazine interacting with ALA2571, PRO2392, ARG2395, ILE2399, and ARG2395; bound to tumerone interacting with THR2068, ARG2065, PHE2064, LEU2038, and MET2030; bound to ungeremine interacting with ASP2542, LEU2538, PRO2537, ARG2886, and LEU2601; bound to astragalin by interacting with LYS1763, GLN1770, VAL1668, SER1669, and GLN395 with the binding energy of −7.85, −5.49, −6.33, −4.74, −6.06, −5.88, −4.40, respectively (Fig. 2). The monoamine oxidase-A bound to huperzine-A interacting with TYR396, ARG40, LYS395, VAL292, TRP386, PHE341 and LYS294; bound to linalool interacting with ARG40, ILE12 and ALA437; bound to norsanguinine interacting with ALA33, VAL233, LEU31 and ILE262; bound to tetrabenazine interacting with TYR396, ILE169,TRY433 and ILE324; bound to tumerone interacting with GLY432, ILE12, CYS395 and ARG40; bound to ungeremine interacting with VAL233 and GLU32; it bound to astragalin by interacting with TYR110, LEU484 and TRP480 with binding energy-7.30, −5.73. −6.86, −6.79, −6.56, −6.62, and −3.01, respectively (Fig. 3). Monoamine oxidase −B proteins bound to huperzine-A interacting with TYR393, ALA35, GLY12, VAL10, VAL235, LYS271, and HIS73; bound to linalool interacting with VAL235, ALA35, HIS273, VAL10, and ILE264; bound to norsanguinine interacting with HIS273, VAL235, and LEU33; bound to tetrabenazine interacting with TYR60, TYR398, CYS172, TYR435, GLY434, and MET436; bound to tumerone interacting with LYS271, ILE264, VAL10, VAL235 and ALA35; bound to ungeremine interacting with VAL235 andGLU34; and it bound to astragalin by interacting with TYR337, TYR301,GLN49, and VAL381 with binding energy −7.58, −5.78, −7.31, −7.20, −7.11, −6.87, and −3.26 respectively (Fig. 4). The binding active site in neuropathological proteins such as the GABA receptor, dopamine receptor, muscarinic acetylcholine receptor, Serotonin receptor, parkin proteins, alpha-synuclein proteins, amyloidal precursor protein, amyloid beta-protein, tau protein, beta secretase interacting with huperzine-A, linalool, norsanguinine, tumerone, ungeremine, tetrabenazine, and astragalin (Figs. 1-10 supporting information). The docking binding energy of neuropathological proteins was presented in Tables 1–14 (supporting information).
Amino acids residues of glutamate receptors interacting with the (A) Huperzine-A, (B) Linalool, (C) Norsanguinine, (D) Tetrabenazine, (E) Tumerone, (F) Ungeremine and (G) Astragalin.
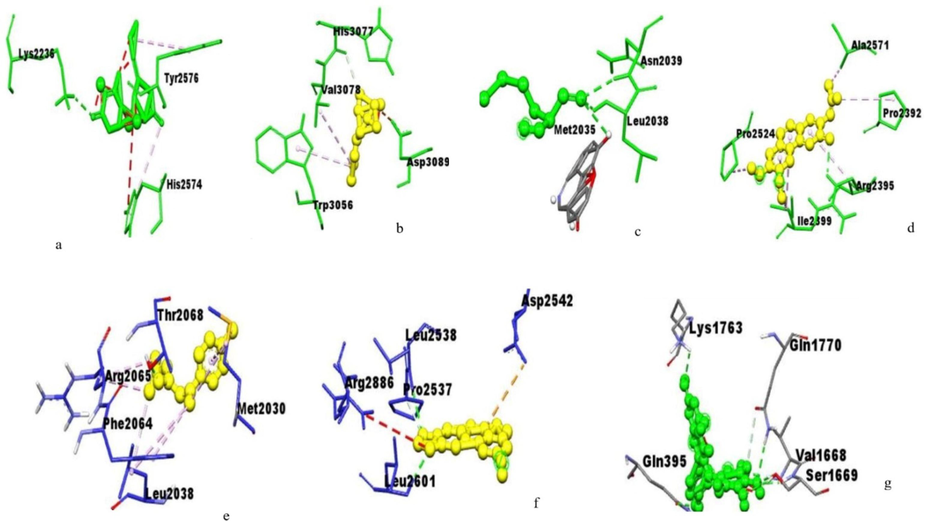
Amino acids residues of huntingtin protein interacting with the (a) HuperzineA,(b) Linalool,(c) Norsanguinine, (d) Tetrabenazine, (e) Tumerone, (f) Ungeremine and (g) Astragalin.
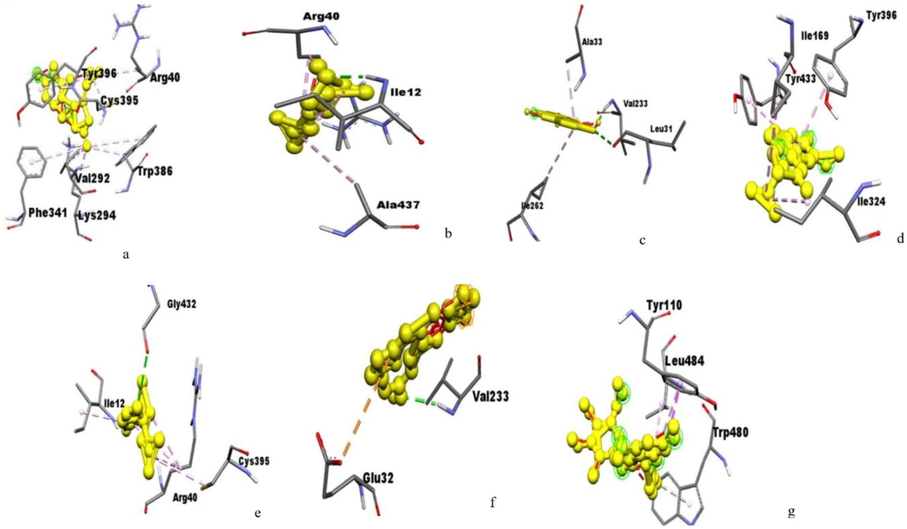
Amino acids residues of monoamine oxidase A interacting with the (a) Huperzine, (b) Linalool,(c) Norsanguinine,(d) Tetrabenazine,(e) Tumerone,(f) Ungeremineand (g) Astragalin.
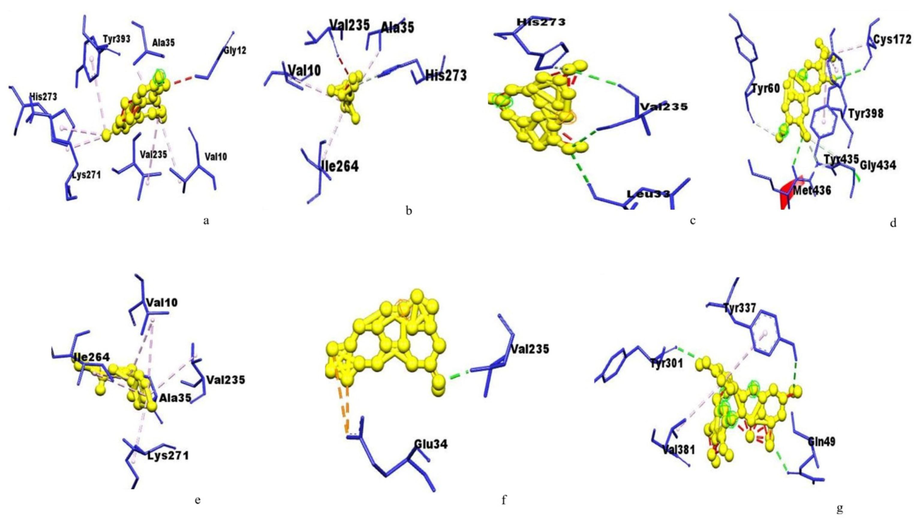
Amino acids residues of monoamine oxidase B interacting with the (a) Huperzine,(b) Linalool,(c) Norsanguinine,(d) Tetrabenazine,(e) Tumerone,(f) Ungeremineand (g) Astragalin.
3.2 In-vitro studies
3.2.1 Effect of Huperzine-A and cadmium chloride on the survival of SH-SY5Y cells
We initially evaluated the data of huperzine-A alone or cadmium chloride alone exposure to the SH-SY5Y cell line. Cells were treated with various concentrations of huperzine-A (5 nM, 10 nM, 20 nM, 50 nM, 100 nM, 200 nM, 500 nM and 1 μM) and cadmium chloride (5 nM, 10 nM, 50 nM, 100 nM and 200 nM) for 24 h and cell survival (Fig. 11 a and b Supporting information). The 50 nM concentrations of huperzine-A in the SH-SY5Y cell viability were observed. However, cell viability was slightly decreased at 100 nM, and highly toxic cell viability was observed in 200 nM, 500 nM, and 1 μM huperzine-A treatment cells. Cadmium chloride treatment (5 nM, 10 nM, 50 nM, 100 nM, and 200 nM for 24 h) showed a dose-dependent change in cell viability, and lethal dose (LD50) was observed at 100 nM. Therefore, cytotoxic exposure to 100 nM cadmium chloride for 24 h was a suitable concentration for further experiments.
3.2.2 Huperzine-A protects against cadmium-induced cytotoxicity
The neuroprotective effect of huperzine-A against cadmium chloride-induced toxicity with cell viability increased (86 ± 3.97 %) that of control in the presence of 50 nM of huperzine-A. Thus, based on the dose-dependent result, 50 nM huperzine-A and 100 nM cadmium chloride treatments were suitable concentrations for further experiments (Fig. 12 a, b, and c Supporting information).
3.2.3 Effect of huperzine-A on the level of biochemical changes in SH-SY5Y cells
The levels of ROS and LDH activity were significantly increased (p < 0.05) in cadmium chloride-treated cells when compared to control cells (Fig. 5 a and b). The levels of MAD, CAT, SOD, and GPx were significantly altered (p < 0.05) in cadmium chloride-treated cells when compared to control cells (Fig. 6a, b, c, and d).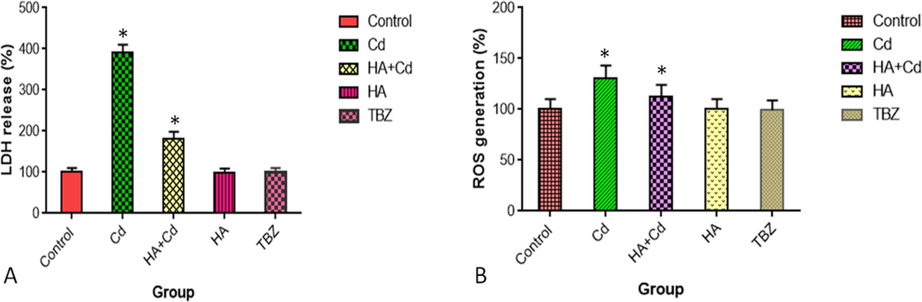
(A) Lactate dehydrogenase release and (B) ROS formation in SH-SY5Y cells after 48 h of exposure in the control and experimental groups. The results were expressed as the mean SEM of three independent. The significances are displayed in comparison to the control.
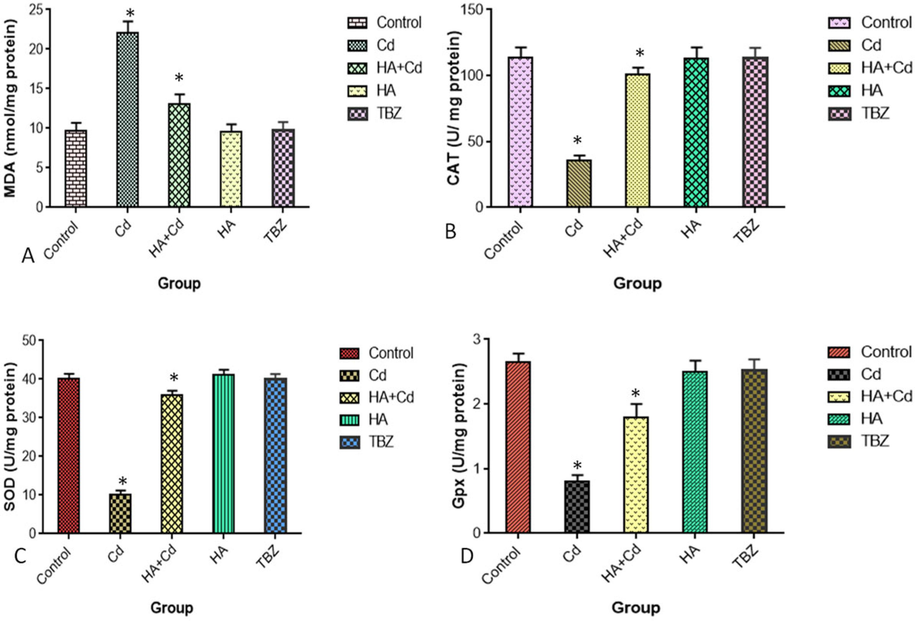
(A) MDA formation, (B) CAT (C) SOD and (D) Gpx in SH-SY5Y cells after 48 h of exposure in the control and experimental groups. The results were expressed as the mean SEM of three independent. The significances are displayed in comparison to the control.MDA- malondialdehyde; CAT-Catalas; SOD-Superoxide Dismutase; and Gpx-Glutathione peroxidase.
3.2.4 Effect of huperzine-A on the levels of mRNA changes in SH-SY5Y cells
The mRNA levels of TNFα, Wnt3a, and CAMK2A were significantly increased (p < 0.05) in cadmium chloride-treated cells when compared to control cells (Fig. 7 a, b, and d).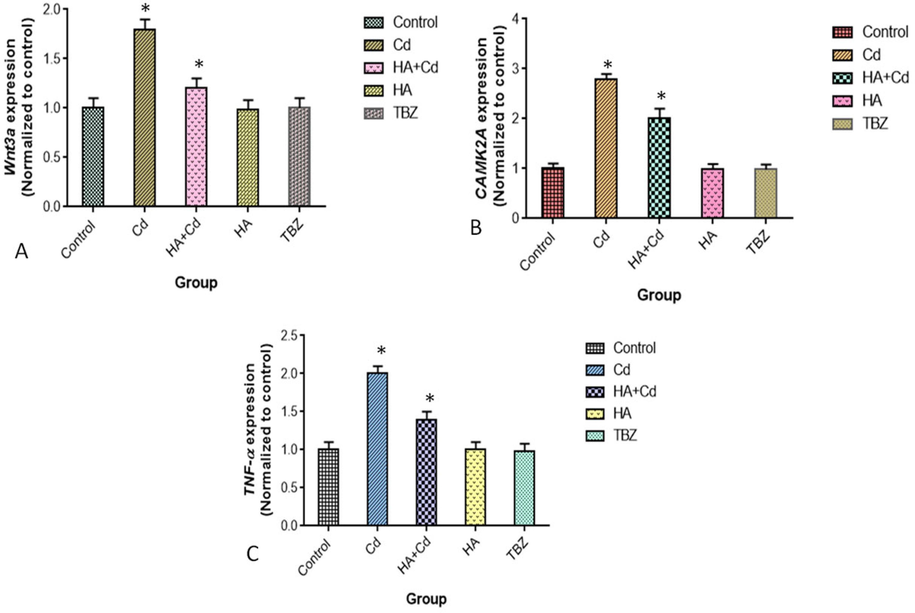
Levels of m-RNA (A) Wnt3a, (B) CAMK2A (C) TNF-α in SH-SY5Y cells after 48 h of exposure in the control and experimental groups. The results were expressed as the mean SEM of three independent. The significances are displayed in comparison to the control.
3.3 In-vivo studies
3.3.1 Effect of huperzine-A on the neuronal behavior and phenotypes change in Drosophila melanogaster
The pattern of neuronal behaviors such as feeding, climbing, and rough eye phenotype were significant changes (p < 0.05) that induced cadmium chloride in flies compared to control flies. Those patterns behaviors were significant changes (p < 0.05) in treated cadmium chloride with huperzine-A in flies when compared to that of the cadmium chloride group (Fig. 8a, b and c).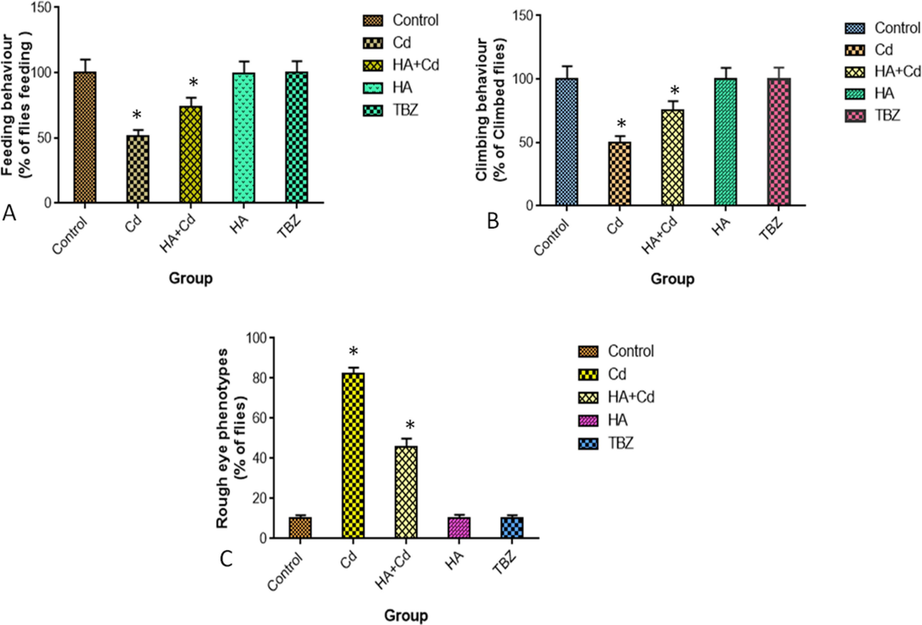
Pattern of (A) Feeding behavior (B) Climbing behavior and (C) Rough eye phenotypes in the control and experimental groups of Drosophila melanogasters. The results were expressed as the mean SEM of three independent. The significances are displayed in comparison to the control.
3.3.2 Effect of huperzine-A on the biogenic amines change in Drosophila melanogaster
The activities of serotonin, dopamine, and acetylcholine esterase were significantly increased (p < 0.05) induced cadmium chloride group of flies. While significantly decreased (p < 0.05) on cadmium with huperzine-A treated flies when compared to the cadmium chloride group of flies (Fig. 9a, b and c).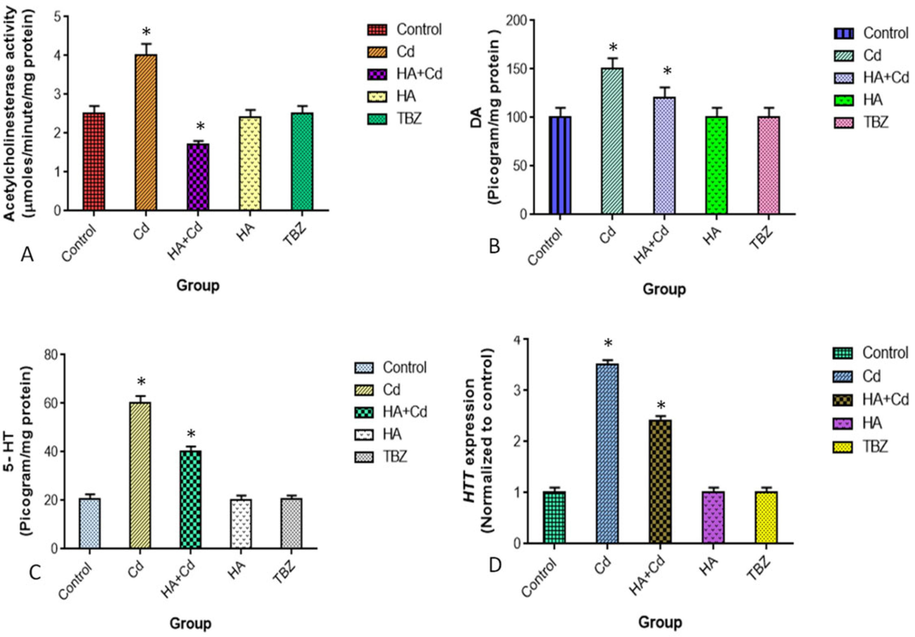
Levels of (A) AchE (B) DA, (C) 5-HT and (D) HTT in the control and experimental groups of brain Drosophila melanogasters. The results were expressed as the mean SEM of three independent. The significances are displayed in comparison to the control. AchE-Acetylcholinesterase-; DA-Dopamine; 5-HT-Serotonin and HTT-Huntingtin.
3.3.3 Effect of huperzine-A on the levels of mRNA change in Drosophila melanogaster
The mRNA level of Htt was significantly increased (p < 0.05) induced cadmium chloride in flies when compared to the respective control group of flies. Their mRNA level was significantly reduced (p < 0.05) in treated cadmium chloride with the huperzine-A group when compared to cadmium chloride-induced flies (Fig. 9d).
3.3.4 Effect of huperzine-A on brain tissue change in Drosophila
Histopathology examination of Drosophila tissue section control and treated groups of brains (Fig.13 Supporting information). The striking lesions are the vacuoles, such as loss of cell bodies (Fig.13 supporting information), and controls exhibit little or no pathology (Fig. 13 Supporting information). Brain structure is preserved, including the outer cellular and neuron cells (Fig.13c Supporting information), whereas treated drosophila brain sections showed mild neuropathology, including vacuolar-like lesions throughout the neuropile (Fig.13d Supporting information).
4 Discussion
Neurotoxicity and cell death may result from exposure to cadmium (Cd). In the area of the brain affected by Huntington's disease (HD), Cd accumulates due to its abundance in the environment, such as soil and water (Kamitsuka et al., 2023). Alternative in vitro and in vivo research is required for target medicine, with the confirmation of a neuroprotective effective medicine screening. Initially, molecular docking studies were carried out on astragalin, linalool, tumerone, ungeremine, huperzine-A, tetrabenazine, and norsanguinine interacting neurophalogical proteins to understand their binding efficiency. The inhibitory gamma-aminobutyric acid neurotransmitter are vital in neuronal behavioral and motor control (Ciranna, 2006). In our study, huperzine A with GABA receptor showed the binding energy of −7.48 kcal and huperzine A could be regulate the normal function of brain in flies.
The two isoforms of monoamine oxidases represented as monoamine oxidases –A and monoamine oxidases-B owing to pharmacological differences. Monoamine oxidases-B plays an important role in oxidizing the dopamine neurotransmitter to metabolizing noradrenaline and serotonin amines in neural cell bodies (Finberg, 2010; De Colibus et al., 2005). The monoamine oxidases-B binds to huperzine- A with TYR393, ALA35, GLY12, VAL10, VAL235, LYS271, and HIS73 amino acids (Fig. 4) as reported by the active site of hMAO-B interact flavonoid (Laloo et al., 2021). It is suggested, huperzine- A regulate the normal levels of biogenic amine pathways in flies. The activity of acetylcholinesterase was significantly increased (p ≥ 0.05) in cadmium induced Drosophila melanogaster (Fig. 9 a). The inhibition of cholinesterases and anti-oxidative properties are potential mechanisms of action of huperzine- A for the minimizing of oxidative stress induced HD.
Further, SH-SY5Y cells exposed to cadmium chloride in the medium, exhibited significantly changes of oxidative stress markers viz., increased in malondialdehyde (p ≥ 0.05) and decreased in CAT (p ≥ 0.05), SOD(p ≥ 0.05) and Gpx (p ≥ 0.05) activities (Fig. 6 a, b, c and d). Huperzine-A offered complete protection against cadmium chloride induced oxidative stress in SH-SY5Y cells.
Feeding is an animal’s behavioral repertoire and forms a mechanistic link between neuronal behavior and molecular neurophysiology (Itskov et al., 2014). Interestingly, flies were exposed to cadmium chloride with huperzine-A exhibited a reduced rough eye phenotype changes and performed better in neuronal behavior (p ≥ 0.05) both suggesting the neuroprotective potential of huperzine-A. Excessive reactive oxygen species cause oxidative damage to deoxyribonucleic acid, proteins, lipids, and antioxidant enzymes, the latter of which has been linked to CAG repeat expansion during DNA repair. Thus, the pathogenesis of neurodegeneration is triggered by the enlarged HTT CAG repeat, leading to clinical symptoms like −Huntington’s disease (Kovtun et al., 2007). The level of m-RNA was significantly increased (p ≥ 0.05) in the cadmium chloride-induced group of flies, and Huperzine-A could maintain the normal level of Htt in the brain of flies.
According to our findings in SH-SY5Y cells, cadmium chloride causes neuronal abnormalities by upregulating Wnt signaling pathways. Cadmium chloride-enhanced Wnt signaling pathways worsen proinflammatory responses, possibly increasing neurotoxicity. Wnts regulate synapse formation and drive the remodeling of mature terminals provoked by neurodegenerative disease (Budnik and Salinas, 2011). The level of m-RNA was significantly increased (p ≥ 0.05) in the cadmium chloride-induced group, and Huperzine-A could maintain the synaptic plasticity in SH-SY5Y cells. We found that cadmium chloride (p ≥ 0.05) increased the mRNA level of CAMK2A in SH-SY5Y cells, which affected neuronal development and function. Cadmium enhanced the mRNA level of CAMK2A in SH-SY5Y cells, perhaps leading to neuronal death (Zalcman et al., 2018). In contrast, cadmium treatment with huperzine-A reduced (p ≥ 0.05) the level of CAMK2A in SH-SY5Y cells, implying that huperzine-A protects neuronal cell death.
Although the exact etiology of Huntington’s disease pathogenesis remains elusive, it is thought to be multifactorial, involving genetic and environmental triggers associated with disease onset. Earlier studies on drug discovery mainly focused on a single target to avoid unnecessary side effects and toxicity (Schneider and Bird, 2016; Yang et al., 2008; Frantz, 2005). Due to the multifaceted nature of neurodegenerative diseases, it is necessary to pursue multiple target approaches to achieve improved therapeutic outcomes. Our data identified huperzine-A as a potential target neuroprotective agent against cadmium-induced degenerative disease symptoms like Huntington's disease in Drosophila melanogaster through its diverse pharmacological effects, including antioxidant and anti-inflammatory (Fig. 10). Future studies are needed in genetic and mammalian Huntington's disease models to validate the neuroprotective effects of huperzine-A for positive outcomes in clinical trials.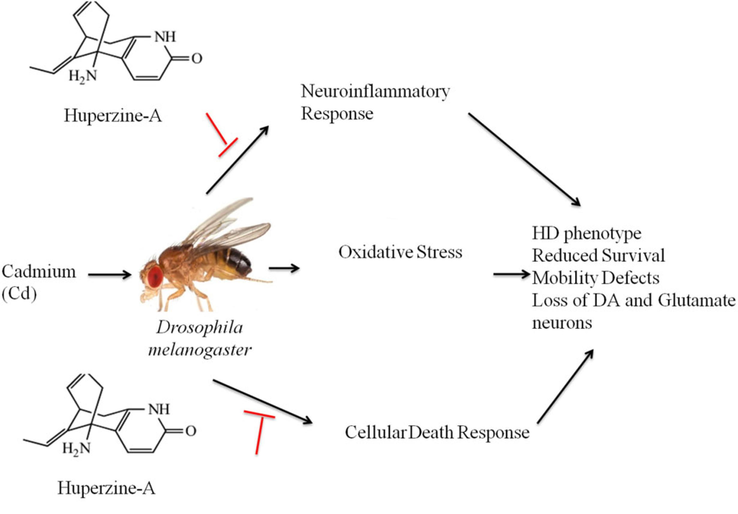
Schematic representation of the neuroprotective effects of huperzine-A on Drosophila melanogaster with cadmium-induced Huntington's disease. (1) Huperzine-A can reduce reactive oxygen species formation and increase antioxidant enzymes like glutathione, reducing oxidative stress. (2) Metal ion toxicity increases oxidative stress in neuronal tissues, which can be reduced by chelating metal ions with huperzine-A. (3) Huperzine-A upregulates heat shock protein activity, which reverses misfolding of proteins and breaks down protein aggregates. (4) Huperzine-A alters genes associated with Huntington's disease, including genes for mitochondrial activity.
5 Conclusion
This study revealed that cadmium chloride can significantly affect Drosophila's serotonin, dopamine, acetylcholine esterase, Htt mRNA expression and functions thereby altering neuronal behaviors. It could be concluded that huperzine-A has the potential to act as a molecular mechanistic agent against cadmium chloride-induced Huntington's disease-like symptoms in Drosophila melanogaster.
CRediT authorship contribution statement
Mamangam Subaraja: Software, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Selvaraj Arokiyaraj: Writing – review & editing, Supervision, Formal analysis. Pratheesh Mathew: Supervision, Methodology, Formal analysis, Data curation, Conceptualization.
Acknowledgments
The authors acknowledge the Tamil Nadu State Council for Science and Technology for funding this research by supporting the project under a grand number (TNSCST/SPS/2021-2022/MS425).
Disclosure of funding
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (TNSCST/SPS/2021-2022/MS425), Tamil Nadu State Council for Science and Technology, Chennai- 600 025, Tamil Nadu, India.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Dopamine and glutamate in Huntington's disease: a balancing act. CNS Neurosci. Ther.. 2010;16:163-178.
- [Google Scholar]
- Apoptotic effects of beta-carotene, alpha-tocopherol and ascorbic acid on PC-3 prostate cancer cells. Hacettepe J. Biol. Chem.. 2020;48(3):211-218.
- [Google Scholar]
- A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem.. 1952;195:133-140.
- [Google Scholar]
- Drosophila as a model for human neurodegenerative disease. Annu. Rev. Genet.. 2005;39:153-171.
- [Google Scholar]
- Wnt signaling during synaptic development and plasticity. Curr. Opin. Neurobiol.. 2011;21(1):151-159.
- [Google Scholar]
- Huntingtin as an essential integrator of intracellular vesicular trafficking. Trends Cell Biol.. 2009;19(4):147-155.
- [Google Scholar]
- Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: implications in physiological functions and in pathology. Curr. Neuropharmacol.. 2006;4:101-114.
- [Google Scholar]
- Selective neuronal degeneration in Huntington's disease. Curr. Top. Dev. Biol.. 2006;75:25-71.
- [Google Scholar]
- De Colibus, L., Li, M., Binda, C., Lustig, A., Edmondson, D.E., Mattevi, A., 2005. Three-dimensional structure of human monoamine oxidase A (MAOA): relation to the structures of rat MAO A and human MAO B. Proc. Nat. Acad. Sci. USA 102, 12684-12689.
- Characterization of a large group of individuals with huntington disease and their relatives enrolled in the COHORT study. PLoS One. 2012;7(2)
- [Google Scholar]
- A new and rapid colorimetric determination of acetyl cholinesterase activity. Biochem. Pharmacol.. 1961;7:88-95.
- [Google Scholar]
- Pharmacology of rasagiline, a new MAO-B inhibitor drug for the treatment of Parkinson’s disease with neuroprotective potential. Rambam Maimonides Med. J. 2010;1(1):e0003
- [Google Scholar]
- Huperzine A and Its neuroprotective molecular signaling in alzheimer's disease. Molecules. 2021;26(21):6531.
- [Google Scholar]
- Automated monitoring and quantitative analysis of feeding behavior in Drosophila. Nat Commun. 2014;5:4560.
- [Google Scholar]
- Defective mitochondrial dynamics and protein degradation pathways underlie cadmium-induced neurotoxicity and cell death in huntington's disease striatal cells. Int. J. Mol. Sci.. 2023;24(8):7178.
- [Google Scholar]
- OGG1 initiates age-dependent AG trinucleotide expansion in somatic cells. Nature 2007:447-452.
- [Google Scholar]
- Laloo, D., Kalita, J.M., Prasad, S.K., 2021. Molecular docking studies of plant-derived bioactive compounds: a comprehensive in silico standardization approach, evidence based validation of traditional medicines, Singapore: Springer 371-404.
- Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265-275.
- [Google Scholar]
- Spectrophotometric assay of superoxide dismutase activity based on the activated autoxidation of a tetracyclic catechol. Anal. Biochem.. 1993;214:442-451.
- [Google Scholar]
- Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med.. 1967;70:158-169.
- [Google Scholar]
- Determination of brain biogenic amines in Cynodon Dactylon Pers. and Cyperus Rotundus L. treated mice. Int. J. Pharmacy Pharm. Sci.. 2009;1(1):190-197.
- [Google Scholar]
- Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev.. 2011;63:411-436.
- [Google Scholar]
- Cognitive and functional decline in Huntington's disease: dementia criteria revisited. Mov. Disord.. 2010;25(9):1163-1169.
- [Google Scholar]
- The incidence and prevalence of Huntington’s disease: a systematic review and meta-analysis. Mov. Disord.. 2012;27:1083-1091.
- [Google Scholar]
- Fluorometric micromethod for the simultaneous determination of serotonin, noradrenaline and dopamine in milligram amounts of brain tissue, pergamon press, printed M Great Britain. Biochem. Pharmacol.. 1974;23:2337-2446.
- [Google Scholar]
- Huntington's disease, huntington's disease look-alikes, and benign hereditary chorea: what's new? Movement disorders clinical. Practice. 2016;27, 3(4):342-354.
- [Google Scholar]
- Fucoidan serves a neuroprotective effect in an Alzheimer's disease model. Front. Biosci. (elite Ed). 2020;12(1):1-34.
- [Google Scholar]
- Molecular docking: from lock and key to combination lock. J. Mol. Med. Clin. Appl.. 2017;2(1) 10.16966/2575-0305
- [Google Scholar]
- The role of natural antioxidants against reactive oxygen species produced by cadmium toxicity: a review. Adv. Pharm. Bull.. 2020;10(2):184-202.
- [Google Scholar]
- Finding multiple target optimal interventions in disease-related molecular networks. Mol. Syst. Biol.. 2008;4:228.
- [Google Scholar]
- CaMKII isoforms in learning and memory: localization and function. Front. Mol. Neurosci.. 2018;11:445.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103319.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







