Translate this page into:
Neurological complications of COVID-19 in children and the associated immunological responses
⁎Corresponding authors. xuemengzhou@zzu.edu.cn (Mengzhou Xue), mushahid@ksu.edu.sa (Shahid Mahboob)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The high spread rate, severe symptoms, psychological and neurological problems, and unavailability of effective medicines are the major factors making Coronavirus disease 2019 (COVID-19) a massive threat to the world. It is thought that COVID-19 causes mild symptoms or mild infectious illness in children. However, we cannot rule out the possibility of serious complications such as the multisystem inflammatory syndrome. COVID-19 induces mild to severe neurological problems in children, such as stroke, encephalopathy, mild shortness of breath, and myalgia. The development of these conditions is associated with pro-inflammatory responses and cytokine storms, which alter the physiology of the blood–brain barrier and allow the virus to enter the brain. Despite the viral entry into the brain, these neurological conditions can also be caused indirectly by severe immune responses. In this article, we describe COVID-19 and the associated neurological and immunological complications in children.
Keywords
COVID-19
Children
Immune
Neurologica
Viral transmission
1 Introduction
Severe Acute Respiratory syndrome coronavirus-2 (SARS-CoV-2) has killed millions across the globe. The virus causes coronavirus disease (COVID-19), which has been reported to harm adults more than children. However, its severity has also been reported in children (Lin et al., 2021). Although the severity of the disease varies among individuals, the COVID-19 development procedure is the same, starting with the virus's entrance to host cells (Shereen et al., 2020). Spike protein has two subunits known as S1 and S2, where S1 facilitates receptor binding and S2 contributes to membrane fusion. Spike proteins have been found to enhance the spreading of the virus by promoting the adhesion of infected cells with adjacent non-infected cells (Li et al., 2003). The entry of SARS-CoV-2 to human host cells requires the cellular receptor angiotensin‐converting enzyme 2 (ACE2) and serine protease TMPRSS2 for spike protein priming (Shereen et al., 2020). TMPRSS2 initiates the process of cleavage, while activated spike protein promotes viral entry into the cells as well as its transmission to neighboring cells. A receptor binding domain, present in the S1 subunit can bind to the human ACE2 with enhanced receptor-spike interactions, and promote infection (Shereen et al., 2020; Li et al., 2003) (see Fig. 1).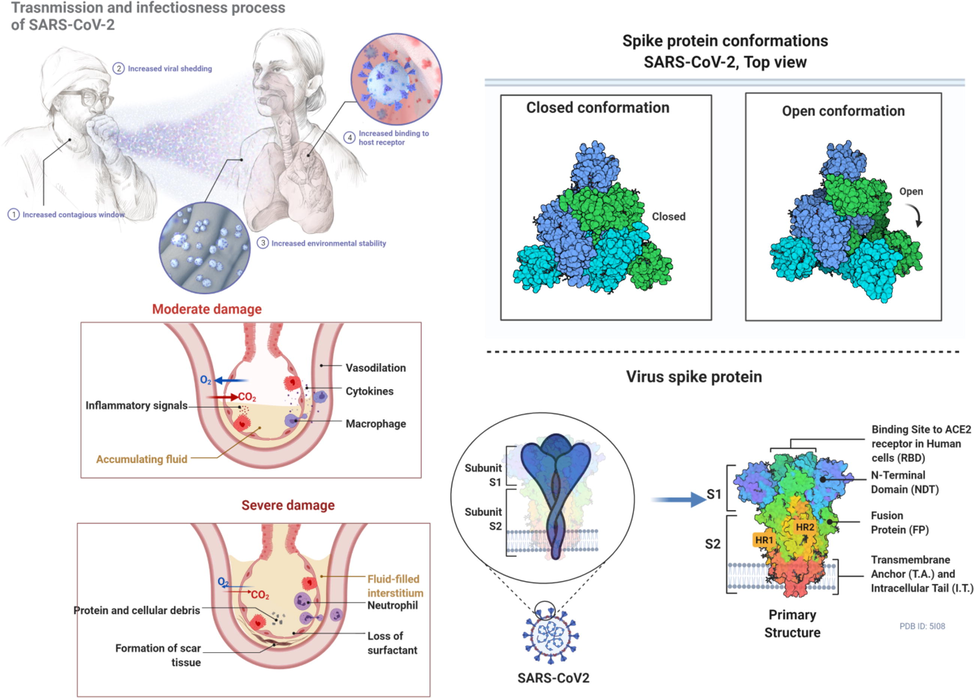
This figure depicts inflammatory signals and the associated damage to the tissues as well as the conformational changes in the spike proteins during the entry and infection of the virus.
Although, it is widely known that ACE2 is highly abundant on alveolar pneumocytes, enabling SARS-CoV-2 to enter and infect these cells. Similarly, evidence indicates that ACE2 is also expressed on neuronal and glial cells in the human CNS (Khan and Gomes, 2020), suggesting that SARS-CoV-2 can enter these cells (Meinhardt et al., 2020). The entry of SARS-CoV-2 to CNS may involve routes/mechanisms such as axonal transport, leukocytes, and CNS endothelia. Nevertheless, investigations have confirmed that COVID-19 has a prominent link with cerebrovascular diseases such as stroke (Tsivgoulis et al., 2020). Although COVID-19 induces neurological complications, however, it is unclear how these complications occur. Nonetheless, one possible reason could be direct viral infection as SARS-CoV-2 has been detected in the brain (Stafstrom and Jantzie, 2020; Ellul et al., 2020). In this paper, we discuss the infection of COVID-19, clinical features, transmission, and associated neurological and immunological consequences in children.
2 Transmission of SARS-CoV-2 in neonates and children and clinical features
Transmission of SARS-CoV-2 requires a minimum dose of replication-competent virus that can be delivered to a vulnerable susceptible host. This transmission is majorly affected by the combination of host, viral, and environmental characteristics. The primary source of emergence and zoonotic transmission has not been confirmed, however, human-to-human transmission has been widely reported. It is now evident that children can easily acquire SARS-CoV-2 from an adult through direct contact; however, the transmission rate from children is comparatively low (Khan et al., 2020a,b). The spread of SARS-COV-2 through respiratory transmission depends on the fine aerosols expelled from the respiratory tract or suspended virions on large droplets, which are typically larger than 5 μm, while aerosols are thought to be smaller than 5 μm (Meyerowitz et al., 2021). Although SARS-CoV-2 RNA has been detected in the stool samples of pediatric patients, there is no convincing evidence indicating fecal-oral transmission of the virus. Among the various transmission media, droplets are considered the most crucial medium of transmission in children (Williams et al., 2020).
Considering the infection in neonates, several reports have outlined the adverse outcomes of COVID-19 during pregnancy. However, none of the reports has confirmed vertical transmission. We have previously reported that SARS-CoV-2 infection can increase the risk of neonatal pneumonia and preterm delivery. We noticed that all neonates except two suspected cases delivered by women infected with COVID-19 were healthy. These results suggest that the intrauterine vertical transmission may not occur. However, SARS-CoV-2 can lead to adverse pregnancy outcomes (Khan et al., 2020). In the case of natural birth, we found similar results to that of Cesarean section. Overall, these reports did not find convincing evidence regarding vertical transmission or maternal-to-neonatal intrapartum transmission of COVID-19 (Khan et al., 2020). As per the literature search, none of the studies has reported convincing evidence (Caparros-Gonzalez, 2020). Therefore, we conclude that there is minimal possibility of vertical transmission of SARS-CoV-2 from infected mothers into infants. Based on these indications, it is recommended that mothers should continue breastfeeding their infants, with proper care and following the hygiene measures (Williams et al., 2020).
In children, the disease can be from asymptomatic to severe illness and life-risking. However, severe illness is not common because children are less likely to have underlying diseases, including hypertension, diabetes, cardiovascular problems. Moreover, conditions such as differential expression of ACE2, co-morbidities, and predisposition to pro-inflammatory states can impact viral entry, replication, inflammation, hypoxia, and tissue injury. It is further aided with the higher efficiency of an innate immune response, which typically declines with age. (Williams et al., 2020). Nevertheless, COVID-19 can pose a life-threatening risk in infants. Therefore, serious attention is needed. Studies have shown that fever is the most common feature of COVID-19, while rhinorrhoea, cough, gastrointestinal symptoms, headache, encephalopathy, mild shortness of breath, and myalgia have also been reported in children (Williams et al., 2020; Yasuhara et al., 2020). Despite multisystem inflammatory syndrome and respiratory symptoms, neurological complications have also been associated with COVID-19 in children, such as headaches, encephalopathy, and altered mental status. Moreover, children diagnosed with multisystem inflammatory syndrome suffered from severe neurological aberrations, including encephalitis, seizure, coma, demyelinating disorders, dysgeusia or ageusia, aseptic meningitis, stroke dysarthria, dysphagia, cerebellar ataxia, axial hypotonia, drowsiness, or moaning, and peripheral neuropathy (Ellul et al., 2020; Fan et al., 2020; Molloy and Bearer, 2020; Diorio et al., 2020).
3 COVID-19 mediated mental health consequences during pregnancy
The COVID-19 can largely affect the mental health of individuals infected with it. This disease especially can also increase the risk of psychological and mental problems in pregnant women, which in turn can affect the health of neonates. Since individuals develop anxiety, anger, fear after pandemics, therefore, the hormonal balance is altered in response to its effect on the hypothalamic-pituitary–gonadal axis. Collectively these complications can lead to adverse pregnancy and neonatal outcomes, which in long term can increase the risk of schizophrenia and associated complications in neonates and children. It is now a well-known fact that maternal psychosocial stress poses adverse effects on the development and maturation of the fetal brain because of elevated maternal cortisol and cytokines levels, which then induce the disruption of serotonin homeostasis. These complications can further adversely microbiome and the ecosystem of the fetus environment, which ultimately leads to the disruption of the fetal gut-brain axis. Persisting gut-brain complications may lead to neurodevelopmental disorders in growing children and neonates (Nabi et al., 2020; Khan et al., 2019).
Evidence indicated that maternal psychosocial stress and mental health complications if left untreated may increase the risk of serious medical complications in neonates, including smaller head circumference, low birth weight, preterm birth, morphological and physiological alteration in fetal brain, and neurobehavioral disorders (schizophrenia, autism, learning disorders, and mood disorders) (Nabi et al., 2020; Khan et al., 2019). Considering the aforementioned details, the current situation of the COVID-19 pandemic can pose higher challenges to children with disabilities, existing mental health issues, and trauma experiences. Therefore, it is necessary to provide timely and promising treatments for mitigating long-term consequences (Fegert et al., 2020). Ignoring psychological outcomes and mental health consequences in pregnant women during the COVID-19 pandemic, ensued by biological functioning and troubled sleeping, may cause serious medical complications. Therefore, researchers and practitioners should perform proper investigations to detect specific psychological problems in children, neonates, and pregnant women so that proper medical assistance can be provided.
4 Neurological aspects of covid-19 in neonates and children
Several neurologic complications in children with COVID-19 are sensory deficits in smell and taste. Moreover, COVID-19 increases the risk of other serious neurological conditions such as stroke, peripheral nervous system disorders, delirium, encephalopathy, and headaches. Reports with neurologic dysfunction in neonates and children infected with SARS-CoV-2 are limited, however, researchers are now focusing on studying these complications.
COVID-19 has been widely studied to induce inflammation of the vascular epithelium, which in turn can increase the risk of strokes (Fan et al., 2020). Although in COVID-19 infected children stroke has not been reported yet, we cannot rule out the possibility that growing variants of SARS-CoV-2 may cause stroke in children. Some of the COVID-19 patients have been presented with encephalitis however, research failed to detect the virus in cerebrospinal fluid. On the other hand, the occurrence rate of seizures in COVID-19 patients is meager. Therefore, a conclusion cannot be made if the infected patients are at higher risk or lower risk. Nonetheless, we cannot ignore the newly emerging variants, which are more aggressive and may widely cause seizures and the associated neurological symptoms.
5 Possible mechanism of neurological involvement
As reports have indicated that ACE2 receptors can be found on vascular endothelial cells in the brain and CNS, thereby suggesting a possible mechanism through which SARS-CoV-2 enters the brain and causes infection. The most important part, in this case, is to determine how this virus can cause infection in the brain and increase the risk of neurological conditions? One of the possible options can that the binding of the virus with ACE2 triggers pro-coagulable and pro-inflammatory reactions, which can disrupt vascular integrity and activate clotting cascade (Meinhardt et al., 2020; Paterson et al., 2020; Grein et al., 2020). This Virus-ACE-2 interaction may adversely impact the autoregulation of blood pressure, which in combination with inflammatory responses, cytokines storm, and coagulative reactions can lead to the occurrence of neurological diseases or complications such as ischemic stroke and related cerebrovascular disorders (Lin et al., 2021). We know that researchers have not determined the neurovirulence at the molecular level, however, the potential mechanisms associated with the infectiousness and causing brain diseases can be unveiled by understanding the neurotropic effect of the virus, post-infection inflammatory responses, prothrombotic impact of the virus on the CNS vasculature, and autoimmune effect mediated by the immune system (Williams et al., 2020; Chen et al., 2020). These details can provide the basis for studying the detailed mechanism, which is essential to develop promising therapeutic options. Recently scientists have indicated that the disruption of the BBB might be one of the potential routes for viral transport and entry to the brain regions. To reach the brain regions, the virus may travel through the systemic circulation and the cerebral circulation (Fig. 2), thereby causing damage to the capillary epithelium. Unluckily, researchers have not reported any convincing evidence that could confirm that SARS-CoV-2 produces sustained or significant viremia. It is evident that the molecules can be transported into the brain through BBB, therefore, attachment of the virus to ACE-2 on BBB can facilitate its transportation into the brain or CNS. This process causes edema and damage to the endothelial layer. On the other hand, COVID-19 induces inflammatory responses, which can cause damage to the BBB, and as a result, BBB becomes dysfunctional, thereby allowing the virus to transmit directly or through activated immune cells. It is also important to note that inflammation is generally exacerbated by the inflammatory cytokines produced and released by neural mast cells and activated glial cells, which can in turn induce the viral flow through lymphatic channels (Stafstrom and Jantzie, 2020). These processes may lead to a breach of the barrier between CSF and blood. This can further facilitate SARS-CoV-2 to enter into CNS or brain. Overall, huge research work is still needed to investigate these processes and mechanisms as no convincing evidence is available that could prove these proposed mechanisms.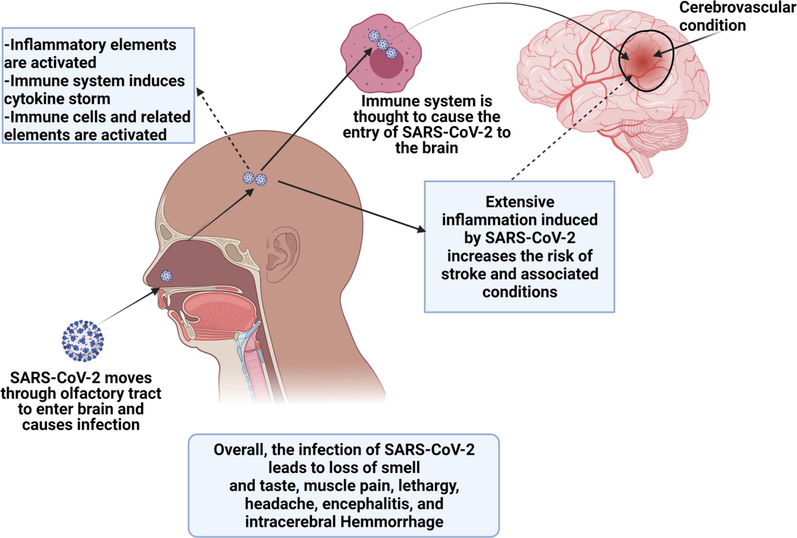
This figure shows the infectiousness of the virus and its transmission to the nervous system through immune cells or nasal passage. Moreover. It also represents the occurrence of stroke after the virus enters and infects the brain.
6 Immunological responses in children
In response to the infection of SARS-CoV-2 immune system is activated and a number of immunological pathways are activated (Molloy and Bearer, 2020) (Fig. 3). Importantly, responses from CD4 and CD8 T cells are produced and virus-specific antibodies are generated (Diorio et al., 2020; Conti et al., 2020; Olbei et al., 2021). Researchers have reported that immune system hyperactivity is associated with increased inflammatory markers levels. These markers include ferritin, IL-6, erythrocyte sedimentation rate, pro-calcitonin, fibrinogen, d-dimer, and most importantly C-reactive protein (CRP). High acute innate inflammatory markers levels have also been found linked with neurological complications because pro-inflammatory cytokines disrupt the BBB, instigate neuroinflammation, and activate glial cells. These phenomena induce neuronal hyperexcitation, fatigue, synapses loss, encephalopathy, and neuron death (Ellul et al., 2020; Khan et al., 2019; Fegert et al., 2020; Fan et al., 2020). The inflammatory cytokines are thought to activate the responses from T-helper cells response. Since their activation is an important event in the activation of specific immunity, therefore, T helper cells have been largely studied in COVID-19. Researchers have reported that the elevated levels of T helper cells in patients with COVID-19 increase the release of cytokines, thereby inhibiting the inflammatory response (Patra et al., 2020; Mannino et al., 2020). Among these cytokines, IL-2R and IL-6 have been indicated positively correlated with the severity of the disease in patients with COVID-19. Generally, critically ill patients are presented with higher serum levels of granulocyte colony-stimulating factor, TNF-α, and macrophage inflammatory protein-1A. These details indicate that there is a positive correlation between cytokine storm and disease severity. Evidence indicated that patients who are suffered from ARDS have a higher mortality rate. ARDS is caused by the interstitial and pulmonary tissue damage in response to nonspecific inflammatory cell infiltration, which is directly linked with the excessive release of cytokines (Mannino et al., 2020; Rello et al., 2020). It is well known that the inflammatory cytokine storm and COVID-19 are closely related in the aspect of progression, initiation and development of ARDS, as indicated by the increased levels of serum cytokines in patients with ARDS. Moreover, this increase in cytokine storm and ARDS is directly related to mortality. On the other hand, the cytokine storm is considered as a key factor associated with extrapulmonary multiple-organ failure, suggesting that the damage to extrapulmonary tissues and organs is caused by inflammatory cytokine storm (Diorio et al., 20202020; Olbei et al., 2021; Gustine and Jones, 2021). Overall, SARS-CoV-2 infection induces inflammatory cytokine storm, which leads to extrapulmonary multiple-organ failure or ARDS and is a key factor associated with COVID-19 exacerbation as well as mortality in COVID-19 patients.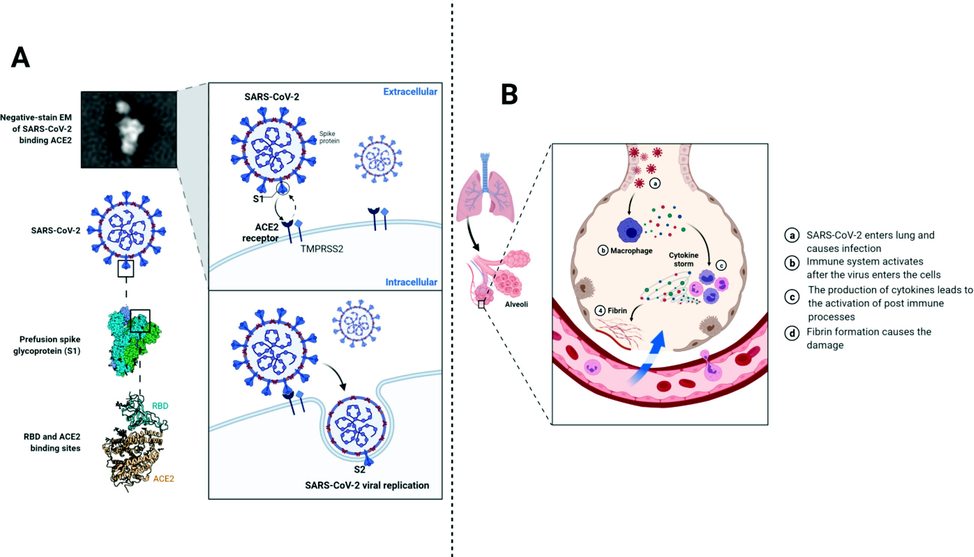
This figure shows the entry process and the mechanism associated with immune responses after the viral entry into the cell.
7 Conclusions
Neonates and children infected with SARS-CoV-2 or COVID-19 infection are thought to be at higher risk of inflammatory responses leading to injury and critical illness. Examination of the peripheral blood may be important for identifying seriously ill patients or patients with severe COVID-19 disease as well as patients with MIS-C. This peripheral blood examination is important as it can allow the investigators to understand the values of neutrophil toxic granulations, burr cells, and schistocytes, and cytokines. Several children have indicated COVID-19 mediated complications including stroke, and encephalopathy, suggesting that COVID-19 can impose a severe impact on children. This severe COVID-19 disease in children is associated with an inflammatory response mediated by the immune system, associated with MIS-C. Considering all these aspects, cytokine storms should be given primary importance while developing the treatment and preventive options against COVID-19 in children.
Acknowledgement
The authors acknowledge operating grant support from the National Natural Science Foundation of China (grants no: 82071331, 81870942, and 81520108011), National Key Research and Development Program of China (grant no: 2018YFC1312200).
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Maternal and neonatal consequences of coronavirus COVID-19 infection during pregnancy: a scoping review. Rev Esp Salud Publica. Spain. 2020;94
- [Google Scholar]
- Insights into neuroimaging findings of patients with coronavirus disease 2019 presenting with neurological manifestations. Front Neurol.. 2020;11:1-9.
- [Google Scholar]
- Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeostat. Agents Italy 2020:327-331.
- [Google Scholar]
- Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J. Clin. Invest.. 2020;130(11):5967-5975.
- [Google Scholar]
- Influence of COVID-19 on cerebrovascular disease and its possible mechanism. Neuropsychiatr. Dis. Treat.. 2020;16:1359-1367.
- [Google Scholar]
- Neurological manifestations in critically Ill patients with COVID-19: A retrospective study. Front Neurol.. 2020;11
- [CrossRef] [Google Scholar]
- Challenges and burden of the Coronavirus 2019 (COVID-19) pandemic for child and adolescent mental health: a narrative review to highlight clinical and research needs in the acute phase and the long return to normality. Child Adolesc. Psychiatry Ment. Health.. 2020;14:20.
- [Google Scholar]
- Compassionate use of remdesivir for patients with severe covid-19. N. Engl. J. Med.. 2020;382(24):2327-2336.
- [Google Scholar]
- Immunopathology of Hyperinflammation in COVID-19. Am. J. Pathol.. 2021;191(1):4-17.
- [Google Scholar]
- Khan S, Gomes J. Neuropathogenesis of SARS-CoV-2 infection. 2020; 1–9.
- (COVID-19): Causative agent, mental health concerns, and potential management options. J. Infect. Public Health.. 2019;2020(13):1840-1844.
- [Google Scholar]
- Emergence of a novel coronavirus, severe acute respiratory syndrome coronavirus 2: biology and therapeutic options. J. Clin. Microbiol.. 2020;58(5)
- [CrossRef] [Google Scholar]
- Coronavirus diseases 2019: Current biological situation and potential therapeutic perspective. Eur. J. Pharmacol.. 2020;886:173447.
- [CrossRef] [Google Scholar]
- Association of COVID-19 with pregnancy outcomes in health-care workers and general women. Clin. Microbiol. Infect.. 2020;26(6):788-790.
- [Google Scholar]
- Impact of COVID-19 infection on pregnancy outcomes and the risk of maternal-to-neonatal intrapartum transmission of COVID-19 during natural birth. Infect. Control Hosp. Epidemiol.. 2020;41(6):748-750.
- [Google Scholar]
- Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450-454.
- [Google Scholar]
- Neurological issues in children with COVID-19. Neurosci. Lett.. 2021;743:135567.
- [CrossRef] [Google Scholar]
- Severe acute respiratory syndrome coronavirus-2 induces cytokine storm and inflammation during coronavirus disease 19: perspectives and possible therapeutic approaches. Front. Pharmacol.. 2020;11:592169
- [Google Scholar]
- Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. United States 2020
- [Google Scholar]
- Transmission of SARS-CoV-2: A review of viral, host, and environmental factors. Ann. Intern. Med.. 2021;174(1):69-79.
- [Google Scholar]
- COVID-19 in children and altered inflammatory responses. Pediatric Res. United States. 2020;88(3):340-341.
- [Google Scholar]
- Nabi G, Siddique R, Xiaoyan W, Ullah R, Xue M, Khan S. COVID-19 induced psychosocial stressors during gestation : possible maternal and neonatal consequences. Curr Med Res Opin [Internet]. Taylor & Francis; 2020; 0: 1–2. Available from: https://doi.org/10.1080/03007995.2020.1815003
- SARS-CoV-2 causes a different cytokine response compared to other cytokine storm-causing respiratory viruses in severely Ill patients. Front Immunol.. 2021;12:1-11.
- [Google Scholar]
- The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104-3120.
- [Google Scholar]
- SARS-CoV-2 spike protein promotes IL-6 trans-signaling by activation of angiotensin II receptor signaling in epithelial cells. PLoS Pathog.. 2020;16(12):e1009128.
- [Google Scholar]
- Update in COVID-19 in the intensive care unit from the 2020 HELLENIC Athens International symposium. Anaesth. Crit. Care Pain Med.. 2020;39(6):723-730.
- [Google Scholar]
- COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. Elsevier 2020
- [Google Scholar]
- Stafstrom, C.E., Jantzie, L.L., 2020. COVID-19: Neurological Considerations in Neonates and Children. Child (Basel, Switzerland). 2020; 7.
- COVID-19 and cerebrovascular diseases: a comprehensive overview. Ther. Adv. Neurol. Disord.. 2020;13
- [CrossRef] [Google Scholar]
- SARS-CoV-2 in children: spectrum of disease, transmission and immunopathological underpinnings. Pathology. 2020;52(7):801-808.
- [Google Scholar]
- Clinical characteristics of COVID-19 in children: A systematic review. Pediatr Pulmonol. United States. 2020;55:2565-2575.
- [Google Scholar]







