Translate this page into:
Nephroprotective potential of Sharbat-e-Bazoori Motadil (sugar-free) in HEK-293 cells and Wistar rats against cisplatin induced nephrotoxicity
⁎Corresponding author at: Bioactive Natural Product Laboratory, Department of Pharmacognosy and Phytochemistry, School of Pharmaceutical Education & Research Jamia Hamdard, New Delhi 110062, India. sahmad_jh@yahoo.co.in (Sayeed Ahmad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Sharbat-e-Bazoori Motadil (SBM) is a traditional Unani syrupy formulation, widely used for the management of kidney diseases. Due to lack of scientific evidence, the present work prospective is aimed to evaluate the protective effect of sugar-free SBM against cisplatin (CP)-induced nephrotoxicity using in vitro and in vivo followed by phytochemical studies. The sugar-free SBM formulation was chromatographically characterized for chemical analysis through HPTLC and evaluated for its metabolic contents. Thereafter, in vitro phytochemicals and free radical scavenging assays were performed to evaluate the total phenols and flavonoid content and antioxidant potential of sugar-free SBM. Human embryonic kidney-293 (HEK-293) cell was used to assess nephroprotective and antioxidant studies of sugar-free SBM. Further, in vivo nephroprotective studies were performed in female Wistar albino rats at different dose (32.1, 64.2, 128.4 mg/kg/day, p.o.) of SBM by assessment of biochemical markers, antioxidants status, inflammatory cytokines, and histopathological analysis. Qualitative and quantitative HPTLC analysis of SBM revealed eleven and eight metabolites at 254 and 366 nm, respectively, while the content of caffeic acid and trans ferulic acid was found as 5.63 ± 0.29 and 12.64 ± 0.71 µg/mg, respectively. In vitro free radical scavenging assays showed the significant antioxidant potential of sugar-free SBM. The in vitro assay for nephroprotective and cellular antioxidant analysis of SBM showed significant (p < 0.001) nephroprotective and antioxidant potential. Additionally, in vivo studies of different doses of SBM showed significant (p < 0.001) amelioration in kidney and liver biomarkers. Besides, it also manifests antioxidant, anti-inflammatory and anti-apoptosis activity confirmed by regulation of CAT, GPx, GSH, SOD, TNF-α, IL-1β, NO and caspase-3 levels. Additionally, normalization in histopathological changes of kidney tissue against cisplatin toxicity was also observed in SBM. The sugar-free SBM significantly ameliorated cisplatin induced nephrotoxicity by exerting normalcy in biochemical markers, antioxidant, and anti-inflammatory activity. These findings indicated an opportunity to develop a sugar-free formulation from SBM composition, as well as scientific validation of its traditional claim in the Unani system of medicine.
Keywords
Sharbat-e-Bazoori
Kidney
Cisplatin
Nephrotoxicity
HEK-293
α-Ketoanalogue
- AKI
-
Acute Kidney Infection
- Alb
-
Albumin
- ALP
-
Alkaline Phosphatase
- ALT
-
Alanine Aminotransferase
- AST
-
Aspartate Aminotransferase
- BU
-
Blood Urea
- Ca
-
Calcium
- CAA
-
Cellular Antioxidant Activity
- CAT
-
Catalase
- CKD
-
Chronic Kidney Disease
- CP
-
Cisplatin
- Cr
-
Creatinine
- DB
-
Direct Bilirubin
- DCFH-DA
-
Dichloro-dihydro-Fluorescein acetate
- DMEM
-
Dulbecco’s Modified Eagle Medium
- DPPH
-
2,2-Diphenyl-1-Picrylhydrazyl
- FBS
-
Fetal Bovine Serum
- FC
-
Folin-Ciocalteu
- Glb
-
Globulin
- GSH
-
Glutathione
- HEK-293
-
Human Embryonic Kidney-293
- HPTLC
-
High Performance Thin Layer Chromatography
- I.P
-
Intraperitoneal
- IAEC
-
Institutional Animal Ethics Committee
- K
-
Potassium
- MDA
-
Malondialdehyde
- MTT
-
3-(4, 5-Dimethylthiazol-2-Yl)-2, 5-Diphenyltetrazolium Bromide
- Na
-
Sodium
- NO
-
Nitric Oxide
- NS
-
Normal Saline
- NSAIDs
-
Non-Steroidal Anti-Inflammatory Drugs
- P
-
Phosphorus
- ROS
-
Reactive Oxygen Species
- SBM
-
Sharbat-e-Bazoori-Motadil
- TB
-
Total Bilirubin
- TP
-
Total Protein
- UA
-
Uric Acid
- WHO
-
World Health Organization
Abbreviations
1 Introduction
Nephrotoxicity is a most common kidney problem is referred as loss of renal function either due to direct exposure to drugs, toxins or environmental chemicals noted as nephrotoxic agents (Alsalame et al., 2018). In 2017, World Health Organization proposed that early identification and diagnosis may prevent, slow down or reverse the loss of kidney function in acute kidney infection (AKI) and chronic kidney disease (CKD) by the use of inexpensive interventions including natural medicine (World Health Organization, 2018).
Aminoglycoside antibiotics, anti-cancer and some non-steroidal anti-inflammatory drugs (NSAIDs) are the most common nephrotoxins (Kovacic and Jacintho, 2012). Cisplatin (CP), a potent anti-neoplastic medication utilized in chemotherapy for the management of hematologic or solid tumors. Several recent studies reported that CP interacts with mitochondrial as well as nuclear DNA inducing nephrotoxicity (Sadeghi et al., 2020). The most relevant mechanism related to CP nephrotoxicity involves oxidative stress, inflammatory reaction, and apoptosis (Sohn et al., 2009; Meng et al., 2017).
Currently, various strategies have been assessed to decrease the CP-induced nephrotoxicity and generate the need for alternative therapy which should be effective, economic and non-toxic for human health. Scientifically validated and standardized herbal drugs may result using the path of reverse pharmacology approach based on traditional data.
Unani medicine is one of the most ancient systems of medicine and originated in Greece and its further spread over Arabic and Asian subcontinent area and is used for management or prevention of numerous diseases and reported to play a strong role in the health care system (Ahmad et al., 2021). Various Unani drugs and formulations claim to possess a safe and effective role in different renal disorders. Despite being widely used in the management of renal disease by Unani practitioners, these formulation have not been scientifically evaluated for pharmacological potential. Only few Unani drugs including Gule Surkh and Beikh Kasni (Rosa damascena and Cichorium intybus) (Khaliq et al., 2015), Kabab Chini (Piper cubeba) (Ahmad et al., 2012) and Jawarish Zarooni Sada (Afzal et al., 2004) have been investigated for their protective role in kidney and its related disorders. Various herbal formulations, which are widely used mainly include Jawarish Zarooni Sada, Himalaya Uri-care, Neeri- KFT and Adel 33 Apo oedema Drop, thus, the increasing interest and popularity in traditional formulation leads to the requirement of scientific evidence to describe the quality potential of these formulations.
Sharbat-e-bazoori Motadil (SBM) is a polyherbal Unani formulation composed of seven medicinal plants Table 1 and has been widely used for the management of kidney disorders in the Unani system of medicine. But no scientific data has been reported on SBM about its therapeutic efficacy till date. The majority of medicinal plants of the formulation including Cichorium intybus (Azhar, 2018), Cucumissativus (Prasanthi and Adikay, 2016), and Cucumis melo (Saleem et al., 2019), have previously been reported to possess nephroprotective potential.
S.N.
Plant Material Name
(Biological Name/Unani Name)Parts Used
Quantity
Voucher No.
1
Cichorium intybus L./Beikh kasni
Root
100 g
BNPL/JH/Ph.D/02/18/01
2
Cichorium intybus L./Tukhme kasni
Seed
50 g
BNPL/JH/Ph.D/02/18/02
3
Foeniculum vulgare Mill./Beikh badiyan
Root
50 g
BNPL/JH/Ph.D/02/18/03
4
Cucumis melo L./Tukhme kakri
Seed
50 g
BNPL/JH/Ph.D/02/18/04
5
Cucumius sativus L./Tukhme khera
Seed
50 g
BNPL/JH/Ph.D/02/18/05
6
Cucumis melo L./ Tukhme kharbuza
Seed
50 g
BNPL/JH/Ph.D/02/18/06
7
Tribulus terresestris L./ Khare khasak khurd
Fruit
50 g
BNPL/JH/Ph.D/02/18/07
Thus, the present study was aimed to evaluate nephroprotective potential of SBM (sugar-free) against CP-induced nephrotoxicity in HEK-293 cells and Wistar albino rats followed by phytochemical analysis. Hope this study could be used as an exemplary for the evaluation of other traditional formulations, which may come out as an effective and safe remedy for CKD.
2 Material and methods
2.1 Chemicals and reagents
Folin-Ciocalteu, aluminum chloride and sodium carbonate reagent (SD Fine Chem Pvt. Ltd, Mumbai), 2,2-Diphenyl-1-picrylhydrazyl (DPPH), 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) (MTT) EZcountTM, Caffeic, ferulic acid and ascorbic acid (Sigma Aldrich USA), Dulbecco’s modified eagle medium (DMEM) and fetal bovine serum (FBS) (GibcoTM, USA), TLC Silica gel 60F254 (Merck KGaA, 64271 Darmstadt, Germany). Rat TNF-α, IL-1β, NO, and Caspase-3 ELISA Kit (EliKine™, USA), Alpha-Keto Analogue (Nephron Star Healthcare Pvt. Ltd). The solvents and chemicals used in the study were of analytical grades.
2.2 Preparation of sugar-free SBM
The plant materials of SBM (Table 1) were procured from the local market of old Delhi, authenticated and prepared as per the Unani Pharmacopoeia India; Anonymous I. Briefly, the crude materials were coarsely powdered and soaked with 1.4 L of water overnight and decoct for 3 h at 60 °C and the process repeated three times with fresh solvent. The extract was filtrated, concentrated, and stored at 4 °C for further analysis.
2.3 Estimation of total phenolic and flavonoid content
Total phenolic and flavonoids content in the SBM was determined by using the Folin Ciocalteu (FC) and aluminum chloride methods, respectively with some modifications (Gaurav et al., 2020), (Chester et al., 2017). The total phenols and flavonoid contents were expressed as milligram (mg) gallic acid equivalent to per gram (g) of the sample (mg GAE/g sample) and milligram (mg) rutin equivalent to per gram (g) of the sample (mg RUT/g sample), respectively.
2.4 Fingerprinting and chemical analysis by HPTLC
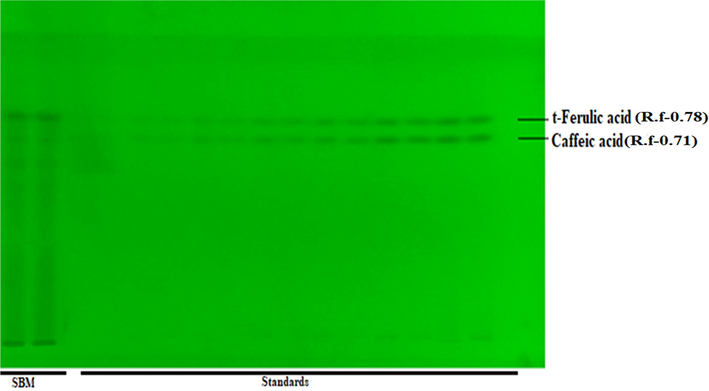
In HPTLC fingerprinting and quantitative analysis was performed with respect to caffeic acid and trans-ferulic acid as per the described protocol with some modification (Zahiruddin et al., 2021). In brief, 10 mg/mL solution of SBM in methanol and stock solution (0.5 mg/mL), of caffeic acid and ferulic acid mixed standard was prepared and applied on a TLC plate. The TLC plate was developed in a pre-saturated development chamber using toluene: ethyl acetate: formic acid (6:3:1, v/v/v) as a mobile phase and scan at 254 and 354 nm.
2.5 Estimation of antioxidant activity
DPPH-free radical scavenging assay, reducing power assay and total antioxidant capacity assay of the formulation were estimated as per reference protocol with some modifications (Zahiruddin et al., 2021), (Khan et al., 2017), (Oyaizu, 1986), (Aliyu et al., 2013). A wide concentration range (31.25–500 μg/mL) was used in each method for the evaluation of the antioxidant activity of SBM.
2.6 In vitro cell culture studies
2.6.1 Cell line
Human embryonic kidney-293 (HEK-293) cells were collected from the National Centre for Cell Science, Pune, India, and cultured in DMEM supplemented with 10% FBS and antibiotics and allowed to grow at 37 °C in a humidified incubator with 5% CO2.
2.6.2 Protective effect of SBM against cisplatin induced nephrotoxicity and oxidative stress in HEK-293 cell line
After cytotoxicity estimation of SBM and cisplatin nephroprotective effect of SBM (3.9–500 µg/mL) was determined against cisplatin induced nephrotoxicity in HEK-293 cells. Briefly, cells were loaded on the 96-well plate and incubated with different concentration (3.9–500 µg/mL) for 24 h at 37 °C. Thereafter, the cells were treated with 100 µL of CP (13 µg/mL) and the plate was incubated for further 24 h. Then MTT reagent was mixed in each well and incubated for an additional 4 h at 37 °C. The formed blue formazan crystals were liquefied with the addition of 100 µL of solubilizing agent and absorbance recorded at 570 nm. Morphological changes in HEK-293 cells were examined before the addition of MTT reagent using EVOS XL Core Cell Imaging System (Kpemissi et al., 2019).
In Cellular antioxidant activity (CAA), intracellular formation of ROS was evaluated by using oxidation sensitive Dichloro-dihydro-fluorescein acetate (DCFH-DA) probes (Grauzdytė et al., 2018). Briefly, the pre-cultured HEK-293 cells in 96 well plates were treated with 100 µL of SBM (62.5–500 μg/mL) and the plate was incubated for 3 h. Thereafter, the washed cells were treated with 100 μL of DCFH-DA (10 μmol/L) after the treatment with cisplatin and fluorescence was recorded at excitation wavelength 485 nm and 530 nm.
2.7 In vivo evaluation of SBM on CP-induced nephrotoxicity
2.7.1 Experimental animal
Wistar albino female rats (200–250 g) were utilized for in vivo studies and these rats obtained from Central Animal House of Hamdard University, approved (Approval Number: 1554) by Institutional Animal Ethics Committee (IAEC), Jamia Hamdard, New Delhi, India (Registration No.: 173/GO/RE/S/2000/CPCSEA).
2.7.2 CP-induced nephrotoxicity
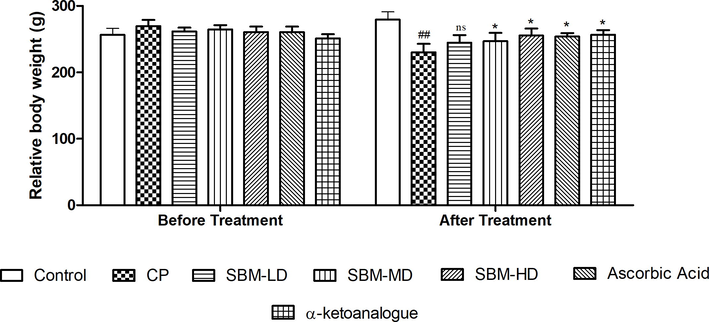
The dose and time schedule of CP administration was adopted in this study were based on the pilot study done by Kumar et al. 2017. CP 7 mg/Kg body weight of through intraperitoneal (i.p.) route was used for the induction of nephrotoxicity. All the rats were divided into seven groups (n = 6 per group). Group 1 received normal saline (NS) and served as Control; Group II received CP (7 mg/kg/day, i.p.) and served as toxic control; Group III received SBM in low dose (LD-SBM, 32.1 mg/kg/day); Group IV received SBM in medium dose (MD-SBM, 64.2 mg/kg/day); Group V received SBM in high dose (HD-SBM, 128.4 mg/kg/day); Group VI received ascorbic acid (10 mg/kg/day, p.o.) and served as positive control; Group VII received α-ketoanalogue (10 mg/kg/day, p.o.) and served as positive control II. The dose of the sugar-free SBM formulation was calculated from the extractive value obtained with respect to the dose mentioned in Unani Pharmacopeia. Group II, III, IV, V, VI and VII received CP (7 mg/kg/day, i.p.) for the last two days of the experimental period i.e., 13th and 14th day. The body weight of all animals was measured pre and post treatment. At the end of the experiment, rats were sacrificed using ether anesthesia. Before scarification, blood samples were collected from retro-orbital and separate serum for biochemical analysis.
After the processing, the animals were sacrificed successively to collect kidney tissue from each animal to assess the antioxidant and histopathological analysis (Rezaee-Khorasany et al., 2020).
2.7.3 Evaluation of biomarkers in serum and urine
Collected sample of urine and serum was used for the investigation of different biochemical analysis. In kidney biomarkers analysis, blood urea (BU), creatinine (Cr), total protein (TP), albumin (Alb), globulin (Glb), total bilirubin (TB), direct bilirubin (DB), uric acid (UA), calcium (Ca), phosphorus (P), sodium (Na) and potassium (K) level was estimated on blood serum and urine. In hepatic biomarkers analysis, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) were performed on serum (Kpemissi et al., 2019).
2.7.4 Assessment of antioxidant status and oxidative stress biomarkers in the kidney
Collected kidney from each animal, homogenates (10% w/v) were made in tris–phosphate buffer (50 mM, pH 7.4) and then centrifuged at 1300 rpm for 10 min at 4 °C. The resultant supernatant were used to assess the glutathione (GSH), glutathione peroxidase (GPx), superoxide dismutase (SOD), malondialdehyde (MDA), catalase (CAT), and nitric oxide (KET900) levels using spectrophotometric assays (Kpemissi et al., 2019).
2.7.5 Determination of proinflammatory cytokines and caspase-3 in the kidney
Tumor necrosis factor-α (KET9007), interleukins-1β (KET900), caspase-3 levels (KET100992) were measured in kidney tissue homogenate as per the manufacturer protocol.
2.7.6 Histopathology
Histopathological studies of kidney tissues were conducted as per the described protocol using Olympus IX 71 research microscope (10×) (Sharma et al., 2017).
2.8 Statistical analysis
Statistical representations of the data were expressed as mean ± SEM using One Way ANOVA followed by Tukey test. The statistical significance difference was represented in terms of p value and summary.
3 Results
Aqueous extract of sugar-free SBM formulation was prepared, successfully. The percentage yield of the formulation was found as 37.23 ± 2.13% (w/w).
3.1 Phenolic and flavonoid contents of SBM
The total phenolic and flavonoid content in the formulation was measured successfully. The results showed total phenolic and flavonoid contents were 33.53 ± 2.45 and 13.39 ± 1.45 mg GAE and RUT/g sample, respectively.
3.2 Fingerprinting and quantitative estimation of chemicals by HPTLC
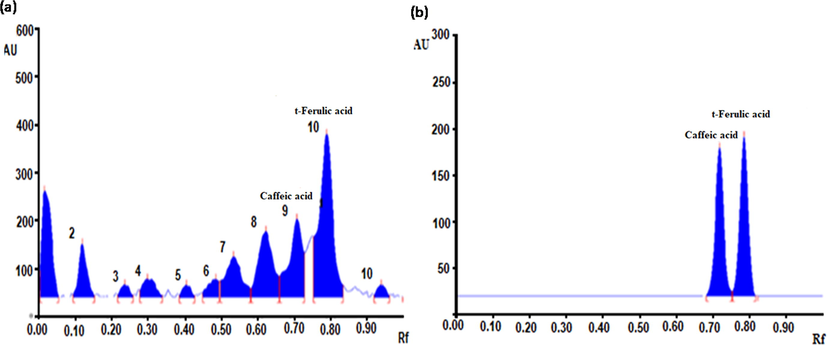
Eleven metabolites were detected after scanning at 254 nm, whereas eight metabolites were detected at 366 nm in SBM (Supplementary Table 1). For the quantitative estimation of polyphenols (caffeic acid and ferulic acid) in SBM, the in house developed solvent system was found reproducible separation results of caffeic acid and ferulic acid at 254 nm. The average quantity of caffeic and trans ferulic acid in SBM was found to be 5.63 ± 0.29 and 12.64 ± 0.71 µg/mg, respectively (Supplementary Fig. 1A, B).
3.3 Antioxidant potential of SBM
In vitro free radical scavenging assay were performed to evaluate the antioxidant characteristic of SBM. The resulting data revealed the DPPH IC50 values of SBM (55.78 ± 4.31 µg/mL) similar to Ascorbic acid (43.93 ± 2.30 µg/mL). To check the antioxidant potency of the sugar-free formulations, we did it to another method that was reducing power. The reducing power IC50 value of SBM (90.72 ± 3.78 µg/mL) was similar to Ascorbic acid (55.85 ± 3.21 µg/mL). The third assay that is total antioxidant activity (TAC) and IC50 value of SBM (35.21 ± 4.80 µg/mL) was comparable to Ascorbic acid (19.81 ± 2.35 µg/mL). The graph of antioxidant assay of SBM are summarized in Fig. 1.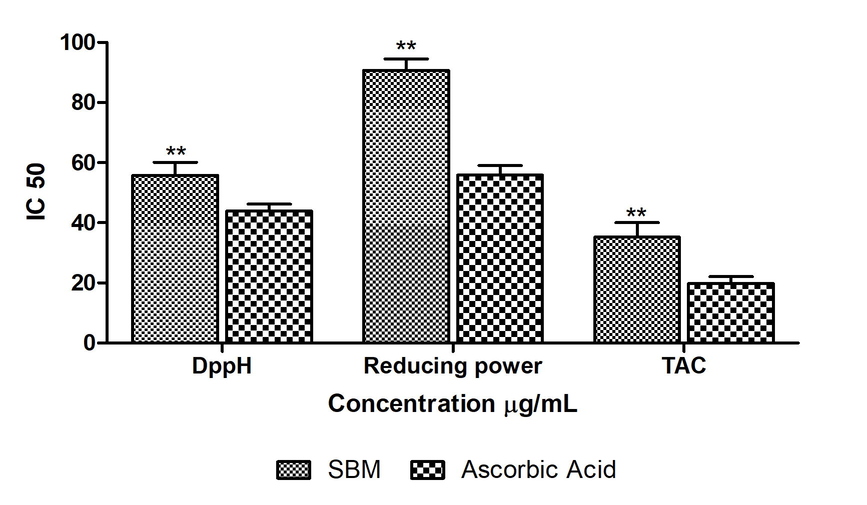
In vitro antioxidant activities of SBM extract against DPPH, Reducing power, Total antioxidant activities. The statistical representation was made as Mean ± SEM (n = 3). The comparison was made with respect to IC50 for each sample. The statistical significance level was expressed at *P < 0.05, **p < 0.01, ***P < 0.001; ns p > 0.5.
3.4 In vitro cell culture studies
3.4.1 Effect of SBM on cytotoxicity of HEK-293 cells
The results showed 500 μg/mL and 13 μg/mL cytotoxic concentrations of SBM and cisplatin. The effect of SBM on cell viability was found in a dose-dependent manner. The graph of cytotoxicity assay of SBM and Ascorbic acid are summarized in Fig. 2a.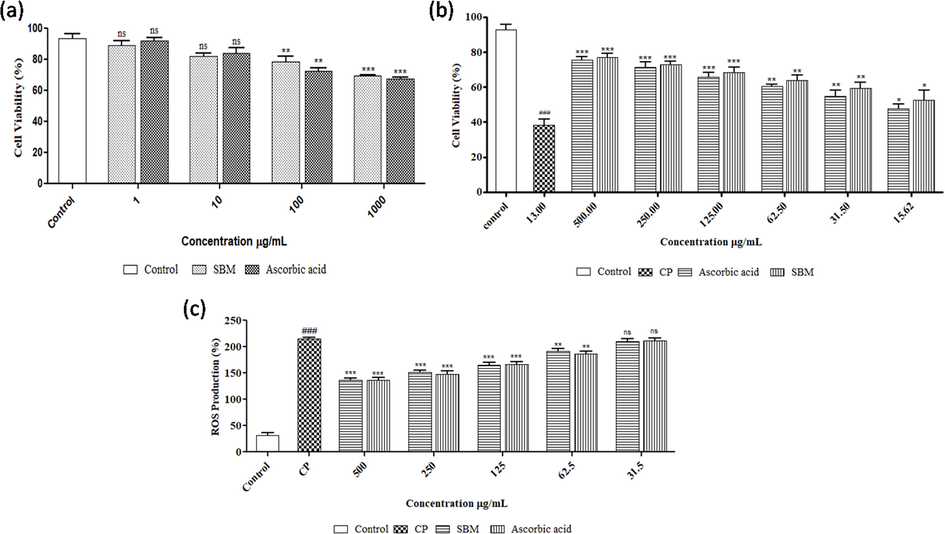
(a) Effect of sugar-free SBM and ascorbic acid on cell viability in HEK-293 cell line. Data are expressed as mean ± SEM (n = 3). One-way ANOVA followed by Tukey's multiple comparisons test. Compared to normal control group: The statistical significance level was expressed at *P < 0.05, **P < 0.01, ***P < 0.001; ns p > 0.5. (b) Nephroprotective potential of SBM on HEK-293 cell line using CP as toxicant. Data are expressed as mean ± SEM (n = 3). One-way ANOVA followed by Tukey's multiple comparisons test. Compared to normal control group: ## P < 0.01, ### P < 0.001; Compared to CP control group: The statistical significance level was expressed at *P < 0.05, **P < 0.01, ***P < 0.001; ns p > 0.5. 2(c) Fig. 6 Effect of SBM on CP- induced ROS production in HEK-293 cell line, the intracellular ROS production was quantified, expressed as percentage of control and show as mean ± SEM (n = 3). Compared to normal control group: ### P < 0.001; Compared to CP control group: The statistical significance level was expressed at **P < 0.01, ***P < 0.001; ns p > 0.5.
3.4.2 Nephroprotective effect of SBM against CP-induced toxicity in HEK-293 cells
The nephroprotective effect of the sugar-free SBM was evaluated against CP-induced cytotoxicity in HEK-293 cells. The resulted data showed that CP treatment significantly (P < 0.001) reduced the cell viability which was reversed after the treatment with SBM. SBM was accompanied with 76.85 ± 1.50% viability at 500 µg/mL against the reduced viability of HEK-293 with 41.20 ± 0.05% by CP. Although the protective effect of SBM against the CP toxicity was found in a dose-dependent manner while no significant changes were observed compared to Ascorbic acid (Fig. 2b).
3.4.3 Cellular antioxidant potential of SBM
In order to evaluate the protective actions of SBM against CP-induced intracellular ROS production in HEK-293 cells, DCFH-DA fluorescent probe was used to measure intracellular ROS level in HEK-293 cells. The experimental findings reveal that level of ROS in HEK-293 cells was significantly increased with incubation of CP compared with the control group (Fig. 2c). Treatment with SBM at different concentrations (150–500 µg/mL) significantly decreases CP-induced ROS production. Ascorbic acid showed no significant difference compared to SBM. (Fig. 2c)
3.4.4 Effect of CP on morphology of HEK-293 cells
Morphological changes in HEK-293 cell lines have been assessed after induction of nephrotoxicity by CP and the results were observed as marked changes in the morphology of HEK-293 cells. The morphological change was assessed by phase-contrast microscopy as shown in Fig. 3.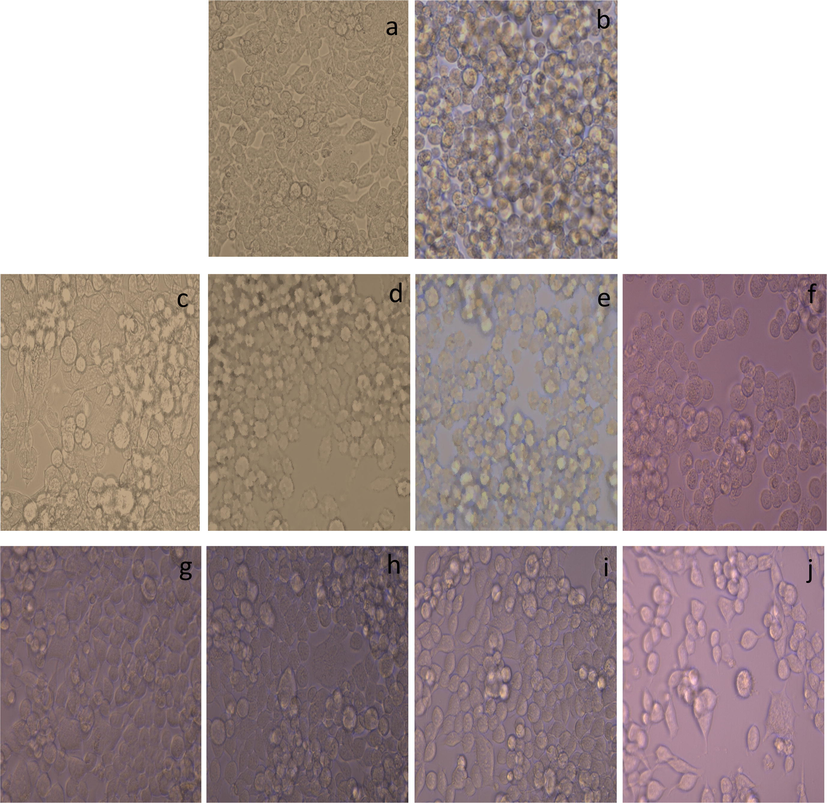
Control cells (a) that shows a small, polygonal-shaped and epithelial-like morphology. In contrast, cells exposed to CP (b) expressing complete distorted architect as compared to control with loss to grow as epithelium-like monolayer and even with condensed intracellular parts and almost all the cells appeared with the spherical shape or rounded phenotype. (c-f) displays treatment groups of cells with SBM, at different concentration (500 C, 125 D, 31.25 E and 7.81 F μg/mL). After the treatment with SBM, figure C-D showing normal architect of cells with least cells death as compared to control cells whereas figure (e and f) showing significant changes in architect of cells with rounded phenotype. Figure K-N displays treatment groups of cells with ascorbic acid at different concentration (500 K, 125 L, 31.25 M and 7.81 N μg/mL). After the treatment with ascorbic acid, figure (g and h) showing normal architect of cells with least cells death as compared to control cells whereas figure (i and j) showing reduced number of cells but no significant changes in architect of cells with least round phenotype.
3.5 Effect of SBM in CP-induced toxicity in Wistar albino rats
3.5.1 Effect of SBM on serum biochemical markers
The resulted data reveals that two days of treatment with cisplatin causes significant changes in biomarkers of the kidney and liver. In contrast, serum levels of creatinine, urea and uric acid in the CP group were significantly increased (P < 0.05) compared to the normal control group, while the levels of total protein (TP), blood urea nitrogen (BUN), albumin (Alb), globulin (Glb), direct bilirubin, total bilirubin Table 2 and electrolytes (calcium, phosphorus, and Magnesium), were significantly (P < 0.05) decreased Table 3. Similarly, electrolytes such as sodium and potassium levels were found as significantly increased (P < 0.05) in CP group as compared to the normal control group. In addition, the serum levels of AST, ALT and ALP enzymes activity were also significantly (P < 0.05) increased in the CP group compared to the normal control. These altered levels of renal function parameters confirmed the induction of nephrotoxicity. However, the treatment with all three doses of SBM resulted in significant (P < 0.05) amelioration of renal and liver biomarkers. Data are expressed as mean ± SEM (n = 6). One-way ANOVA followed by Tukey's multiple comparisons test. Compared to normal control group: ### P < 0.001; Compared to CP control group: *P < 0.05, **P < 0.01, ***P < 0.001; ns p > 0.5. SBM LD-Sharbat-e-Bazoori Low Dose; SBM MD- Sharbat-e-Bazoori Medium Dose; SBM HD- Sharbat-e-Bazoori High Dose Data are expressed as mean ± SEM (n = 6). One-way ANOVA followed by Tukey's multiple comparisons test. Compared to normal control group: ### P < 0.001; Compared to CP control group: *P < 0.05, **P < 0.01, ***P < 0.001; ns p > 0.5. SBM LD-Sharbat-e-Bazoori Low Dose; SBM MD- Sharbat-e-Bazoori Medium Dose; SBM HD- Sharbat-e-Bazoori High Dose.
KFT Parameter
Normal Control
Toxic Control
SBM LD
SBM MD
SBM HD
Ascorbic acid
α-Keto Analogues
Creatine (mg/dL)
0.37 ± 0.01
1.90 ± 0.03###
1.82 ± 0.09 ns
1.70 ± 0.07**
1.72 ± 0.08**
1.78 ± 0.10*
0.47 ± 0.05***
Urea (mg/dL)
20.78 ± 1.10
58.23 ± 2.57###
51.97 ± 2.47*
52.23 ± 1.28*
25.70 ± 1.36***
35.23 ± 1.32**
33.37 ± 1.63***
Uric acid (mg/dL)
2.40 ± 0.18
4.09 ± 0.16###
3.34 ± 0.13*
2.98 ± 0.09**
2.33 ± 0.15***
2.38 ± 0.18***
2.99 ± 0.16**
Total protein (gm/dL)
6.50 ± 0.11
3.79 ± 0.02###
4.11 ± 0.05*
5.09 ± 0.04**
6.18 ± 0.14***
6.19 ± 0.09***
6.23 ± 0.08***
BUN (mg/dL)
28.70 ± 1.52
40.45 ± 1.76###
39.45 ± 0.66 ns
34.85 ± 0.82**
33.95 ± 0.64**
32.18 ± 1.54**
31.62 ± 1.79**
Albumin (gm/dL)
2.52 ± 0.06
5.35 ± 0.05###
4.89 ± 0.02*
4.85 ± 0.01*
2.70 ± 0.02***
3.26 ± 0.05***
2.78 ± 0.03***
Globulin (gm/dL)
3.40 ± 0.33
6.75 ± 0.25###
5.18 ± 0.24*
3.26 ± 0.04***
4.60 ± 0.17**
3.70 ± 0.08***
3.14 ± 0.01***
Total Bilirubin (mg/dL)
0.11 ± 0.01
0.25 ± 0.06###
0.23 ± 0.02 ns
0.18 ± 0.01*
0.12 ± 0.08**
0.17 ± 0.05*
0.14 ± 0.04**
Direct Bilirubin (mg/dL)
0.03 ± 0.03
0.09 ± 0.08###
0.08 ± 0.07 ns
0.07 ± 0.004 ns
0.06 ± 0.007*
0.05 ± 0.003*
0.04 ± 0.005**
Oxidative maker
Normal Control
Toxic Control
SBM LD
SBM MD
SBM HD
Ascorbic acid
α-Keto Analogues
ALP (U/L)
138.21 ± 3.62
186.81 ± 2.68###
175.61 ± 3.05*
172.44 ± 3.16*
139.11 ± 4.26**
171.33 ± 0.85**
125.65 ± 1.46***
ASAT (U/L)
160.12 ± 3.09
212.73 ± 3.66###
208.23 ± 2.69 ns
193.5 ± 1.25*
183.71 ± 1.89**
180.52 ± 0.66**
182.71 ± 1.09**
ALAT (U/L)
24.38 ± 1.49
68.40 ± 1.47###
65.11 ± 2.33 ns
60.78 ± 1.80*
43.99 ± 0.28**
47.47 ± 0.33**
44.82 ± 0.71**
Calcium (mg/dL)
34.90 ± 1.75
23.92 ± 1.43###
22.83 ± 0.45 ns
28.43 ± 0.67**
27.25 ± 0.75**
29.52 ± 0.51**
30.68 ± 0.69***
Phosphorus (mg/dL)
5.73 ± 0.19
2.75 ± 0.26###
2.34 ± 0.51 ns
4.71 ± 0.14**
5.28 ± 0.22***
4.88 ± 0.15**
5.53 ± 0.15***
Magnesium (mg/dL)
34.40 ± 1.52
23.92 ± 1.43###
26.83 ± 0.45*
28.43 ± 0.67**
29.42 ± 0.45**
27.68 ± 0.69***
28.22 ± 0.75**
Sodium (mmoI/I)
146.91 ± 3.93
105.44 ± 1.42###
103.02 ± 1.91*
118.3 ± 2.07**
121.52 ± 0.91**
129.11 ± 1.46**
145.63 ± 1.28***
Potassium(mmoI/I)
4.65 ± 0.13
2.48 ± 0.13###
2.92 ± 1.22 ns
3.30 ± 0.17*
3.99 ± 0.15***
3.55 ± 0.03***
3.37 ± 0.15**
3.5.2 Effect of SBM on urine biochemical markers
Administration of CP resulted in significant (p < 0.05) alteration in kidney function urine biomarkers such as creatinine, urea, uric acid and electrolytes compared to the control group. Treatment of all three doses of SBM exhibited significant (p < 0.05) normalization of all the kidney function parameters as compared to CP group (Table 4). Data are expressed as mean ± SEM (n = 6). One-way ANOVA followed by Tukey's multiple comparisons test. Compared to normal control group: ### P < 0.001; Compared to CP control group: *P < 0.05, **P < 0.01, ***P < 0.001; ns p > 0.5. SBM LD-Sharbat-e-Bazoori Low Dose; SBM MD- Sharbat-e-Bazoori Medium Dose; SBM HD- Sharbat-e-Bazoori High Dose.
Urine Parameter
Normal Control
Toxic Control
SBM LD
SBM MD
SBM HD
Ascorbic acid
α-Keto Analogues
Creatine (mg/dL)
2.51 ± 0.20
0.53 ± 1.19###
0.52 ± 0.09*
0.60 ± 0.03*
1.61 ± 0.08***
0.55 ± 0.04*
1.39 ± 0.05***
Urea (mg/dL)
63.33 ± 0.65
22.22 ± 0.42###
20.17 ± 1.07 ns
36.00 ± 1.73**
54.10 ± 0.79***
34.98 ± 1.71**
58.83 ± 1.01***
Uric (mg/dL)
2.60 ± 0.16
8.15 ± 0.38###
6.18 ± 0.04*
6.41 ± 0.11*
5.32 ± 0.15**
6.21 ± 0.09*
5.60 ± 0.09**
Calcium (mg/dL)
3.56 ± 0.15
7.46 ± 0.16###
6.0.42 ± 0.12*
6.51 ± 0.06*
5.44 ± 0.13**
5.38 ± 0.14***
5.32 ± 0.06**
Phosphorus (mg/dL)
1.52 ± 0.05
4.36 ± 0.01###
3.91 ± 0.12*
3.25 ± 0.02**
2.53 ± 0.05***
3.45 ± 0.01**
2.73 ± 0.05***
Magnesium (mg/dL)
24.85 ± 0.90
51.22 ± 1.42###
50.88 ± 1.55 ns
50.85 ± 2.31 ns
36.33 ± 1.28**
50.85 ± 2.58 ns
36.50 ± 0.30**
Sodium (mmoI/I)
83.32 ± 0.95
128.71 ± 0.49###
116.76 ± 0.98*
115.33 ± 1.11*
92.25 ± 0.77***
114.95 ± 1.05*
90.08 ± 1.11***
Potassium (mmoI/I)
2.91 ± 0.10
1.44 ± 0.13###
1.46 ± 0.08 ns
1.78 ± 0.05*
2.80 ± 0.15***
1.68 ± 0.16*
1.80 ± 0.03**
3.5.3 Effect on antioxidant oxidative stress parameter
Rat Kidney treated with CP had significantly higher (p < 0.05) MDA and NO levels, while SOD, CAT, GPx and GSH activities were significantly lower compared to the control group. Furthermore, pre-oral administration of all three doses of SBM, ascorbic and α-keto-analogue increase CAT, GPx, GSH and SOD (p < 0.05) level compare with CP group. Whereas, the treatment of ascorbic acid, α-ketoanalogue and all three doses of SBM significantly (p < 0.05) decrease the level of MDA and NO (Fig. 4a–f).![Effect of SBM on GPx [a], GSH [b], CAT [c], SOD [d], MDA [e] and NO [f] in CP-induced nephrotoxicity. Data are expressed as mean ± SEM (n = 6). One-way ANOVA followed by Tukey's multiple comparisons test. Compared to normal control group: ### P < 0.001; Compared to CP control group: The statistical significance level was expressed at *P < 0.05, **P < 0.01, ***P < 0.001; ns p > 0.5.](/content/185/2022/34/3/img/10.1016_j.jksus.2022.101839-fig4.png)
Effect of SBM on GPx [a], GSH [b], CAT [c], SOD [d], MDA [e] and NO [f] in CP-induced nephrotoxicity. Data are expressed as mean ± SEM (n = 6). One-way ANOVA followed by Tukey's multiple comparisons test. Compared to normal control group: ### P < 0.001; Compared to CP control group: The statistical significance level was expressed at *P < 0.05, **P < 0.01, ***P < 0.001; ns p > 0.5.
3.5.4 Effect on inflammatory markers in kidney
CP treated rat showed significant (p < 0.05) higher levels of TNF-α, IL-1β and caspase-3 as compared with the normal control group. However, SBM potentially ameliorates the CP induces inflammatory stress. While the group co-administered ascorbic acid and α-ketoanalogue showed significant (p < 0.05) amelioration in oxidative stress induced by CP (Fig. 5a–c).![Effect of SBM on TNF-α [a], IL-1β [b], and Caspase-3 [c], in CP-induced nephrotoxicity. Data are expressed as mean ± SEM (n = 6). One-way ANOVA followed by Tukey's multiple comparisons test. Compared to normal control group: ### P < 0.001; Compared to CP control group: The statistical significance level was expressed at *P < 0.05, **P < 0.01, ***P < 0.001; ns p > 0.5.](/content/185/2022/34/3/img/10.1016_j.jksus.2022.101839-fig5.png)
Effect of SBM on TNF-α [a], IL-1β [b], and Caspase-3 [c], in CP-induced nephrotoxicity. Data are expressed as mean ± SEM (n = 6). One-way ANOVA followed by Tukey's multiple comparisons test. Compared to normal control group: ### P < 0.001; Compared to CP control group: The statistical significance level was expressed at *P < 0.05, **P < 0.01, ***P < 0.001; ns p > 0.5.
3.5.5 Effect of SBM on renal histological changes in Wistar albino rats
After administration of SBM, the altered renal morphology was found to be significantly restored to normal. The morphology of Bowman's capsule, proximal tubule, and distal tubule de-structured brush border cells and epithelial cells drastically changed after two days of cisplatin therapy. SBM, ascorbic acid and α-ketoanalogue restored the kidney architecture against CP group (Fig. 6).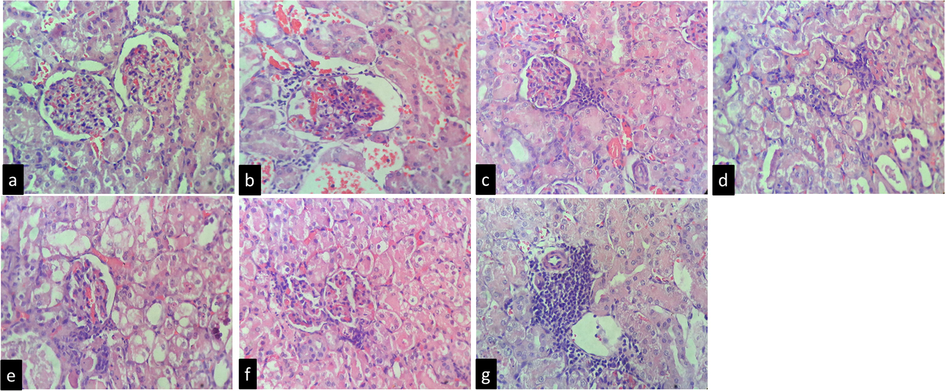
Histopathological study of renal tissues of experimental animals (n = 6). No changes were noticed in the normal control group (a); The kidney samples of the CP (toxic control group) showed periglomerular mild infiltrate in inflammatory cells of few glomeruli. (b); The renal treated with low dose of SBM cortex showed numerous normal glomeruli having normal capillary loops, vascular endothelial cell swelling, mild interstitial congestion and edema, and multifocal mixed inflammatory infiltrates (c); kidney sample treated from medium dose of SBM, cortex showed numerous normal glomeruli having normal capillary loops, mesangial cells and mesangial matrix deposition is normal and mild infiltrate in inflammatory cells (d); The renal treated with high dose of SBM showed normal glomeruli, mesangial cells and mesangial matrix deposition is normal. (e); The kidney samples of ascorbic acid group cortex showed numerous normal glomeruli having normal capillary loops, mesangial cells and mesangial matrix deposition is normal. Proximal (f); The kidney section of α-ketoanalogue cortex showed numerous normal glomeruli having normal capillary loops, mesangial cells are not increased and mesangial matrix deposition is normal. Many glomeruli showed periglomerular mild infiltrate in inflammatory cells. Focal areas in cortex (near PCT) showed mild inflammatory cells infiltrate (g).
4 Discussion
This is the first study that investigated the nephroprotective effects of sugar-free SBM formulation against CP-induced nephrotoxicity along with it its quality control evaluation and generated scientific evidence for its regulatory purpose. This preliminary study was carried out to characterize the formulation based on their metabolomic profile including phenolic and flavonoid and the results showed that SBM is enriched in polyphenols. Polyphenols play a supportive role in nephroprotection, antioxidant and immunomodulatory effects and improve the kidney function (Zahiruddin et al., 2022), (Gautam et al., 2021). Our results for fingerprinting of SBM by HPTLC revealed about 8–11 metabolites at different wavelengths, where few of them, namely caffeic acid and t-ferulic acid was the major metabolites.
Moreover, the protective effect of SBM was evaluated against CP-induced nephrotoxicity and oxidative stress on HEK-293 cells. The results of the study showed that SBM has a strong protective effect in HEK-293 cells against cisplatin-induced nephrotoxicity and oxidative stress. It is reported, antioxidant drug quenches the action of free radicals induced by oxidative stress (Grauzdytė et al., 2018). Furthermore, it can be suggested that cisplatin-induced intracellular ROS production on HEK-293 cells was significantly reversed by SBM which was estimated by DCF fluorescence analysis.
In morphological analysis, CP leads to several phenotypic changes on HEK-293 cells such as cell shrinkage, rounded phenotype, poor cell adhesion, and cell number reduction similarly as reported in previous findings (Mi et al., 2018). SBM exhibits strong reversal effect against CP toxicity. In vitro verification of the antioxidant activity of plant is more important before in vivo verification (Yu et al., 2021). In our experimental findings, the sugar-free formulation of SBM has strong efficacy as antioxidants due to enriched polyphenols as confirmed in our preliminary experimental studies on HEK-293 cells. The findings support our DPPH, reducing power and TAC potential of sugar-free SBM. The previous finding reveals that caffeic acid and ferulic acid are strong candidates to quench the free radicals induced by oxidative stress along with reduces inflammation induced by oxidative stress (Dhanisha et al., 2021). Our finding of ferulic acid in the formulation also support the previous finding, reduction in oxidative stress and increases the antioxidative status of the SBM (Stompor-Gorący and Machaczka, 2021).
Moreover, in vivo experimental studies for the nephroprotective potential of SBM were evaluated against nephrotoxicity induced by CP in Wistar rats. Two days treatment with CP caused severe nephrotoxicity as evident by biochemical and histological studies. Our result revealed that CP causes significant reduction in the body weight, this might be due to low intake of food, breakdown of muscle and tissue protein as indicated by increased levels of creatinine, urea, uric acid, BUN, total protein, albumin and electrolytes (Alsuhaibani, 2018). The administration of SBM significantly increase the experimental weight loss with decrease in the levels of ALT, AST, ALP, TB, and BD in CP treated group. SBM significantly reduced acute CP-induced nephrotoxicity by restoring the altered levels of electrolytes (magnesium, calcium and phosphorus) in urine and serum (Kpemissi et al., 2019).
Our study revealed that CP induced oxidative stress by increased MDA and NO levels and decreased (p < 0.05) in CAT, GPx, GSH and SOD levels in renal tissue. MDA is formed by ROS-induced lipid peroxidation and is commonly used as a biomarker of oxidative stress, increased MDA level indicated damage to kidney tissue and altered membrane function. NO is a biological mediator involved in several physiological functions, increased NO in response to CP exposure suggests induction of nitrosative stress response, likely due to upregulation of Nos2. GSH is a cellular tripeptide decrease in renal GSH level in response to CP exposure and inhibition of accumulation of MDA. The antioxidant enzymes such as SOD, CAT, GSH and GPx play a fundamental role in the elimination of reactive oxygen and nitrogen species, which is responsible for cellular oxidative damage. CP decreased in SOD, CAT, GSH and GPx levels and increased in MDA and NO levels due to renal damage by oxidative stress. However oral administration of SBM (128.4 mg/kg and 64.2 mg/kg), ascorbic acid and α-keto analogue reverses the functional ability of SOD, CAT, GSH and GPx against CP-induced toxicity while SBM (32.1 mg/kg) was not found significantly effective against toxicity.
The increase in levels of TNF-α, IL-1β and caspase-3 play an imperative role in pathophysiological changes in the kidney (Barnett and Cummings, 2019). The experimental outcome suggests significant elevated level of TNF-α, IL-1β, and caspase-3 in CP group, whereas the treatment with SBM was found to restore the elevated level of inflammatory markers (Topcu-Tarladacalisir et al., 2016). Thus, our results along with prior evidences confirmed the involvement of multiple nephroprotective mechanism including antioxidant, anti-inflammatory and anti-apoptosis activity of SBM against CP-induced nephrotoxicity (Zaman et al., 2017). Furthermore, the examination of histopathological studies confirms cellular degeneration, inflammatory cell infiltrate in the cortex and tubular cast indicates impair glomerular filtration and periglomerular mild inflammatory cells infiltrate. In contrast, the oral administration of SBM (128.4 mg/kg) strongly reversed the deleterious effect of CP to nearly normal. All the experimental findings confirm significant nephroprotective potential of sugar-free SBM against acute and or chronic kidney dysfunction by ameliorating oxidative and inflammatory stress.
5 Conclusion
Sugar-free SBM was found as an effective formulation in CP-induced nephrotoxicity established in both in vitro and in vivo models. Our finding also suggests that sugar-free SBM can be considered as a safe nephroprotective supplement during CP chemotherapy. Sugar-free formulation of SBM can be considered, as a therapeutic option in diabetic nephropathy and in CKD but still a deeper scientific justification is necessary to validate its beneficial properties.
Acknowledgement
The author would like to acknowledge Indian Council Medical Research (ICMR) for providing scholarship to Grant No. 3/1/2/126/2019-(Nut) to carry out their research work. The corresponding author is also thankful to Ministry of AYUSH, Govt of India for providing funding for infrastructure at Bioactive Natural Product Laboratory, Jamia Hamdard.
Conflicts of interest
The authors declare no conflicts of interest.
References
- Diuretic and nephroprotective effect of Jawarish Zarooni Sada - A polyherbal unani formulation. J. Ethnopharmacol.. 2004;91(2-3):219-223.
- [CrossRef] [Google Scholar]
- Nephroprotective effect of Kabab chini (Piper cubeba) in gentamycin-induced nephrotoxicity. Saudi J. Kidney Dis. Transpl.. 2012;23(4):773.
- [CrossRef] [Google Scholar]
- Pharmacological Evaluation of Safoof-e-Pathar Phori- A Polyherbal Unani Formulation for Urolithiasis. Front. Pharmacol. 2021
- [CrossRef] [Google Scholar]
- Aliyu, A.B., Ibrahim, M.A., Musa, A.M., Musa, A.O., Kiplimo, J.J., Oyewale, A.O., 2013. Free radical scavenging and total antioxidant capacity of root extracts of Anchomanes Difformis ENGL. (ARACEAE). Acta Pol. Pharm. - Drug Res. 70,115-21. https://doi.org/ 236274968/0001-6837.
- Protective role of alchoholic extract of fennel seed in nephrotoxicity induced by cisplatin in male rabbits. Biochem. Cell. Arch.. 2018;18(1–6)
- [Google Scholar]
- Effect of nigella sativa against cisplatin induced nephrotoxicity in rats. Ital. J. Food Saf.. 2018;7(2):7242.
- [CrossRef] [Google Scholar]
- Azhar, M., 2018. Effect of herbal unani formulation on nephrotic syndrome: A case study. Indian J. Tradit. Knowl. 17,807-810. . https://doi.org/10.1016/j.etap.2017.06.026.
- Cellular and molecular mechanisms of kidney toxicity. Semin. Nephrol.. 2019;39(2):141-151.
- [CrossRef] [Google Scholar]
- Chester, K., Zahiruddin, S., Ahmad, A., Khan, W., Paliwal, S., Ahmad, S., 2017. Bioautography-based Identification of Antioxidant Metabolites of Solanum nigrum L. and Exploration Its Hepatoprotective Potential against D-Galactosamine-induced Hepatic Fibrosis in Rats. Pharmacogn. Mag. 62,104-110. https://doi.org/10.4103/pm.pm.
- Dhanisha, S., Drishya, S., Mony, R., Guruvayoorappan, C., 2021. Polyphenolic-rich fraction of Pithecellobium dulce attenuates methotrexate-induced oxidative stress and associated tissue injury by regulating the TNF-α, IL-1β and IL-6 pro-inflammatory cytokines . Int. J. Funct. Nutr. https://doi.org/10.3892/ijfn.2021.17.
- TLC-MS bioautography-based identification of free-radical scavenging, α-amylase, and α-glucosidase inhibitor compounds of antidiabetic tablet BGR-34. ACS Omega.. 2020;5(46):29688-29697.
- [CrossRef] [Google Scholar]
- A systematic review on nephron protective AYUSH drugs as constituents of NEERI-KFT (A traditional Indian polyherbal formulation) for the management of chronic kidney disease. Saudi J. Biol. Sci. 2021
- [CrossRef] [Google Scholar]
- Protective effects of Phyllanthus phillyreifolius extracts against hydrogen peroxide induced oxidative stress in HEK293 cells. PLoS One. 2018;13(11):e0207672.
- [CrossRef] [Google Scholar]
- Khaliq, T., Mumtaz, F., Zia-ur-Rahman, Javed, I., Iftikhar, A., 2015. Nephroprotective potential of Rosa damascena mill flowers, Cichorium intybus linn roots and their mixtures on gentamicin-induced toxicity in albino rabbits. Pak. Vet. J. 35(1) 43–47. https://doi.org/ 0253-8318/ 270824099.
- Hypoglycemic potential of aqueous extract of Moringa oleifera leaf and in vivo GC-MS metabolomics. Front. Pharmacol.. 2017;8:577.
- [CrossRef] [Google Scholar]
- Mechanisms of carcinogenesis focus on oxidative stress and electron transfer. Curr. Med. Chem.. 2012;8:773-796.
- [CrossRef] [Google Scholar]
- Nephroprotective activity of Combretum micranthum G. Don in cisplatin induced nephrotoxicity in rats: In-vitro, in-vivo and in-silico experiments. Biomed. Pharmacother.. 2019;116:108961.
- [CrossRef] [Google Scholar]
- The renoprotective activity of hesperetin in cisplatin induced nephrotoxicity in rats: Molecular and biochemical evidence. Biomed. Pharmacother.. 2017;89:1207-1215.
- [CrossRef] [Google Scholar]
- Ameliorative effect of daidzein on cisplatin-induced nephrotoxicity in mice via modulation of inflammation, oxidative stress, and cell death. Oxid. Med. Cell. Longev.. 2017;2017:1-10.
- [CrossRef] [Google Scholar]
- The protective effects of maltol on cisplatin-induced nephrotoxicity through the AMPK-mediated PI3K/Akt and p53 signaling pathways. Sci. Rep.. 2018;8(1)
- [CrossRef] [Google Scholar]
- Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr.. 1986;44:307-315.
- [CrossRef] [Google Scholar]
- Prasanthi, D., Adikay, S., 2016. Amelioration of cisplatin and gentamicin -induced nephrotoxicity by seeds of Cucumis sativus. Int. J. Pharma Bio 7(4):245-53. Sci.https://doi.org/10.22376/ijpbs.2016.7.4.p245-253.
- Rezaee-Khorasany, A., Razavi, B.M., Taghiabadi, E., Yazdi, A.T., Hosseinzadeh, H., 2020. Effect of crocin, an active saffron constituent, on ethanol toxicity in the rat: Histopathological and biochemical studies. Iran. J. Basic Med. Sci. https://doi.org/10.22038/IJBMS.2019.37133.8845.
- Antioxidant and protective effect of Stachys pilifera Benth against nephrotoxicity induced by cisplatin in rats. J. Food Biochem.. 2020;44
- [CrossRef] [Google Scholar]
- HPLC analysis and in vivo renoprotective evaluation of hydroalcoholic extract of cucumis melo seeds in gentamicin-induced renal damage. Med.. 2019;55(4):107.
- [CrossRef] [Google Scholar]
- Antiurolithiasis activity of bioactivity guided fraction of Bergenia ligulata against ethylene glycol induced renal calculi in rat. Biomed. Res. Int.. 2017;2017:1-11.
- [CrossRef] [Google Scholar]
- Sohn, S.H., Lee, H., Nam, J. young, Kim, S.H., Jung, H.J., Kim, Y., Shin, M., Hong, M., Bae, H., 2009. Screening of herbal medicines for the recovery of cisplatin-induced nephrotoxicity. Environ. Toxicol. Pharmacol. 28,206-212. https://doi.org/10.1016/j.etap.2009.04.005.
- Recent advances in biological activity, new formulations and prodrugs of ferulic acid. Int. J. Mol. Sci.. 2021;22:12889.
- [CrossRef] [Google Scholar]
- Curcumin counteracts cisplatin-induced nephrotoxicity by preventing renal tubular cell apoptosis. Ren. Fail.. 2016;38(10):1741-1748.
- [CrossRef] [Google Scholar]
- World Health Organization, 2018. Bulletin of world health organisation 96, 369-440.
- Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep.. 2021;11
- [CrossRef] [Google Scholar]
- Metabolomic Profiling and Immunomodulatory Activity of a Polyherbal Combination in Cyclophosphamide-Induced Immunosuppressed Mice. Front. Pharmacol.. 2022;12:647244
- [CrossRef] [Google Scholar]
- TLC-Based Metabolite Profiling and Bioactivity-Based Scientific Validation for Use of Water Extracts in AYUSH Formulations. Evid.-Based Complementary Altern. Med. 2021 Article ID 2847440
- [CrossRef] [Google Scholar]
- Zaman, R., Alam, A., Jafri, M.A., Sofi, G., Ahmad, G., 2017. Nephroprotective effect of Beekh Kasni (Roots of Cichorium intybus) in the form of methanolic and aqueous extract in Gentamycin induced rat models 6, 337–341. https://doi.org/ 337730901.
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.101839.
Appendix A
Supplementary data
The following are the Supplementary data to this article: