Nanomedicine potential of Cymbopogon citratus Linn. –Biogenic synthesized silver nanoparticles: A study on antimicrobial and anticancer efficacy
⁎Corresponding authors at: Department of Botany and Microbiology, College of Science, King Saud University, P.O. Box – 2455, Riyadh 11451, Saudi Arabia (P. Karuppiah); Department of Biomedical Science, Faculty of Science, University Tunku Abdul Rahman, Jalan University, Bandar Barat, Kampar 31900, Malaysia (S. Djearamane). pkaruppiah@ksu.edu.sa (Ponmurugan Karuppiah), sinouvassane@utar.edu.my (Sinouvassane Djearamane)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Green synthesis methods for making nanoparticles using plant extracts have gained considerable attention in recent years, particularly because of their potential applications in nanomedicine. Plant biosynthesized nanoparticles have shown noteworthy biomedical uses in comparison. The silver nanoparticle (AgNP) synthesized using extracts from the leaves and flowers of Cymbopogon citratus Linn was investigated for its antibacterial and anticancer properties. The biosynthesized AgNPs were characterized using UV–Vis, FTIR, DLS, EDX and SEM. The presence of alcohol, alkene, and phytochemicals in the AgNPs was confirmed using FTIR analysis. The investigation using SEM with EDX spectroscopy confirmed that the AgNPs were both pure and in the form of nanocrystals. Furthermore, AgNPs demonstrated significant antibacterial activity by efficiently suppressing bacterial growth. The biosynthesized AgNPs exhibited a concentration-dependent reduction in the lung cancer-A549 cell growth. The ethanolic extracts of C. citratus flower-synthesized AgNPs contain a wide range of phytochemical constituents. These components have been shown to effectively modulate antibacterial and anticancer activities, superior to the effects of leaf extracts synthesized AgNPs. Consequently, additional research is required to investigate the potential biomedical applications of these biosynthesized AgNPs.
Keywords
Cymbopogon citratus
Green biosynthesis
Silver nanoparticles
Antibacterial activity
Anticancer activity
1 Introduction
Nanoparticles (NPs) are employed in a wide range of applications, with molecular imaging, health management, pollution remediation and detection, biosensors, aquaculture, and agriculture (Raza et al., 2023). NPs display innovative biomedical properties due to their greater surface-to-volume ratio, chemical stability, conductivity, and distinct size (Hussein et al., 2022). NPs are classified into organic or inorganic. An organic NP can be composed of polymers, dendrimers, liposomes, or micelles, whereas an inorganic NP can be composed of metals and quantum dots, among others. The antimicrobial, photocatalytic, physical, and chemical properties of metallic nanoparticles have attracted considerable attention (Balciunaitiene et al., 2021; George et al., 2022). Currently, scientists have shown great attention to silver nanoparticles (AgNPs) due to their diverse biological activities including significant antibacterial properties. AgNPs are widely used in numerous applications including sensors, optics, and antimicrobial agents (Anjum et al., 2019).
AgNPs can be synthesized via several methods, including reducing, thermal decomposition, microwave, laser, and biological regeneration methods (Sellami et al., 2021). Recently, there has been significant interest in the green production of NPs using living organisms and natural resources viz. plants, fungi and bacteria (Ovais et al., 2016). The synthesis platform of NPs that utilizes plants provides a more efficient and eco-friendly approach, eliminating the need for toxic or expansive substances (Ovais et al., 2016). The presence of diverse biomolecules in plants enhances the stability of NPs synthesized from plants and assists as a protective layer for these NPs, resulting in significant effects on microbial pathogens (Anand et al., 2020). Additionally, this method is cost-effective, more efficient than chemical synthesis, and widely available. It significantly reduces the use of toxic chemicals (eco-friendly) and biomolecules derived from plant extracts, including organic ligands, polysaccharides, and proteins, to enhance biological activity, including antibacterial, antifungal, and anticancer activities (Makarov et al., 2014). Several secondary metabolites found in plants, including flavonoids, proteins, and terpenes, are mainly responsible for contributing to eco-friendly, nonhazardous processes for the biosynthesis of AgNPs (Guilger-Casagrande and de Lima, 2019).
The aromatic plants used as a remedy for various alignments have gradually grown worldwide in primary healthcare systems (Theodoridis et al., 2023), and identifying novel plant components has been shown not to harm agriculture, animals, or human systems (Vaou et al., 2021). C. citratus, commonly referred to as lemongrass, Cochin grass, and Malabar grass, is a medicinal plant belonging to the Poaceae family. It has a long past of use as a traditional medicine for many centuries (Gautam and Agrawal, 2017). Tropical regions such as India, Bangladesh, Burma, and Sri Lanka are the primary producers of this substance. It has a long-standing traditional use in treating fevers, specifically typhoid and malaria, as well as mental disease and gastrointestinal ailments. Additionally, it establishes anti-inflammatory, anticancer, antioxidant, and sedative features (Tibenda et al., 2022). Citronellal is the primary phytocompound present in C. citratus, and it is mostly responsible for the lemon fragrance seen in different plants. A number of other chemicals have been identified in C. citratus such as neochlorogenic acid, quinic acid, chlorogenic acid, luteolin and p-coumaric acid (Costa et al., 2015). In addition, infusions made from the leaf and petals of C. citratus are utilized to treat a range of conditions, including skin infections, migraines, and hepatitis. Furthermore, essential oils derived from C. citratus are employed in the practice of aromatherapy (Ekpenyong et al., 2015). This study aimed to assess the antibacterial and anticancer activities of AgNPs produced through green biosynthesis from C. citratus ethanolic extracts of leaves and flowers.
2 Materials and methods
2.1 Preparation of plant extracts
Healthy C. citratus plants were collected from India. The plant leaves and petals were meticulously sliced into little fragments and left to desiccate at 37 °C. The samples were turned into powders and analyzed. The ethanolic extract was prepared according to the standard protocol proposed by the Indian Pharmacopoeia. The extraction was performed in 1-liter ethanol using Soxhlet. The extracted solution was removed and dried under reduced pressure (40–50 °C) (Harborne, 1973). It was subsequently stored in airtight vessels in a refrigerator.
2.2 Biosynthesis and characterization of AgNPs
A conical flask was used to mix 750 mL of 1 mg/mL silver nitrate with 7.5 mL of the preserved C. citratus ethanolic extracts. It was subsequently exposed to sunlight to facilitate the formation of AgNPs. When the sample combination was exposed to sunlight, it changed colour, and turned brown and the particles in the mixture settled at the bottom of the flasks. The colour change observed was indicative of the formation of AgNPs. The biosynthesized AgNPs were characterized by UV–Vis spectroscopy, FTIR, Zeta potential analysis, DLS, EDX, and SEM.
2.2.1 UV–Vis spectrometric study of synthesized AgNPs
The biosynthesized AgNPs were studied with a dual-beam UV–Vis 2000 spectrophotometer (Lab India, Chennai) which had a resolution of 0.5 nm and operated within the wavelength range of 200–800 nm. It is applied to characterize and confirm the existence of AgNPs. The absorption spectra of the biosynthesized AgNPs were attained and plotted.
2.2.2 FTIR study of the AgNPs
The biosynthesized AgNPs were examined with FTIR (Perkin Elmer, Frontier, USA). This study aimed to verify the presence of functional groups. The AgNPs were analyzed using transmission mode spectroscopy.
2.2.3 SEM and EDX examination
The structure of biosynthesized AgNPs was determined through SEM which obtained higher resolution images (Jeol, Tokyo, Japan). Additionally, the AgNPs were analyzed with EDX; JSM-6360, JEOL, Japan to obtain elemental and quantitative compositional data.
2.2.4 Dynamic light scattering (DLS) analyses of AgNPs
AgNPs were characterized using DLS and Zeta potential measurements to determine their surface charge, size, and stability. The Zetasizer Pro (Nanophox, Symantec GmbH, Germany) was used to obtain data on the polydispersity and Z-average size of the biosynthesized AgNPs.
2.3 Antibacterial efficacy of synthesized AgNPs
The antibacterial efficacy of AgNPs was determined with standard CLSI guidelines (CLSI, 2024). The culture medium was prepared using nutrient agar (NA). The bacterial strains Pseudomonas aeruginosa MTCC1035 and Staphylococcus aureus MTCC 1144 were procured from Gene Bank (MTCC), India. The molten NA was carefully poured into sterile Petri plates (20 mL) and allowed to solidify. The bacterial strains P. aeruginosa and S. aureus were swabbed homogeneously into NA plates. Then, the 6 mm agar wells were made with sterile cork borers. The initial well was loaded with 100 µL of plant extract (1 mg/mL), the second well was loaded with 100 µL of AgNPs (1 mg/mL), and the third was loaded with 100 µL of silver nitrate (1 mg/mL) solution. Finally, 10 µL of 1 % streptomycin was added to the fourth well as a positive control. The NA plates were then incubated at 37 °C for 24 h. After, incubation, a circular area devoid of growth, known as a zone of inhibition, was noticed surrounding each perforation and was measured in millimeters (mm). A negative control well was prepared for each experiment by filling it with only solvent. The values of the zones of inhibition of every treatment were compared to those of the negative control.
2.4 MTT assay
The lung cancer cell line (A549) was obtained from the National Centre for Cell Sciences Cell Repository in Pune, India. The cells were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10 % fetal bovine serum (FBS), 100 mg/mL streptomycin, and 100 U/mL penicillin. After that, the culture was maintained at 37 °C in a CO2 incubator with 5 % CO2. To facilitate cell adhesion, viable cells (104 cells/well) were carefully seeded into 96-well plates and then incubated in a CO2 incubator for 24 h. Later, during the 24-hour incubation period, the A549 lung cancer cells were treated to varying concentrations (2.5–12.5 μg/mL) of biosynthesized AgNPs. A 5 mg/mL solution of MTT was then added to each well, and the cells were incubated at 37 °C for an additional 4 h. A plate reader set at 540 nm was used to determine cell viability following incubation with purple formazan crystals. The % of live cells was calculated against the control.
2.5 Statistical study
In the statistical analysis, Duncan's multiple range test was used after a one-way analysis of variance (ANOVA, SPSS, USA) utilized the Statistical Package of Social Sciences (SPSS) software in Windows version 12.0. The five samples were considered from every group. The mean ± standard error (SE) was used to calculate the values. Significant hypotheses were selected at P < 0.05.
3 Results and discussion
3.1 Depiction of biosynthesized AgNPs
3.1.1 Structural analysis
The biosynthesized AgNP solutions were observed for colour change from yellowish green to brown when exposed to sunlight (Fig. 1). Fig. 1A and C show the ethanolic extracts of the leaves and flowers, which ranged from light yellowish green and dark green to brown, confirming the formation of AgNPs (Fig. 1B and D). The time required for a color change and the thickness of the NPs vary from plant to plant. Fig. 1 shows the time required for our study and the change in the pH from 4.0 to 4.6. The silver ions undergo a reduction inside the solution when mixed with plant extracts, which results in the formation of silver hydrosol and spherical structures (Fig. 1E and F). This reduction process occurs once silver ions are in contact with plant extracts, as evidenced by the change in color to brown (Putheti et al., 2008).

- Extraction and green synthesis of silver nanoparticles from C. citratus ethanolic solvent; (A and C) synthesis of silver nanoparticles using C. citratus leaf ethanolic extract; (B and D) synthesis of silver nanoparticles using C. citratus flower ethanolic extract; (E and F) synthesis of silver nanoparticle crystal structures in C. citratus leaf and flower ethanolic extracts.
Due to their characteristic features, their increased permeability, and antibacterial potential of AgNPs are very intriguing and helpful for a variety of applications (Bruna et al., 2021). Research has also revealed that silver is less dangerous for mammalian cells but more toxic to microorganisms than other metals (Oladeji et al., 2019). AgNPs have recently been found to be effectively synthesized environmentally as a viable substitute for chemical or physical processes. Biosynthesized AgNPs were characterized using FTIR, SEM, and UV–Vis spectroscopy.
According to the reaction mixture's color shift, C. citratus flower extracts may reduce silver nitrate through a variety of phytochemicals. The biosynthesis of AgNPs in this study was tentatively established by observing the colour shift of the reaction mixture. Following the addition of AgNO3, a colour shift was seen, and as time went on, the intensity grew, giving the mixture a brown hue. Ajayi and Afolayan, 2017; Chen et al., 2019; and other scientists on C. citratus reported similar results. Hence, the colour shift indicated the conclusion of the interaction between the C. citratus extracts and AgNO3.
3.1.2 UV–Vis spectroscopic analysis
The UV–Vis spectrum is shown in Fig. 2A and B, revealing that the ethanolic extract of C. citratus leaves produced AgNPs with an absorbance peak at 400 nm, whereas the ethanolic extract of C. citratus flowers resulted in an absorbance peak at 460 nm. The plant extracts combination changed color to brown upon the addition of metal ions at different concentrations, which may have been caused by the AgNPs' surface plasmon vibrations being excited (Kelly et al., 2003). In the current study, the UV–Vis spectrum revealed broad absorption peaks at 400 nm (leaf extract) and 460 nm (flower extract), which were specific to AgNPs. Consistent with our results, several studies have reported that the size of the AgNPs biosynthesized using C. citratus typically falls within the range of 435–470 nm range (Ajayi and Afolayan, 2017), 440 nm (Riyanto et al., 2022), 450 nm (Keshari et al., 2020), and 469 nm (Rakib-Uz-Zaman et al., 2022). In metallic particles, AgNPs form as a result of surface plasmon resonance, which is caused by collective oscillations of electrons. NPs' sizes, shapes, doses, agglomeration types, and refractive index determine the precise wavelength range of absorption in the visible region (Ider et al., 2017).
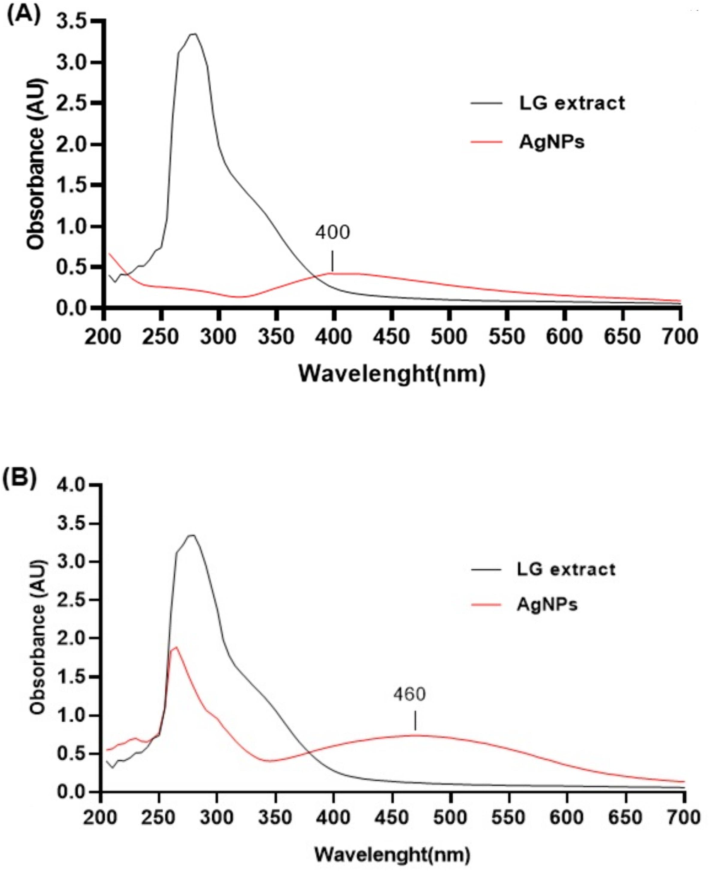
- Ultraviolet–visible spectroscopic analysis of C. citratus silver nanoparticles: (A) spectrum of silver nanoparticles synthesized from C. citratus leaf extract; (B) spectrum of silver nanoparticles synthesized from C. citratus flower extract.
3.1.3 FTIR analysis
The FTIR spectra of the ethanolic extracts from C. citratus leaves and flowers, as well as the corresponding AgNPs are presented in Fig. 3. The spectral analysis data showed various peaks, indicating the presence of active functional groups in the ethanolic extracts of C. citratus leaves and flower (Fig. 3A and B). The intense peaks were detected at 3400.43, 2339.17, 1629.36, 1417.63, 1043.17, 666.97, 571.00 and 410.96 cm−1 in the flower ethanolic extract and at 3423.95, 2079.96, 1637.21, 1404.73, 1105.47, 1017.17, 667.06 and 450.50 cm−1 in the leaf ethanolic extract. Leaf extract-synthesized AgNPs were detected at 3402.11, 2927.48, 2072.01, 1609.61, 1513.15, 1449.57, 1382.17, 1284.09, 1198.76, 1113.53, 880.61, 760.89, 657.52, 619.63 and 471.47 cm−1. There were very sharp, wide peaks at 3426.20, 2925.74, 2426.27, 1601.78, 1383.57, 1269.02, 924.10, 776.48 and 620.22 cm−1 for the flower extract-synthesized AgNPs compared to the leaf extract-synthesized AgNPs. The bands at 3426.20, 3402.11, and 3400.43 cm−1 indicated O–H stretching vibrations of alcohol, while the peaks at 2,927.48, 2426.27 and 2072.01 cm−1 indicated C–H in the plane band of alkenes. The peaks at 1609.61, 1601.78, 1449.57, 1383.57, and 1062.83 cm^-1 correspond to C-O and C-N stretching vibrations. The peaks at 880.61, 790.89, 776.48, 620.22, and 619.63 cm^-1 represent C–H and C-Br stretching vibrations associated with alkyl halides, which may indicate the presence of carboxylic acids, esters, ethers, aliphatic amines, and alcoholic groups. The unique occurrence of these IR peaks suggests the presence of polyphenols. The persistence of these IR bands indicates the presence of amide (I and II) groups, suggesting the presence of proteins and flavonoids that could contain compounds with pharmacological properties.
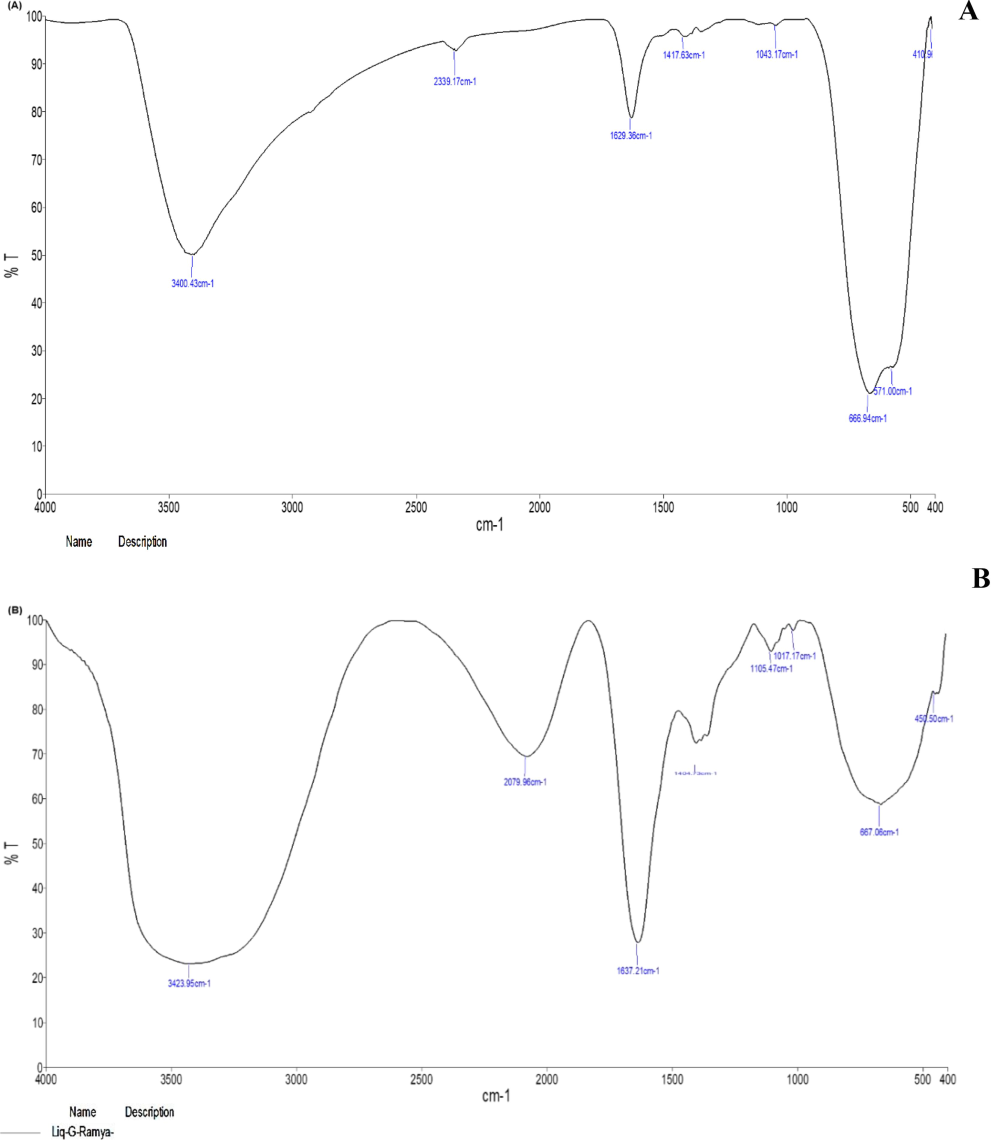
- FTIR spectral analysis of C. citratus leaf and flower ethanolic extracts and synthesized AgNPs; (A) C. citratus leaf extract; (B) C. citratus flower extract; (C) C. citratus leaf extract–silver nanoparticles; (D) C. citratus flower extract–silver nanoparticles.
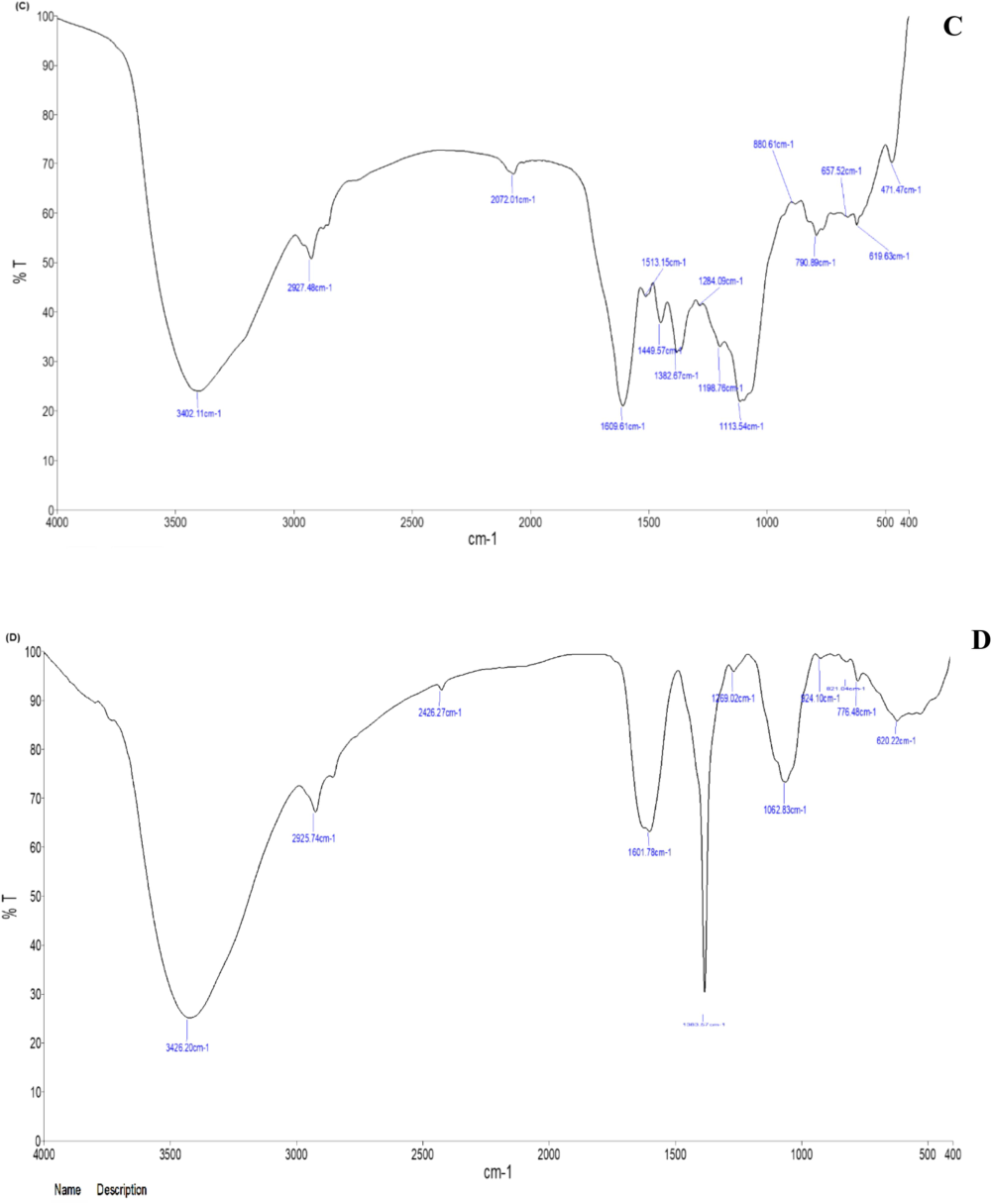
- FTIR spectral analysis of C. citratus leaf and flower ethanolic extracts and synthesized AgNPs; (A) C. citratus leaf extract; (B) C. citratus flower extract; (C) C. citratus leaf extract–silver nanoparticles; (D) C. citratus flower extract–silver nanoparticles.
The FTIR spectra of the C. citratus extract and the intense peak corresponding to the AgNPs proposed the presence of alcohol, alkenes, alkyl halides, carboxylic acids, esters, ethers, and aliphatic amines in the biosynthesized AgNPs. Additionally, IR peaks showed the presence of polyphenols, flavonoids, and proteins. Following our study, FTIR studies on C. citratus AgNPs have revealed the presence of ethylene, amides, and alcohols (Ajayi and Afolayan, 2017). Al-Otibi et al. (2023) reported that C. citratus contains alcohol, alkenes, nitro compounds, halo compounds, alkanes, amines, and allenes. An SEM analysis revealed the size and form of AgNPs and the nanocrystal morphology. A previous study supported the idea that nanocrystals exhibit a very high penetration potential in bacteria and exhibit more antibacterial effects than nano-cubes (Fang et al., 2018).
3.1.4 SEM and EDX
As shown by the SEM images, biosynthesized AgNPs were intense and silver nanostructures were formed. Fig. 4A displays SEM images of the biosynthesized AgNPs showing their size, shape, and dispersion. The AgNPs exhibited spherical and crystalline forms in the leaves extract and nanospheres in the floral extract, with a particle size of ∼ 100 nm. According to DLS measurements, the average diameter of the particles was 28.18 nm, and their polydispersity index (PDI) was 0.236 nm (Fig. 5A and B). The single peak indicated that the quality of the nanoparticles was good (the zeta potential was −4.09 mV, the zeta deviation was 5.87 mV, and the conductivity was 1.62 mS/cm, as shown in Fig. 5), and two potential peaks appeared for various-sized nanoparticles. EDX analysis recognized the presence of Ag, C, and O, verifying the occurrence of AgNPs in the flower extract (Fig. 6A, B, and Table 1). AgNPs synthesized from green sources are reported to be highly toxic to multidrug-resistant bacteria and might be useful in biomedical applications (Velusamy et al., 2015).
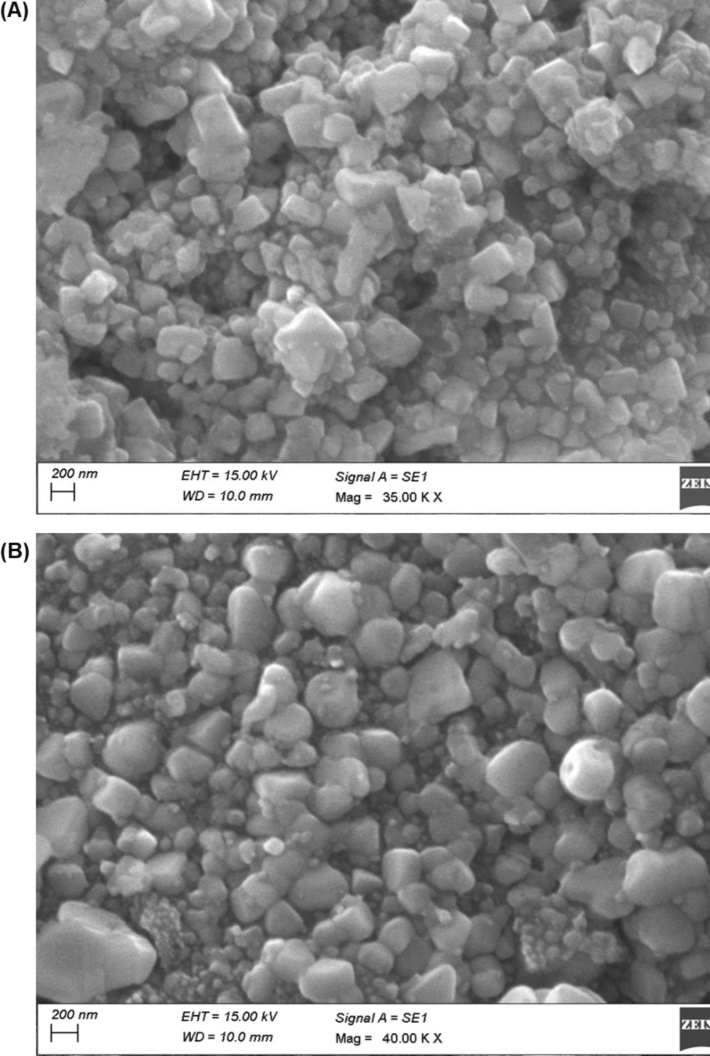
- SEM analysis; (A) representative images of silver nanoparticles- C. citratus leaves extract; (B) representative images of silver nanoparticles-C. citratus flower extract.
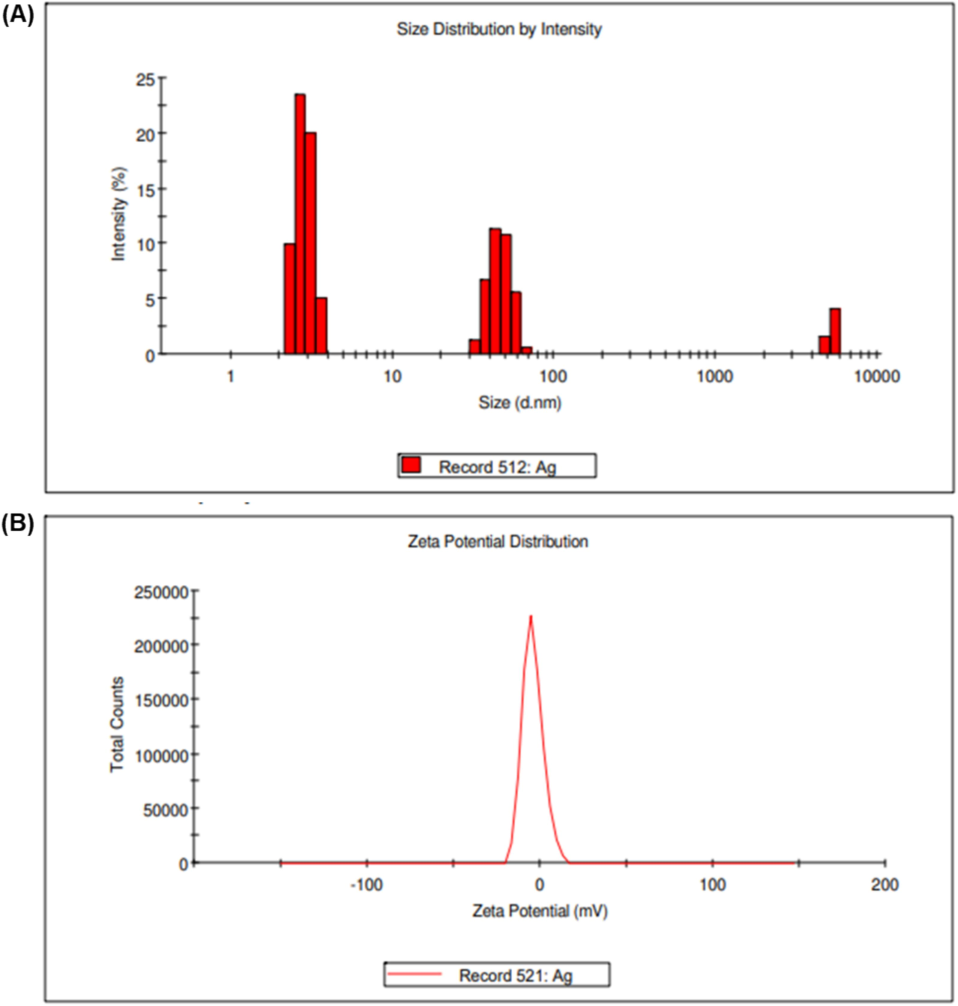
- Zeta potential analysis of C. citratus flower ethanolic extract–AgNPs; (A) Particle size distribution of C. citratus flower extract–silver nanoparticles; (B) Zeta potential analysis of C. citratus flower extract–silver nanoparticles.
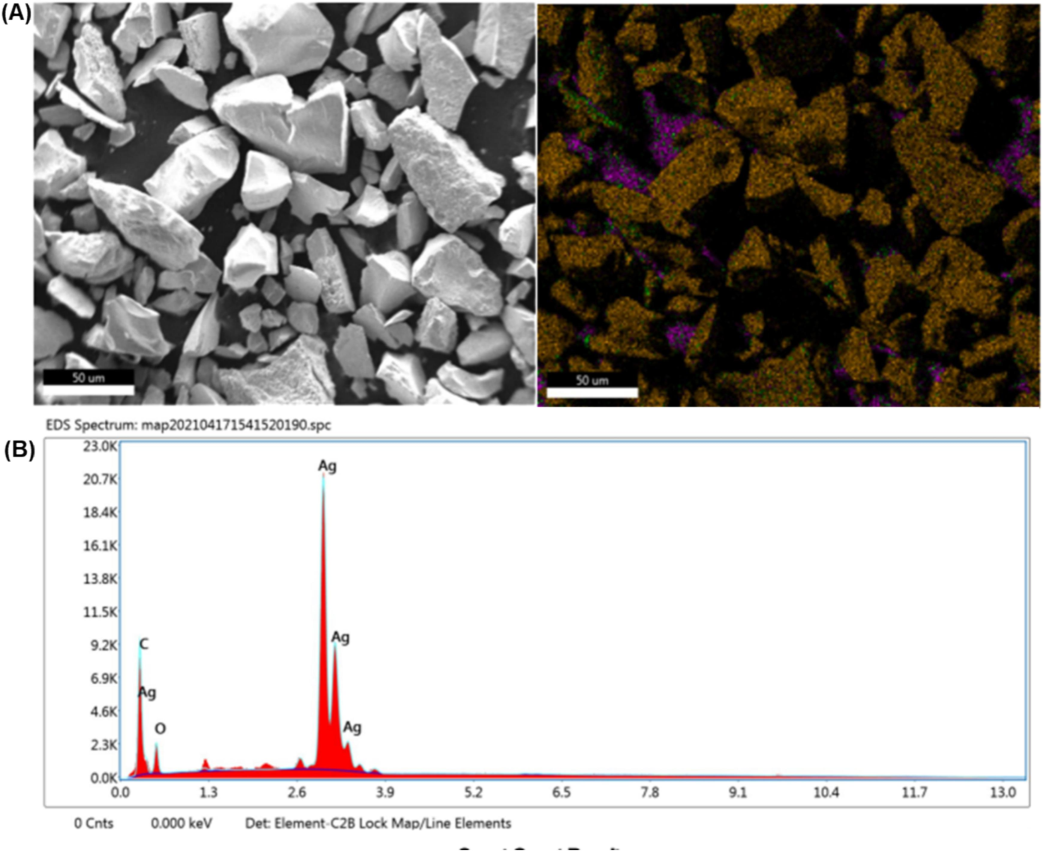
- SEM with 3D-EDX analysis of C. citratus flower extract–silver nanoparticles; (A) SEM 3D crystallography image; (B) elemental distribution of C. citratus flower extract–silver nanoparticles.
| Element | Weight % | Atomic % | Net Int. | Error % | K ratio | Z | A | F |
|---|---|---|---|---|---|---|---|---|
| CK | 10.27 | 38.38 | 550.61 | 4.11 | 0.1021 | 1.3551 | 0.7337 | 1.000 |
| OK | 10.17 | 28.52 | 154.65 | 11.28 | 0.0174 | 1.3037 | 0.1313 | 1.000 |
| AgL | 79.56 | 33.10 | 2639.25 | 1.52 | 0.7422 | 0.9091 | 1.0262 | 1.000 |
3.2 Antimicrobial activity
The preliminary results of AgNPs biosynthesized from leaves were not significant compared with flower AgNPs. Henceforth, the antimicrobial competence study was continued with biosynthesized AgNPs from flower (mg/mL) and the agar well diffusion technique was applied against two bacterial strains, namely S. aureus and P. aeruginosa. The inhibition zone of the biosynthesized AgNPs were 7.00 ± 0.52 and 15.00 ± 1.20 mm for S. aureus and 8.00 ± 0.60 and 16.00 ± 0.98 mm for P. aeruginosa at 100 µl of 1 mg/mL of flower extract and biogenic AgNPs from flower extract were reported respectively (Table 2). AgNPs synthesized from medicinal plants have been reported to be highly toxic to various pathogenic bacteria (Moosa et al., 2015). In this study, the AgNPs from C. citratus had the substanial antibacterial effects on the human pathogens tested, including S. aureus and P. aeruginosa.
| Bacterial Pathogens | Concentration (1 mg/ml) / Zone of inhibitions (mm) | |||
|---|---|---|---|---|
| AgNO3 | Flower extract | AgNPs from flower extract | Streptomycin | |
| 100 µl | 100 µl | 100 µl | 10 µL | |
|
S. aureus MTCC 1144 |
10.00 ± 0.83 | 7.00 ± 0.52 | 15.00 ± 1.20 | 20.05 ± 0.20 |
|
P. aeruginosa MTCC 1035 |
12.00 ± 0.92 | 8.00 ± 0.60 | 16.00 ± 0.98 | 19.50 ± 0.98 |
Bacterial infections are considered severe health issues that result in infectious diseases and death worldwide. Furthermore, bacterial infections' resistance to antimicrobial drugs is a global and national problem (MacDougall et al., 2005). The hunt for novel antibiotics that can fight antibiotic-resistant bacteria of S. aureus and P. aeruginosa is on as current medications are ineffective against bacterial illnesses. Recently, it has been reported that AgNPs possess antimicrobial effects on various human pathogens and multidrug-resistant bacteria (Siddiqi et al., 2018). In this study, it was observed that AgNPs synthesized from C. citratus extracts inhibited the growth of S. aureus and P. aeruginosa. According to studies, AgNPs bind to bacteria more effectively due to their increased surface-area-to-mass ratio. Additionally, these AgNPs disrupt bacterial cell membranes, alter respiration and metabolism, and increase the production of reactive oxygen species, thereby enhancing their antibacterial effects. (Cao et al., 2020). Additionally, it has been noted that AgNPs prevent the development of S. aureus biofilms. In antibiotic-resistant bacteria, AgNPs exert antibacterial effects by inducing reactive oxygen species production, stimulating lipid peroxidation, disrupting bacterial membrane disintegration, inhibiting cell wall synthesis, and intercalating with DNA (Bekele and Alamnie, 2022).
3.3 Anticancer activity
In vitro, the cytotoxicity of both AgNPs was tested in lung cancer A549 cells using the MTT assay. It's interesting to note that both AgNP treatments progressively reduced A549 cell viability in a dose-dependent way (Fig. 7A–D). The findings demonstrated that, at doses of 10 and 12.5 µg/mL, the ethanolic flower-extracted AgNPs exhibited more cytotoxic effects on lung cancer-A549 cells than did the ethanolic AgNPs synthesized from C. citratus leaf extract. The arrows indicate that both AgNP treatments caused cell death and fragmentation. In this study, both flower and leaf extracts of AgNPs exhibited significant cytotoxic activities with IC50 of 7.2 ± 0.54 and 8.6 ± 0.72 µg/mL respectively, comparatively flower extract showed more effective results. AgNPs were found to exert anticancer activity and reduce cell viability by engaging in physicochemical interactions between silver atoms and the functional groups of cellular proteins, as well as the nitrogenous base and phosphate groups of DNA molecules, as observed in a similar study (Satyavani et al., 2012). According to Chen et al. (2019), C. citratus‐AgNPs effectively improved proapoptotic proteins (Bax and Caspases) and reduced the expression of antiapoptotic proteins (Bcl‐2 and Bcl‐xL), thereby inducing apoptosis in the A549 cell line.
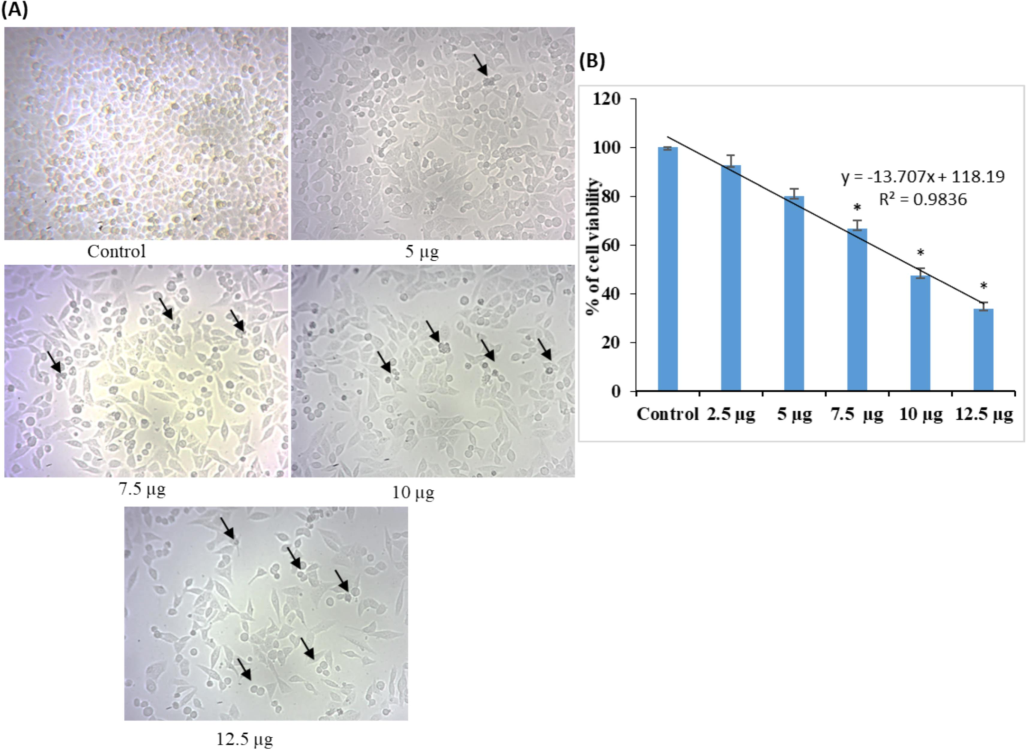
- Cytotoxicity study of AgNPs synthesized from C. citratus extracts; (A) representative images of A549 cells treated with different concentrations of C. citratus (leaf extract) AgNPs; (B) quantitative data from MTT assays showing the inhibitory concentrations of AgNPs extracted from leaves. The calculated IC50 value of C. citratus leaf extract-synthesized AgNPs was 8.6 ± 0.72. (C) Representative images of A549 cells treated with different concentrations of C. citratus (flower extract) AgNPs. (D) Quantitative MTT assay data showing the inhibitory concentrations of AgNPs. The calculated IC50 value of the AgNPs synthesized from C. citratus flower extract was 7.2 ± 0.54; the arrows indicate the presence of dead cells. The values were calculated as the mean ± standard error (SE). A level of significance of p< 0.05 was chosen based on three replicate experiments.
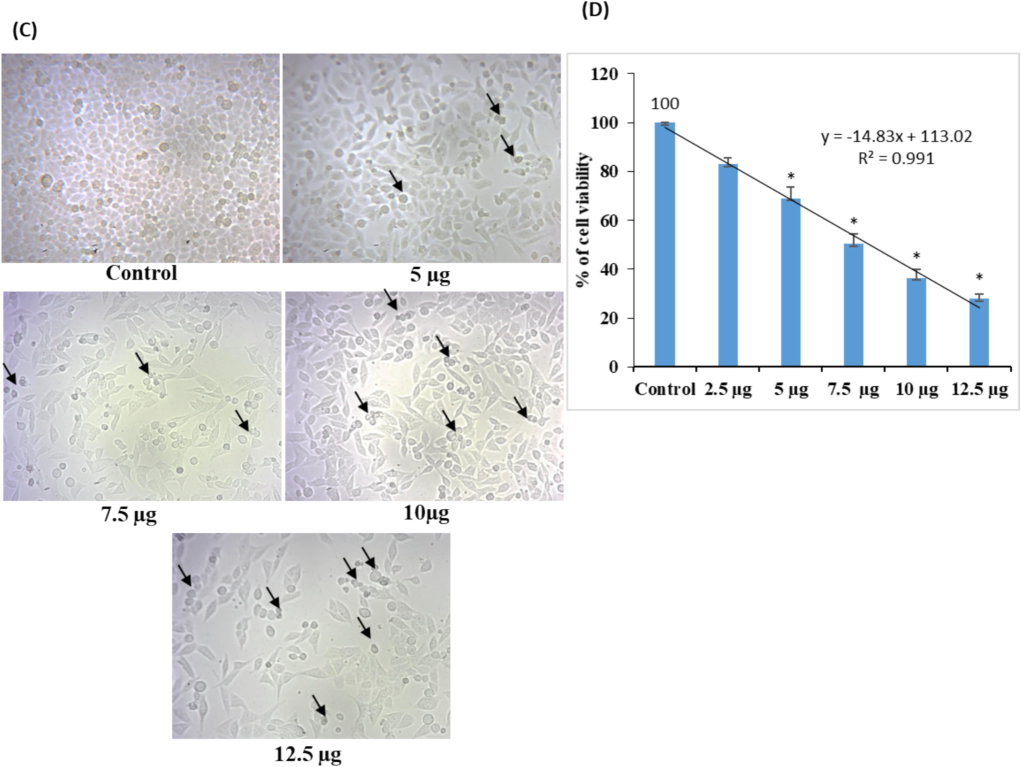
- Cytotoxicity study of AgNPs synthesized from C. citratus extracts; (A) representative images of A549 cells treated with different concentrations of C. citratus (leaf extract) AgNPs; (B) quantitative data from MTT assays showing the inhibitory concentrations of AgNPs extracted from leaves. The calculated IC50 value of C. citratus leaf extract-synthesized AgNPs was 8.6 ± 0.72. (C) Representative images of A549 cells treated with different concentrations of C. citratus (flower extract) AgNPs. (D) Quantitative MTT assay data showing the inhibitory concentrations of AgNPs. The calculated IC50 value of the AgNPs synthesized from C. citratus flower extract was 7.2 ± 0.54; the arrows indicate the presence of dead cells. The values were calculated as the mean ± standard error (SE). A level of significance of p< 0.05 was chosen based on three replicate experiments.
4 Conclusions
The biosynthesis of AgNPs using C. citratus was examined through various techniques, with UV–Vis, FTIR, DLS, and SEM-EDX analysis. The study results indicated that the primary compounds of C. citratus acted as reducing and capping agents, facilitating the bio-reduction of silver and contributing to the agglomeration and stabilization of the nanoparticles. The antibacterial assay showed that the biosynthesized AgNPs significantly inhibited the two clinical pathogens namely S. aureus and P. aeruginosa. Interestingly, AgNPs effectively decreased the proliferation of A549 cells, indicating enhanced anticancer potential. Therefore, the biosynthesized AgNPs exhibited antimicrobial and anticancer activity, warranting further investigation via a variety of biomedical assays.
CRediT authorship contribution statement
Manikandan Ramasamy: Methodology, Investigation, Conceptualization. Ponmurugan Karuppiah: Writing – review & editing, Resources. Harini Ranganathan: Writing – original draft, Data curation. Sinouvassane Djearamane: Validation, Formal analysis. Ezhumalai Muthukrishnan: Writing – original draft, Data curation. Saminathan Kayarohanam: Writing – original draft, Data curation. Natarajan Arumugam: Writing – review & editing, Funding acquisition. Abdulrahaman I. Almansour: Writing – review & editing. Ling Shing Wong: Formal analysis, Data curation.
Acknowledgment
The project was funded by Researchers Supporting Project number (RSP2024R143), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Green synthesis, characterization, and biological activities of silver nanoparticles from alkalinized Cymbopogon citratus Stapf. Adv. Natural Sci: Nanosci. Nanotech.. 2017;8(1):015017
- [Google Scholar]
- Al-Otibi, F., Albulayhid, L.S., Alharbi, R.I., Almohsen, A.A., AlShowiman, G.M., 2023. Biological Activity of Biosynthesized Silver Nanoaggregates Prepared from the Aqueous Extract of Cymbopogon citratus against Candida spp. Nanomater. (Basel, Switzerland). 13(15), 2198.
- Structural and optical properties of nickel oxide nanoparticles: Investigation of antimicrobial applications. Surf. Interfaces.. 2020;18:100460
- [Google Scholar]
- Investigation of the herbal synthesis of silver nanoparticles using Cinnamon zeylanicum extract. Emergent Mater.. 2019;2:113-122.
- [Google Scholar]
- Green synthesis of silver nanoparticles using extract of Artemisia absinthium L., Humulus lupulus L., and Thymus vulgaris L., physicochemical characterization, antimicrobial and antioxidant activity. Processes.. 2021;9(8):1304.
- [Google Scholar]
- Treatment of antibiotic-resistant bacteria by nanoparticles: Current approaches and prospects. Ann. Adv. Chem.. 2022;6:001-009.
- [Google Scholar]
- Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci.. 2021;22(13):7202.
- [Google Scholar]
- Nonantibiotic antimicrobial agents to combat biofilm-forming bacteria. J. Glob. Antimicrob. Resist.. 2020;21:445-451.
- [Google Scholar]
- Anticancer activity of green synthesized AgNPs from Cymbopogon citratus (LG) against lung carcinoma cell line A549. IET Nanobiotech.. 2019;13(2):178-182.
- [Google Scholar]
- CLSI 2024. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 12th ed. CLSI guideline – M07. Clinical and Laboratory Standards Institute.
- Validation of an RP-HPLC method for quantitation of phenolic compounds in three different extracts from Cymbopogon citratus. Res. J. Med. Plant.. 2015;9(7):331-339.
- [Google Scholar]
- Ethnopharmacology, phytochemistry, and biological activities of Cymbopogon citratus (DC.) Stapf extracts. Chinese. J. Nat. Med.. 2015;13(5):321-337.
- [Google Scholar]
- Differential Pd-nanocrystal facets demonstrate distinct antibacterial activity against gram-positive and gram-negative bacteria. Nat. Commun.. 2018;9(1):129.
- [Google Scholar]
- Influence of metals on essential oil content and composition of lemongrass (Cymbopogon citratus (D.C.) Stapf.) grown under different levels of red mud in sewage sludge amended soil. Chemosphere.. 2017;175:315-322.
- [Google Scholar]
- Photocatalytic effect of CuO nanoparticles flower-like 3D nanostructures under visible light irradiation with the degradation of methylene blue (MB) dye for environmental application. Environ. Res.. 2022;203:111880
- [Google Scholar]
- Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotech.. 2019;7:287.
- [Google Scholar]
- Phytochemical methods: A guide to modern techniques of plant analysis. Published by Chapman and Hall, an Imprint of Thomson Science. 1973;1973:60-70.
- [Google Scholar]
- Harnessing endophytic fungi for biosynthesis of selenium nanoparticles and exploring their bioactivities. AMB Express. 2022;12(1):68.
- [Google Scholar]
- Silver metallic nanoparticles with surface plasmon resonance: synthesis and characterizations. J. Clust. Sci.. 2017;28:1051-1069.
- [Google Scholar]
- The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J. Phys. Chem.. 2003;107:668-677.
- [Google Scholar]
- Fabrication and characterization of biosynthesized silver nanoparticles using Cymbopogon citratus and evaluation of its antioxidant.; free radicals and reducing power activity. Nanomed. Res. J.. 2020;5:132-142.
- [Google Scholar]
- Pseudomonas aeruginosa, Staphylococcus aureus, and fluoroquinolone use. Emerg. Infect. Dis.. 2005;11(8):1197-1204.
- [Google Scholar]
- Green nanotechnologies: synthesis of metal nanoparticles using plants. Acta Nat.. 2014;6(1):35-44.
- [Google Scholar]
- Process parameters for green synthesis of silver nanoparticles using leaves extract of Aloe vera plant. Int J Mlutidicip. Curr. Res.. 2015;3:966-975.
- [Google Scholar]
- Phytochemistry and pharmacological activities of Cymbopogon citratus: A review. Sci. Afr.. 2019;6:e00137.
- [Google Scholar]
- Ovais M, Khalil AT, Raza A, Khan MA, Ahmad I, Islam NU, et al., 2016. Green synthesis of silver nanoparticles via plant extracts: beginning a new era in cancer theranostics. Nanomed. (London, England). 11(23), 3157–3177.
- Nanotechnology importance in the pharmaceutical industry. Afr. J. Pure Appl. Chem.. 2008;2:27-31.
- [Google Scholar]
- Biosynthesis of Silver Nanoparticles from Cymbopogon citratus Leaf Extract and Evaluation of Their Antimicrobial Properties. Challenges.. 2022;13(1):18.
- [Google Scholar]
- In-Vivo Bactericidal Potential of Mangifera indica Mediated Silver Nanoparticles against Aeromonas hydrophila in Cirrhinus mrigala. Biomedicines.. 2023;11(8):2272.
- [Google Scholar]
- Direct synthesis of lemongrass (Cymbopogon citratus L.) essential oil-silver nanoparticles (EO-AgNPs) as biopesticides and application for lichen inhibition on stones. Heliyon.. 2022;8(6):e09701.
- [Google Scholar]
- Toxicity Study of Silver Nanoparticles Synthesized from Suaeda monoica on Hep-2 Cell Line. Avicenna J. Med. Biotech.. 2012;4(1):35-39.
- [Google Scholar]
- Green Synthesis of Silver Nanoparticles Using Olea europaea Leaf Extract for Their Enhanced Antibacterial, Antioxidant, Cytotoxic, and Biocompatibility Applications. Int. J. Mol. Sci.. 2021;22(22):12562.
- [Google Scholar]
- A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotech.. 2018;16(1):14.
- [Google Scholar]
- Evaluating natural medicinal resources and their exposure to global change. Lancet Planet. Health.. 2023;7(2):e155-e163.
- [Google Scholar]
- Review of phytomedicine, phytochemistry, ethnopharmacology, toxicology, and pharmacological activities of Cymbopogon genus. Front. Pharmacol.. 2022;13:997918
- [Google Scholar]
- Toward advances in medicinal plant antimicrobial activity: a review study on challenges and future perspectives. Microorganisms.. 2021;9(10):2041.
- [Google Scholar]
- Greener approach for the synthesis of antibacterial silver nanoparticles using an aqueous solution of neem gum. Ind. Crops Products.. 2015;66:103-109.
- [Google Scholar]







