Translate this page into:
Nanomedicine for ovarian cancer: Enhancing pharmacokinetics and biodistribution
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Ovarian cancer continues to pose a substantial healthcare obstacle, demanding the development of novel strategies to improve therapeutic results. Nanomedicine’s introduction has created novel opportunities for the targeted delivery of drugs in the treatment of ovarian cancer. This review article presents a thorough examination of the pharmacokinetics and biodistribution of nanoparticles designed to target ovarian cancer, emphasizing their capacity to significantly transform therapeutic approaches. Furthermore, we investigate the function of nanoparticles as vehicles for precise pharmaceutical administration, placing particular emphasis on the benefits they offer in comparison to traditional methodologies. We explore the intricacies of biodistribution, including the influence of formulations, surface modifications, and nanoparticle size on their internal distribution. In addition, we provide a comprehensive analysis of the importance of pharmacokinetics in the enhancement of drug delivery. This includes a synopsis of critical parameters including absorption, distribution, metabolism, and excretion, and their connection to the therapeutic effectiveness of nanoparticle-based approaches for ovarian cancer. A fundamental component of this review is a critical evaluation of the approaches utilized to improve the biodistribution and pharmacokinetics of nanoparticles designed to target ovarian cancer. Moreover, we have highlighted significant research studies and clinical trials of the in vivo behavior of nanoparticles and their practical applications. Nevertheless, we refrain from obfuscating over the extant obstacles and constraints, such as possible toxicity issues and impediments to the process of clinical translation. The paper concludes by engaging in a discussion on emerging technologies and future prospects, with a specific emphasis on the potential of personalized medicine in the context of ovarian cancer treatment.
Keywords
Nanomedicine
Ovarian cancer
Pharmacokinetics
Biodistribution
Therapeutic strategies
1 Introduction
Ovarian cancer is a significant health concern that has profound implications for women's well-being (Arriba et al., 2010.; Ahmed-Lecheheb and Joly, 2016.; Nicoletta et al., 2017). Due to its silent progression, ovarian cancer often remains undetected until it reaches an advanced stage, resulting in a significant mortality rate (Roland et al., 2013; Stewart et al., 2019). Consequently, ovarian cancer is currently ranked as the fifth most common cause of cancer-related deaths among women. The gravity of the situation can be attributed, in large part, to the absence of effective and universally recognized screening methods for early detection. In contrast to breast and cervical cancers, which benefit from the availability of mammograms and Pap smears respectively, ovarian cancer lacks an equivalent method for early detection (Butow, 2014; Ezendam, 2014; Zhou, 2016). Due to the absence of dependable screening methods, the identification of the disease during its initial and more manageable phases poses a challenge. The disease is usually diagnosed in advanced stages (III or IV), as early symptoms, such as abdominal bloating, pelvic pain, and urinary disturbances, are often mistaken for benign conditions. Approximately 90 % of ovarian cancers are of epithelial origin, originating from the surface of the ovary or fallopian tube, with high-grade serous carcinoma being the most common and aggressive subtype.
The available treatment modalities for advanced or recurrent ovarian cancer are constrained, underscoring the significance of continuous research endeavors aimed at the development of more efficacious therapeutic interventions (Abdoul-Latif et al., 2023). Several risk factors contribute to the complexity of the situation, including a familial predisposition to the disease, specific genetic mutations (e.g., BRCA1 and BRCA2), and an increased risk for women who have not experienced childbirth (Petrucelli et al., 2010). In addition to the clinical obstacles, a notable deficiency in public awareness regarding ovarian cancer is often observed (Palma et al., 2006). This phenomenon may lead to a prolonged period before a diagnosis is made and a reduction in financial resources allocated towards research and the development of treatment alternatives. Ovarian cancer, in contrast to other prominent malignancies like breast and lung cancer, does not garner equivalent levels of attention and support (Daly, 2010). Due to the inherent unpredictability of the disease, the potential adverse effects of treatment, and the scarcity of medical interventions available for advanced cases, patients and their families often encounter distress, uncertainty, and a substantial emotional burden.
Ovarian cancer is recognized for its aggressive nature. Prior to the onset of symptoms, the spread of the condition often occurs within the pelvic and abdominal cavities (Bharwani et al., 2011). The swift advancement of the condition not only presents challenges in terms of treatment, but also diminishes the likelihood of long-term survival due to the decreased efficacy of therapy in advanced stages of the disease. The current standard of care includes debulking surgery followed by platinum-based chemotherapy, often in combination with paclitaxel. Although many patients initially respond well to chemotherapy, resistance frequently develops, leading to disease recurrence in over 70 % of cases. This resistance, along with the systemic toxicity of conventional treatments, underscores the urgent need for more targeted and effective therapies. In recent years, significant efforts have been made to develop novel treatment strategies, particularly in the field of nanotechnology, which holds promise for addressing several key challenges in ovarian cancer treatment.
Recently, nanotechnology represents a rapidly advancing field in ovarian cancer treatment, offering innovative approaches to overcome the limitations of conventional therapies. By enhancing drug delivery, reducing toxicity, and enabling precision medicine, nanotechnology holds the potential to significantly improve outcomes for ovarian cancer patients (Levit and Tang, 2021). Nanotechnology involves the design and manipulation of materials at the nanoscale, typically between 1 and 100 nm. This technology allows for the development of drug delivery systems that can precisely target cancer cells, reducing off-target toxicity and enhancing therapeutic efficacy. Nanoparticles can be engineered to improve drug solubility, enhance bioavailability, and allow for sustained release, making them ideal candidates for cancer therapy. Several types of nanocarriers, including liposomes, polymeric nanoparticles, dendrimers, and micelles, have been explored for the treatment of ovarian cancer. Among the most prominent examples of nanotechnology in ovarian cancer treatment is liposomal doxorubicin (Doxil), which has been approved for use in patients with recurrent ovarian cancer.
Targeted drug delivery involves the precise and controlled administration of therapeutic agents to the specific location of the tumor, thereby minimizing damage to healthy tissues and enhancing the efficacy of the treatment. The surface of ovarian cancer cells contains a variety of markers and receptors that can be selectively identified and bound to by nanoparticles that have had their surfaces modified, such as by adding ligands or antibodies. Due to the presence of permeable and disordered vasculature in ovarian tumors, nanoparticles exhibit enhanced accumulation within the tumor tissue (Bertrand et al., 2014). Drugs with low solubility can be encapsulated by nanoparticles to increase their solubility and bioavailability. The comprehension of nanoparticle biodistribution and pharmacokinetics are crucial in order to optimize the therapeutic efficacy of drugs by facilitating the delivery of a substantial portion of the therapeutic payload to the desired target (Wolfram and Ferrari, 2019).
For ovarian cancer-targeted nanoparticles, the primary goal is to ensure that the majority of the therapeutic payload is delivered to the tumor site (Wang et al., 2021). Understanding the biodistribution and pharmacokinetics of nanoparticles is essential because it helps guarantee that a significant portion of the drug reaches the intended target (Wang and Jia, 2016). Pharmacokinetics, on the other hand, is how the body processes drugs or nanoparticles including the absorption, distribution, metabolism, and excretion. Additionally, pharmacokinetics provides insights into potential drug interactions, toxicity, and the development of resistance over time (Zhang et al., 2019). By fine-tuning the pharmacokinetics of targeted nanoparticles, researchers can work to extend drug circulation times in the bloodstream, enhance drug stability, and control drug release rates at the tumor site. This not only ensures that an effective concentration of the therapeutic agent is maintained over time but also minimizes the risk of toxicity or adverse reactions. Therefore, this review paper focuses on the biodistribution, including how formulations, surface modifications, and nanoparticle size affect internal distribution. This manuscript also presents how pharmacokinetics improves drug delivery, absorption, distribution, metabolism, and excretion and their impact on nanoparticle-based ovarian cancer treatment. This review critically evaluates methods used to improve ovarian cancer nanoparticle biodistribution and pharmacokinetics. However, we do not ignore current challenges like toxicity, clinical translation, emerging technologies and future prospects, focusing on personalized medicine for ovarian cancer treatment.
2 Nanoparticle-based drug delivery in ovarian cancer
Conventional drug delivery methods for treating ovarian cancer face several challenges that limit their effectiveness. Ovarian cancer is often diagnosed at an advanced stage, making it particularly aggressive and difficult to treat (Milewska et al., 2021). The main challenges in conventional drug delivery for ovarian cancer include limited drug specificity, systemic toxicity, drug resistance, and inadequate penetration into tumor tissues. Firstly, conventional chemotherapy drugs used in ovarian cancer treatment are typically administered systemically, meaning they circulate throughout the body. This lack of specificity results in healthy cells being exposed to the toxic effects of the drug, leading to severe side effects, such as nausea, hair loss, and fatigue (Perez-Fidalgo et al., 2021). Moreover, the high toxicity limits the maximum tolerated dose, reducing the drug's effectiveness against the cancer cells. Secondly, ovarian cancer can develop resistance to chemotherapy over time, leading to treatment failure. The cancer cells can adapt and become less responsive to the drugs, requiring the use of increasingly potent and toxic agents, further exacerbating side effects and compromising the patient's quality of life (Lutgendorf, 2017).
Due to their unique characteristics, nanoparticles have garnered significant interest as potential carriers for targeted therapy in the context of ovarian cancer. These nanostructures, typically ranging in size from one to one hundred nanometers, can be engineered to encapsulate chemotherapy drugs.
Poly (ethylene glycol)–poly (lactic acid) (PEG–PLA) nanoparticles are widely used in drug delivery systems due to their biocompatibility, biodegradability, and ability to improve the pharmacokinetic profile of drugs. One critical factor in assessing the effectiveness of these nanoparticles is their ζ-potential, which measures the surface charge of the particles. The ζ-potential plays a significant role in the stability of nanoparticle suspensions and their interaction with biological environments. Nanoparticles with a high absolute ζ-potential (either highly positive or negative) are more likely to repel one another, preventing aggregation, and maintaining colloidal stability, which is crucial for consistent drug delivery.
In drug delivery, a neutral to slightly negative ζ-potential (typically between −10 mV to −30 mV) is often desirable for PEG-PLA nanoparticles. This balance helps avoid rapid clearance by the immune system (e.g., macrophages) and enhances circulation time, improving the likelihood that the drug will reach the tumor or target site. A too highly charged nanoparticle may cause unintended interactions with cells or proteins in the bloodstream, whereas too low a charge can result in aggregation or rapid clearance.
Previously, researchers have employed a method wherein angiogenesis inhibitors, specifically TNP-470, were enclosed within nanoparticles composed of PEG–PLA that were modified with an APRPG (Ala-Pro-Arg-Pro-Gly) peptide. The ζ-potential of the nanoparticles was measured to be −14.3 mV, indicating a negative charge. Due to its high selectivity, a reduced number of healthy cells were subjected to exposure, thereby mitigating the occurrence of adverse effects (Wang, 2014). The nanoparticles' ability to release cargo over an extended period of time allows for minimal systemic toxicity and controlled drug delivery to cancer cells. One example of this phenomenon is the effective simultaneous delivery of Paclitaxel (PTX) and Cisplatin (CDDP) using nanocarriers as part of a combined therapeutic approach for ovarian cancer. The in vitro elimination of ovarian cancer cells was achieved through the utilization of an optimized drug loading ratio and the implementation of a programmed faster release of PTX in the nanoformulation, which demonstrated superior efficacy compared to CDDP (Cai et al., 2015). Additionally, the drug release analyses showed that Albendazole (ABZ); an anti-parasite compound was released from BSA-ABZ nanoparticles measuring 10 nm approximately 93 % of the time, while ABZ was released from nanoparticle albumin bound (Nab-ABZ) nanoparticles measuring 200 nm only 83 % of the time. At a pH of 7.4, this release happened over the course of eight days. These results imply that the traditional way of administering these nanoparticles might be less effective (Noorani et al., 2015).
Encapsulating drugs within nanoparticles can protect them from degradation and clearance, allowing for a longer circulation time and better drug delivery to the tumor site. These particles can also be designed to penetrate physiological barriers, such as the blood–brain barrier, further expanding their applications in ovarian cancer treatment (Lutgendorf, 2017). Despite undergoing maximal cytoreductive surgery and platinum-based chemotherapy as part of conventional treatment, patients frequently encounter chemoresistance and disease recurrence. The clinical intervention known as immune checkpoint blockade (ICB) aims to modulate T-cell activity (Fig. 1) within the anti-tumor microenvironment (TME) in order to achieve stabilization. The lack of significant therapeutic efficacy demonstrated by ICB is a source of disappointment, despite the established association between tumor infiltrating lymphocytes and improved survival outcomes in ovarian cancer (Johnson et al., 2021). Their ability to enhance drug specificity, reduce systemic toxicity, and overcome drug resistance makes them an attractive option for targeted therapy.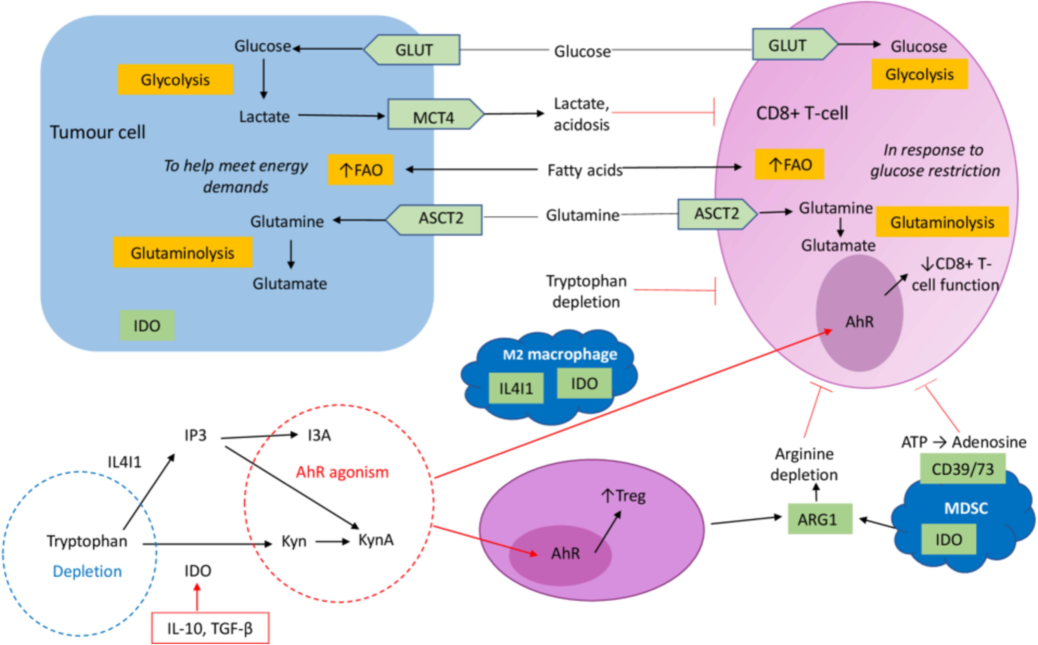
The effect of immune cell differentiation on changes in metabolism within the tumor microenvironment (TME). Increased tumor aerobic glycolysis, which is driven by an upregulation of GLUT-mediated glucose provision, causes lactate accumulation in the TME. Cancer cells receive energy from this process to proliferate rapidly. Furthermore, tumor cells use fatty acid oxidation (FAO) as a ()
Source of energy for growth, which prompts glucose-starved CD8 + T-cells to increase FAO Johnson et al., 2021.
3 Biodistribution of ovarian cancer-targeted nanoparticles
The term “biodistribution” refers to the movement of a substance, such as a drug or a nanoparticle, from one part of the body to another as it travels through the body (de Sousa Cunha et al., 2019). Because it determines where in the body the therapeutic agent will be found and how much of it will be there, biodistribution is an extremely important part of the drug delivery process. It is essential to have a solid understanding of the biodistribution of drug-loaded nanoparticles in order to make the most of their therapeutic potential and minimize any potential side effects (Zhai, 2018).
There are many reasons why studies on biodistribution are beneficial to the field of drug delivery research. To begin, they are instrumental in the production of nanoparticles that are able to specifically target the target site, which in this case are the tumors caused by ovarian cancer (Haber, 2020). There is a discernible relationship between the diffusion coefficients of particles with sizes 15, 40, and 100 nm, but there is also a discernible difference in volume, which impacts the ability of the particle to transport drugs. This has a number of effects, one of which is translocation and flux, which enhance the therapeutic effect (Florence, 2012). Important characteristics of the poly(d,l-lactide)-block-poly(ethylene glycol) polymeric nanoparticles (PLA-b-PEG PNPs) include the ability to load drugs, the potential for chemical targeting, and the enhancement of circulation time, all of which make this material very appealing in the drug delivery field. Furthermore, PLGA-b-PEG PNPs can lengthen the time of circulation. Even though these nanoparticles have good qualities, much more work needs to be done to enhance control over their charge surface, size, and polydispersity as well as to make it easier to repeat these features throughout the synthesis stages. Many parameters are presently being researched in an effort to enhance the drug-loading capacity as well as the drug release profile. Despite their excellent qualities, using PLGA polymeric nanoparticles for in vivo applications remains an open challenge (Rehman et al., 2022). This is caused by a variety of factors, such as their large diameter (150–200 nm), poor stability in water, and the liver and spleen's removal of these nanocarriers from the blood stream, which significantly lowers the concentration of drugs in tumor tissue (Locatelli and Comes Franchini, 2012).
In addition, these studies help reduce the systemic toxicity of the drug while simultaneously increasing the drug's concentration at the site where it is most needed (the tumor). This is accomplished by designing the nanoparticles to concentrate in the tumor (Yao, 2018). The plasma half-lives of drugs can be lengthened through the use of nanoparticle encapsulation, which makes sustained release possible and improves therapeutic outcomes. The chemotherapeutic agent utilized was PTX, while the therapeutic radioisotope was yittrium-90 (90Y) (Fig. 2). Since the majority of ovarian cancers overexpress the folate receptor, folate was used as the targeting ligand. Studies on the characterization of nanoparticles revealed monodispersed particles with regulated PTX release. NPs were taken up by tumor cells through the action of a folate targeting ligand. When compared to folate-targeted NPs containing a single therapeutic or any non-targeted NP therapeutics, in vitro efficacy studies showed that folate-targeted NPs containing chemoradiotherapy was the most effective therapeutic (Werner, 2011).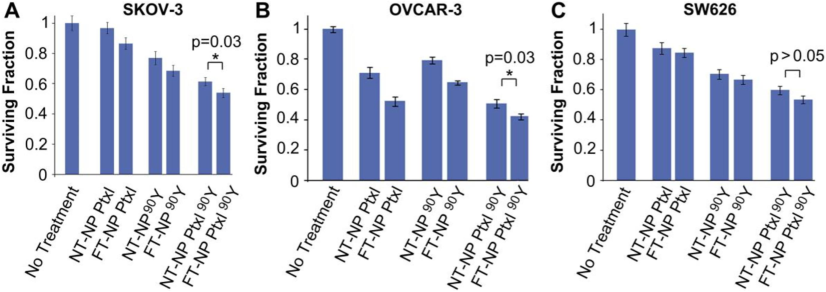
Effectiveness of ChemoRad NPs in vitro. Clonogenic assay results of cells treated with different NP therapeutics: SKOV-3 (A), OVCAR-3 (B), and SW626 (C). cells given 50 ug/mL of NPs containing either 20 ug of PTX or 50 uCi of Yttrium-90 for one hour. Using the Student's T test, *p = 0.03 (Werner, 2011).
The process of optimizing nanoparticle formulation for biocompatibility and effective drug delivery is made easier by having a thorough understanding of biodistribution. In addition, the controlled delivery of drugs that is made possible by nanoparticles enables doctors to provide care that is precise and individualized according to the requirements of each patient. PTX targeted drug delivery systems can improve the efficacy of immunotherapy by ensuring that immunotherapeutic agents are delivered to the microenvironment of the tumor.
3.1 Factors affecting nanoparticle biodistribution
The size of nanoparticles plays a significant role in their biodistribution, with smaller nanoparticles exhibiting enhanced tumor penetration and accumulation, leading to improved drug delivery efficacy (Liu, 2012). For instance, gold nanoparticles (Au NPs), in particular, serve as attractive materials for nucleic acid delivery applications because of various advantages (Kim et al., 2013). Early research used anionic mercaptoundecanoic acid (MUA)-functionalized gold NPs with a 2 nm core to study NP–protein interactions (Fischer et al., 2002). The protease chymotrypsin (ChT) was selectively interacting with MUA-gold NPs, which led to an inhibition of enzymatic activity (apparent inhibition constant, Ki = 10.4 ± 1.3 nM). The biodistribution and targeting properties of nanoparticles can also be affected by surface modifications. In Fig. 3, for instance, gold particles modified with Shells of Ceragenin CSA-131 were categorized as crystalline due to the visibility of circles indexed with planes corresponding to the face-centered cubic (fcc) structure of Au. Using FT-Raman spectroscopy analysis, the accuracy of the functionalization of peanut-shaped gold nanoparticles (AuP NPs) with CSA-131 was examined. Thereafter, it was discovered that the physicochemical characteristics of nanoparticles significantly impacted the pharmacokinetics, biodistribution, intratumoral penetration, and tumor bioavailability (Piktel, 2021).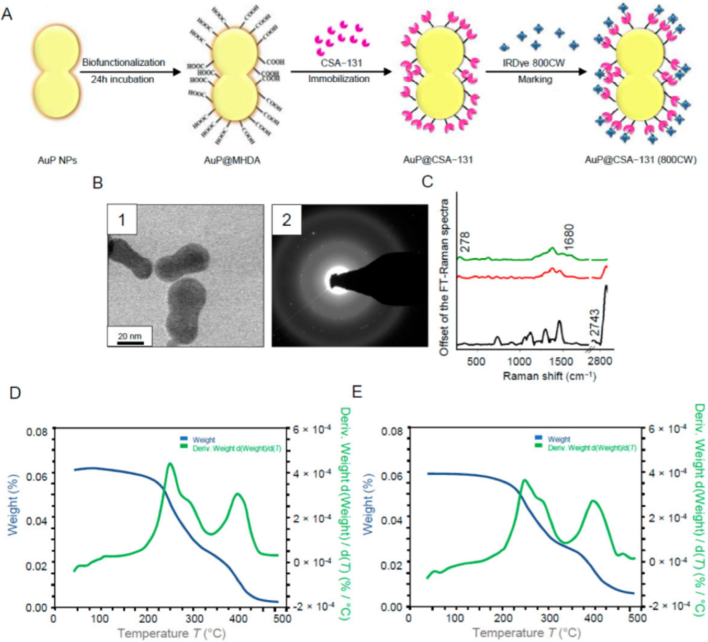
Diagram showing the biofunctionalization of AuP NPs, the immobilization of ceragenin (CSA), and the IRDye® 800CW. (A) labeling of AuP@CSA-131. STEM image of the produced AuP NPs (B1) and the gold nanoparticles' SEAD patterns (B2) utilized in the creation of nanosystems. CSA-131 immobilized on the AuP NPs surface (red spectrum), MHDA's unenhanced FT-Raman spectra (black spectrum), and CSA-131 immobilized on the AuP NPs surface marked with IRDye® 800CW (green spectrum) (C). TGA data of AuP@CSA-131 (D) and AuP@CSA-131 (800CW) (E) demonstrating the product's solvent removal and breakdown (Piktel, 2021).
Functionalizing the surface of nanoparticles with targeting ligands or antibodies can enhance their specificity for ovarian cancer cells, leading to improved biodistribution and therapeutic outcomes (Marques et al., 2020). Optimized formulations from nanotechnology platforms promote therapeutic drug delivery and offer advantages such as biocompatibility, which can impact biodistribution within the body. In a related study the cases of H9C2 versus MDA-MB-231 and IMR-90 versus MDA-MB-231, the experimental optimal therapeutic window has shown to be close to the feedback system control (FSC) prediction with high p values (0.78 and 0.47, respectively) (Wang, 2015). Several case studies have demonstrated the effectiveness of nanoparticle-based drug delivery systems for ovarian cancer. For example, nanoparticle encapsulation has been shown to enhance accumulation in patient tumors and increase plasma half-life compared with free doxorubicin, demonstrating the impact of formulation on biodistribution and therapeutic efficacy (Chen, 2021). Overall, understanding and optimizing the factors affecting nanoparticle biodistribution are essential for the development of effective drug delivery systems for the treatment of ovarian cancer.
3.2 Factors affecting nanoparticle pharmacokinetics
Particle size, in conjunction with surface composition, is critical to the biodistribution of long-circulating nanoparticles and achieving therapeutic efficacy (Fig. 4), as demonstrated by physiological parameters such as hepatic filtration, tissue extravasation, tissue diffusion, and kidney excretion (Liu et al., 2010). However, the temperature effect on ovarian elimination revealed a notably accelerated uptake of the 50 nm polystyrene nanoparticles at lower temperatures (Firdessa et al., 2014). Similarly, previous studies have provided evidence indicating that the dimensions of the nanoparticle play a crucial role in determining the extent of protein absorption. There was a notable correlation observed between particle size and protein absorption in poly(methoxy-polyethyleneglycol cyanoacrylate-co-n-hexadecyl cyanoacrylate) (PEGylated PHDCA) nanoparticles of varying sizes (small, medium, and large) when incubated with serum protein for a duration of two hours (Fang et al., 2006). Furthermore, cross-linked micelles incubated in PBS (pH 7.4) for more than 3 days at 37 °C did not show significant increases in size and polydispersity compared to non-cross-linked micelles (PD ∼ 0.5 after 10 h). Because of the low rate, stable micelles had a long circulation half-life of 8 h. Micelles were found to accumulate in the upper layer of affected ovarian cancer cells, similar to the findings with small liposomes (<100 nm) (Rijcken et al., 2007).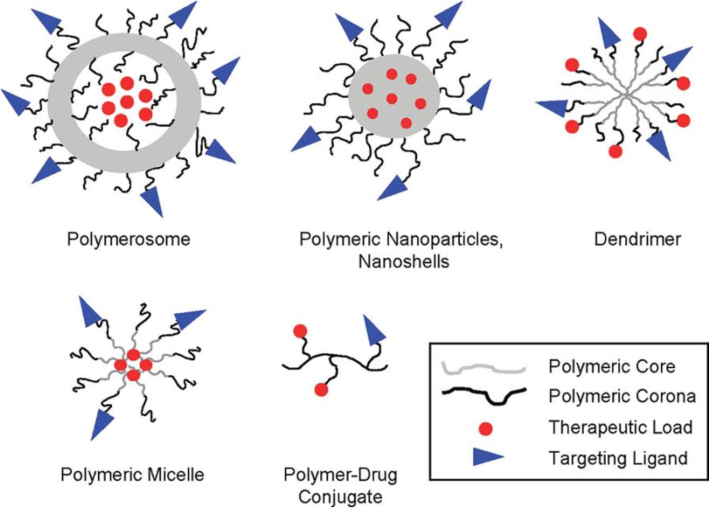
The utilization of nanoparticles as drug delivery platforms. The defining characteristics of polymeric nanoparticle platforms, including polymersomes, solid polymeric nanoparticles, nanoshells, dendrimers, polymeric micelles, and polymer-drug conjugates, are their physicochemical structures (Alexis et al., 2008).
The temperature-dependent deformation is exhibited by nanoparticles with Shell crosslinked knedel-like nanoparticles (SCKs) which include partially hydrochlorinated poly(isoprene) cores. To assess the impact of the polymeric core's rigidity on in vivo biodistribution, eighty-four SCK nanoparticles with comparable physiochemical properties (size, approximately 20 nm; ζ, approximately − 25 mV) and a low glass transition temperature (Tg) with a fluid-like poly(methyl acrylate) (PMA) core or a high Tg with a glassy poly(styrene) (PS) core were created. When compared to nanoparticles made of PMA with a low Tg, those with a high Tg, with a core material composed of PS exhibited a noticeably longer duration of presence in the bloodstream. It is expected that the application of a low Tg core will lead to increased surface interactions and improved flexibility between the nanoparticles and the surrounding biological milieu and tissues (Rijcken et al., 2007). However, it was still unclear if the blood residence time was affected by the polymers' hydrophobicity or other physicochemical characteristics, such as their relative stiffness. The results show that the makeup of the core has a major impact on how long blood stays in the area (Tang et al., 2012). At this point, it was found that the PEG surface modifications had no appreciable effect on the kidney's ability to accumulate and eliminate nanoparticles.
Various strategies have been employed to enhance the biodistribution and pharmacokinetics of nanoparticles targeted for ovarian cancer. Surface modification techniques, such as PEGylation, improve stability and circulation time, reducing clearance and potential immunogenicity (Hussain et al., 2019). Concurrently, conjugation of targeting ligands enhances specific recognition of ovarian cancer cells, improving tumor targeting and cellular uptake. Size and shape optimization impact circulation time and tumor accumulation, with small size enhancing tissue penetration and non-spherical shapes improving cellular internalization (Pantshwa et al., 2020). Responsive nanoparticles, sensitive to factors like pH or temperature, enable controlled drug release in the tumor microenvironment. Both passive targeting, utilizing the enhanced permeability and retention (EPR) effect, and active targeting, involving ligands recognizing ovarian cancer cell markers, contribute to enhanced accumulation in tumors (Fig. 5). Multifunctional nanoparticles facilitate combination therapy and simultaneous imaging and therapy (theranostics) (Liu, 2015).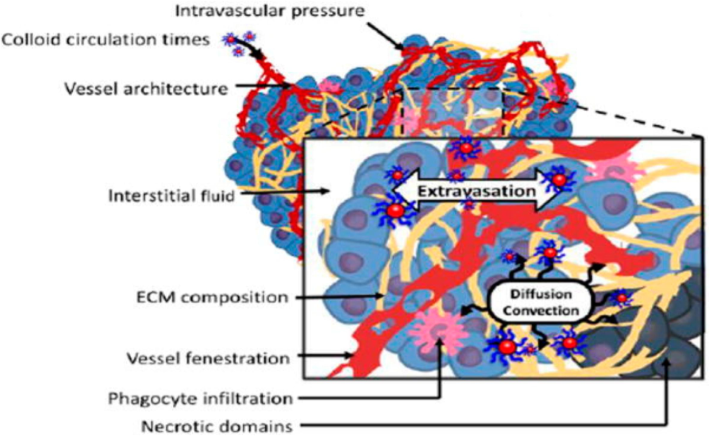
The enhanced permeation and retention (EPR) of the nanoparticles are caused by two phenomena: extravasation and later diffusion and convection of the colloid in the extracellular matrix (Subhan et al., 2021).
Biodegradable nanoparticles release their payload gradually, minimizing systemic toxicity, while cell-penetrating peptides enhance cellular uptake. Intraperitoneal administration allows direct delivery to the peritoneal cavity, particularly beneficial for ovarian cancer in the abdominal region. Nanoparticle coatings for immune evasion, such as stealth coatings, prolong circulation time by reducing immune recognition (Padmakumar et al., 2018). Responsive drug release strategies enable controlled and targeted drug release in response to stimuli in the tumor microenvironment. Additionally, incorporating imaging agents into nanoparticles provides real-time monitoring of biodistribution. These strategies collectively aim to optimize the performance of ovarian cancer-targeted nanoparticles, offering improved therapeutic outcomes while minimizing off-target effects and systemic toxicity. Comparison of various strategies employed to improve the biodistribution and pharmacokinetics of ovarian cancer-targeted nanoparticles is addressed in Table 1. PEGylation improves stability and circulation time. Conjugation of targeting ligands enhances specific recognition of ovarian cancer cells. Prolongs circulation time, reducing clearance. Enhanced tumor targeting and cellular uptake. Potential immunogenicity of PEG. Ligand conjugation complexity. Modulating size impacts circulation time and tumor accumulation. Non-spherical shapes may enhance tumor penetration and cellular uptake. Small size improves tissue penetration. Improved cellular internalization. Larger particles may have prolonged circulation. Complexity in synthesis and characterization. pH-sensitive nanoparticles exploit the acidic tumor microenvironment. Temperature-sensitive nanoparticles release payload in response to elevated temperatures. Controlled drug release in response to tumor pH. Precision in drug release at elevated temperatures. Stability concerns in neutral environments. Limited applicability to certain tumors. Passive targeting utilizes the EPR effect for tumor accumulation. Active targeting involves ligands recognizing ovarian cancer cell markers. Enhanced accumulation in tumors with leaky vasculature. Specific binding to cancer cells, improving selectivity. Heterogeneity in the EPR effect among patients. Ligand stability and potential off-target effects. Combination therapy with multiple agents. Theranostic nanoparticles for simultaneous imaging and therapy. Synergistic effects for improved therapeutic outcomes. Real-time monitoring of treatment response. Increased complexity in formulation and characterization. Challenges in integrating imaging and therapeutic components.
Strategy
Description
Advantage
Challenge
Ref
Surface Modification
(Correa, 2020)
Size and Shape Optimization
(Li et al., 2019)
Responsive Nanoparticles
(Fathi et al., 2020)
Active and Passive Targeting
(Li et al., 2017)
Multifunctional Nanoparticles
(Chen, 2019)
Biodegradable Nanoparticles
Release payload gradually, reducing systemic toxicity.
Minimizes long-term toxicity and potential accumulation.
Controlled degradation may be challenging.
(Sánchez-Ramírez, 2020)
Cell-Penetrating Peptides
Enhance cellular uptake of nanoparticles.
Improved internalization into cancer cells.
Potential cytotoxicity and non-specific uptake.
(Vale, 2020)
Intraperitoneal Administration
Direct delivery to the peritoneal cavity for improved drug distribution in the abdomen.
Enhanced drug exposure to ovarian cancer in the peritoneal cavity.
Limited to abdominal cancers; potential toxicity concerns.
(Cao, 2017)
Nanoparticle Coating for Immune Evasion
Stealth coatings for immune system evasion.
Prolongs circulation time by reducing immune recognition.
Challenges in maintaining coating integrity.
(Sood, 2006)
Responsive Drug Release
Triggered release in response to stimuli in the tumor microenvironment.
Controlled and targeted drug release.
Limited stimuli in certain tumor types.
(Lin, 2016)
In Vivo Imaging
Incorporation of imaging agents for real-time monitoring of biodistribution.
Provides valuable insights into nanoparticle behavior.
Additional complexity and potential toxicity of imaging agents.
(Jokerst et al., 2012)
4 Recent innovations and implications
Recent innovations in ovarian cancer research have led to significant advancements with promising implications for diagnosis, treatment, and prognosis. Notably, precision medicine approaches have gained traction, utilizing genomic and molecular profiling to identify specific genetic alterations and guide targeted therapies (Sivapalan et al., 2021; Haselmann et al., 2022; Trinidad et al., 2020). Preclinical studies involving innovative nanoparticle-based drug delivery systems have shown potential for improved treatment outcomes. For instance, studies exploring the use of multifunctional nanoparticles for combination therapy, incorporating chemotherapeutic agents and targeted therapies, have demonstrated enhanced efficacy in preclinical models of ovarian cancer (Mendes, 2022; Kemp et al., 2016; Di Lorenzo et al., 2018). Additionally, the development of immunotherapeutic strategies, such as immune checkpoint inhibitors and adoptive T-cell therapy, is showing promise in preclinical studies by harnessing the body's immune system to target ovarian cancer cells.
Recent clinical trials have looked into the effectiveness of Poly(ADP-ribose) polymerase (PARP) inhibitors, like |Niraparib and Olaparib, especially in patients who have BRCA mutations. By specifically targeting cancer cells with compromised DNA repair mechanisms, these inhibitors take advantage of the notion of synthetic lethality (Paunovska et al., 2022; Sachdev et al., 2019; Jiang et al., 2019). The success of PARP inhibitors has led to their inclusion in the standard of care for ovarian cancer patients, showcasing a significant advancement in personalized medicine. Moreover, ongoing clinical trials are exploring the potential of novel anti-angiogenic agents, immunotherapies, and targeted therapies that aim to improve patient outcomes and quality of life. For instance, immune checkpoint inhibitors like pembrolizumab are being evaluated in clinical settings to assess their effectiveness in ovarian cancer patients. Additionally, the development of circulating tumor DNA (ctDNA) as a liquid biopsy for monitoring disease progression and treatment response represents a non-invasive and potentially transformative approach. Recent innovations in ovarian cancer research include advances in precision medicine, nanoparticle-based drug delivery systems, immunotherapies, and targeted therapies (Table 2). Preclinical studies highlight the potential of these innovations in improving treatment efficacy, while clinical trials demonstrate their translation into tangible benefits for patients, offering new avenues for personalized and more effective ovarian cancer management.
Innovation
Implications
Preclinical Studies
Clinical Studies
Pharmacokinetics
Biodistribution
Precision Medicine
Tailoring treatments based on genomic and molecular profiling.
Identification of specific genetic alterations guiding targeted therapies.
Clinical trials evaluating the efficacy of PARP inhibitors (e.g., Olaparib, Niraparib) in patients with BRCA mutations.
Individualized drug metabolism, clearance, and therapeutic drug monitoring.
Assessment of genomic and molecular markers in tumors for personalized targeting.
Nanoparticle-Based Drug Delivery
Improved drug delivery for enhanced treatment outcomes.
Multifunctional nanoparticles for combination therapy, demonstrating efficacy in preclinical models.
Ongoing trials assessing the safety and efficacy of nanoparticle-based drug delivery systems in ovarian cancer patients.
Investigation of nanoparticle size, surface modification, and ADME characteristics.
Study of how nanoparticles distribute throughout the body, targeting tumors.
Immunotherapy
Harnessing the immune system to target ovarian cancer cells.
Preclinical studies exploring immune checkpoint inhibitors and adoptive T-cell therapy.
Clinical trials investigating the efficacy of immune checkpoint inhibitors (e.g., pembrolizumab) in ovarian cancer patients.
Monitoring immunotherapeutic agent levels and persistence in the bloodstream.
Examination of how immune-modulating agents interact with tissues and tumors.
PARP Inhibitors
Targeting cancer cells with defective DNA repair mechanisms.
Preclinical validation of synthetic lethality in ovarian cancer cells.
Inclusion of PARP inhibitors (e.g., olaparib, niraparib) in standard care for patients, with ongoing trials exploring combinations.
Pharmacokinetic profiling of PARP inhibitors, including absorption, distribution, metabolism, excretion (ADME) characteristics.
Investigation of how PARP inhibitors distribute in tumors and healthy tissues.
Anti-Angiogenic Agents
Inhibiting the formation of new blood vessels to limit tumor growth.
Preclinical evaluation of novel anti-angiogenic agents in ovarian cancer models.
Clinical trials assessing the efficacy of anti-angiogenic agents in ovarian cancer patients.
Examination of pharmacokinetic parameters such as half-life and clearance.
Study of how anti-angiogenic agents distribute in the tumor microenvironment.
Liquid Biopsy (ctDNA)
Non-invasive monitoring of disease progression and treatment response.
Preclinical development and validation of circulating tumor DNA (ctDNA) as a liquid biopsy.
Ongoing clinical trials evaluating the utility of ctDNA as a biomarker in ovarian cancer management.
Investigation of ctDNA clearance and persistence dynamics.
Exploration of ctDNA distribution and release into circulation
5 Conclusion
The persistence of ovarian cancer as a major healthcare obstacle calls for the development of novel strategies to improve treatment results. The development of nanomedicine has created new opportunities for the targeted delivery of drugs for the treatment of ovarian cancer. The pharmacokinetics and biodistribution of nanoparticles designed specifically to target ovarian cancer are thoroughly examined in this review article.
Nanomedicine represents a transformative approach in the treatment of ovarian cancer, offering substantial potential for improving therapeutic outcomes by addressing critical limitations in traditional cancer therapies. Through the development of nanotechnology-based drug delivery systems, nanomedicine enhances pharmacokinetics—the absorption, distribution, metabolism, and excretion of drugs by optimizing the release and targeting of chemotherapeutic agents. This approach mitigates the toxicity to healthy tissues while maximizing the therapeutic concentration of drugs within tumor cells.
In ovarian cancer, where early diagnosis is challenging and the disease often recurs after standard treatments, nanomedicine offers new hope by improving biodistribution. Nanocarriers, such as liposomes, dendrimers, and polymeric nanoparticles, are engineered to preferentially accumulate in tumor tissues via the enhanced permeability and retention (EPR) effect. They can be further functionalized with targeting ligands to improve precision, ensuring that drugs reach the cancer cells with minimal systemic exposure. Such targeted delivery not only enhances the therapeutic index but also reduces side effects, improving patients' quality of life during treatment. Furthermore, nanomedicine offers the potential for multifunctional platforms that combine therapy with diagnostic imaging, known as theranostics. These systems enable real-time monitoring of treatment response and disease progression, allowing clinicians to tailor therapies to individual patients, ushering in a new era of personalized medicine for ovarian cancer.
Despite the promising advances, challenges remain in translating nanomedicine from preclinical studies to widespread clinical use. Issues such as large-scale manufacturing, long-term toxicity, regulatory hurdles, and the complexity of ovarian cancer biology need to be addressed. However, ongoing research continues to make significant strides in overcoming these obstacles, fostering optimism that nanomedicine will soon play an integral role in the management of ovarian cancer.
In conclusion, nanomedicine stands at the forefront of innovation in cancer therapy, offering a strategic advantage in overcoming the limitations of conventional chemotherapy for ovarian cancer. By enhancing pharmacokinetics and biodistribution, it promises not only more effective treatments but also a future where cancer management is more personalized, precise, and patient-centered. With continued research and development, nanomedicine holds the potential to significantly improve survival rates and quality of life for ovarian cancer patients, heralding a new era in cancer care.
CRediT authorship contribution statement
Salina Saddick: Writing – review & editing, Writing – original draft.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- “An overview of cancer in djibouti: current status, therapeutic approaches, and promising endeavors in local essential oil treatment”. Pharmaceuticals. 2023;16(11)
- [CrossRef] [Google Scholar]
- Ovarian cancer survivors’ quality of life: a systematic review. J. Cancer Surviv.. 2016.;10(5):789-801.
- [Google Scholar]
- Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm.. 2008;5(4):505-515.
- [Google Scholar]
- A review of issues surrounding quality of life among women with ovarian cancer. Gynecol. Oncol.. 2010.;119(2):390-396.
- [Google Scholar]
- Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev.. 2014;66:2-25.
- [Google Scholar]
- Ovarian cancer management: the role of imaging and diagnostic challenges. Eur. J. Radiol.. 2011;78(1):41-51.
- [Google Scholar]
- Caring for women with ovarian cancer in the last year of life: a longitudinal study of caregiver quality of life, distress and unmet needs. Gynecol. Oncol.. 2014;132(3):690-697.
- [Google Scholar]
- Telodendrimer nanocarrier for co-delivery of paclitaxel and cisplatin: a synergistic combination nanotherapy for ovarian cancer treatment. Biomaterials. 2015;37:456-468.
- [Google Scholar]
- Intraperitoneal administration of neural stem cell–nanoparticle conjugates targets chemotherapy to ovarian tumors. Bioconjug. Chem.. 2017;28(6):1767-1776.
- [Google Scholar]
- A multifunctional-targeted nanoagent for dual-mode image-guided therapeutic effects on ovarian cancer cells. Int. J. Nanomed.. 2019;vol. 14(null):753-769.
- [Google Scholar]
- Preclinical evaluation of PEGylated liposomal doxorubicin as an effective radiosensitizer in chemoradiotherapy for lung cancer. Strahlenther. Onkol.. 2021;197(12):1131-1142.
- [Google Scholar]
- Tuning nanoparticle interactions with ovarian cancer through layer-by-layer modification of surface chemistry. ACS Nano. 2020;14(2):2224-2237.
- [Google Scholar]
- “Genetic/familial high-risk assessment: breast and ovarian,” (in English) Journal of the National Comprehensive Cancer Network J Natl Compr Canc Netw.. 2010;8(5):562-594.
- [Google Scholar]
- Development of nanoparticulate systems with action in breast and ovarian cancer: nanotheragnostics. J. Drug Target.. 2019;27(7):732-741.
- [Google Scholar]
- “Imaging and therapy of ovarian cancer: clinical application of nanoparticles and future perspectives,” (in eng) Theranostics. 2018;8(16):4279-4294.
- [Google Scholar]
- Chemotherapy-induced peripheral neuropathy and its impact on health-related quality of life among ovarian cancer survivors: results from the population-based PROFILES registry. Gynecol. Oncol.. 2014;135(3):510-517.
- [Google Scholar]
- In vivo tumor targeting of tumor necrosis factor-α-loaded stealth nanoparticles: effect of MePEG molecular weight and particle size. Eur. J. Pharm. Sci.. 2006;27(1):27-36.
- [Google Scholar]
- Methotrexate-conjugated chitosan-grafted pH- and thermo-responsive magnetic nanoparticles for targeted therapy of ovarian cancer. Int. J. Biol. Macromol.. 2020;154:1175-1184.
- [Google Scholar]
- Identification of multiple cellular uptake pathways of polystyrene nanoparticles and factors affecting the uptake: relevance for drug delivery systems. Eur. J. Cell Biol.. 2014;93(8):323-337.
- [Google Scholar]
- Inhibition of chymotrypsin through surface binding using nanoparticle-based receptors. Proc. Natl. Acad. Sci.. 2002;99(8):5018-5023.
- [Google Scholar]
- “Targeting” nanoparticles: the constraints of physical laws and physical barriers. J. Control. Release. 2012;164(2):115-124.
- [Google Scholar]
- Specific targeting of ovarian tumor-associated macrophages by large, anionic nanoparticles. Proc. Natl. Acad. Sci.. 2020;117(33):19737-19745.
- [Google Scholar]
- V. Haselmann, M. Hedtke, and M. Neumaier, “Liquid profiling for cancer patient stratification in precision medicine – current status and challenges for successful implementation in standard care,” vol. 46, no. 4, pp. 225-236, 2022.
- PEGylation: a promising strategy to overcome challenges to cancer-targeted nanomedicines: a review of challenges to clinical transition and promising resolution. Drug Deliv. Transl. Res.. 2019;9(3):721-734.
- [Google Scholar]
- Current status and future prospects of PARP inhibitor clinical trials in ovarian cancer. Cancer Manag. Res.. 2019;vol. 11(null):4371-4390.
- [Google Scholar]
- “Barriers to immunotherapy in ovarian cancer: metabolic, genomic, and immune perturbations in the tumour microenvironment”. Cancers. 2021;13(24)
- [CrossRef] [Google Scholar]
- Gold nanorods for ovarian cancer detection with photoacoustic imaging and resection guidance via raman imaging in living mice. ACS Nano. 2012;6(11):10366-10377.
- [Google Scholar]
- “Combo” nanomedicine: Co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv. Drug Deliv. Rev.. 2016;98:3-18.
- [Google Scholar]
- The role of surface functionality in determining nanoparticle cytotoxicity. Acc. Chem. Res.. 2013;46(3):681-691.
- [Google Scholar]
- “Polymeric nanoparticle delivery of combination therapy with synergistic effects in ovarian cancer”. Nanomaterials. 2021;11(4)
- [CrossRef] [Google Scholar]
- Anti-tumor efficacy of folate modified PLGA-based nanoparticles for the co-delivery of drugs in ovarian cancer. Drug Des. Devel. Ther.. 2019;vol. 13(null):1271-1280.
- [Google Scholar]
- “Be active or not: the relative contribution of active and passive tumor targeting of nanomaterials,” (in eng) Nanotheranostics. 2017;1(4):346-357.
- [Google Scholar]
- Integrated self-assembling drug delivery system possessing dual responsive and active targeting for orthotopic ovarian cancer theranostics. Biomaterials. 2016;90:12-26.
- [Google Scholar]
- Enhanced antitumor efficacy, biodistribution and penetration of docetaxel-loaded biodegradable nanoparticles. Int. J. Pharm.. 2012;430(1):350-358.
- [Google Scholar]
- Ultrasound-mediated destruction of paclitaxel and oxygen loaded lipid microbubbles for combination therapy in ovarian cancer xenografts. Cancer Lett.. 2015;361(1):147-154.
- [Google Scholar]
- “Long-circulating targeted nanoparticles for cancer therapy”. Curr. Nanosci.. 2010;6(4):347-354.
- [Google Scholar]
- Biodegradable PLGA-b-PEG polymeric nanoparticles: synthesis, properties, and nanomedical applications as drug delivery system. J. Nanopart. Res.. 2012;14(12):1316.
- [Google Scholar]
- Quality of life among long-term survivors of advanced stage ovarian cancer: a cross-sectional approach. Gynecol. Oncol.. 2017;146(1):101-108.
- [Google Scholar]
- Functionalizing nanoparticles with cancer-targeting antibodies: a comparison of strategies. J. Control. Release. 2020;320:180-200.
- [Google Scholar]
- Current trends and challenges in pharmacoeconomic aspects of nanocarriers as drug delivery systems for cancer treatment. Int. J. Nanomed.. 2021;vol. 16(null):6593-6644.
- [Google Scholar]
- Impact of recurrence of ovarian cancer on quality of life and outlook for the future. International Journal of Gynecologic Cancer. 2017;27(6):1134.
- [Google Scholar]
- Albumin nanoparticles increase the anticancer efficacy of albendazole in ovarian cancer xenograft model. J. Nanobiotechnol.. 2015;13(1):25.
- [Google Scholar]
- Intraperitoneal chemotherapy for ovarian cancer using sustained-release implantable devices. Expert Opin. Drug Deliv.. 2018;15(5):481-494.
- [Google Scholar]
- BRCA1 and BRCA2: the genetic testing and the current management options for mutation carriers. Crit. Rev. Oncol. Hematol.. 2006;57(1):1-23.
- [Google Scholar]
- “Nanodrug delivery systems for the treatment of ovarian cancer”. Cancers. 2020;12(1)
- [CrossRef] [Google Scholar]
- “Drug delivery systems for RNA therapeutics,” (in eng) Nat Rev Genet. 2022;23(5):265-280.
- [Google Scholar]
- Systemic treatment of newly diagnosed advanced epithelial ovarian cancer: from chemotherapy to precision medicine. Crit. Rev. Oncol. Hematol.. 2021;158:103209
- [Google Scholar]
- Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2. Genet. Med.. 2010;12(5):245-259.
- [Google Scholar]
- “Peanut-shaped gold nanoparticles with shells of ceragenin CSA-131 display the ability to inhibit ovarian cancer growth in vitro and in a tumor xenograft model”. Cancers. 2021;13(21)
- [CrossRef] [Google Scholar]
- Polymeric nanoparticles-siRNA as an emerging nano-polyplexes against ovarian cancer. Colloids Surf.B: Biointerfaces. 2022;218:112766
- [Google Scholar]
- Hydrolysable core-crosslinked thermosensitive polymeric micelles: synthesis, characterisation and in vivo studies. Biomaterials. 2007;28(36):5581-5593.
- [Google Scholar]
- A literature review of the social and psychological needs of ovarian cancer survivors. Psychooncology. 2013;22(11):2408-2418.
- [Google Scholar]
- PARP inhibition in cancer: an update on clinical development. Target. Oncol.. 2019;14(6):657-679.
- [Google Scholar]
- Biodegradable photoresponsive nanoparticles for chemo-, photothermal- and photodynamic therapy of ovarian cancer. Mater. Sci. Eng. C. 2020;116:111196
- [Google Scholar]
- Molecular profiling of ctDNA in pancreatic cancer: opportunities and challenges for clinical application. Pancreatology. 2021;21(2):363-378.
- [Google Scholar]
- Stress hormone–mediated invasion of ovarian cancer cells. Clin. Cancer Res.. 2006;12(2):369-375.
- [Google Scholar]
- “Recent advances in tumor targeting via EPR effect for cancer treatment”. Journal of Personalized Medicine. 2021;11(6)
- [CrossRef] [Google Scholar]
- Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv. Mater.. 2012;24(12):1504-1534.
- [Google Scholar]
- Reducing ovarian cancer mortality through early detection: approaches using circulating biomarkers. Cancer Prev. Res.. 2020;13(3):241-252.
- [Google Scholar]
- Cell-penetrating peptides in oncologic pharmacotherapy: A review. Pharmacol. Res.. 2020;162:105231
- [Google Scholar]
- Specific cell targeting with APRPG conjugated PEG–PLGA nanoparticles for treating ovarian cancer. Biomaterials. 2014;35(3):983-992.
- [Google Scholar]
- Mechanism-independent optimization of combinatorial nanodiamond and unmodified drug delivery using a phenotypically driven platform technology. ACS Nano. 2015;9(3):3332-3344.
- [Google Scholar]
- Ovarian cancer targeted hyaluronic acid-based nanoparticle system for paclitaxel delivery to overcome drug resistance. Drug Deliv.. 2016;23(5):1810-1817.
- [Google Scholar]
- Emerging targeted drug delivery strategies toward ovarian cancer. Adv. Drug Deliv. Rev.. 2021;178:113969
- [Google Scholar]
- Folate-targeted nanoparticle delivery of chemo- and radiotherapeutics for the treatment of ovarian cancer peritoneal metastasis. Biomaterials. 2011;32(33):8548-8554.
- [Google Scholar]
- Development and evaluation of novel tumor-targeting paclitaxel-loaded nano-carriers for ovarian cancer treatment: in vitro and in vivo. J. Exp. Clin. Cancer Res.. 2018;37(1):29.
- [Google Scholar]
- Paclitaxel-loaded self-assembled lipid nanoparticles as targeted drug delivery systems for the treatment of aggressive ovarian cancer. ACS Appl. Mater. Interfaces. 2018;10(30):25174-25185.
- [Google Scholar]
- Ovarian carcinoma biological nanotherapy: comparison of the advantages and drawbacks of lipid, polymeric, and hybrid nanoparticles for cisplatin delivery. Biomed. Pharmacother.. 2019;109:475-483.
- [Google Scholar]
- Health-related quality of life in ovarian cancer survivors: results from the american cancer society's study of cancer survivors — I. Gynecol. Oncol.. 2016;141(3):543-549.
- [Google Scholar]







