Translate this page into:
Multiple metal resistance and Cr6+ reduction by bacterium, Staphylococcus sciuri A-HS1, isolated from untreated tannery effluent
⁎Corresponding author. rehman.mmg@pu.edu.pk (Abdul Rehman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A chromium-resistant bacterium was isolated indigenously from tannery effluent and identified as Staphylococcus sciuri. The optimum growth was observed at 37 °C and pH 7. S. sciuri was able to resist Cr6+ (25 mM) and other heavy metals such as As2+ (19 mM), Pb2+ (18.5 mM), Zn2+ (17 mM), Cu2+ (2.5 mM), Cd2+ (3 mM), and Ni2+ (1.5 mM). Biochemical characterization of chromate reductase revealed its optimal pH as 8.0 and temperature as 40 °C. The enzyme activity was stimulated only by Mg2+ among other heavy metals tested. S. sciuri showed chromium biosorption proficiency (q) after 2, 4, 6 and 8 days as 42, 73, 85 and 31 mM/g, respectively. Cr6+ did not stimulate activities of APOX, SOD and CAT significantly, however, only POX showed marked production (86%). Also, enhanced production of glutathione and other non-protein thiols significantly combat metal induced oxidative stress. Pilot study verified that S. sciuri was able to remove 93% Cr6+ from tannery effluent within 6 days of incubation. Thus, S. sciuri could act as a potential candidate for the bioremediation of hexavalent chromium contaminated environmental sites.
Keywords
Chromium
S. sciuri A-HS1
Chromate reductase
Antioxidant enzymes
Glutathione
Bioremediation
1 Introduction
Advancement in technology and industrial globalization led to indiscriminate emission of hazardous industrial wastes into the biosphere, consequently inflict serious damage to the environment. The industrial effluents usually consist of a huge amount of various toxic heavy metals at an elevated concentration, when discharged directly into the environment without any prior treatment; severely contaminate the land and fresh water bodies (Nissim et al., 2018).
Anthropogenic inputs of chromium has been increased due to its widespread use in industries like electroplating, tanning of hides and skins, resistant stainless steel alloys production, textile dye productions (Cheunga and Gu, 2007), nuclear coolants (i.e., biocide) and mining activities. Thus, huge amount of Cr-containing wastes has been released in the industrial effluents that contaminate the ecosystem. When gets in water bodies, Cr is toxic to the plants and animals due to its mutagenic, carcinogenic, and teratogenic effects.
Tanning industry generate huge varieties of toxins such as salinity, inorganic and organic load (chemical and biological oxygen demand), dissolved, suspended solids, specific pollutants (chromium, sulfide, sodium, chloride and other salt residues) and heavy metals etc. (Katz and Salem, 1994; Zahoor and Rehman, 2009). Tanning process utilizes huge quantities of water out of which 90% water get discarded as effluent. Also solid and gaseous waste byproducts generated during leather processing contaminate the environment. Chrome tanning procedure discharges at least 40% unused chromium salts in the final effluents, posing a serious threats to the environment (Leghouichi et al., 2009; Garg et al., 2012).
As a transition metal, Cr exists in various valence states i.e., −2 to +6, with the hexavalent (Cr6+) and trivalent (Cr3+) being the predominant chromium species in the natural aquifers and municipal wastewater rich in organic contaminants, respectively (Cheunga and Gu, 2007). Dominancy of Cr6+ in the aquifer is due to its high solubility in water in the full pH range, making it bioavailable in the ecosystem. In contrast, Cr3+ displays a high affinity for organics resulting in the formation of complexes that precipitate as amorphous hydroxide (Sawyer et al., 1994). Because of its persistence in the environment, anthropogenic release of Cr6+ is a matter of concern (Katz and Salem, 1994; Cheunga and Gu, 2007).
Heavy metals, non-degradable in nature, easily get accumulated in the environment without limitations, hence removing them mechanically is not only an expensive approach but also time consuming. Thus, biological removal of heavy metals from the effluents has been utilized for the bioremediation purposes as it primarily depends on the ability of microbes to uptake and/or reduce the metal (Mala et al., 2015).
Ability of microorganisms to survive and proliferate within the vicinity of excruciating toxic metals has helped the scientists to develop several strategies for the bioremediation of hazardously polluted environments. Thus, the discovery of chromate reducing microorganisms proved to be a potential candidate for in situ and on-site bioremediation approaches (Garg et al., 2012; Mangaiyarkarasi and Geetharamani, 2014).
The main objective of this study was to screen and characterize chromate resistant and reducing bacteria from the metal polluted environment, and to determine the chromium reduction potential of the isolated strain as a potential candidate for the chromate bioremediation.
2 Materials and methods
2.1 Sample collection and screening of chromium tolerant bacterial strain
Nine tannery effluent samples were collected from different locations of long term chromium contaminated sites of Sheikhupura and Qasoor, Lahore (Pakistan). At the time of sample collection, some physiochemical characteristics of effluent samples (color, pH, temperature) were noted. For cultivation of Cr6+ tolerant bacteria, serial dilutions of the wastewater samples were prepared, and plated onto modified Luria-Bertani (LB) agar [g/l; 10, trypton; 5, yeast extract; 5, NaCl; 15, agar] supplemented with 2 mM K2Cr2O7, and incubated at 37 °C for 24–72 h. Bacterial isolates screening was done by their potential to survive on higher concentrations of hexavalent chromium (K2Cr2O7,). Only one Cr6+ resistant bacterial strain, A-HS1, was selected for further study on the basis of its resistance and pathogenicity (Fig. S1).
2.2 Biochemical and molecular characterization
The purified isolated strain A-HS1 was characterized by routine biochemical tests (Gram’s staining, Catalase, Mannitol fermentation). Genomic DNA was isolated (Masneuf-Pomarade et al., 2007) and 16S rRNA gene was amplified by using the universal bacterial primers RS1 and RS3 through PCR (Rehman et al., 2007). PCR product was purified by Fermentas purification kit (#K0513) and Genetic analysis system model CEQ-800 (Beckman) Coulter Inc. Fullerton, CA, USA was used to analyze the sequence. The sequences were identified through BLAST analysis. Moreover, a dendrogram was constructed on the basis of homology using MEGA7 program.
2.3 Determination of optimum growth conditions
The temperature and pH optima were tested for the bacterial isolate. The strain was cultured in (LB) liquid medium (pH 7) at 20 °C, 30 °C, 37 °C, 45 °C and 55 °C. For the pH experiment, cultures (100 ml LB in 250 flasks) were adjusted to pH 5, 6, 7, 8, 9 and 10 and incubated at the optimum temperature. For growth pattern, bacterial isolate was grown in MS broth [g/l: FeSO4.7H2O 0.015 g, KH2PO4 4.7 g, MgSO4.7H2O 1 g, CaCl2.2H2O 0.01 g, Na2HPO4 0.12 g, NH4NO3 4 g, MnSO4.4H2O 0.01 g, glucose 10 g and yeast extract 5 g (pH 7–7.2)] without metal (control) and with 2 mM K2Cr2O7 (experimental) at 37 °C for 24 h. Optical density at 600 nm (OD600nm) was employed as the function of bacterial growth.
2.4 Minimum inhibitory concentrations (MICs) of Cr6+ and other heavy metals
MIC of Cr6+ and other heavy metals was determined in modified M9 broth [g/l: Na2HPO4, 0.65 g; KH2PO4, 1.5 g; NH4Cl, 0.5 g; NaCl, 0.25 g; MgSO4.7H2O, 0.12 g; Casamino acid, 10 g; Glucose, 5 g (pH 6.9)]. To check MIC for Cr6+, different concentrations of K2Cr2O7 (0.05–30 mM) was supplemented to the 100 ml modified M9 medium and incubated at 37 °C in rotary shaker at 120 rpm for one week and cell growth was determined at OD600. Similarly, to evaluate MICs for other heavy metals, different concentrations of CdCl2, NiCl2.6H20, ZnSO4.7H2O, CuSO4.5H2O and PbNO3, ranging from 1 to 15 mM, were added separately to the modified M9 medium. All metal solutions were prepared in deionized water and filter sterilized.
2.5 Chromate reductase assay
2.5.1 Crude enzyme preparation
The bacterial isolate was cultivated in 100 ml MSM amended with 2 mM K2Cr2O7 and incubated at 37 °C rotary shaker for 5 days. Cells were centrifuged at 6000 rpm for 10 min. The supernatant and pellet were separated, the pellet was washed thrice (phosphate buffer), and sonicated for 15 pulses with an interval of 60 s for 3 times. The sonicated pellet was centrifuged for 10 min at 14,000 rpm and the supernatant was used as intracellular crude enzyme. Ammonium sulphate was used to saturate the supernatant up to 60% and placed at −20 °C overnight. The mixture was centrifuged for 10 min, the upper 10 ml supernatant was discarded, and the pellet was used for extracellular enzyme. Chromate reductase activity, both extra and intra -cellular was assayed according to the method of Sarangi and Krishnan (2008).
2.5.2 Chromate reductase characteristics
The optimal pH value was scrutinized for maximum chromate reductase activity by using different buffers of pH values ranging from 4.0 to 9.0. The reaction mix contained 50 mM buffer of the specific pH with 20 µM Cr6+, 0.1 mM NADH and crude enzyme (0.1 ml), and incubated for 30 min. For optimum temperature, enzyme assay was done at various incubation temperatures i.e., 30, 40, 50, 70, 90 °C. The metal ions effect was assessed by adding 0.1 mM concentration of metal ion solutions i.e., NaCl, MnCl2, MgCl2, ZnCl2, HgCl2, NiCl2, CuSO4. The reaction mixture with no added metal served as control, and all the enzyme activities were evaluated by Sarangi and Krishnan (2008).
2.6 Quantification of antioxidant enzymes
To assay antioxidant enzymes, A-HS1 was cultivated in 100 ml MSM medium at 37 °C for 24 h. After 24 h growth, medium was supplemented with 2 mM Cr6+ and incubated again. After 48 h of incubation, cells were harvested at 14,000 rpm for 10 min, and pellet was weighed and resuspended in phosphate buffer. Pellet was sonicated and centrifuged, and the supernatant was used for the estimation of antioxidants.
Glutathione transferase (GST) activity was assayed according to Habig et al. (1974). Ascorbate peroxidase (APOX) activity was determined according to Israr et al. (2006). Peroxidase (POX) activity was determined by the method of Reuveni et al. (1992) and catalase activity was determined according to Beers and Sizer (1952). Superoxide dismutase (SOD) activity was measured indirectly as described by Ewing and Janero (1995).
2.7 Quantification of glutathione and cysteine levels in metal stress
To estimate levels of reduced glutathione (GSH), oxidized glutathione (GSSG), and other non-protein thiol in bacterial cell lysate under Cr6+ stress conditions, method of Shamim and Rehman (2013) was followed. Briefly, bacterial strain was cultivated for 24 h and then 2 mM K2Cr2O7 was added in two flasks while the third one without metal stress served as control. The flasks were incubated again for 48 h. Cells were pelleted, washed thrice with phosphate buffer and pellet was weighed and re-suspended in 1 ml of 5% sulfosalicylic acid. Pellet was sonicated and centrifuged at 14,000 rpm at 4 °C for 10 min. The supernatant was divided into two equal portions, one was used to determine level of glutathione and the other was used for non-protein thiol estimation.
2.8 Metal uptake potential of the bacterial isolate
To assess metal uptake potential of the bacterium, A-HS1 was cultured in 100 ml LB broth with 2 mM Cr6+ stress. As a control in one flask medium was only supplemented with 2 mM Cr6+ but without bacterial culture. All the flasks were incubated in shaking incubator (120 rpm) at 37 °C. An aliquot of 5 ml was withdrawn from each flask after regular intervals (2, 4, 6 and 8 days of growth). Samples were spun down at 6000 rpm for 10 min and, both the pellets and supernatants were used for Cr6+ estimation by atomic absorption spectrophotometer (Zahoor and Rehman, 2009).
2.8.1 Tannery effluent Cr 6+ bioremediation; a mini-pilot assay
A-HS1 potential to reduce Cr6+ in tannery effluents was determined. A set of three plastic containers was used; the first container carried only tannery wastewater (10 L) (control), second container carried 10 L distilled water with 1.5 L culture, while the third container carried 10 L wastewater with 1.5 L culture. Each container was amended with 2 mM Cr6+ and incubated at room temperature (25 ± 2 °C). Ten milliliter samples were taken after regular intervals (2, 4, 6, 8 days of incubation), and growth was centrifuged for 10 min at 4000 rpm. The samples supernatants were analyzed for Cr6+ estimation by DPC method. Change in Cr6+ concentration was determined by the standard curve made under the same experimental conditions using standards of Cr6+ solution.
2.9 Use of bacterial treated effluent for plant growth
Effect of bacterially treated tannery wastewater on plant growth was investigated. In this regard, small pots were prepared using autoclaved soil and six seeds of Vigna radiata (mung beans) were sown in each pot. The experimental pot was watered with wastewater treated with bacterial isolate A-HS1 while the other two controls were watered with tap water and original wastewater (untreated). The plants were cultivated for 10 days under 1:1 light and dark period. Any difference in plants growth watered with microbially treated wastewater and untreated wastewater, was observed
2.10 Bacterial protein isolation
The bacterium was cultivated in MSM broth with and without 2 mM Cr6+. SDS PAGE was performed according to Laemmli (1970).
2.11 Fourier-transform infrared and energy dispersive X-ray analysis
Infrared spectra for A-HS1 cells were obtained using a Fourier Transform Infrared Spectrometer (Bruker, alpha). The method of Deokar et al. (2013) was employed to prepare bacterial samples. To ascertain the binding mechanism between the metal ions and microbial cells, it is imperative to identify the location of the chromium species (Cr6+, Cr3+) relative to the bacterial cells. Elemental analysis of hexavalent chromium reduction was determined by EDXA (energy dispersive X-ray analysis) to evaluate the compound distribution before and after the biosorption of heavy metal.
2.12 Statistical analysis
For each experiment, three independent measurements were taken and data shown are average values of means ± standard deviation (SD). Significance testing between samples was calculated by performing paired Student’s t-test. Control group was treated identically without exposure to any treatment.
3 Results
3.1 Physiochemical characteristics
It was observed that the temperature of all the samples ranged from 22 to 34 °C, and pH range was 6.0–10.0. Samples were diluted and plated on LB agar plates amended with 2 mM Cr6+. Concentration of Cr6+ was increased regularly, and one isolate who survived at the highest concentration of Cr6+ (25 mM) was selected for this study, labelled as A-HS1.
3.2 Identification of bacterial isolate
The organism is gram and catalase positive and characteristics of the isolate are given in Table S1. 16S rRNA gene ribotyping revealed 93% homology with Staphylococcus sciuri (Accession number JQ916905) already submitted to NCBI database. A dendrogram was constructed on the basis of similarity with other S. sciuri strains using MEGA7 program taking bootstrap value 500 (Fig. 1a).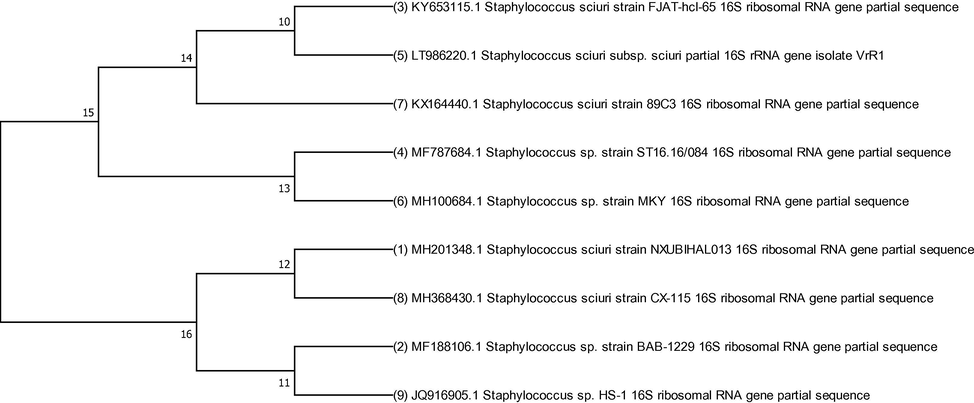
The evolutionary relationship of S. sciuri A-HS1 was inferred with already reported strains. The evolutionary history was deduced using the Neighbor-Joining method with 500 bootstrap test value in MEGA7 software.
3.3 Optimum growth conditions
S. sciuri showed maximum growth at 37 °C and pH 7. Hexavalent chromium presence (2 mM Cr6+) in the medium significantly decreased the bacterial growth (Fig. 1b).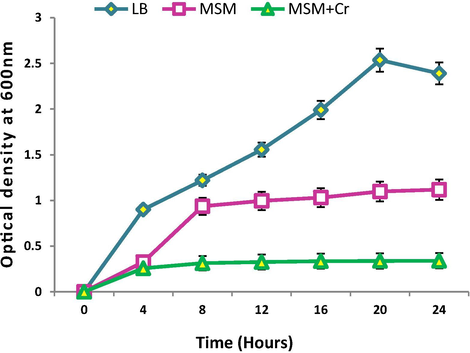
Growth curves of Staphylococcus sciuri A-HS1 in LB medium (control), mineral salt medium (MSM) and MSM supplemented with 2 mM K2Cr2O7 (treated) incubated at 37 °C. Optical density was taken at 600 nm after regular time interval.
3.4 Cross metal resistance
S. sciuri was capable to tolerate Cr6+ up to 25 mM and also showed resistance to other heavy metals, viz., 17 mM (Zn2+), 18.5 mM (Pb2+), 2.5 mM (Cu2+), 3 mM (Cd2+), 19 mM (As3+) and 1.5 mM (Ni2+). The order of resistance regarding heavy metals concentration was Cr6+ > As3+ > Pb2+ > Zn2+ > Cd2+ > Cu2++ > Ni2+.
3.5 Characterization of chromate reductase
Characterization of chromate reductase of S. sciuri A-HS1 demonstrated optimum pH for the enzymatic activity was 8.0 (Fig. 2a) and optimum temperature was 40 °C, as the enzyme was inhibited significantly at 70 °C (87% of inhibition) (Fig. 2b). Effect of different metal cations on the enzyme activity (Fig. 2c) reveal that all the metal ions tested inhibit the Cr6+ reductase activity except for Mg2+ (13%).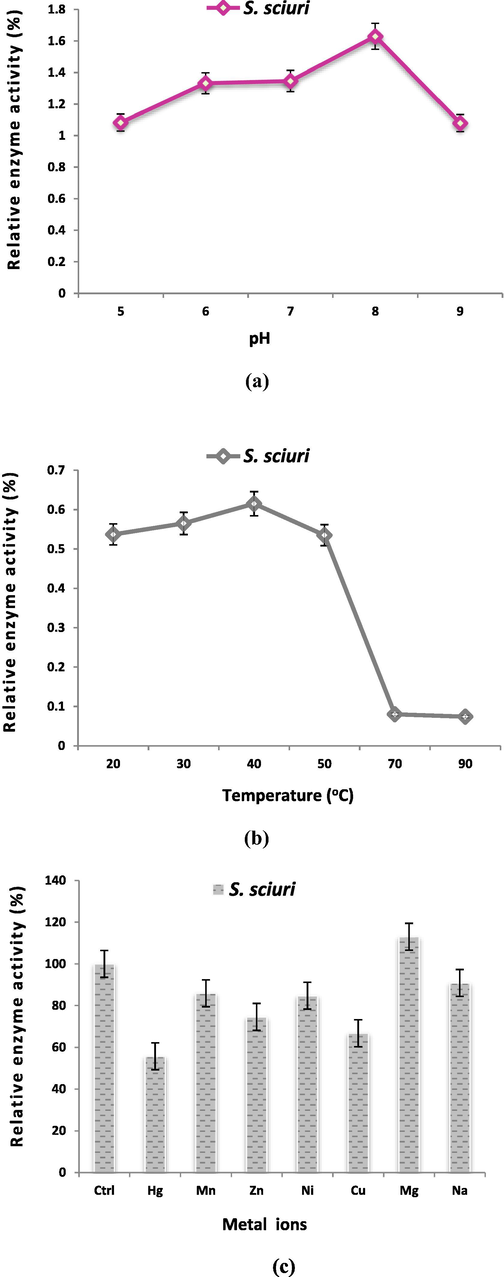
Effect of pH (a) and temperature (b) on enzyme activity (c) metal ions on the chromate reductase activity.
3.6 Quantification of antioxidant enzymes
Estimation of the antioxidant enzymes in the presence of Cr stress unveils very interesting results. Cr6+ did not stimulate activities of APOX, SOD and CAT in significant amount, however, a decrease in activities of these enzymes was observed i.e., APOX (11%), SOD (8%), and CAT (3%), respective to the normal growth conditions. The Cr toxicity stimulated S. scuiri A-HS1 to express a relatively higher level of GST. The only antioxidant which expressed itself in significantly higher level was POX (86%) under Cr6+ stress condition (Fig. 3).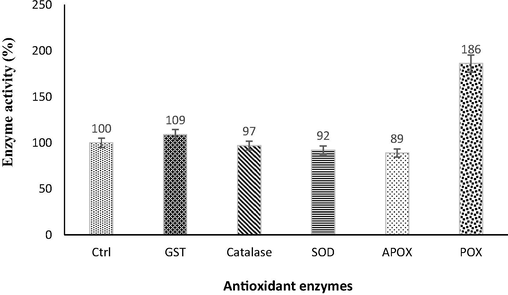
Changes in antioxidant enzymes activity profile, exhibited by S. sciuri A-HS1 upon exposure of 2 mM Cr6+.
3.7 Quantification of GSH and non-protein thiols
An alternative level of GSH and GSSG was estimated in the presence of Cr6+ in S. sciuri A-HS1 (Table 1). When the bacterium was supplemented with 2 mM Cr6+ stress, an increase level of 230.8% in GSH was determined as compared to the control. Moreover, a significant increased level of non-protein thiols was also recorded in the presence of chromium (432.7%).
CrConc.(mM)
GSH(mMg−1 FW)
GSSG (mMg−1 FW)
GSH + GSSG (mMg−1 FW)
GSH/GSSG ratio
% increase in GSH
Non-protein thiols
% increase in Non-protein thiols
0.0
17.526
7.573
25.099
2.314
230.8
18.392
432.7
2.0
19.834
11.540
31.374
1.719
22.719
3.8 Chromium processing ability of bacterial isolate
3.8.1 Biosorption of Cr6+
Biosorption ability of S. sciuri A-HS1 was evaluated by culturing it in LB broth containing 2 mM Cr6+ (Fig. 4a). The biosorption efficiency (q) of S. sciuri A-HS1 was as 42, 73, 85 and 31 mM Cr6+/g after 2, 4, 6 and 8 days of incubation. The amount of Cr6+ up taken by bacterial cells after 2, 4, 6 and 8 days was 14, 16, 14 and 6 mM/g, respectively. While 22, 47, 63 and 16 mM/g of Cr6+ were estimated to be adsorbed on the surface of bacterium after the incubation of same time period.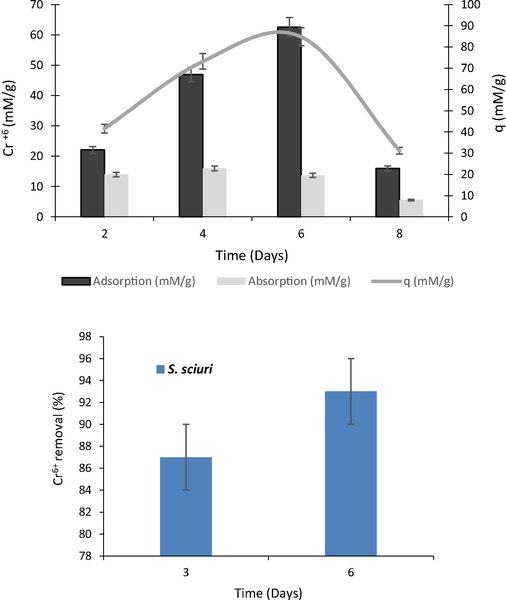
(a) Biosorption of Cr6+ by S. sciuri A-HS1 at lab scale incubated for 8 days. (b) Cr6+ reduction by S. sciuri A-HS1 from tannery effluent after 3 and 6 days of incubation at room temperature.
3.8.2 Pilot scale Cr6+ bioremediation
S. sciuri A-HS1 ability to reduce Cr6+ was checked at pilot scale, where 10 L of tannery wastewater was used and the efficiency of the organism was determined by DPC method. Assay results revealed that at pilot scale S. sciuri A-HS1 was able to remove 93% Cr6+ from tannery effluent after 6 days of incubation when Cr6+ concentration was maintained at 2 mM in tannery effluent (Fig. 4b).
3.9 Phytotoxicity study
In the present study phytotoxicity results showed good germination rate of Vigna radiata in the bacterial processed tannery wastewater and in tap water. The noxious tannery wastewater in the presence of bacterial strain has become nontoxic due to toxic Cr6+ reduction into less toxic Cr3+ (Fig. 5).
Growth of Vigna radiata (mung beans) in microbial treated wastewater (with S. sciuri A-HS1), tap water and untreated wastewater after 10 days of incubation under 1:1 light and dark period.
3.10 Protein analysis
Changes in total protein profiles of S. sciuri A-HS1 in the presence and absence of Cr6+ stress was checked by polyacrylamide gel electrophoresis. SDS-PAGE analysis revealed the over-expression of some proteins in the presence of Cr6+. A protein band of about 65 kDa was observed in control culture sample but was absent in the culture which was challenged with 2 mM Cr6+ (Fig. S2).
3.11 FTIR and EDX analysis
FTIR spectrum of the control (without chromium) and treated (with chromium) bacterial strain S. sciuri A-HS1 is shown in Fig. 6a. When grown in LB broth medium without Cr6+, the infrared absorption peaks of S. sciuri A-HS1 display characteristic absorption peaks of amino, carboxyl, hydroxyl, and sulphonate groups representing the presence of moieties on the cell surface. While a shift in characteristic absorption peaks was observed under the stress of 2 mM Cr6+. Under the stress of Cr6+, S. sciuri A-HS1 displayed changes in the region of 1800–1000 cm−1 and 3280 cm−1. EDX analysis also confirmed the accumulation of Cr6+ in the cell cytoplasm (Fig. 6b).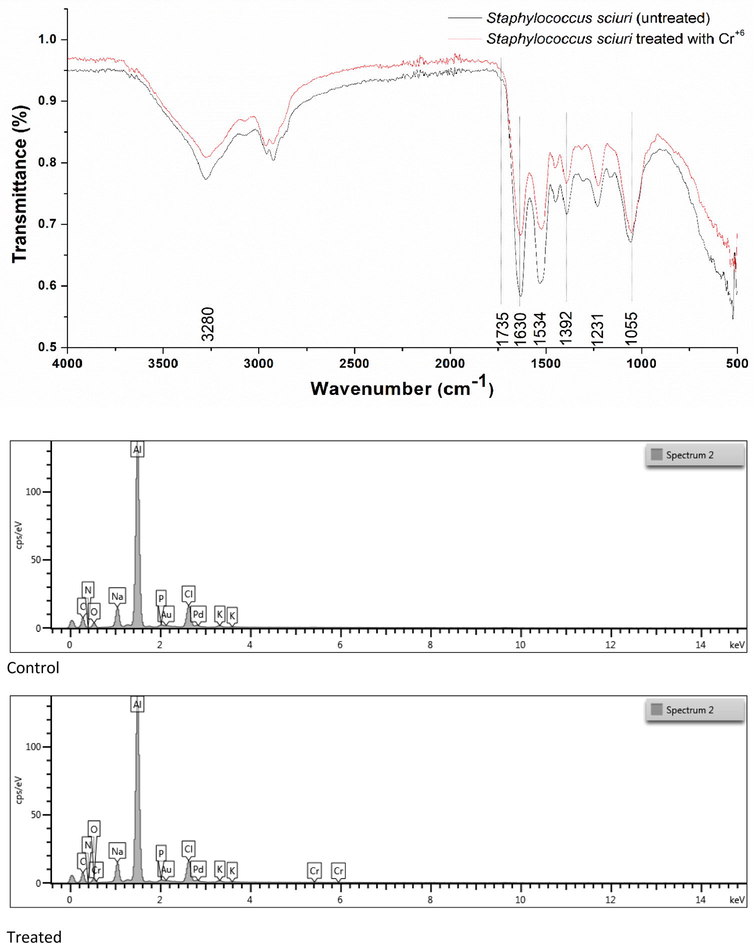
(a) FTIR spectra of S. sciuri A-HS1 in the presence and absence (control) of hexavalent chromium stress (2 mM Cr6+). (b) Energy dispersive X-ray spectroscopy through SEM of S. sciuri A-HS1 (A) control and (B) Cr6+-treated. (c) Proposed Cr6+ resistance and reduction mechanism in gram positive bacterium, S. sciuri 43C. (a) Cr6+ reduces into Cr3+ extracellularly by chromate reductase. (b) Cr6+ enters into cell cytoplasm and changes into Cr3+ by intracellular chromate reductase. Some portion of Cr6+ r changes into its intermediate species. (c) Cr5+ and Cr4+ are involved in the generation of ROS. The oxidative stress resulted by the formation of ROS is combated by two ways; (d) antioxidant enzymes and (e) antioxidant molecules. After certain level of accumulation Cr6+ expel out through efflux system.
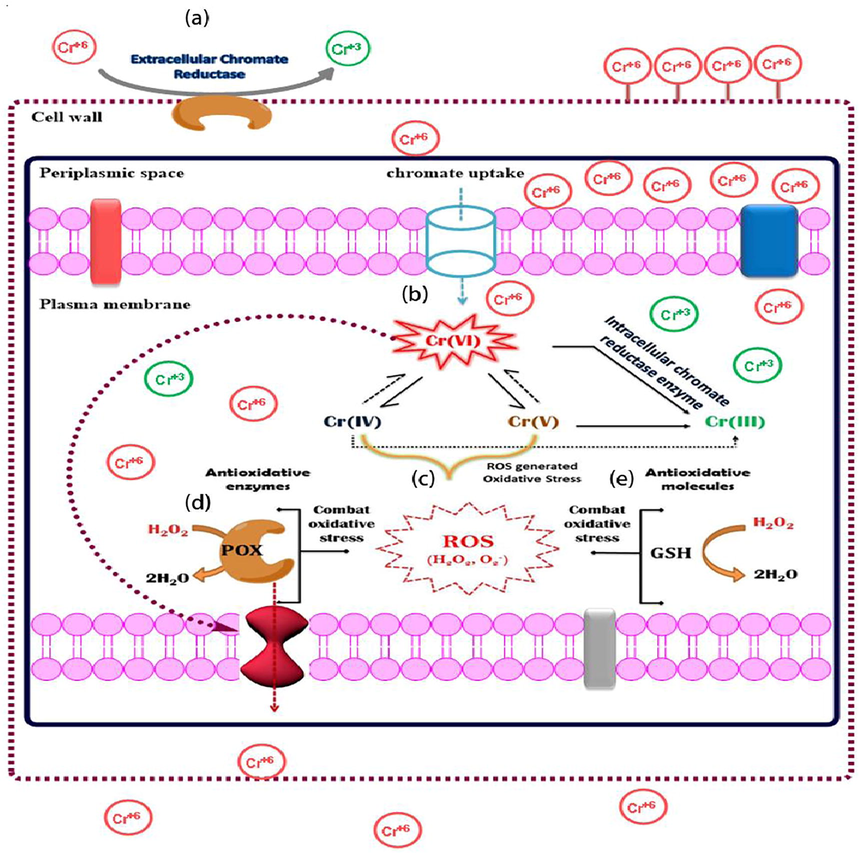
(a) FTIR spectra of S. sciuri A-HS1 in the presence and absence (control) of hexavalent chromium stress (2 mM Cr6+). (b) Energy dispersive X-ray spectroscopy through SEM of S. sciuri A-HS1 (A) control and (B) Cr6+-treated. (c) Proposed Cr6+ resistance and reduction mechanism in gram positive bacterium, S. sciuri 43C. (a) Cr6+ reduces into Cr3+ extracellularly by chromate reductase. (b) Cr6+ enters into cell cytoplasm and changes into Cr3+ by intracellular chromate reductase. Some portion of Cr6+ r changes into its intermediate species. (c) Cr5+ and Cr4+ are involved in the generation of ROS. The oxidative stress resulted by the formation of ROS is combated by two ways; (d) antioxidant enzymes and (e) antioxidant molecules. After certain level of accumulation Cr6+ expel out through efflux system.
4 Discussion
Several investigations have been reported on chromate resistant and reducing bacteria, isolated from metal contaminated as well as non-contaminated environments (Zahoor and Rehman, 2009; Mistry et al., 2010; Kouadjo and Zeze, 2011; Sagar et al., 2012) (Table S2) under aerobic and anaerobic conditions (Wani et al., 2007; Tripathi and Garg, 2014; Iyengar and Usha; 2016). In this study, a bacterial strain, S. sciuri A-HS1 was isolated that could resist Cr6+ stress up to 25 mM. Chromate resistant Staphylococci species have also been reported previously in Cr6+ reduction (Mangaiyarkarasi and Geetharamani, 2014; Iyengar and Usha; 2016; Duttaa et al., 2017).
Bioaccumulation comprises two stages. In the first step, initial uptake of metal take place through direct physical contact, that does not require energy, and this passive accumulation of metal at cell surface is thought to be mediated by adsorption, chelation, ion-exchange, complexation, and microprecipitation (Bharagava and Mishra, 2017). In the second stage, the initial rapid physical adsorption transforms into slow phase of chemical bondage of Cr6+ to high affinity sites. After bioaccumulation, Cr6+ could also get reduced to Cr3+ by serving as terminal electron acceptor before binding chemically to the cell wall (Srinath et al., 2001; Cheunga and Gu, 2007). It has been reported that there is a chemical analogy between sulphate and chromate oxyanions, therefore, active transportation of chromate ions across biological membranes could be mediated through sulphate channels (Cervantes and Campos-Gracia, 2007).
Once get inside the cell, Cr6+ rapidly takes part in a number of processes causing high toxicity in the cytoplasm (Bharagava and Mishra, 2017). Reduction of Cr6+ into Cr3+ occurs through enzymatic as well as non-enzymatic reactions under aerobic as well as anaerobic conditions via electron transport system containing cytochromes. Aerobically, reduction of Cr6+ occur through the production of toxic short lived intermediates such as of Cr5+ and Cr4+ (Bharagava and Mishra, 2017). However, it is not known that the conversion of Cr5+ to Cr4+ and Cr4+ to Cr3+ is either enzyme mediated or spontaneous. In Cr6+ reduction, electron donors (NADH, NADPH) and electron from the endogenous reserve are also involved (Varadhan and Mohan, 2017). Tripathi et al. (2011) reported that B. cereus was able to bioremediate 74.5% Cr6+ after 48 h of incubation. In the present investigation, pilot scale study of Cr6+ bioremediation also replicated the results as the maximum removal of Cr6+ was noted after 6 days of incubation in case of real tannery effluent (93% Cr6+ removal).
To combat the heavy metal and oxidative stress in the cell, ROS generation imbalances the reducing environment within the cell, several proteins are produced by the defensive system to resist chromate stress (Ramirez-Diaz et al., 2008; Thatoi et al., 2014). However, ROS generated oxidative stress is largely combated by the antioxidant enzymes, that scavenge ROS by converting them into non harmful radicals, like superoxide dismutase (SOD), glutathione transferase, catalase, etc. (Ackerley et al., 2004; Hussein and Joo, 2013). Our results revealed that among antioxidants, the induction of APOX (11%), SOD (8%), and CAT (3%) is less than POX (86%) enzyme under Cr6+ stress. Peroxidases, widely known as stress enzymes, produced under various stress conditions such as drought-stress (Zhang and Kirkham, 1994), water stress (Zhang and Kirkham, 1994), salinity (Mittal and Dubey, 1991), gamma-radiation (Hussein and Joo, 2013) and under the toxic level of heavy metals such as Cu, Al, Cd, Zn (Chaoui et al., 1997; Hussein and Joo, 2013). Our results are in agreement with Chen et al. (2000) and Lee and Shin (2003) who have reported a slight increase in catalase activity after Cd stress. Our results are also consistent with Shamim and Rehman (2013), who revealed that the stress of Cd could not able to induce higher production of SOD, APOX, and GR, however, an increase in glutathione was estimated (Fig. 6c).
Energy Dispersive X-ray analysis confirmed the presence and accumulation of Cr6+ in the bacterial cell. FTIR spectroscopy was performed to identify the functional groups present on the bacterial cell surface. The peaks appearing between 3450–3200 cm−1 are characteristic of the hydroxal groups, and —NH groups moieties on the cell surface (Park et al., 2005; Mungasavalli et al., 2007). The intense bands within 1100–1000 cm−1 represent C—O bond, attributed to the characteristic peak for polysaccharides (Das and Guha, 2007). The representative band region at 1100–1030 characterizes the presence of orthophosphate (PO43−). However, the same wavelength in the absorption spectrum i.e., 1350–1000 cm−1, is also representing C-N stretching (Mungasavalli et al., 2007). Under the stress of Cr6+, S. sciuri A-HS1 displayed changes in the region of 1800–1000 cm−1 and 3280 cm−1. The shift in characteristic absorption peaks suggested that hexavalent chromium ions were sequestered by certain functional groups present on the cell surface (Bueno et al., 2008).
Utilization of bacterially treated wastewater for plantation purposes revealed that the seeds watered with the treated tannery wastewater showed a marked increase in plant growth as compared to the one watered with the original wastewater. The plants, watered with untreated wastewater, show delayed and hindered growth. Thus, bacterially treated wastewater could be safely used for the watering of plants in real time scenario.
5 Conclusions
S. sciuri showed high resistance against multiple heavy metals and was also capable to remove nearly 93% Cr6+ from the tannery wastewater over a period of 6 days. Moreover, bacterially treated tannery wastewater, which was found safe for the plantation, could be used at-least for crops irrigation. In coming years, it can become an attractive environmental tool for green chemistry after exploring its molecular biology.
Acknowledgement
This is to acknowledge the support of University of the Punjab, Lahore, Pakistan to accomplish this research work.
Conflicts of interest
None.
References
- Chromate-reducing properties of soluble flavoproteins from Pseudomonas putida and Escherichia coli. Appl. Environ. Microbiol.. 2004;70(2):873-882.
- [Google Scholar]
- A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem.. 1952;195:133-140.
- [Google Scholar]
- Hexavalent chromium reduction potential of Cellulosimicrobium sp. isolated from common effluent treatment plant of tannery industries. Ecotoxicol. Environ. Saf.. 2017;147:102-109.
- [CrossRef] [Google Scholar]
- Biosorption of lead (II), chromium (III) and copper (II) by R. opacus: equilibrium and Kinetic Studies. Miner. Engin.. 2008;21:65-75.
- [Google Scholar]
- Reduction and efflux of chromate by bacteria. Mol. Microbiol. Heavy Met.. 2007;6:407-419.
- [Google Scholar]
- Cadmium and Zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in Bean (Phaseolus vulgaris L.) Plant. Sci.. 1997;127:139-147.
- [Google Scholar]
- Copper toxicity in rice seedlings: Changes in antioxidative enzyme activities, H2O2 level, cell wall peroxidase activity in roots. Bot. Bull. Acad. Sin.. 2000;41:99-103.
- [Google Scholar]
- Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: a review. Int. Biodeterior. Biodegrad.. 2007;59:8-15.
- [Google Scholar]
- Biosorption of chromium by Termitomyces clypeatus. Colloids Surf. B.. 2007;60:46-54.
- [Google Scholar]
- Single-walled carbon nanotube coated antibacterial paper: preparation and mechanistic study. J. Mater. Chem. B.. 2013;1:2639-2646.
- [CrossRef] [Google Scholar]
- Isolation of indigenous Staphylococcus sciuri from chromium-contaminated paddy field and its application for reduction of Cr (VI) in rice plants cultivated in pots. Biorem. J.. 2017;21(1):1-8.
- [Google Scholar]
- Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal. Biochem.. 1995;232:243-248.
- [Google Scholar]
- Strategies for chromium bioremediation of tannery effluent. Rev. Environ. Contam. Toxicol.. 2012;217:75-140.
- [Google Scholar]
- Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem.. 1974;249:7130-7139.
- [Google Scholar]
- Heavy metal resistance of bacteria and its impact on the production of antioxidant enzymes. African J. Microbiol. Res.. 2013;7(20):2288-2296.
- [CrossRef] [Google Scholar]
- Cadmium accumulation and antioxidant responses in the Sesbania dummondii callus. Arch. Environ. Contam. Toxicol.. 2006;50:121-127.
- [Google Scholar]
- Removal of chromium by Staphylococcus saprophyticus subsp. bovis strain 1. Biologia. 2016;62(1):1-8.
- [Google Scholar]
- The Biological and Environmental Chemistry of Chromium. New York: VCH; 1994.
- Chromium tolerance and reduction potential of Staphylococci species isolated from a fly ash dumping site in South Africa. Afr. J. Biotechnol.. 2011;10(69):5587-5594.
- [CrossRef] [Google Scholar]
- Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-685.
- [Google Scholar]
- Cadmium-induced changes in antioxidant enzymes from the marine alga Nannochloropsis oculata. J. Appl. Phycol.. 2003;15:13-19.
- [Google Scholar]
- Evaluation of chromium contamination in water, sediment and vegetation caused by the tannery of Jijel (Algeria): a case study. Environ. Monit. Assess.. 2009;153:111-117.
- [Google Scholar]
- Inducible chromate reductase exhibiting extracellular activity in Bacillus methylotrophicus for chromium bioremediation. Microbiol. Res.. 2015;170:235-241.
- [Google Scholar]
- Bio absorption of chromium employing microorganism isolated from tannery effluent. SIRJ-BES.. 2014;1(9):29-36.
- [Google Scholar]
- Molecular typing of wine yeast strains Saccharomyces bayanus var. uvarum using microsatellite markers. Sys. Appl. Microbiol.. 2007;30:75-82.
- [Google Scholar]
- Hexavalent chromium reduction by Staphylococcus sp. isolated from Cr (VI) contaminated land fill. Int. J. Biotechnol. Biochem.. 2010;6(1):117-129.
- [Google Scholar]
- Behaviour of peroxidases in rice: changes in enzyme activity and isoforms in relation to salt tolerance. Plant Physiol. Biochem.. 1991;29:31-40.
- [Google Scholar]
- Biosorption of chromium from aqueous solutions by pretreated Aspergillus niger: batch and column studies. Colloids Surf. A.. 2007;301:214-223.
- [Google Scholar]
- Phytoremediation of sewage sludge contaminated by trace elements and organic compounds. Environ. Res.. 2018;164:356-366.
- [Google Scholar]
- Studies on Hexavalent chromium biosorption by chemically-treated biomass of Ecklonia sp. Chemosphere. 2005;60:1356-1364.
- [Google Scholar]
- Mechanisms of bacterial resistance to chromium compounds. Biometals. 2008;21(3):321-332.
- [Google Scholar]
- Resistance and biosorption of mercury by bacteria isolated from industrial effluents. Pakistan J. Zool.. 2007;39(3):137-146.
- [Google Scholar]
- Peroxidase activity as a biochemical marker for resistance of muskmelon Cucumis melo to Pseudopernospora cubensis. Phytopathol.. 1992;82:749-753.
- [Google Scholar]
- Hexavalent chromium reduction and plant growth promotion by Staphylococcus arlettae strain Cr11. Chemosphere. 2012;86:847-852.
- [Google Scholar]
- Comparison of in vitro Cr (VI) reduction by CFEs of chromate resistant bacteria isolated from chromate contaminated soil. Bioresour. Technol.. 2008;99:4130-4137.
- [Google Scholar]
- Chemistry for Environmental Engineering (fourth ed.). New York: McGraw-Hill; 1994.
- Antioxidative enzyme profiling and biosorption ability of Cupriavidus metallidurans CH34 and Pseudomonas putida mt2 under cadmium stress. J. Basic Microbiol.. 2013;53:1-8.
- [Google Scholar]
- Isolation of hexavalent chromium reducing facultative anaerobes from tannery effluent. J. Gen. Appl. Microbiol.. 2001;47:307-312.
- [Google Scholar]
- Bacterial chromate reductase, a potential enzyme for bioremediation of hexavalent chromium: a review. J. Environ. Manage.. 2014;146:383-399.
- [Google Scholar]
- Isolation and growth characteristics of chromium (VI) and pentachlorophenol tolerant bacterial isolate from treated tannery effluent for its possible use in simultaneous bioremediation. Ind. J. Microbiol.. 2011;51(1):61-69.
- [Google Scholar]
- Dechlorination of chloroorganics, decolorization, and simultaneous bioremediation of Cr6+ from real tannery effluent employing indigenous Bacillus cereus isolate. Environ. Sci. Pollut. Res. Int.. 2014;21(7):5227-5241.
- [Google Scholar]
- Selection and use of efficient bacterial strains for chromium biosorption in tannery effluent. Int. J. Recent Sci. Res.. 2017;8(3):16230-16233.
- [Google Scholar]
- Chromate reduction by Burkholderia cepacia MCMB-821, isolated from the pristine habitat of alkaline Crater lake. Appl. Microbiol. Biotechnol.. 2007;75:627-632.
- [Google Scholar]
- Isolation of Cr (VI) reducing bacteria from industrial effluents and their potential use in bioremediation of chromium containing wastewater. J. Environ. Sci.. 2009;21:814-820.
- [Google Scholar]
- Drought stress induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol.. 1994;35:785-791.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jksus.2018.07.016.
Appendix A
Supplementary data







