Translate this page into:
Morpho- physiological status of fenugreek seedlings under NaCl stress
⁎Corresponding author. bardees_mickky@mans.edu.eg (Bardees M. Mickky)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract

Abstract
Salinity is one of the main factors affecting seed germination and seedling growth. An in vitro experiment was conducted to assess the ability of fenugreek (Trigonella foenum-graecum L.) to germinate and cope with salt stress. Fenugreek seeds were germinated in presence of 0, 50, 100, 150 and 200 mM NaCl for 2 and 5 days; and the seedlings were evaluated for their morpho- physiological features. Data revealed that seedling dry mass and seedling mass vigor index (SMVI) were non- significantly affected by salinity at both ages, seedling fresh mass was adversely affected only at the first age, while germination percent, germination index, seedling length and seedling length vigor index were all suppressed by salinity at both ages. Salinity could also hit the seedlings cellular membranes as indicated by enhanced membrane lipid peroxidation and membrane injury with subsequent less membrane stability at the two ages. As a strategy to withstand stress, water extracts of the seedlings had higher osmotic pressure under salinity; with increased amount of total soluble sugars, proline, citric acid as well as sodium and chloride especially at the first age. Induced activity of some antioxidant enzymes like catalase, peroxidase, ascorbic peroxidase and polyphenol oxidase were also recorded at both ages under most of the checked salt concentrations. Moreover, correlation coefficient was determined between each parameter of the estimated morpho- physiological criteria and SMVI. Also, the degree by which the estimated criteria contributed to overall seedling performance was statistically computed.
Keywords
Fenugreek seedlings
Salinity
Morphology
Physiology
Correlation
Contribution
1 Introduction
Seed germination is the first stage in plant life cycle; and it is therefore one of the most critical phases that determine not only the degree of plant establishment in control habitats but also its ability to survive under stressful conditions (Mickky and Aldesuquy, 2017). Subsequent seedling vigor could then indicate the plant performance whether under optimum or elevated environmental circumstances. At such juvenile stage, the plant is most amenable to abiotic stresses particularly salinity that originates due to the presence of excessive salts in the surrounding medium causing limited plant growth and development (Mansour, 2013). The ill impact of salinity on plant performance is usually associated with low osmotic potential of the growing medium, nutritional imbalance, specific ion toxicity or possibly a combination of these factors (Attia et al., 2011). Moreover, there is an evidence that salinity could induce oxidative stress via the over- production of reactive oxygen species (ROS) that attack cellular macromolecules and biomembranes (Mansour, 2013). Plants tending to withstand salinity can usually accumulate various kinds of osmolytes to maintain osmotic balance within the cell and re-establish cell redox balance (Szabados et al., 2011). In addition, some plants can defend against stress- induced ROS accumulation by an array of antioxidant enzymes such as superoxide dismutase, catalase, peroxidase, ascorbic peroxidase, polyphenol oxidase and glutathione reductase (Mickky, 2016a).
Fenugreek (Trigonella foenum-graecum L.) is an annual leguminous herb regarded as a multi- purpose crop cultivated mainly as forage or medicinal plant. In addition, the use of different parts of fenugreek as flavoring agents, stabilizers, adhesives or for cosmetics, papers and paints industry is also documented (Ahmad et al., 2016). Fenugreek grows worldwide most obviously in arid and semi- arid regions where elevated level of salts is among the obvious features of soil in those habitats (Hasni et al., 2009). In this context, Gupta (2016) pointed out to the suitable performance of fenugreek in saline soils where no other legume is profitable. However and up to our knowledge, little is known about the precise mechanisms by which fenugreek can tolerate salinity at germination and early juvenile stage. So, the present study aims not only at assessing the different tactics exerted by fenugreek to germinate and grow under various levels of salinity, but also at applying statistical indices to determine which of the estimated mechanisms would contribute more to the overall performance of fenugreek germinated under salt stress.
2 Materials and methods
2.1 Experimental design
Homogeneous lot of fenugreek (Trigonella foenum-graecum L. var. Giza 3) seeds was surface sterilized using 4% NaOCl for 10 min, washed thoroughly by sterile water and hydro- primed for 8 h to activate the embryo. Seeds were then allowed to germinate in dark- painted plastic boxes on 8- layered cheesecloth at 25 ± 2 °C. When required, the seeds were sprayed with NaCl solution at 0, 50, 100, 150 or 200 mM and the samples were taken as 2- and 5- day old seedlings. Chemical analysis of the tap water used for seed presoaking, control treatment and preparation of salt solutions showed that it had an electric conductance of 0.4 mS with 35 ppm Na+, 16 ppm Ca++ and 7 ppm K+.
2.2 Estimation of germination parameters
At the two considered ages, germination percent, seedling length and seedling biomass were determined. Also, seedling water content and some indices were calculated as following:
Seedling water content = (fresh mass–dry mass)/fresh mass (Mickky, 2016b).
Seedling mass vigor index (SMVI) = (seedling dry mass × germination percent)/100 (Kharb et al., 1994).
Seedling length vigor index (SLVI) = (seedling length × germination percent)/100 (Kharb et al., 1994).
2.3 Estimation of membrane features
Membrane features estimated herein in fresh fenugreek seedlings included lipid peroxidation, membrane stability index and membrane injury index.
2.3.1 Determination of membrane lipid peroxidation
One g of the samples was macerated in 5 ml of 0.1% trichloroacetic acid (TCA) then centrifuged at 10,000 rpm for 5 min. Four ml of 20% TCA containing 0.5% thiobarbituric acid was added to every ml of the supernatant, incubated at 95 °C for 30 min then cooled, centrifuged again and the absorbance (A) was measured at 532 and 600 nm. Readings at A600 were subtracted from those at A532 with using an extinction coefficient of 155 × 10−3 µM−1 cm−1 to express malondialdehyde (MDA) content in μ mol g−1 f wt (Heath and Packer, 1968).
2.3.2 Determination of membrane injury index
In two sets, 0.2 g of seedlings was cut into uniform small pieces and transferred into test tubes containing 20 ml of distilled water. The first set was incubated at 40 °C for 30 min while the second was incubated at 100 °C for 15 min then EC of each set was measured. Membrane injury index (MII) could be calculated following Deshmukh et al. (1991) as: where EC1 and EC2 refer to the electric conductance at 40 and 100 °C; respectively.
2.3.3 Determination of membrane stability index
A simple method was implied to directly derive membrane stability index (MSI) as the value designated for MII subtracted from 100.
2.4 Estimation of osmotic regulation
Osmolytes were determined in plant- water extracts prepared by incubating 0.1 g of dry tissue powder in 25 ml of distilled water at 90 °C for 60 min followed by centrifugation. The pellet was re-extracted twice and the combined supernatants were raised up.
2.4.1 Determination of osmotic pressure
The EC of the plant- water extracts was measured to directly express osmotic pressure.
2.4.2 Determination of total soluble sugars
For 10 min, 3 ml of anthrone reagent was reacted with 0.1 ml of extract in a boiling water bath then reading the cooled samples at 625 nm (Irigoyen et al., 1992).
2.4.3 Determination of proline
For an hour, 1 ml of each of extract, glacial acetic acid and ninhydrin reagent were reacted in boiling water bath then 1 ml of the acid was introduced followed by cooling, raising up to 5 ml with the acid and measuring at 510 nm (Bates et al., 1973).
2.4.4 Determination of citric acid
To 5 ml of the extract, 15 ml of a deproteinizing solution of (3 g of each of HgCl2 and ZnSO4 in 100 ml of water) was added and left overnight then filtered. Four ml of 10 N HCl and 1 ml of 6.2% FeCl3 were added, mixed and measured at 445 nm (Snell and Snell, 1949).
2.4.5 Determination of ionic contents
According to Chapman and Pratt (1982), sodium and potassium were determined using flame photometer, while calcium and magnesium were determined by titration against EDTA using muroxide and erichrome black T indicators; respectively. As described by Hansen and Munns (1988), chlorides were determined by titration against AgNO3.
2.5 Estimation of enzymatic antioxidant system
The activity of antioxidant enzymes was determined in the extracts prepared by cold homogenization of 2 g of fresh tissue in 20 ml of 0.1 M phosphate buffer followed by cold centrifugation at 10,000 rpm for 20 min. Buffer at pH 6.8 was used to extract catalase, peroxidase, polyphenol oxidase and glutathione reductase; and pH 7.8 for ascorbic peroxidase and superoxide dismutase (Agarwal and Shaheen, 2007).
2.5.1 Assay of catalase (CAT; EC 1.11.1.6.)
One ml of extract, 2 ml of 0.1 M H2O2 and 3 ml of 0.1 M phosphate buffer were mixed, incubated at 27 °C for 5 min, 1 ml of 0.7 N H2SO4 was added and the residual H2O2 was then titrated against 0.01 N KMnO4 till the pink color persisted. A blank titration was carried out with the acid introduced at zero time (Devi, 2007).
2.5.2 Assay of POX (EC 1.11.1.7.)
Three ml of 0.05 M pyrogallol was mixed with 0.5 ml of 1% H2O2 and 0.1 ml of the extract then the increase in absorbance at 420 nm was recorded (Devi, 2007).
2.5.3 Assay of APX (EC 1.11.1.11.)
Two and half ml of 0.5 mM ascorbic acid, 0.4 ml of 2 mM H2O2 and 0.1 ml of the extract were mixed then the decrease in absorbance at 290 nm was recorded (Barka, 2001).
2.5.4 Assay of PPO (EC 1.14.18.1.)
One ml of 0.05 M pyrogallol, 2 ml of 0.02 M phosphate buffer at pH 7 and 1 ml of the extract were mixed then the increase in absorbance at 420 nm was recorded (Devi, 2007).
2.5.5 Assay of SOD (1.15.1.1.)
One ml of a working reagent (50 mM phosphate buffer at pH 8.5: 1 mM nitroblue tetrazolium: 1 mM NADH mixed in 10: 1: 1 vol ratio), 0.1 ml of the extract and 0.1 ml of 0.1 mM phenazine methosulphate were mixed then the increase in absorbance at 560 nm was recorded (Nishikimi et al., 1972).
2.5.6 Assay of GR (EC 1.8.1.7.)
Exactly 0.05 ml of the extract, 1 ml of 100 mM phosphate buffer at pH 7.5 containing 1 mM EDTA, 0.1 ml of 50 mM glutathione and 0.1 ml of 2 mM NADPH were mixed then the decrease in absorbance at 340 nm was recorded (Goldberg and Spooner, 1983).
2.6 Statistical analysis
Ten replicas were taken to determine germination parameters, while three were chosen for other investigations; and only the mean values with standard deviations were represented. A test at p ≤ 0.05 was performed using CoHort/CoStat software with one way completely randomized analysis of variance. In addition, Pearson correlation coefficient (r) and coefficient of determination (r2) were determined between each of the estimated morpho- physiological criteria and SMVI. Contribution coefficient (CC) could be then calculated for each estimated criterion as the average of 100 × r2 for the included individual parameters then contribution index (CI) was derived as the relative percent of CC (Mickky (2016a).
3 Results and discussion
Salinity is one of the most common environmental constraints limiting plant growth and development. Although plant performance is known to be altered under salt stress at almost all of its growth stages; seed germination and seedling emergence are usually more amenable to salinity (Soughir et al., 2013). In the present study, gradual levels of NaCl up to 200 mM applied to 2- and 5- day old fenugreek seedlings had non- significant impact on seedling dry mass and seedling mass vigor index (SMVI) at p ≤ 0.05. Although salinity had similar non- significant impact on seedling fresh mass at the second age, salinity could decrease seedling fresh mass at the first age. For germination percent, germination index, seedling length and seedling length vigor index (SLVI), these were all suppressed by salinity at both ages (Table 1). Similar effects of salinity on fenugreek were recorded by Soughir et al. (2013) as well as Gupta (2016).
Seedling age
NaCl concentration (mM)
Seedling fresh mass (mg)
Seedling dry mass (mg)
Seedling water content (mg H2O mg−1 f wt)
Seedling length (mm)
Germination percent (%)
Germination index
Seedling mass vigor index (SMVI)
Seedling length vigor index (SLVI)
2-day old
0
19.6e ± 4.8
12.5b ± 1.27
0.32d ± 0.19
31.8e ± 2.0
98c ± 0
1a ± 0
12.25c ± 1.24
31.16e ± 1.95
50
25.0de ± 7.5
13.2ab ± 1.48
0.44c ± 0.13
24.6f ± 1.6
97d ± 0
0.99b ± 0
12.80abc ± 1.43
23.86f ± 1.60
100
25.4de ± 7.0
13.3ab ± 2.26
0.46c ± 0.09
23.8 fg ± 1.0
96e ± 0
0.98c ± 0
12.77abc ± 2.17
22.85 fg ± 0.99
150
27.7de ± 7.5
13.3ab ± 2.16
0.50c ± 0.11
20.5 h ± 1.3
94f ± 0
0.97d ± 0
12.64bc ± 2.05
19.48 h ± 1.21
200
29.9d ± 6.8
13.9ab ± 1.37
0.51c ± 0.13
17.7i ± 1.3
93 g ± 0
0.95e ± 0
12.93abc ± 1.27
16.46i ± 1.24
5-day old
0
81.6a ± 20.8
14.5a ± 2.55
0.82a ± 0.02
71.6a ± 2.4
100a ± 0
1a ± 0
14.50a ± 2.55
71.60a ± 2.37
50
74.5a ± 11.7
13.8ab ± 2.10
0.81a ± 0.02
54.1b ± 2.4
99b ± 0
0.99b ± 0
13.66abc ± 2.08
53.56b ± 2.35
100
62.6b ± 10.0
14.7a ± 2.58
0.76a ± 0.02
40.9c ± 1.4
97d ± 0
0.97d ± 0
14.26ab ± 2.51
39.67c ± 1.33
150
53.6b ± 9.6
13.7ab ± 2.31
0.74ab ± 0.04
33.5d ± 2.6
98c ± 0
0.98c ± 0
13.43abc ± 2.27
32.83d ± 2.58
200
42.8c ± 6.2
14.1ab ± 2.02
0.67b ± 0.04
22.6 g ± 1.8
97d ± 0
0.97d ± 0
13.68abc ± 1.96
21.92 g ± 1.78
LSD
9.0
1.83
0.09
1.7
4.7e-8
4.4e-8
1.78
1.62
DSV
***
ns
***
***
***
***
ns
***
The negative influence of salt stress recorded herein on germination percent, and consequently on germination index, can be attributed to (i) osmotic effects of salinity that impede seeds ability to imbibe water and (ii) ionic effects of salinity that result from sodium and chlorides uptake in excess amounts (Attia et al., 2011). In this context, salinity was recorded to (i) alter the utilization and mobilization of materials stored within seeds resulting in inhibited embryonic growth (Rahman et al., 2008), (ii) disrupt electron transport resulting in the accumulation of harmful reactive oxygen species (ROS) (Groß et al., 2013) and (iii) cause mitochondrial oxidative damage retarding seed respiration with consequent inhibition of energy production (Zheng et al., 2009). These alterations can also account for salinity- induced decrease in fenugreek seedlings fresh mass recorded herein at the second age as well as the decrease in seedling length and SLVI both recorded at the two ages.
In addition, salinity markedly increased membrane lipid peroxidation indicated by higher malondiladehyde (MDA) content at the two considered ages of fenugreek seedlings. So, marked increase in membrane injury index (MII) with corresponding decrease in membrane stability index (MSI) were reported for the studied seedlings as a result of most applied salt concentrations at p ≤ 0.05 (Table 2). Upgraded lipid peroxidation was intensively recorded in plants subjected to salinity (Malik et al., 2011). Also, upgraded electrolyte leakage indicated by higher MII and/ or lower MSI were extensively employed to refer to increased membrane permeability under salinity (Ashraf and Ali, 2008; Tiwari et al., 2010). The reverse impact of salinity on the cellular membranes of fenugreek seedlings can be ascribed to the negative effects of ROS that accumulate under stress with consequent injury to the essential cellular components; the most obviously- attacked of which are the cell membranes (Mansour, 2013).
Seedling age
NaCl concentration (mM)
Lipid peroxidation (μ mol MDA g−1 f wt)
Membrane injury index (MII) (%)
Membrane stability index (MSI) (%)
2-day old
0
0.129i ± 0.001
18.92e ± 1.05
81.08a ± 1.05
50
0.398f ± 0.007
20.31e ± 0.32
79.69a ± 0.32
100
1.021e ± 0.017
26.02c ± 2.22
73.98c ± 2.22
150
2.262d ± 0.024
29.17ab ± 2.13
70.83de ± 2.13
200
3.598b ± 0.016
30.07ab ± 1.14
69.93de ± 1.14
5-day old
0
0.187 h ± 0.007
22.68d ± 1.23
77.32b ± 1.23
50
0.327 g ± 0.014
28.25bc ± 0.98
71.75 cd ± 0.98
100
2.282d ± 0.027
30.88a ± 1.43
69.12e ± 1.43
150
2.527c ± 0.010
31.08a ± 1.54
68.92e ± 1.54
200
3.792a ± 0.010
29.92ab ± 0.50
70.08de ± 0.50
LSD
9.0
0.026
2.35
DSV
***
***
***
In the present study, it seems that fenugreek seedlings could not ameliorate the ill impact of most NaCl concentrations on their membranes at both ages; and it was also noticed that the recorded alterations in membrane features of the studied seedlings were in parallelism with the salt concentration. Only 50 mM NaCl caused non- significant change in MII and MSI in the 2- day old seedlings at p ≤ 0.05; indicating possible ability of such seedlings to reverse salinity effects on their cellular membrane probably by enhanced activity of some of their antioxidant enzymes and/ or their potentiality to accumulate certain osmo- protestants that can detoxify ROS and stabilize bio- membrane as shown later on. Even for salt concentrations causing adverse effects on fenugreek membrane, it seems that such effects may be not so intensive since the fenugreek seedlings could produce reasonable dry matter as indicated by the values of SMVI.
Regarding the capacity of the concerned fenugreek seedlings for osmotic regulation, most of the applied salt concentrations brought about significant increase in the osmotic pressure of seedlings water extracts as inferred from their electric conductivity with significant increase in the amount of some organic osmolytes (e.g., total soluble sugars, proline and citric acid) as well as significant increase in the amount of some inorganic osmolytes (e.g., chloride, sodium, potassium, calcium and magnesium) at p ≤ 0.05 (Table 3). In accordance with these results, Nair et al. (2017) recorded that fenugreek seedlings could accumulate total soluble sugars and proline in response to salinity. Also, Dadresan et al. (2015) found that salt- stressed fenugreek plants could accumulate sodium as compared with their unstressed synonyms.
Seedling age
NaCl concentration (mM)
Osmotic pressure (EC in mS)
Total soluble sugars (mg g−1 d wt)
Proline (mg g−1 d wt)
Citric acid (mg g−1 d wt)
Sodium (mmol g−1 d wt)
Potassium (mmol g−1 d wt)
Calcium (mmol g−1 d wt)
Magnesium (mmol g−1 d wt)
2-day old
0
0.25i ± 0.01
146.69f ± 2.32
2.59f ± 0.48
0.41 h ± 0.25
0.58 g ± 0.01
0.71d ± 0.02
0.195a ± 0.05
0.20 cd ± 0
50
0.37 h ± 0.01
193.60c ± 6.61
3.21 cd ± 0.05
11.41de ± 0.89
0.75e ± 0.02
0.82b ± 0.01
0.160ab ± 0.06
0.19 cd ± 0
100
0.52f ± 0.03
226.00a ± 2.71
4.84a ± 0.04
16.00b ± 2.11
0.83d ± 0.01
0.77c ± 0.03
0.096c ± 0
0.32a ± 0.06
150
0.62d ± 0
183.90d ± 7.99
3.03de ± 0.09
6.53f ± 0.68
0.90c ± 0.02
0.54e ± 0.01
0.192a ± 0
0.16d ± 0.06
200
0.62d ± 0.02
152.91ef ± 6.57
2.49f ± 0.33
9.65e ± 2.20
0.93bc ± 0.01
0.83b ± 0.01
0.098c ± 0
0.23bcd ± 0.11
5-day old
0
0.47 g ± 0.02
124.20 g ± 5.51
2.11 g ± 0.07
3.61 g ± 0.61
0.69f ± 0.02
0.77c ± 0.01
0.096c ± 0
0.29ab ± 0
50
0.59e ± 0.02
177.50d ± 2.79
3.63b ± 0.18
15.21bc ± 0.74
0.80d ± 0.03
0.91a ± 0.05
0.128bc ± 0.06
0.26abc ± 0.06
100
0.65c ± 0
154.10ef ± 2.27
3.46bc ± 0.18
16.17b ± 2.48
0.90c ± 0.02
0.82b ± 0
0.192a ± 0
0.19 cd ± 0
150
0.71b ± 0.02
157.50e ± 4.25
2.72ef ± 0.23
13.09 cd ± 1.12
0.94b ± 0
0.73 cd ± 0.06
0.096c ± 0
0.29ab ± 0
200
0.78a ± 0.01
203.90b ± 1.83
1.15 h ± 0.05
19.62a ± 0.27
0.99a ± 0.03
0.88a ± 0.01
0.096c ± 0
0.19 cd ± 0
LSD
0.03
8.13
0.04
2.35
0.03
0.05
0.04
0.08
DSV
***
***
***
***
***
***
***
**
Osmotic regulation is one of the main mechanisms that enable plants to withstand salinity tolerance since osmolytes allow the continuation of cell expansion during stress with ultimate better plant growth and development. Osmolytes can also ameliorate the oxidative damage caused by ROS over- produced under stress and thus protect cellular structures (Szabados et al., 2011). In this regard, sugars, proline and citric acid were documented as the main organic osmolytes accumulated in plants as a salinity tolerance strategy (Farissi et al., 2011). Soluble sugars, that contribute by almost 50% in the osmotic regulation of salt- stressed glycophytes, act as not only osmo-protectants but also as carbon- storing skeletons and efficient sequesters of some free radicals (Ashraf and Harris, 2004). For proline and beside being potent osmolyte, it was found to play an important role in maintaining well- controlled pH (Ottow et al., 2005) and stabilizing cellular membranes (Ashraf and Harris, 2004). Also, citric acid is another vital osmolyte that facilitates iron transport within the plants body and serves as an antioxidant source for carbon skeleton and cellular energy (Zhao et al., 2015).
However and for energetic reasons, it was documented that osmotic regulation cannot be achieved by organic solutes alone. In addition to organic osmolytes, energetically cheap inorganic ions (e.g., chlorides, sodium, potassium, calcium and magnesium) can be accumulated in the vacuoles to help achieving osmotic regulation (Glenn et al., 1999). In this connection, some plants can evolve mechanisms for selective sodium and chlorides sequestration into their vacuoles not only to avoid the adverse effects of these ions on cytosol and organelles, but also to maintain a sufficient uptake of potassium ions resulting in high cytosolic potassium/sodium ratio which is a key indicator for plant salt tolerance (Slama et al., 2015).
Results of the current study may thus indicate that the overall capability of fenugreek seedlings of coping with most of the applied salt levels especially at their elder age (5- day old) may at least in part be ascribed to their capacity for osmotic regulation. Citric acid in particular was recorded to highly accumulate in salt- stressed seedlings; and such increase is enough by itself to reverse the ill effects of salinity on the studied fenugreek seedlings.
Regarding the enzymatic antioxidant defence system of fenugreek seedlings subjected to salt stress, the obtained results manifested that superoxide dismutase (SOD) activity decreased by salinity in the 2- day old seedlings, while it generally increased by salinity in their 5- day old synonyms (Table 4). In other studies, both increased and decreased SOD activity were reported in plants in response to salinity; and it seems that the change in SOD activity varies depending on stress level as well as plant species, organ and age (Lechno et al., 1997; Kamiński et al., 2012). Enhanced SOD activity was documented as a strategy exerted by tolerant plants to scavenge ROS over- produced under stress; where SOD catalyzes the dismutation of superoxide anion radicals into molecular oxygen and hydrogen peroxide. Nonetheless, it was postulated that water stress could inhibit SOD activity due to the negative effects of stress on protein synthesis and/ or to the deficiency in Cu, Zn, Mn and/or Fe metals required for enzyme activation (Mickky and Aldesuquy, 2017).
Seedling age
NaCl concentration (mM)
Catalase activity (Unit g−1f wt)
Peroxidase activity (delta A 420 nm)
Polyphenol oxidase activity (delta A 420 nm)
Ascorbic peroxidase activity (delta A 290 nm)
Glutathione reductase activity (Unit ml−1)
Superoxide dismutase activity (Unit ml−1)
2-day old
0
2.153d ± 0.039
0.080d ± 0.010
0.016e ± 0.005
0.002d ± 0.001
8.04 g ± 4.02
181.45ab ± 12.1
50
2.969bc ± 0.196
0.098c ± 0.004
0.036a ± 0.008
0.006 cd ± 0.004
41.53bcd ± 11.60
44.35e ± 18.48
100
2.289d ± 0.104
0.106c ± 0.002
0.031abc ± 0.007
0.008c ± 0.002
29.47ef ± 4.64
64.51e ± 6.99
150
2.811c ± 0.208
0.114bc ± 0.001
0.022de ± 0.006
0.013b ± 0.001
34.83cde ± 2.32
116.93d ± 13.97
200
1.836e ± 0.236
0.101c ± 0.002
0.022de ± 0.001
0.005 cd ± 0.003
24.11f ± 0
125.00 cd ± 42.48
5-day old
0
2.743c ± 0.079
0.130b ± 0.005
0.027bcd ± 0.003
0.005 cd ± 0.001
32.15def ± 6.96
125.00 cd ± 6.98
50
3.151b ± 0.079
0.152a ± 0.012
0.033ab ± 0.003
0.009c ± 0.002
48.23b ± 6.96
165.32abc ± 18.48
100
2.312d ± 0
0.129b ± 0.005
0.037a ± 0.005
0.006 cd ± 0.003
60.29a ± 4.02
157.26abcd ± 43.62
150
3.627a ± 0.039
0.154a ± 0.026
0.037a ± 0.005
0.016ab ± 0.004
32.15def ± 8.04
201.61a ± 13.97
200
1.564f ± 0.118
0.108c ± 0.003
0.024cde ± 0.004
0.018a ± 0.003
44.21bc ± 4.02
141.13bcd ± 45.80
LSD
0.23
0.017
0.008
0.004
10.38
45.45
DSV
***
***
***
***
***
***
At the same time, most of the applied NaCl concentrations induced the activity of catalase (CAT), peroxidase (POX), ascorbic peroxidase (APX), polyphenol oxidase (PPO) and glutathione reductase (GR) at both ages (Table 4). Enhanced activity of antioxidant enzymes was previously recorded in fenugreek plants exposed to stress (Kapoor et al., 2013; Behairy et al., 2017). It seems from the results of the present study that fenugreek seedlings might tolerate most of the studied salinity levels (as indicated by SMVI) probably by inducing marked activity of some of its antioxidant enzymes. Hydrogen peroxide generated as a result from SOD canalisation reaction and from others can be detoxified by CAT into water and oxygen. Another route for hydrogen peroxide decomposition is through ascorbate/ glutathione cycle in which ascorbate and glutathione can be oxidized and reduced by the action of APX and GR; respectively. Also, POX catalyzes hydrogen peroxide decomposition in presence of co- substrate such as phenolics. In addition, PPO catalyzes phenolics oxidation into their corresponding quinones (Mickky, 2016a).
By studying the impact of gradual concentrations of NaCl on germination parameters, membrane features, osmotic regulation and enzymatic antioxidants of fenugreek seedlings when they were 2- and 5- day old, it was critical to correlate each of the investigated morpho- physiological criteria with the most indicative seedling vigor; SMVI. Among the investigated membrane features, MSI was found to correlate positively with SMVI while MMI correlate negatively with SMVI; referring to direct relationship between the overall seedlings performance and the integrity of their cellular membranes although such correlations were weak (Fig. 1). At the same time, the osmotic pressure of seedlings water extracts was found to moderately and positively correlate with SMVI; referring to higher effect of salinity on osmotic regulation than that on membrane features (Fig. 1).
Correlation coefficient (r) and coefficient of determination (r2) of the estimated morpho- physiological criteria of NaCl- stressed fenugreek seedlings in relation to their mass vigor index (SMVI).
Regarding enzymatic antioxidant defence system, all the assayed enzymes showed positive correlation with SMVI but only POX and GR related strongly with SMVI, while APX related moderately with SMVI (Fig. 1). This possibly indicates reasonable contribution of the enzymatic antioxidant defence system of fenugreek seedlings in the overall tolerance of seedlings to salt stress. Supporting this assumption, values of contribution coefficient (CC) in Fig. 2 and contribution index (CI) in Fig. 3 revealed that the antioxidant enzymatic defence system could contribute to SMVI with a CI value of about 51%, followed by the osmotic regulatory system whose CI reached 27% and finally came membrane features that contributed by only 22% to SMVI.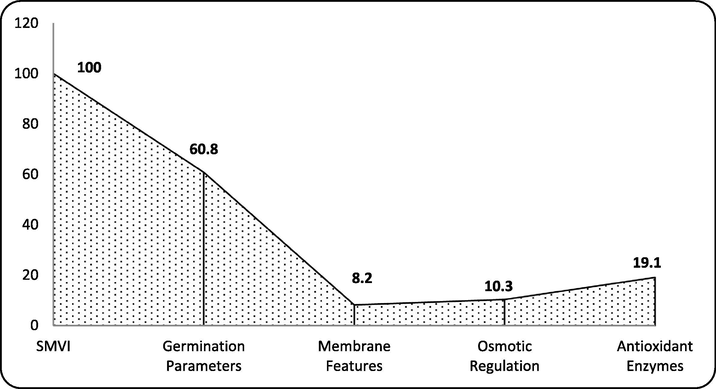
Contribution coefficient (CC in %) of the estimated morpho- physiological criteria of NaCl- stressed fenugreek seedlings in relation to their mass vigor index (SMVI).
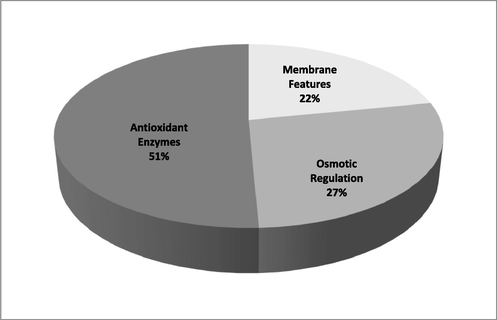
Contribution index (CI) of the estimated morpho- physiological criteria of NaCl- stressed fenugreek seedlings in relation to their mass vigor index (SMVI).
4 Conclusions
Therefore, it could be concluded from the present study that application of salt stress up to 200 mM NaCl to fenugreek at early germination phase might have slight impact on its morpho- physiological performance since the seedlings were found to tolerate salinity by stimulating its enzymatic antioxidant system along with efficient osmotic regulation.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of interest
None.
References
- Stimulation of antioxidant system and lipid peroxidation by abiotic stresses in leaves of Momordica charantia. Braz. J. Plant Physiol.. 2007;19:149-161.
- [Google Scholar]
- Fenugreek a multipurpose crop: potentialities and improvements. Saudi J. Biol. Sci.. 2016;23:300-310.
- [Google Scholar]
- Relative membrane permeability and activities of some antioxidant enzymes as the key determinants of salt tolerance in canola (Brassica napus L.) Environ. Exp. Bot.. 2008;63:266-273.
- [Google Scholar]
- Potential biochemical indicators of salinity tolerance in plants. Plant Sci.. 2004;166:3-16.
- [Google Scholar]
- Effect of salt stress on gene expression of superoxide dismutases and copper chaperone in Arabidopsis thaliana. Biol. Plant.. 2011;55:159-163.
- [Google Scholar]
- Protective enzymes against reactive oxygen species during ripening of tomato (Lycopersicon esculentum) fruits in response to low amounts of UV-C. Aust. J. Plant Physiol.. 2001;28:785-791.
- [Google Scholar]
- Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205-207.
- [Google Scholar]
- Alleviation of salinity stress on fenugreek seedling growth using salicylic acid, citric acid and proline. Middle East J. Agric. Res.. 2017;6:474-483.
- [Google Scholar]
- Methods of analysis for soils, plants and waters. In: University of California Division of Agricultural Sciences. Chapman Publisher; 1982.
- [Google Scholar]
- Effect of salinity stress and surfactant treatment on physiological traits and nutrient absorption of fenugreek plant. Commun. Soil Sci. Plant Anal.. 2015;46:2807-2820.
- [Google Scholar]
- Measurement of ion leakage as screening technique for drought resistance in wheat genotypes. Indian J. Plant Physiol.. 1991;34:89-91.
- [Google Scholar]
- Principles and methods in plant molecular biology, biochemistry and genetics (4th edition). India: Agrobios; 2007.
- Agro-physiological responses of Moroccan alfalfa (Medicago sativa L.) populations to salt stress during germination and early seedling stages. Seed Sci. Technol.. 2011;39:389-401.
- [Google Scholar]
- Salt tolerance and crop potential of halophytes. Crit. Rev. Plant Sci.. 1999;18:227-255.
- [Google Scholar]
- Methods of Enzymatic Analysis (3rd edition). 1983.
- Nitric oxide, antioxidants and prooxidants in plant defence responses. Front. Plant Sci.. 2013;4:419.
- [Google Scholar]
- Effect of salt stress on seed germination and seedling growth of Trigonella foenum-graecum. Int. J. Mendel.. 2016;33:3-4.
- [Google Scholar]
- Effect of CaSO4 and NaCl on mineral content of Leucaena leucocephala. Plant Soil. 1988;107:101-105.
- [Google Scholar]
- Physiological characteristics of salt tolerance in fenugreek (Trigonella foenum-graecum L.). Davis: International Plant Nutrition Colloquium, University of California; 2009.
- Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys.. 1968;196:385-395.
- [Google Scholar]
- Water stress induced changes in concentration of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant.. 1992;84:55-60.
- [Google Scholar]
- Kamiński, P., Koim-Puchowska, B., Puchowski, P., Jerzak, L., Wieloch, M., Bombolewska, K., 2012. Enzymatic antioxidant responses of plants in saline anthropogenic environments. W: “Plant Science”, Nabin Kumar Dhal (Red.), InTechOpen, Rijeka, Croatia.
- Antioxidative defense to salt stress in Trigonella foenum-graecum L. Curr. Disc.. 2013;2:123-127.
- [Google Scholar]
- Prediction of field emergence through heritability and genetic advance of vigour parameters. Seed Sci. Technol.. 1994;22:461-466.
- [Google Scholar]
- Salt stress-induced responses in cucumber plants. J. Plant Physiol.. 1997;150:206-211.
- [Google Scholar]
- Response of antioxidant enzymes to high NaCl concentration in different salt-tolerant plants. Biol. Plant.. 2011;55:191-195.
- [Google Scholar]
- Plasma membrane permeability as an indicator of salt tolerance in plants. Biol. Plantarum. 2013;57:1-10.
- [Google Scholar]
- Impact of osmotic stress on seedling growth observations, membrane characteristics and antioxidant defense system of different wheat genotypes. Egypt. J. Basic Appl. Sci.. 2017;4:47-54.
- [Google Scholar]
- Could sodium benzoate enhance broad bean salinity tolerance? I. Seedling vigor, membrane features, antioxidant enzymes and osmolytes. J. Chem. Biol. Phys. Sci.. 2016;6:313-328.
- [Google Scholar]
- Could sodium benzoate enhance broad bean salinity tolerance? II. Germination parameters, carbohydrates, proteins, nucleic acids and hydrolytic enzymes. J. Chem. Biol. Phys. Sci.. 2016;6:351-367.
- [Google Scholar]
- Evaluation of abiotic stress induced physiological and biochemical changes in Trigonella foenum-graecum. J. Biotechnol. Biochem.. 2017;3:89-97.
- [Google Scholar]
- The occurrence of super oxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun.. 1972;46:849-854.
- [Google Scholar]
- Populus euphratica displays apoplastic sodium accumulation, osmotic adjustment by decreases in calcium and soluble carbohydrates, and develops leaf succulence under salt stress. J. Plant Physiol.. 2005;139:1762-1772.
- [Google Scholar]
- Effects of NaCl salinity on wheat (Triticum aestivum L.) cultivars. World J. Agric. Res.. 2008;4:398-403.
- [Google Scholar]
- Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot.. 2015;115:433-447.
- [Google Scholar]
- Colorimetric Methods of Analysis. New Yourk: D Van Nostrand Co Inc; 1949.
- The effect of NaCl priming on emergence, growth and yield of fenugreek under saline conditions. Cercetări Agronomice în Moldova. 2013;46:73-83.
- [Google Scholar]
- Plants in extreme environments: importance of protective compounds in stress tolerance. Adv. Bot. Res.. 2011;57:105-150.
- [Google Scholar]
- Effect of salt stress on cucumber: Na+/ K+ ratio, osmolyte concentration, phenols, chlorophyll content. Acta Physiol. Plant.. 2010;32:103-114.
- [Google Scholar]
- Differential metabolic responses of two tall fescue genotypes to heat stress. Acta Pratacult. Sin.. 2015;24:58-69.
- [Google Scholar]
- Exogenous nitric oxide improves seed germination in wheat against mitochondrial oxidative damage induced by high salinity. Environ. Exp. Bot.. 2009;67:222-227.
- [Google Scholar]







