Translate this page into:
Moringa olifera leaf extract increases physio-biochemical properties, growth and yield of Pisum sativum grown under salinity stress
⁎Corresponding authors. sibgha.noreen@bzu.edu.pk (Sibgha Noreen), melsheikh@ksu.edu.sa (Mohamed El-Sheikh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The soil salinity is a dominant abiotic problem in arid and semi-arid region of the world. It occurs naturally due to low rainfall and accumulation of salts to soil surface, aided by human activities; excessive irrigations, over-use of fertilizers and poor drainage. The study was aimed to investigate the mitigating role of Moringa olifera leaf extract (MLE) in pea grown under salinity stress. Pea (Pisum sativum) variety-Pea-2009 was grown under control and salt (100 mM NaCl) stress. The MLE extract (3%) was applied as a seed priming and foliar spray. Salinity stress caused a significant (P<0.001) decrease in plant growth (fresh and dry biomass of shoot and root s), leaf relative water contents, chlorophyll a and b, carotenoid, TFAAs, proline, K+ and antioxidants (POD, APX, and CAT), whereas the application of MLE either (seed priming/foliar spray) enhanced all studied attributes under both control and saline conditions. However, Na+, MDA, and H2O2 contents were enhanced under salinity stress. Whereas the application of MLE (seed priming/ foliar spray) enhanced shoot and root fresh and dry weights as compared to saline treatment. While on the other hand the application of MLE (seed priming/ foliar spray) enhanced leaf relative water contents, total chlorophyll contents, total soluble proteins, total free amino acids, catalase, peroxidase, ascorbate peroxidase, proline, K+ contents of shoot and root while lowered H2O2, MDA, Na+ contents of root and shoot compared to their respective saline treatments. Similarly, MLE also enhanced pea seed yield to 36% (priming) and 33 (foliar spray) under salinity stress which was reduced to 56% under salinity stress. The exogenous application of MLE either seed priming or foliar spray, potentially enhanced growth and yield of economically important crops grown under salinity stress by maintaining better physio-biochemical indices.
Keywords
Moringa leaf extract
Superoxide dismutase
Total soluble protein
Proline
Seed weight
1 Introduction
The world population continues to grow, increasing the demand on resources including food, water and energy. It is predicted that human population will mount to 9.7 billion by 2050 which is a serious problem to food security (Nadathur et al., 2017). There must be a major concern of all nations to develop proper strategy to cope with upcoming food insecurity through better use of agricultural soils which are becoming barren due to increase in the intensity of environmental threats like drought and salinity. Soil salinity is the major environmental stress that is spreading speedly due to excessive use of saline water that converts valuable agricultural arable land into a salinized wasteland (Kaya et al., 2020).
Plants grown in saline soils are forced to accumulate salts to toxic levels into their cellular compartments which is the prime cause of oxidative stress leading to accumulation of reactive oxygen species (ROS) to toxic levels, which hampers plants’ normal growth by upsetting physio-biochemical processes including photosynthesis (Mansoor et al., 2022). Although roots are surrounded by water still are unable to take up their adequate amount due to the presence of salts. This phenomenon is called physiological drought that leads to; i) lowered root water potential; ii) phytotoxicity by excessive Na+ and Cl− and iii) nutritional imbalance through hindring in the uptake of other nutrients i.e. K+, Ca++, N, P etc (Akhter et al., 2021), thus hampering plant normal vegetative and reproductive growth.
The synthesis of ROS is natural process in plants however, under adverse environmental conditions like salinity the production of ROS like superoxide radicle, singlet oxygen, H2O2 and OH– radicle is augumented (Mansoor et al., 2022). These increased ROS level stimulate the phytotoxic reaction in plants such as protein degradation, membrane depolarization, lipid peroxidation, DNA mutation, and protein degradation (Mansoor et al., 2022). Plants under such adversities has develop a counter defense mechanism through activation of antioxidant (Ahanger et al., 2020). Catalase (CAT), peroxidases (POD), superoxide dismutase (SOD), ascorbate peroxidase (APX) and proline are the main elements of this plant’s defence system that detoxifies the negative effects of ROS (Mansoor et al., 2022). Similarly, amino acids, proteins and sugars also supports the plants for efficient detoxification of salinity-induced ROS (Ahanger et al., 2020).
The application of plant bio-stimulants is being used strategy to attain maximum crop productivity. These products, derived from biological materials, enhance plant growth and productivity due to the presence of growth regulators, vital nutrients and protective compounds. However, the stimulatory effect of these bio-stimulators is totally based on source, dose and stage at which the extract was applied to of plant. In this regard, Moringa (Moringa oleifera) leaf extract is rich source of vitamins i.e. A and C, phenolics, calcium, iron and carotenes (Ademiluyi et al., 2018). Despite having high level of antioxidants, total free amino acids (TFAAs), soluble sugars and sufficient amount of phytohormones like cytokinins (Nouman et al., 2016), but there are scanty reports which focusing on amelioration of salt stress by combined application (foliar and seed priming) of moringa extract in vegetable crops.
Pisum sativum L., the common pea (locally known as garden or field pea), is an herbaceous annual and cool season crop in the Fabaceae (formerly Leguminosae) family native to Mediterranean basin and near east (Macák et al., 2020). In Pakistan, pea important vegetable and was cultivated at 10,479 hectors yielding upto 71,793 tons with an average of 6.9 tons/hec. In Pakistan pea is ranked as 3rd familiar crop and Punjab is the major contributor (71 %) of the total production (Aslam et al., 2000; Ullah et al., 2020). The current study was aimed to focus on: (1) impact of salinity stress on growth attributes, mineral uptake, photosynthetic pigments, compatible solutes, antioxidant level and relative water contents (RWC) of pea plants, (2) role of MLE in amelioration of salinity stress in pea (3) to compare the response of MLE (foliar applied and seed soaking) to salt stressed pea.
2 Materials and methods
The seeds of Pea (Pisum sativum var. Pea-2009) were collected from Ayub Agriculture Research Institute (AARI), Faisalabad and experiment was carried out at IP and AB Bahauddin Zakariya University Multan, Pakistan in grwoing season October – December 2019–20. The average growing conditions (October – December 2019–20) were recorded as rainfall 10 mm, wind 8 Kmph, relative humidity 30 %, clouds 9 %, minimum temperature 19 °C and maximum temperature 30 °C. The arrangement of plastic pots for this experiment is given in Fig. 1. The pots were filled with 8 kg river sand (double washed with distilled water) and holes with diameter of 1.5 cm were made at the bottem of each pot in order to remove extra water. The healthy seeds were surface seterlized with sodium hypochlorite for 15 min follwed by drying in the shade. Seven seeds per pot were dibbed (1 inch) in wet sand and after complete germination, seedlings were thinned to three plants per pot. The plants were irrigated with Hoagland and Arnon (1950) nutrient solution in order to ensure the availability of all the nutrients required for optimum plant growth.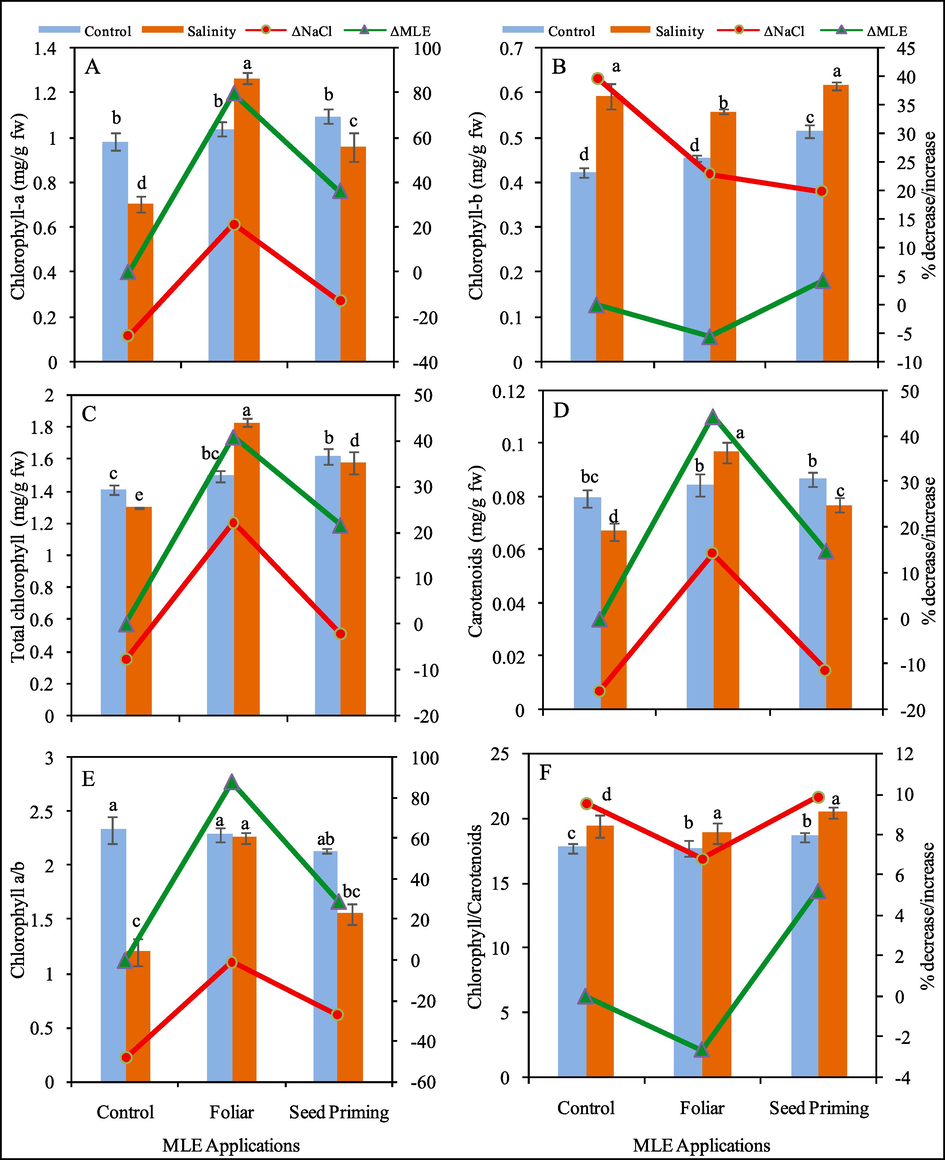
Effect of MLE as seed priming and foliar spray on chlorophyll-a (A), chlorophyll-b (B), total chlorophyll (C), carotenoid (D) contents (mg/g fw), chlorophyll a/b (E) and chlorophyll / carotenoid (F) ratio of Pea 2009 (Pisum sativum L.) at 0 and 100 mM NaCl stress. ΔNaCl and ΔMLE corresponds to percentage decrease / increase with respect to control after application of NaCl and MLE respectively.
2.1 Moringa oleifera leaf (MLE) extraction method
Leaves of Moringa from tender branches (18 g) were collected, washed with tap water and dried at room temperature. The leaves were grinded in pestle and motor by adding 5 mL distilled water to make a paste. Filtered the paste via Whatman No.1 filter paper to obtain a clear MLE extract. Finally, 30 mL MLE was added to 970 mL distilled (1:30) to make ready to use MLE. To ensure optimal penetration in leaf tissues during foliar spray, surfactant tween-20 was used @ 100 mL/ 1000 mL MLE solution.
2.2 Pre-sowing seed priming with MLE
MLE extract (3 mL) was diluted with addition of 97 mL distilled water in a beaker to make a final volume of 1L. Seeds were dipped in beaker containing 3 % MLE solution, covered with aluminium foil and placed overnight. Soaked seeds were sown on next day after treatment of 24 h.
2.3 Foliar application with MLE
Solution prepared (3 mL MLE / 97 mL distilled water) was foliarly applied to young plants at 3rd week of sowing. To ensure an equal level of foliar spray (10 mL for each seedling), a plant sprayer with an equal sized nozzle tip was used.
2.4 Application of salinity stress
The salt treatment was applied to plant at 21st day of sowing. The salinity stress was developed by adding weighed quanitity of NaCl (National Refined) to measured volume of tap water (For 100 mM salt stress 5.84 g of NaCl was added to 1 litter water).
Following data was collected during the course of experimentation.
2.5 Morphological attributes
When the plants were of 48 days, a plant was carefuuly uprooted from each pot to record shoot and root length and fresh weights. The dry biomass of shoot and root was recorded after oven drying of these samples.
2.6 Leaf relative water contents (LRWC)
A mature leaf from each replicate was trimmed and fresh weights (FW) were recorded immediately. The leaf samples were placed in distilled water from their cut side and turgid weights (TW) were recorded after 10 h. These leaves were then oven dried, and dry weights (DW) were recorded Smart and Bingham (1974) method. Following formula was used for measuring LRWC.
2.7 Photosynthetic pigments
Acetone based extraction; detection at 480, 645 and 663 nm using spectrophotometer and calculating with equations devised by Lichtenthaler (Lichtenthaler and Wellburn, 1983) was used for estimation of concentrations of photosynthetic pigments.
2.8 Total soluble protein (TSP)
The fresh flag leaf samples (0.5 g) from ach replicate were homogenized in sodium phosphate buffer (8 mL, pH 7.8) and centrifuged at 15000 rpm for 12 min at 4 °C. The 0.1 mL of supernatant thus removed was poured in test tubes containing 5 mL Bradford reagent. The reading was taken at 595 nm using spectrophotometer after 15 min. of incubation at room temperature (Bradford, 1976). TSP contents were determined using the standard curve.
2.9 Proline
Plant samples (0.5 g) were grounded in 3 % sulfosalicylic acid solution (10 mL) and were filtration with Whatman filter paper. 2 mL of this filtrate was added to 2 mL each of ninhidrin and glacial acetic acid solution in test tubes and water bathed at 100 °C for 60 min. followed by immediate cooling in ice. After cooling, 4.0 mL of toluene was poured in these test tubes, mixed vigorously and kept at room temperature till two layers were formed. The absorbance of upper colored layer was taken at 520 nm (Bates et al., 1973).
2.10 Hydrogen peroxide (H2O2) contents
To assay H2O2 contents plant material (0.25 g) was homogenized in 5 mL TCA (0.1 %) solution and centrifuged for 15 min. at 12,000 rpm. The supernatant (0.5 mL) was mixed with 0.5 mL sodium phosphate buffer and 1 mL of potassium iodide (KI) solution in test tubes. Test tubes were vortexed and absorbance was read at 390 nm (Velikova et al., 2000).
2.11 Malondialdehyde (MDA) contents
The MDA contents were estimated using Heath and Packer (Cakmak and Horst, 1991) methodology. 1 mL of supernatant (same as used in protein estimation) was mixed to 1 mL TBA (0.5 %) solution prepared in 20 % TCA solution in test tubes and were water bathed for 30 min. at 95 °C. The tubes were then ice bathed for 5 min. followed centrifugation at 6,000 rpm. The absorbance was recorded at 532 nm and 600 nm. The extension coefficient (155 mM−1 cm−1) was used for MDA contents calculation.
2.12 Antioxidants
The SOD activities was determined through quantifying the inhibition in photo reduction of nitrobluetetrazolium (NBT), the protocol devised by Beauchamp and Fridovich (Beauchamp and Fridovich, 1971). The reaction solution for POD contained 100 µL 30 mM H2O2, 100 µL guaiacol and 100 µL of enzyme extract (supernatant) into 2.7 mL sodium phosphate buffer (Chance and Maehly, 1955). For estimation of CAT activity same reaction solution as for POD (except guaiacol) was used (Aebi, 1984). The absorbance of POD and CAT samples was observed on time scan (0–60 s) at 470 and 240 nm respectively using spectrophotometer. The activity of APX was determined using Nakano and Asada (Nakano and Asada, 1981) methodology. The reaction solution contained 100 µL ascorbate solution (10 mM), 100 µL H2O2 (30 %) and 100 µL enzyme extract (supernatant) into 2.7 mL of sodium phosphate buffer. After a gentle shake, the absorbance was read at 290 nm with on time scan (0–60 s) using spectrophotometer.
2.13 Sodium (Na+) and potassium (K+) estimation
Oven dried plant samples were taken in digestion flasks containing 2 mL of digestion mixture and were kept overnight at 25 °C. The flasks were then transferred to hot plate set at 250 °C. Samples were heated till fume formation. At this point 0.5 mL of HClO4 was added in each flask and again transferred these flasks to hot plate at 250 °C for almost 2 h until discoloration of samples. After digestion the samplrs were filtred and volume was raised to 50 mL with dH2O to be used for estimation of ionic concentration (Allen et al., 1985). The Na+ and K+ concentrations in samples were estimated using flame photometer (Jenway- PFP7, United Kingdom).
2.14 Yield attributes
At maturity all the pods from each plant / pot were removed and the data for number of pods per plant, weight of pods per plant, number of seed per plant and weight of seeds per plant were recorded.
2.15 Statistical analysis
The statistical analysis (Two way ANOVA) was performed using SPSS-20 (SPSS Inc. Chicago, IL, USA). To conclude the influence of various treatments, R-sutudio (v 4.0.4) was employed. The correlation matrix was designed for estimation of overall relationship of different traits of pea plants under different regimes. Moreover, the data was subjected to principle component analysis (PCA) to decipher influence of different treatments on various plant parameters using Origin (v.2021). The clustered heatmaps between growth, yield and various plant physiological parameters were then constructed to assess the association among studied traits.
3 Results
3.1 Growth parameters
ANOVA for biomass accumulation (shoot and root fresh and dry biomass) of pea displayed a significant (P < 0.001) reduction in these parameters under salt stress, the effect of MLE was also highly significant (P < 0.001) on growth of pea plants (Table 1). Salinity stress reduced shoot fresh biomass (33%), shoot dry biomass 52%), root fresh biomass (35%) and root dry biomass (37%) when compared to non-saline pea plants. However, the application of MLE either as seed priming or foliar application enhanced growth under control and saline conditions. Under NaCl stressed conditions, the foliar application of MLE enhanced fresh and dry biomass of shoot (26% and 67%) and root (21% and 35%) respectively. Similarly, seed priming with MLE also enhanced fresh and dry weights of shoot (13% and 47%) and root (14% and 20%) respectively (Table 1). It has been observed that the impact of foliar application of MLE was more promising in enhancing growth. Each value is the mean ± SE of different treatments with n number of replicates (n = 4). Different letters on values represents a statistically significant difference among different treatments in common pea (Pisum sativum L.) plants at 5% level of probability by Duncan’s Multiple Range Test (DMRT). *,**,*** = significant at 0.05, 0.01, 0.001 % level of probability respectively.
Salinity (NaCl)
Treatments
Shoot fresh weight
Shoot dry weight
Root fresh weight
Root dry weight
0 mM
Control
14.6a ± 0.3
1.9a ± 0.03
2.3a ± 0.06
0.2a ± 0.01
Foliar
18.3b ± 0.1
2.5b ± 0.07
2.6b ± 0.05
0.3b ± 0.02
Seed priming
17.6b ± 0.2
2.4b ± 0.04
2.6b ± 0.02
0.3b ± 0.02
100 mM
Control
9.8a ± 0.6
0.9a ± 0.03
1.5a ± 0.02
0.1a ± 0.01
Foliar
12.3b ± 0.3
1.5b ± 0.05
1.8b ± 0.03
0.2b ± 0.01
Seed priming
11.1b ± 0.2
1.3b ± 0.08
1.7b ± 0.04
0.2b ± 0.01
ANOVA
SOV
df
Salinity
1
160.1 ***
3.1 ***
3.9 ***
0.3 ***
MLE
2
7.6 ***
0.4 ***
0.09 ***
0.05 ***
Salinity × MLE
2
0.7 ns
0.08 ***
9.1 ns
4.5 ns
Error
18
0.5
0.05
0.03
2.7
Total
23
Salinity (NaCl)
Treatments
Number of pod plant−1
Weight of pods plant−1
Number of seeds plant−1
Weight of seeds plant−1
0 mM
Control
9.5a ± 0.3
15.9a ± 0.5
41.3a ± 1.0
10.3a ± 0.5
Foliar
10.8b ± 0.3
17.8ab ± 0.6
60.1c ± 2.1
14.1b ± 0.6
Seed priming
10.5b ± 0.2
16.5a ± 0.6
50.5b ± 2.2
15.4bc ± 0.7
100 mM
Control
6.5a ± 0.3
7.8a ± 0.6
24.8a ± 2.1
4.5a ± 0.4
Foliar
12.8c ± 0.5
12.8bc ± 0.2
27.8a ± 0.5
6.0b ± 0.2
Seed priming
8.2b ± 0.2
7.9a ± 0.5
26.5a ± 0.7
6.1bc ± 0.5
ANOVA
SOV
df
Salinity
1
7.0 ***
310.8 ***
3528.4***
369.3 ***
MLE
2
26.5 ***
28.8 ***
236.5***
30.2 ***
Salinity × MLE
2
16.7 ***
7.5 **
124.1***
4.2 *
Error
18
0.4
0.9
8.3
0.8
Total
23
3.2 Mineral elements
A significant (P < 0.001) increase in shoot and root Na+ contents was perceived, while K+ contents were reduced significantly (P < 0.001). The application of MLE either foliar spray or seed priming reduced Na+ contents while enhanced K+ contents in roots and shoots of pea plant (Table 2). Salinity stress enhanced root and shoot Na+ contents to 42% and 212% respectively, while MLE application reduced Na+ contents to 28% (foliar) and 35 % (priming) in roots and 142% (foliar) and 191% (priming) in shoots respectively. The root and shoot K+ contents were decreased to 62% and 6% respectively with NaCl treatment, on the other hand MLE application enhanced K+ contents to 57% (foliar) and 65% (priming) in roots and 18% (foliar) and 30% (priming) in shoots respectively. Each value is the mean ± SE of different treatments with n number of replicates (n = 4). Different letters on values represents a statistically significant difference among different treatments in common pea (Pisum sativum L.) plants at 5% level of probability by Duncan’s Multiple Range Test (DMRT).
Salinity
Treatments
Root Na+
Shoot Na+
Root K+
Shoot K+
Root / Shoot Na+
Root / Shoot K+
0 mM
Control
35.9b ± 1.44
13.2c ± 0.12
16.2a ± 0.29
16.7a ± 0.44
2.7b ± 0.25
1.0a ± 0.08
Foliar
20.9a ± 1.52
11.1a ± 0.37
20.4c ± 0.22
17.8ab ± 0.63
1.9b ± 0.17
1.1b ± 0.05
Seed priming
22.7a ± 0.79
12.3b ± 0.51
18.7b ± 0.29
19.1b ± 0.39
1.8a ± 0.30
1.0a ± 0.08
100 mM
Control
51.1c ± 0.55
41.1c ± 0.36
17.0c ± 0.34
15.7a ± 0.17
1.2b ± 0.07
1.1b ± 0.06
Foliar
36.7b ± 0.99
26.9b ± 0.90
10.8a ± 0.26
18.6b ± 0.69
1.4b ± 0.27
0.6a ± 0.04
Seed priming
33.3a ± 1.04
35.9a ± 0.84
13.5b ± 0.28
20.5c ± 0.40
0.9a ± 0.06
0.7a ± 0.05
ANOVA
SOV
df
Salinity
1
1156.3 ***
3018.9 ***
1057.8 ***
0.7 ns
118.9 **
283.8 **
MLE
2
607.5 ***
135.2 ***
54.9 ***
26.2 ***
269.4 ns
186.1 *
Salinity × MLE
2
16.3 **
75.5 ***
3.1 *
3.0 *
132.2 **
151.3 ns
Error
18
3.6
1.0
0.7
0.7
6.0
3.5
Total
23
3.3 Photosynthetic pigments
The data showed that Chl-a, Chl-b, T. Chl. and carotenoid contents were lowered in pea plants under salinity stress. As compared to control salinity stress lowered Chl.a (28%), T. Chl. (8%) and carotenoids (15%) contents, however Chl.b contents were enhanced (39%) under salinity stress (Fig. 2). The foliar application of MLE enhanced Chl.a (22%), Chl.b (23%), T.Chl. (22%) and carotenoids (14%) contents under salinity stress as compared to control. But on the other hand, seed priming with MLE caused a considerable reduction. Under salinity Chl.a (13%), T.Chl. (3%) and carotenoids (11%) contents when compare to salinity stressed plants (Fig. 2). The Chl. a/b concentration was remarkably decreased (48% and 27%) under salinity stress and when MLE was applied as seed priming respectively, while foliar spray of MLE maintained Chl. a/b ratio under control as well as saline environment. The chlorophyll / carotenoid were enhanced to 9%, 7% and 10% with salinity treatment, foliar and seed priming MLE under salinity stress respectively (Fig. 2).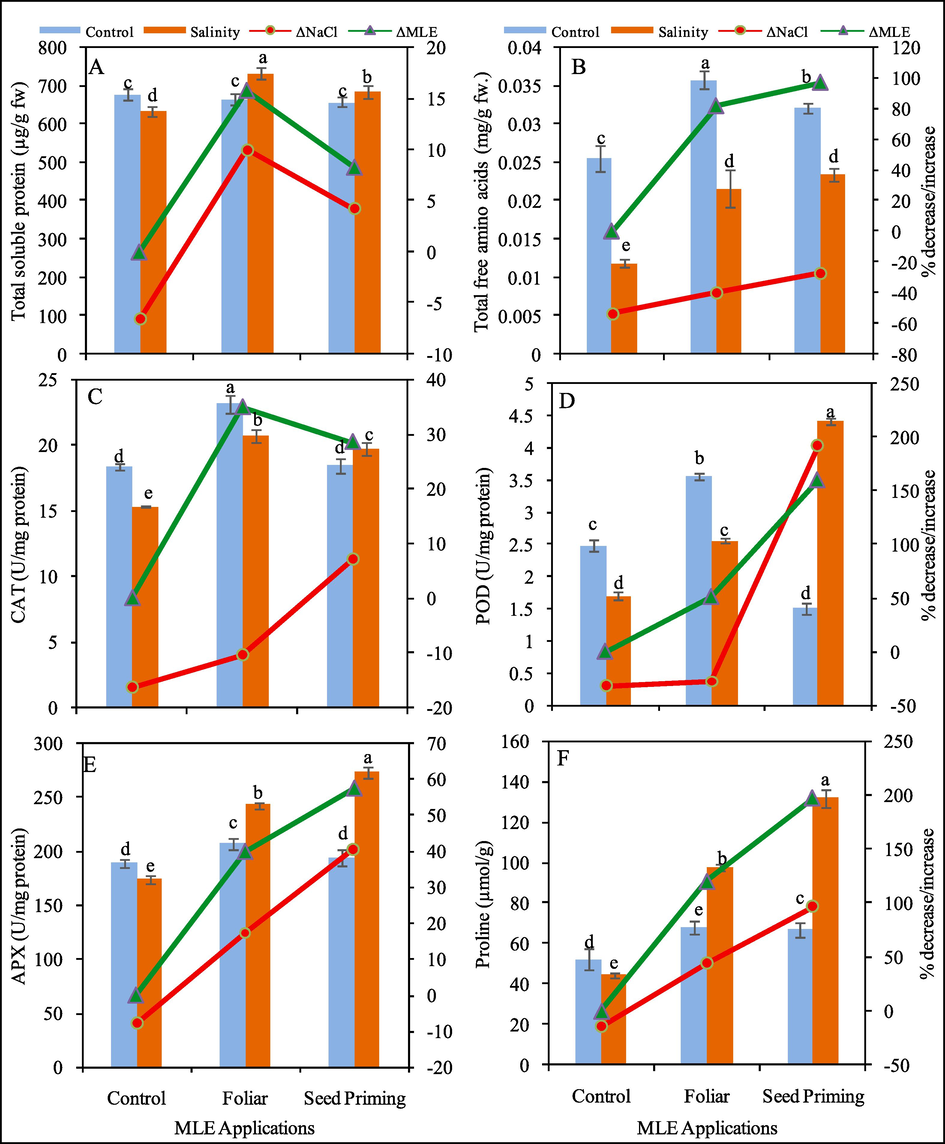
Effect of MLE as seed priming and foliar spray on total soluble protein (mg/g fw) (A), total free amino acid (mg/g fw) (B), CAT (U mg−1 protein fw) (C), POD (U mg−1 protein fw) (D), APX (U mg−1 protein fw) (E) and proline (U mg−1 protein fw) (F) of Pea 2009 (Pisum sativum L.) at 0 and 100 mM NaCl stress. ΔNaCl and ΔMLE corresponds to percentage decrease / increase with respect to control after application of NaCl and MLE respectively.
3.4 Total soluble protein (TSP) and free amino acid (TFAA) contents
The application of MLE caused a remarked enhancement in TSP and TFAA in pea plants. Though, the response of TSP to salt stress was non-significant however, TFAA showed significant effect (Fig. 3). The data revealed that TSP contents were reduced 6% with NaCl application while MLE application enhanced these contents to 10% and 4% with foliar and seed priming with MLE respectively under salinity stress. However, TFAA contents were declined (53%) due to salinity stress, however, the application of MLE either applied foliar or seed priming enhanced these contents to 82% and 97% respectively in pea plants (Fig. 3).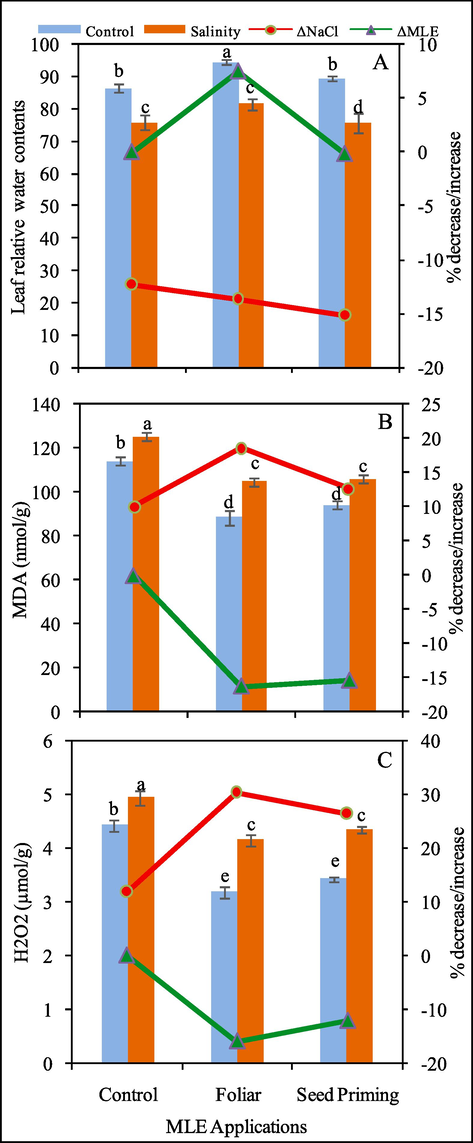
Effect of MLE as seed priming and foliar spray on leaf relative water contents (A), MDA (nmol/g fw) (B) and H2O2 (µmol/g fw) (C) of Pea 2009 (Pisum sativum L.) at 0 and 100 mM NaCl stress. ΔNaCl and ΔMLE corresponds to percentage decrease / increase with respect to control after application of NaCl and MLE respectively.
3.5 Antioxidant response
The data depicted that salinity stress significantly (P < 0.001) effect the accumulation of different antioxidants (CAT, POD, APX, proline) in pea plants. The ameliorative effect of MLE was also significant (P < 0.001) showing a positive enhancement in the accumulation of these antioxidants (Fig. 4). The salinity-induced decrease in CAT (16%), POD (31%), APX (10%) and proline (15%) contents was observed in leaves of pea plants. Under non-saline conditions MLE fertigation caused enhancement in CAT (26% (foliar) and 0.5% (seed priming); POD (43% (foliar), APX (10% (foliar) and 3% (priming) and proline (29% (foliar) and 28% (priming) contents in leaves of pea plants. On the other hand, MLE fertigation either as foliar spray and seeds priming enhanced CAT (35%, 29%), POD (51%, 160%), APX (40%, 57%) and proline (119%, 197%) contents respectively in pea plants under salinity stress (Fig. 3).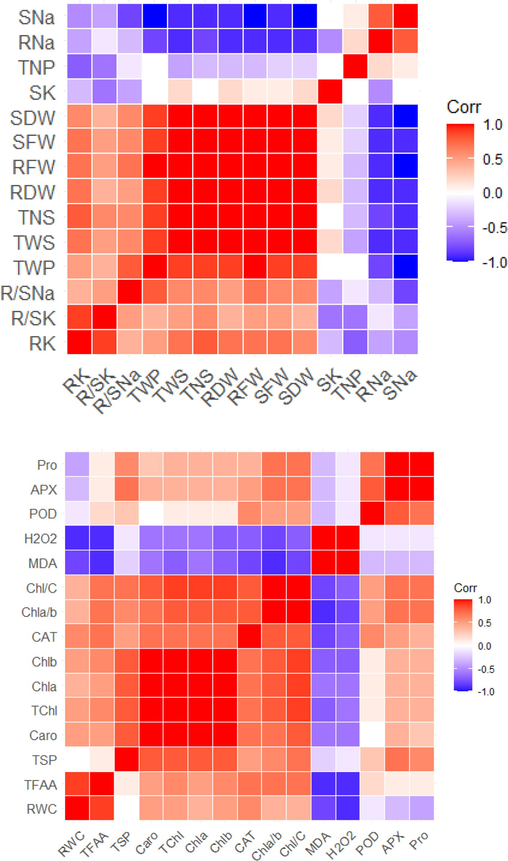
Correlation matrix representing relationship of (A) growth, yield and shoot ionic contents (B) physio-biochemical attributes of Pea 2009 (Pisum Sativum L.) grown under saline environment (S1-salinity 0 mm, S2-salinity 100 mm) with Moringa leaf extract applications (T0-control, T1-foliar application, T2- seed priming).
3.6 Leaf relative water content (LRWC)
The imposition of salinity to growing media lowered (12%) relative water contents of pea leaves. However, these contents were enhanced (7%) by foliar application of MLE while the impact of MLE as seed priming was not significant when compared to control plants (Fig. 4).
3.7 Malondialdehyde (MDA) contents
The ANOVA for MDA contents revealed that these contents were significantly enhanced under salinity stress but were reduced with fertigation of MLE either as foliar spray or seed priming (Fig. 4). MDA contents were enhanced to 10% with NaCl treatment while MLE application reduced these contents to 16% and 15% when applied as foliar or seed priming respectively (Fig. 4).
3.8 Hydrogen peroxide (H2O2) contents
A pronounced rise in H2O2 contents was observed in pea under salinity stress. The increase in H2O2 contents was 12%, 30% and 26% under control, foliar and seed priming (MLE) conditions respectively (Fig. 5). However, when compared to salinity stress alone the foliar spray caused 16% and seed priming caused (12%) decrease in MDA contents under salinity stress (Fig. 4).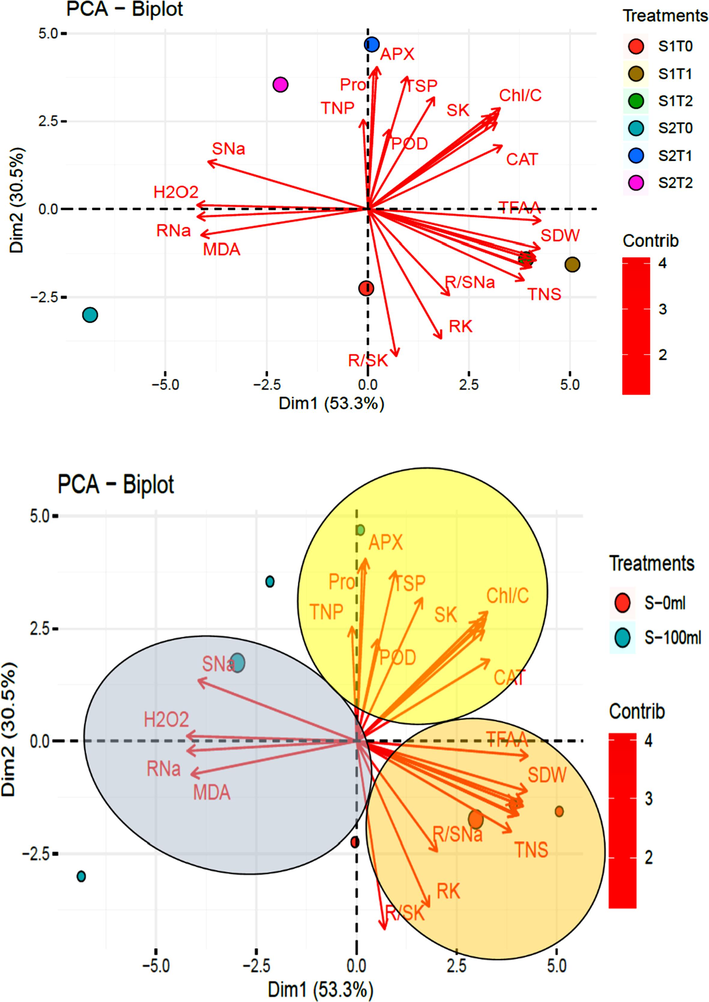
Principle component analysis representing influence of different treatments on morphological and physiological attributes of Pea 2009 (Pisum sativum L.): (A) Overall impact of Moringa leaf extract (Foliar and seed priming) and salinity on different parameters, (B) Eclipses are formed to group parameters on the basis of salinity and mode of treatments (S-0 mM, S-100 mM).
3.9 Yield attributes
The statistical analysis revealed that factors, salinity and MLE application caused remarked effect on yield (number of pods plant−1; pod weight plant−1, number of seeds plant−1 and wt. of seeds plant−1) of pea plants. The effect of salinity × MLE showed that MLE caused a differential effect of yield of pea plants at salinity levels (Table 1). Highest yield either under control or saline conditions was produced by foliar application of MLE showing that foliar spray posed more promising effect on yield (Table 1).
3.10 Multivariate analysis
3.10.1 Correlation matrix
A correlation matrix among morphological attributes and shoot ionic contents represent significant correlation (P ≤ 0.01). A strong positive correlation was found between TWP: RFW, TWP: SFW, TWS: SFW, RDW: PH and TNS: TWP, whereas a week associations was seen among R/SNa: TNS, R/SNa: TWP and R/SK: PH. The SK, TNP, RNa and SNa showed negative correlations (Fig. 5A). In case of correlation drawn among physiological attributes, Pro: APX, TChl: Chla, Chla: Chla/C showed positive correlation, while MDA, H2O2, POD and RWC represent negative correlation (Fig. 5B).
3.10.2 Principal component analysis (PCA-Biplot)
Principal component analysis showed a strong influence of MLE foliar application as well as seed soaking under control environment (S1T1 and S1T2) on PH, TFAA and TNS of Pisum sativum, while RNa affected H2O2 and MDA level of plants subjected to saline condition (S2T0). The SNa imposed strong impact on TNP and Pro content of foliar treated slots (S2T1), while Sk had strong influence on POD, TSP, APX and Chl/C in seed priming slots (S2T2) growing under stressed environment (Fig. 6A).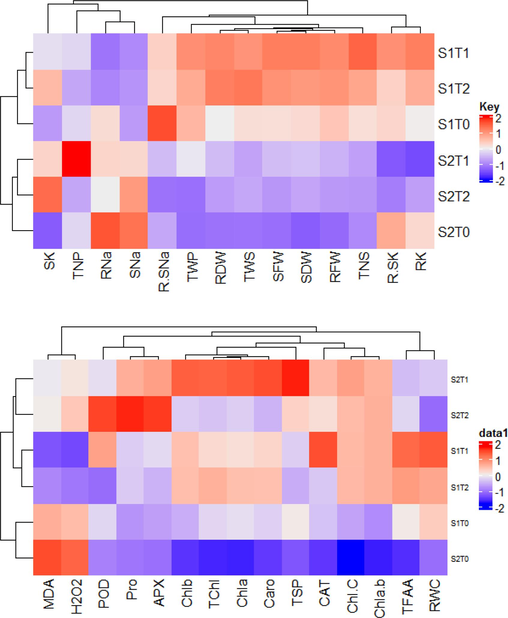
Clustered heatmaps representing overall response of (A) growth, yield and shoot ionic contents (B) physiobiochemical attributes of Pea 2009 (Pisum sativum L.) grown under saline environment (S0-salinity 0 mm, S1-salinity 100 mm) with Moringa leaf extract applications (T0-control, T1-foliar appli-cation, T2- seed priming).
Principal component analysis based on mode of application represented two distinct groups (Fig. 6B). Salinity controlled plants (S-0 %) showed association with TFAA, PH, TNS, R/SNa and RK. Salinity treated plants (S-100 %) exhibited close association with SNA, H2O2, RNa and MDA. Traits like POD, SK, TSP, APX, Pro, TNP, Chl/C and CAT were influenced in both control and salinity treated plants.
3.10.3 Clustered heatmaps
A clustered heatmap was established among morphological and shoot ionic contents to assess their responses under different salinity and Moringa leaf extract (MLE) treatments. The TWP, SFW, SDW, RFW and PH showed positive association and strong clustering in S1T1, RDW and TWS in S1T2, whereas RNa and SNa showed high clustering in S2T0 treatment (Fig. 7A). The clustered heatmap on physiological attributes represented close association of Chla, Chlb, TChl, Caro and TSP in S2T1, APX, Pro and POD in S2T2, while TFAA and RWC in S1T1 treatment (Fig. 7B).
The arrangement of pots used in this stud. The distance of pots between each treatment was 18 cm while among treatment 12 cm.
4 Discussion
Increasing soil salinity is creating alarming situation for agricultural system across the world. According to current soil salinization rate, it may reduce up to 50 % crop production in coming future (Singh, 2022). In order to cope with the situation different short term approaches may be employed to improve crop productivity especially in soils facing abiotic stresses (Noreen et al., 2021). The application of bio-stimulants like micronutrients, leaf extracts, hormones, vitamins and osmoprotectants are commonly used to induce abiotic stress tolerance in crop plants (Zouari et al., 2019). The current study revealed that application of pea plants and seeds with Moring leaf extract (MLE) as foliar and seed priming can potentially mitigate salinity stress. It has been previously studied by several researchers that MLE can potentially mitigate salinity stress and tends to maintain optimum growth and productivity (Arif et al., 2022; Khan et al., 2020; Yasmeen et al. 2013a). MLE used as potential bio-stimulator or bio-enhancer in having some important minerals, phenolics, alkaloids, sugars and vitamins that support plants plant growth and developmental process (Arif et al., 2022). The current study was aimed to examine the possible mechanism adopted by pea plants in response to salt stress via foliar spray and seed priming with MLE in a saline environment.
A major reduction in growth (biomass production) of pea was observed under salinity stress that was linked to reduced yield (number and weight of pods) attributes. The decrease in the activities of cell division and cell elongation due to excessive production of reactive oxygen species (ROS) in cellular system is obvious under salinity stress. Application of MLE as foliar and seed priming not only lessened the salinity’s noxious effects on growth and yield attributes but also enhanced growth rate considerable when applied alone or in combination of salt stress. As it was observed in earlier reports of (Howladar, 2014) in Phaseolus vulgaris and Yasmeen et al. (2013a) in primed wheat seeds to Moringa leaf extract (MLE) under saline condition. Plants extracts (fresh and/or dry) also have stimulatory impacts on growth by upregulating enzymes activity, metabolic contents and water use efficiency (Arif et al., 2022).
Sodium content significantly increased whereas K+ content decreased in root and shoot of pea plants under salinity stress (Table 2). However, application of MLE as foliar and seed priming reduced Na+ content and root/shoot Na+/K+ ratio while enhanced K+ content in root and shoot under stress as compared to control. These results are in line of findings of (Nouman et al., 2012; Ragab et al., 2022; Yasmeen et al. 2013a). The K+ efflux from plant organs and Na+ accumulation are consequences of salt prone conditions, which significantly influence the cytosolic ion homeostasis and plant survival, which regarded as fundamental salt tolerance mechanism of plants (Desoky et al., 2019; Fu et al., 2022).
Salinity-induced reduction in photosynthetic pigment synthesis is subjected to several factors including mineral imbalance and reduction in the activity of several photosynthetic enzymes (Ondrasek et al., 2022). However, MLE fertigation either as foliar or as seed priming enhanced the chlorophyll (chla, chlb, Tchl, chl a/b and Tchl/caro) and carotenoid contents under both controlled and saline condition which is attributed to better mineral uptake and stabilized activities of photosynthetic enzyme. Generally, foliar application of MLE on pea plants was found more effective in comparison of seed priming, and this finding strongly agreed with previous reports of (Attanayaka and Harris, 2019) on okra and (Yaseen and Takacs-Hajos, 2022) on lettuce plants plants revealed that MLE application as foliar pose positive effect on photosynthesis under both normal as well as saline condition. Moreover, enhanced carotenoid content and Total chlorophyll/ carotenoid ratio due to MLE application (foliar and seed priming) again play critical role in protecting macromolecules included DNA, proteins and RNA from free radicals produced in response to osmotic stress (Khan et al., 2020; Nouman et al., 2012).
A substantial increase in hydrogen peroxide (H2O2) and malonaldehyde (MDA) contents was observed under salinity stress in pea plants. Similarly, increased level of total soluble protein (TSP), total free amino acid (TFAA) and proline in response to foliar applied and/or seed priming with MLE could be crucial for osmoregulation and membrane stability under salt stress, as reported by (Basu et al., 2022). Osmoprotectant accumulation is considered as initial protective strategy of plants in order to prevent from high salinity (Kaya et al., 2020). Presence of phenolic, alkaloids and other active ingredients with in MLE may additionally prevent from membrane leakage and structures de-stability in consequence of lipid peroxidation as was observed in common beans (Howladar, 2014).
Salinity-induced ROS stimulates oxidative damage and lipid peroxidation (Mansoor et al., 2022) in plants which can be radially controlled by increasing activity of antioxidants (enzymatic and non-enzymatic) via application of bio-stimulant or growth promoters. Among enzymatic antioxidants CAT, POD, and APX worked as front line defense rescue plants from stressful conditions like salinity and drought (Noreen et al., 2021). In response to salt stress, the removal of extra ROS, through MLE application as foliar and/ or seed priming cause further increase in activity of APX, CAT and POD in order to strength the defense system in plants via removal of reactive ROS (Yasmeen et al., 2013b). Both APX and CATare vital for empowering stress tolerance, as they quickly quench the H2O2 and ultimately lead to protection of membrane functioning. So the high level of APX, CATand POD APX in MLE treated pea plants (foliar and seed primed) indicated the improved stress tolerance to oxidative damage (Hasanuzzaman and Fujita, 2022). Such upraise in enzymetic antioxidant activity by foliar and/or seed priming of MLE could be beneficial for growth maintenance through rapid quenching of ROS. In concluding, the application of MLE as foliar as well as seed priming further strengthened the both enzymetic and non-enzymetic antioxidant in Pisum sativum and similar remarks have also been represented by Zulfiqar et al. (2020) and Merwad (2018) in plant species subjected to salt stress.
5 Conclusions
Moringa leaf extract (MLE) can potentially develop better tolerance to salinity stress in Pea (Pisum sativum) as was observed in this experiment. Although pea is sensitive to salinity stress still the application of MLE either as seed priming or foliar spray enhanced growth and yield of pea plants especially under salinity stress. The production of excessive ROS, accelerated lipid peroxidation (MDA), accumulation of Na+ to toxic levels in plant tissues and disturbance in biochemical (antioxidant) response of pea reduced the photosynthetic ability of plats resulting in reduced growth and development are inevitable events under salinity stress. However, MLE balanced ionic contents, regulated antioxidant response and stabilized photosynthetic activity to cope the adversities of salinity induced ROS. Therefore, MLE fertigation either as seed priming or foliar spray is recommended as remedy for plants grown under saline conditions.
CRediT authorship contribution statement
Sibgha Noreen: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft. Sehrish Saleem: Conceptualization, Data curation. Ummar Iqbal: Writing – original draft, Writing – review & editing. Seema Mahmood: Formal analysis, Project administration. Muhammad Salim Akhter: Investigation. Noor Akbar: Data curation. Mohamed El-Sheikh: Resources, Funding acquisition, Project administration. Prashant Kaushik: Software, Visualization.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2023R182), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Drying alters the phenolic constituents, antioxidant properties, α-amylase, and α-glucosidase inhibitory properties of Moringa (Moringa oleifera) leaf. Food Sci. Nutr.. 2018;6:2123-2133.
- [Google Scholar]
- Aebi H (1984) Catalase in vitro. In: Methods in enzymology, vol 105. Elsevier, pp 121-126.
- Influence of exogenous salicylic acid and nitric oxide on growth, photosynthesis, and ascorbate-glutathione cycle in salt stressed Vigna angularis. Biomolecules. 2020;10(1):42.
- [CrossRef] [Google Scholar]
- Akhter MS, Noreen S, Mahmood S, Ashraf M, Alsahli AA, Ahmad P (2021) Influence of salinity stress on PSII in barley (Hordeum vulgare L.) genotypes, probed by chlorophyll-a fluorescence Journal of King Saud University-Science 33:101239.
- Moringa oleifera Extract as a Natural Plant Biostimulant. J. Plant Growth Regul. 2022:1-16.
- [Google Scholar]
- Inoculation approach to legume crops and their production assessment in Pakistan-A review. Pak. J. Biol. Sci.. 2000;3:193-195.
- [Google Scholar]
- Effect of foliar application of moringa (Moringa oleifera) leaf extract with recommended fertilizer on growth and yield of okra (Abelmoschus esculentus) J. Agric. Sci. AGRIEAST. 2019;13:38-54.
- [Google Scholar]
- Basu S et al. (2022) Micronutrient and redox homeostasis contribute to Moringa oleifera-regulated drought tolerance in wheat Plant Growth Regulation:1-12.
- Rapid determination of free proline for water-stress studies. Plant and Soil. 1973;39:205-207.
- [Google Scholar]
- Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem.. 1971;44:276-287.
- [Google Scholar]
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72:248-254.
- [Google Scholar]
- Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max) Physiol. Plantarum. 1991;83:463-468.
- [Google Scholar]
- Chance B, Maehly A (1955) [136] Assay of catalases and peroxidases.
- Desoky E-SM, ElSayed AI, Merwad A-RM, Rady MM (2019) Stimulating antioxidant defenses, antioxidant gene expression, and salt tolerance in Pisum sativum seedling by pretreatment using licorice root extract (LRE) as an organic biostimulant Plant physiology and biochemistry 142:292-302.
- Vacuolar H+-pyrophosphatase HVP10 enhances salt tolerance via promoting Na+ translocation into root vacuoles. Plant Physiol.. 2022;188:1248-1263.
- [Google Scholar]
- Hasanuzzaman M, Fujita M (2022) Plant Oxidative Stress: Biology, Physiology and Mitigation vol 11. MDPI.
- Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil Circular California agricultural experiment station 347.
- A novel Moringa oleifera leaf extract can mitigate the stress effects of salinity and cadmium in bean (Phaseolus vulgaris L.) plants. Ecotoxicol. Environ. Saf.. 2014;100:69-75.
- [Google Scholar]
- Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol. Plantarum. 2020;168(2):256-277.
- [CrossRef] [Google Scholar]
- Khan S, Basra S, Nawaz M, Hussain I, Foidl N (2020) Combined application of moringa leaf extract and chemical growth-promoters enhances the plant growth and productivity of wheat crop (Triticum aestivum L.) South African Journal of Botany 129:74-81Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Portland Press Ltd.
- Macák M et al. (2020) The Influence of Different Fertilization Strategies on the Grain Yield of Field Peas (Pisum sativum L.) under Conventional and Conservation Tillage Agronomy 10:1728.
- Using Moringa oleifera extract as biostimulant enhancing the growth, yield and nutrients accumulation of pea plants. J. Plant Nutr.. 2018;41:425-431.
- [Google Scholar]
- Nadathur S, Wanasundara J, Scanlin L (2017) Feeding the globe nutritious food in 2050: Obligations and ethical choices. In: Sustainable Protein Sources. Elsevier, pp 409-421.
- Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol.. 1981;22:867-880.
- [Google Scholar]
- Foliar fertigation of ascorbic acid and zinc improves growth, antioxidant enzyme activity and harvest index in barley (Hordeum vulgare L.) grown under salt stress. Plant Physiol. Biochem.. 2021;158:244-254.
- [Google Scholar]
- Profiling of polyphenolics, nutrients and antioxidant potential of germplasm’s leaves from seven cultivars of Moringa oleifera Lam. Indus. Crops Prod.. 2016;83:166-176.
- [Google Scholar]
- Ondrasek G et al. (2022) Salt Stress in Plants and Mitigation Approaches Plants 11:717.
- The effect of foliar application of zinc oxide nanoparticles and Moringa oleifera leaf extract on growth, biochemical parameters and in promoting salt stress tolerance in faba bean. Afr. J. Biotechnol.. 2022;21:252-266.
- [Google Scholar]
- Soil salinity: A global threat to sustainable development. Soil Use Manage.. 2022;38:39-67.
- [Google Scholar]
- Pulses production in Pakistan: status, constraints and opportunities. Int. J. Plant Prod.. 2020;14:549-569.
- [Google Scholar]
- Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci.. 2000;151:59-66.
- [Google Scholar]
- Yaseen AA, Takacs-Hajos M (2022) Evaluation of moringa (Moringa oleifera Lam.) leaf extract on bioactive compounds of lettuce (Lactuca sativa L.) grown under glasshouse environment Journal of King Saud University-Science 34:101916.
- Exogenous application of moringa leaf extract modulates the antioxidant enzyme system to improve wheat performance under saline conditions. Plant Growth Regulation. 2013;69:225-233.
- [Google Scholar]
- Yasmeen A, BASRA S, AHMED M, WAHID A, NOUMAN W, REHMAN HU (2013a) Exploring the potential of Moringa oleifera leaf extract (MLE) as a seed priming agent in improving wheat performance Turkish Journal of Botany 37:512-520.
- Zouari M, Hassena AB, Trabelsi L, Rouina BB, Decou R, Labrousse P (2019) Exogenous proline-mediated abiotic stress tolerance in plants: Possible mechanisms. In: Osmoprotectant-mediated abiotic stress tolerance in plants. Springer, pp 99-121.
- An overview of plant-based natural biostimulants for sustainable horticulture with a particular focus on moringa leaf extracts. Plant Sci.. 2020;295:110194
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.103056.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







