Translate this page into:
Molecular target prediction and docking of anti-thrombosis compounds and its activation on tissue-plasminogen activator to treat stroke
⁎Corresponding author at: Department of Medicine, College of Medicine and Health Science, Jigjiga University, Ethiopia. senthilkumarsubramanian@jju.edu.et (Subramanian Senthilkumar),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The main aim of this study is to analyze effective thrombolytic drugs of natural origin for the treatment of stroke. Thrombolytic activity of natural sources has been reported and active molecules have been isolated and characterized. In this study, a total of ten compounds from nature were selected for docking studies. Caesalpinine c, caesalpinine a, vanillylamine, terpinen-4-Ol, dihydrocapsaicin and 3-carene showed fibrinolytic properties on analysis with PASS server. The present work is based on computer aided molecular modeling and the strength of the ligand was validated using binding energy. Caesalpinine c exhibited good docking score and this compound showed potent thrombolytic activity than other compounds. The drug likeliness varied based on the chemical and physical properties of the ligand. Lipinski’s rule of five was accepted by all of the selected compounds. Pfizer’s rule, and GSK rule were accepted in caesalpinine C. The drug likeliness properties of the all ten selected ligands were accepted in most of the cases and few rejections were observed mostly in Golden Triangle rule. However further in vitro or in vivo trials are required to validate thrombolytic potential of caesalpinine c and vanillylamine.
Keywords
Natural compounds
Stroke, cardiovascular diseases
Thrombolytic
Activator
1 Introduction
Blood clusters occur in aspiratory or cerebral, coronary vessels, they can be highly dangerous-coronary thrombi are the main reason for dead tissue in myocardium, cerebrovascular thrombi involved in strokes and pneumonic thromboembolic can prompt heart and respiratory disappointment. Thrombosis is the blood clot formation within the blood vessel, affecting the blood flow through the circulatory system. Thrombus may form if vein or an artery or surrounding tissue is severely damaged. The thrombi can affect the flow of blood through artery or vein if the thrombi detached from the endothelium of the blood vessel and lodges in vital organs such as brain, lungs and it can become serious issue (Lyaker et al., 2013). Imbalances in the homeostatic mechanisms lead to the development of thrombus in the circulatory system. This is one of the critical events in the cardiovascular diseases including, pulmonary emboli, venous thromboembolic disorders, heart attacks, strokes, and deep vein thrombosis disorders that account for sudden mortality and morbidity (Nicolini et al., 1992). Fibrinolytic agents such as, streptokinase, urokinase, anistreplase, alteplase and t-PAA are widely used for the treatment of cardiovascular diseases, including heart attack and stroke (Dwivedi, 2007; Collen, 1990). Thrombosis is the lysis of blood clots by using thrombolytic agents and this process is commonly called clot busting. It functions by inducing fibrinolysis by the enzyme plasmin through infusion of tissue plasminogen activator (tPA), activates plasmin. Thrombolysis involves the application of various fibrinolytic drugs, which generally dissolve blood clots (Chowdhury et al., 2015). The main purpose of thrombolytic drugs is to dissolve thrombin in coronary arteries thereby to improve blood flow to ischemic myocardium, to limit necrosis and to enhance prognosis (Morcos et al., 2005). For the treatment of stroke and other cardiovascular diseases, various fibrinolytic agents are applied. However, t-PA is highly effective and showed fewer side effects than streptokinase or urokinase type activators. It could be noted that almost all thrombolytic agents still have lot of deficiencies, including, less specificity, required maximum doses and less specificity to fibrin. Hence, increased attention has been paid to develop recombinant variants of these thrombolytic drugs in order to reduce these side effects and improve substrate specificity (Nicolini et al., 1992; Marder, 1993; Paul et al, 2017; Lijnen et al., 1991; Adams et al., 1991). The first generation thrombolytic drugs (urokinase and streptokinase) were commonly used to treat thrombosis, however major side effects such as, haemorrhage, systemic fibrinolysis, anaphylactic reaction have been reported (Mannan et al., 2011). Moreover, immunogenicity is another serious problem which avoids the use of multiple doses of streptokinase (Furie and Furie, 2008).
In recent years, natural sources are widely used to stimulate t-PA activity and plant extracts are widely applied to discover novel drugs (Lahlou, 2013). In developing countries, traditional or alternate medicines have been used to treat various ailments. Traditional medicines are used to treat cardiovascular diseases, including stroke, nervine tonic, nervous debility, piles, fever, back pain, arthritis, headache, toothache, and stomachache (Chowdhury et al., 2008). Traditional medicines are used to improve cardiovascular diseases and to remove cholesterol in the arteries. Medicinal plants contain rich sources of calcium, thus improved bone and teeth health. Drug-target interaction studies by computational stimulations using molecular dynamics and in silico approaches are widely used for the screening and the rational design of various drugs (Jorgensen, 2004). In silica molecular analysis predicts the relationship between to particles. Molecular docking incorporates algorithms like molecular stimulation, molecular dynamic, and fragment based analysis. Molecular docking is wildly utilized to predict the interactions of two different particles and to predict the most suited ligand for various pharmaceutical and other applications (Hsu et al., 2011; Glaab, 2016; Friesner et al., 2004; Bissantz et al., 2000). ADME/T test is widely used to determine the predicted physicochemical properties such as, distribution, absorption, excretion, metabolism and toxicity analysis within the body after drug administration (McInnes, 2007; Ekins et al., 2007).
2 Materials and methods
2.1 Preparation of protein and in-silico molecular docking
Three-dimensional structure of catalytic domain of t-PA complex of human was obtinaed from the available protein data bank athttp://www.rcsb.org as described by Berman et al. (2000). Schrödinger-Maestro v12.5, a protein preparation wizard was used to prepare and refined the protein structure. In this study, bond orders and charges were assigned and hydrogen atoms were added, water molecules were removed, selenomethionines were further converted to methionines. During the process of minimization step, the water molecule less than 3H-bonds to non-water were removed. Force field OPLSe3 was used minimization was performed setting maximum heavy atom root-mean-square-deviation to 0.30 Å.
2.2 Prepartion of ligand
The structure of 10 selected compounds such as, caesalpinine C (CID: 100949741), vanillylamine (CID: 70966), caesalpinine A (CID: 5458904), sabinene (CID: 18818), terpinen-4-Ol (CID: 11230), dihydrocapsaicin (CID: 107982), dihydrocarveol (CID: 12072), beta-Pinene (CID: 14896), camphene (CID: 6616) and 3-Carene (CID: 26049) were obtained from PubChem database (http://www.pubchem.ncbi.nlm.nih.gov). The ligands were further prepared using LigPrep tool embedded in Maestro 2020, enabling the generation of all possible states at pH 7.0 ± 2.0 using Epik and minimized by force field OPLS2005. Desalting process, tautomer generation was enabled with stereoisomer computation set to retain the chirality of selected compounds.
2.3 Grid generation for the selected receptor
Receptor grids were evaluated for the prepared proteins such that many ligand bind at the predicted active site during docking. Grids were developed in Glide tool and keeping the constant factors of van der Waals scaling factor to 1.00 and charge cut off to 0.25 subjected to OPLS 2005 force field. A cubic box with suitable dimensions centered in the active site of residue was developed for the receptor. Also, boundary box was arranged for molecular docking.
2.4 Glide standard precision (SP) and ligand docking
Standard precision flexible ligand docking was performed using Schrödinger-Maestro v12.5 (Friesner et al., 2004). For ligand atoms, partial charge cutoff and van der Waals scaling factor 0.15 and 0.80 respectively were selected. The constant of torsion number is 2, maximum successive failure is 110 and the maximum minimization steps are about 300. Final score analysis was carried out on energy-minimized states and the results were expressed as Glide score. The ligand with lowest glide score value was considered was recorded
2.5 Drug likeness characters and ADME/toxicity analysis
Drug likeness and ADME/toxicity analysis were performed for the selected ligand. The structure of the selected ligand was screened to predict whether the ligands obey Lipinski’s rule of five. The rules of Golden triangle, GSK, Pfizer were also verified. The physicochemical properties of the selected ligands were predicted and the analysis was performed using ADMETlab 2.0 programme ( https://admetmesh.scbdd.com/service/evaluation). The pharmacodynamics properties such as, Caco-2 permeability, mutagenicity, carcinogenicity, CytochromeP (CYP) inhibitory capability, human intestinal absorption, and blood brain barrier permeability were analyzed.
2.6 PASS prediction
In this study a total of ten naturally available compounds were selected for PASS prediction. These include caesalpinine C, vanillylamine, caesalpinine A, sabinene, Terpinen-4-Ol, dihydrocapsaicin, dihydrocarveol, beta-pinene, camphene and 3-carene. Pass prediction analysis estimates the available biological properties, hyper active probability (Pa) and inactive probability (Pi) values and were assessed using the PASS online program ( http://www.way2drug.com/passonline/services.php).
3 Results
3.1 Physicochemical properties of the ligands
The physiochemical properties of the ligands were described in Table 1. Three dimensional structure of tissue Plasminogen activator (tPA) was depicted in Fig. 1. In this study, Ramachandran plot was developed and the protein model was illustrated. The generated protein model was further validated using SAVES tool and ERRAT score was obtained. The Z-score was obtained and plotted with ProSA serve against NMR structures and X-day diffraction and the result was described in Fig. 2. All the selected ligand molecules docked effectively to the target and the result was described in Fig. 3. Of the 10 selected molecules, caesalpinine C showed the lowest binding energy of −6.36 kcal/mol and the result was tabulated in Table 2. The selected ligand caesalpinine C interacted with 18 residues within the binding pocked of the receptor of tPA (Fig. 3). Interactions of two hydrogen bonds were predicted between the Glu 296 residues and Gln182 residue of the receptor. Hydrogen bond interactions were observed in dihydrocarveol, and dihydrocapsaicin. In the case of dihydrocapsaicin hydrogen bond interactions were observed with Hem5, Asp304, and Arg307. Analysis revealed a pi-cation interaction with Arg30 between the ligand and the receptor for the ligand, dihydrocapsaicin. Dihydrocarveol interacted with protein by hydrogen bond on the Glu296 residue of the protein.
Ligands
Molecular Weight
LogS
LogD
LogP
No. of H Acceptors
No. of H Donors
No. of Rotatable Bonds
TPSA
Heavy Atom Count
Caesalpinine C
405.24
−1.894
2.963
2.321
5
2
4
61.44
30
Vanillylamine
153.08
0.479
0.057
0.063
3
3
2
55.48
11
Caesalpinine A
421.24
−1.785
1.375
1.01
6
3
3
81.67
31
SABINENE
136.13
−3.657
3.483
3.274
0
0
1
0
10
Terpinen-4-Ol
154.14
−2.371
2.017
3.06
1
1
1
20.23
11
Dihydrocapsaicin
307.21
−3.425
3.857
3.937
4
2
11
58.56
22
Dihydrocarveol
154.14
−2.285
2.674
2.579
1
1
1
20.23
11
BETA-PINENE
136.13
−4.389
3.668
3.625
0
0
0
0
10
CAMPHENE
136.13
−4.713
3.701
3.781
0
0
0
0
10
3-Carene
136.13
−4.652
3.762
4.36
0
0
0
0
10
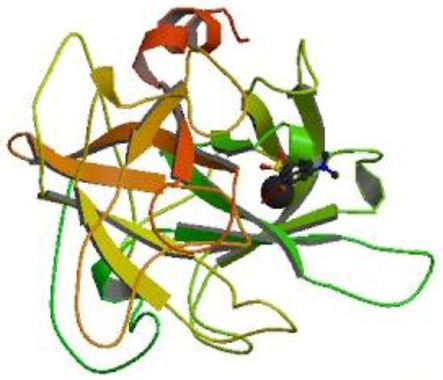
Three dimensional structure of tissue Plasminogen activator (tPA).
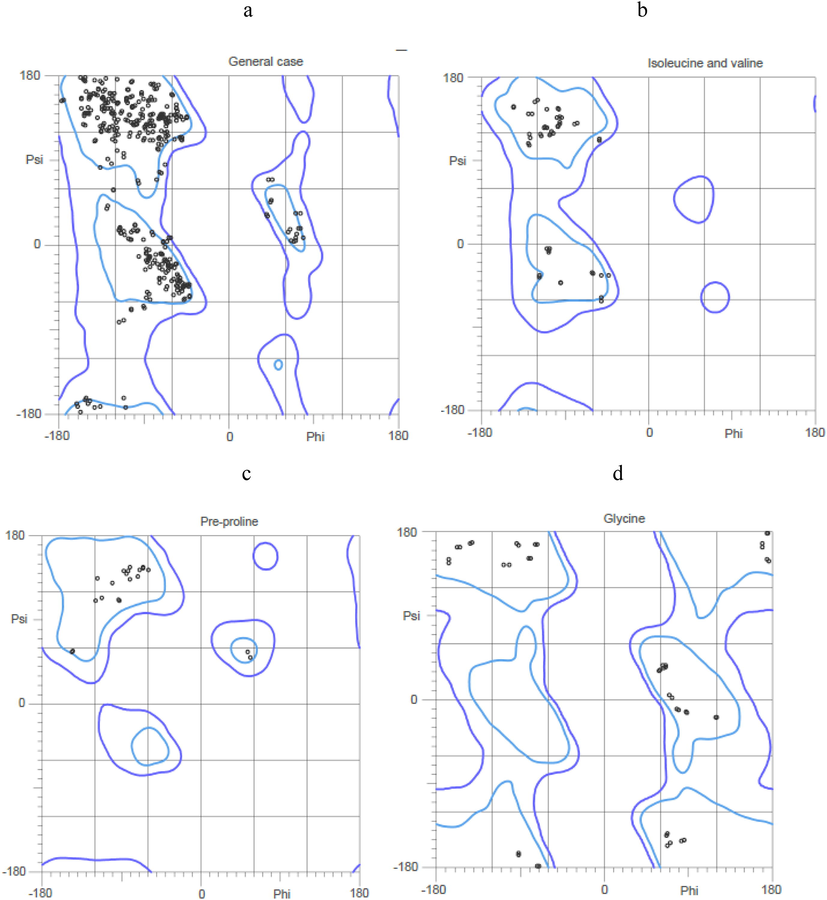
Ramachandran plot for the homology model of the tissue plasminogen activator protein (a-f). Interaction of ligands with protein and the residues involved in the interaction with various binding properties. (A) 3-Carene, (B) Beta-Pinene, (C) Camphene, (D) Caesalpinine A, (E) Caesalpinine C, (F) Terpinen-4-Ol, (G) Dihydrocapsaicin, (H) Sabinene, (I) Vanillylamine, (J) Dihydrocarveol.
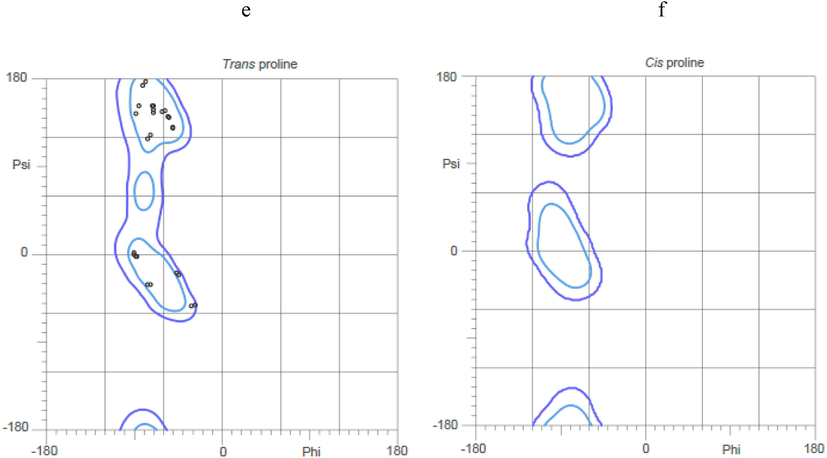
Ramachandran plot for the homology model of the tissue plasminogen activator protein (a-f). Interaction of ligands with protein and the residues involved in the interaction with various binding properties. (A) 3-Carene, (B) Beta-Pinene, (C) Camphene, (D) Caesalpinine A, (E) Caesalpinine C, (F) Terpinen-4-Ol, (G) Dihydrocapsaicin, (H) Sabinene, (I) Vanillylamine, (J) Dihydrocarveol.
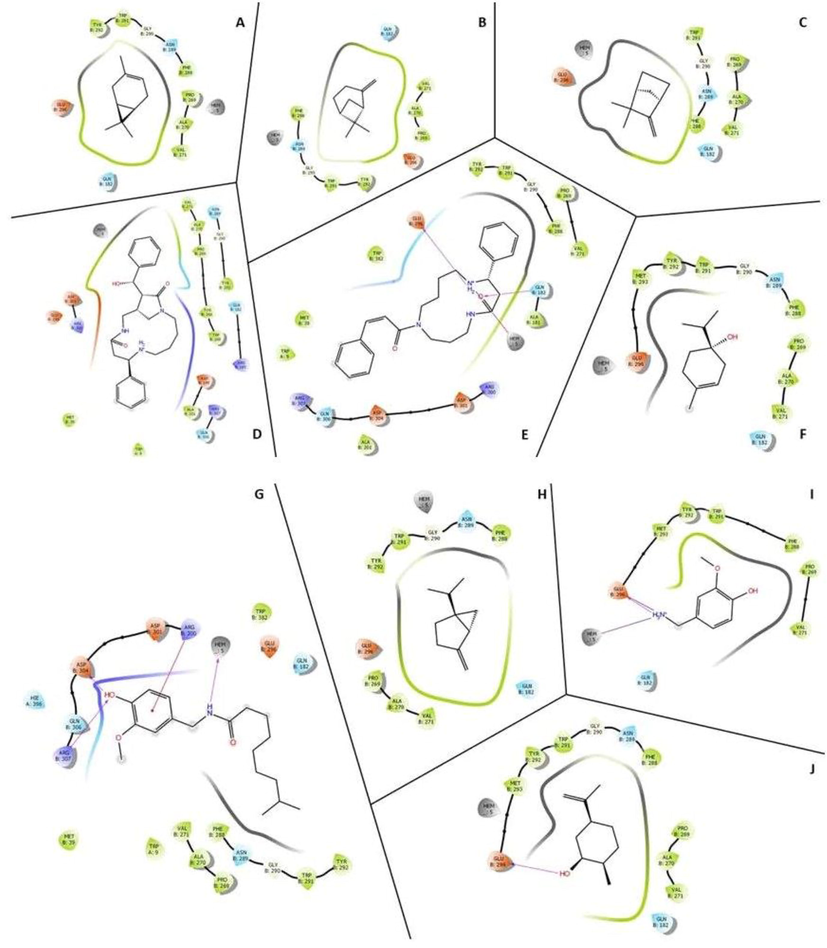
Three dimentional docked structure of caesalpinine C (a) and vanillylamine to tPA active site.
Ligands
Pubchem CID
Molecular Formula
Glide rotatable bonds
Docking score
Glide ligand efficiency
Glide hbond
Glide energy
Glide einternal
Caesalpinine C
100,949,741
C25H31N3O2
4
−6.362
−0.212
−0.435
−49.788
11.416
Vanillylamine
70,966
C8H11NO2
3
−6.211
−0.565
−0.334
−31.277
2.741
Caesalpinine A
5,458,904
C25H31N3O3
4
−5.848
−0.189
0
−56.485
5.629
SABINENE
18,818
C10H16
1
−5.545
−0.554
0
–23.385
5.789
Terpinen-4-Ol
11,230
C10H18O
2
−5.489
−0.499
0
–23.403
5.861
Dihydrocapsaicin
107,982
C18H29NO3
12
−5.448
−0.248
−0.195
−41.759
7.63
Dihydrocarveol
12,072
C10H18O
2
−5.417
−0.492
−0.151
−21.537
6.247
BETA-PINENE
14,896
C10H16
0
−5.398
−0.54
0
−19.424
0
CAMPHENE
6616
C10H16
0
−5.394
−0.539
0
−13.545
0
3-Carene
26,049
C10H16
0
−5.347
−0.535
0
−21.684
0
3.2 Drug likeliness
The drug likeliness is based on the chemical and physical properties of ligand. Lipinski’s rule of five was accepted by all the selected compounds. Pfizer’s rule, and GSK rules were accepted in caesalpinine C. In the case of canillylamine, Lipinski’s Rule, Pfizer Rule and GSK Rule were accepted. The ligands, vanillylamine, and terpinen-4-Ol passed Lipinski’s Rule, Pfizer Rule and GSK Rule. The drug likeliness properties of the all ten selected ligands were accepted in most of the cases and were rejected mostly in Golden Triangle rule. The most favorable drug of choice in the docking analysis was caesalpinine C and passed the rules of Pfizer, Lipinski and Golden triangle but failed to pass GSK’s rule. The least favorable compound was 3-carene and was rejected in all except the basic Lipinski’s rule of five. With the likeliness measured with the QED scoring, caesalpinine C has the highest of 0.768 which is highly desirable (>0.67) whereas 3-carene showed very least QED value (0.449) which is less desirable.
3.3 ADMET analysis
ADME/T test of all ten selected ligand was described in Table S1. Almost all selected ligands except vanillylamine can penetrate the blood brain barrier. Intestinal absorption analysis revealed good absorption by caesalpinine c and a. Caesalpinine c showed P-glycoprotein inhibitory effect and the other legands did not have this effect. The analyzed molecules, caesalpinine C and A penetrated Caco2 cell lines. The selected ligands showed cytochrome P family inhibitory activity and the results were described in the Table S1. Caesalpinine C showed inhibitory activity against CYP3A4 and CYP2D6 enzymes. Of the all ten ligands tested, terpinen-4-Ol was highly susceptible for carcinogenic activity and the probability was about 60%. Vanillylamine showed highest respiratory toxicity and caesalpinine c showed the maximum score in skin sensitivity. Vanillylamine showed maximum half-life and highest clearance than other nine tested ligands. The tested ligand did not show any AME toxicity
3.4 Prediction of biological property
Caesalpinine C, vanillylamine, terpinen-4-Ol, dihydrocapsaicin and 3-carene showed fibrinolytic properties on analysis with PASS server. The predicted fibrinolytic compound vanillylamine showed Pa value of 0.396 and Pi value of 0.158, whereas Terpinen-4-Ol with Pa and Pi values of 0.729. Dihydrocapsaicin with Pa and Pi values 0.675 and 0.033; 3-carene with Pa and Pi values of 0.646 and 0.048 were predicted as fibrinolytic property. Most of the ligands predicted showed antieczematic properties and the results were described in Table S2.
4 Discussion
In silico molecular docking is a computational approach which clearly predicts the bonding between two different particles. This method included, algorithms like molecular stimulation, molecular dynamics and fragment based methods. In recent years, many research works have been focused on the development of antithrombolytic (antiplatelet and anticoagulant) activity however in silico molecular docking of these compounds were limited to develop drugs against cardiovascular diseases such as stroke. Molecular docking studies revealed good docking score, glide energy and glide model. Caesalpinine C and vanillylamine showed maximum docking score. The maximum negative glide energy is highly favourable and the docking score of caesalpinine C showed great compliances (Adams et al., 1991; Banerjee et al., 2004; García-Godoy et al., 2015). Prediction of interaction energies between receptor and ligand are the important task in molecular docking (Babaheydari et al., 2013). Virtual screening uses docking and docking scores of each and every analyzed compound. The method applied is based on predicting the binding affinities and binding modes of selected compound using docking to an X-ray crystallographic structure to the target. Glide-based docking, for example SP-glide based docking is mainly based on binding mode of the selected ligands on the target. The ligands bind on the amino acids in the active loop of the selected protein. To predict and identify the thrombolytic lead molecule from the natural sources for the treatment of stroke, docking analysis was performed on the active site of t-PA. Glide docking studies revealed the significant binding property of the selected compounds on the active site of t-PA. Docking score revealed that Caesalpinine C and vanillylamine affinity on t-PA. The interaction of ligand with protein was described in lines between protein residues and ligand atoms. Plant phytochemicals contain various compounds, including flavonoids, steroids, phenols form complex with various soluble proteins. Glycosides in the plant extract reduced blood pressure and showed cardioprotective property (Gong et al., 2011; Cheng et al., 2003; Okwu, 2001). A number of works have been carried out by various research groups to evaluate the natural food sources and herbs and their food supplements have antiplatelet and anticoagulant properties and there is strong evidence that consuming such phytochemical rich food leads to prevention of stroke and coronary heart diseases (Bazzano et al., 2002). Molecular docking is high efficient method and this method has been used to determine the affinity of drug of choice and the macromolecular target site. The glide scoring method was based on hydrophobic interaction, Van der Waal’s forces and electrostatic attraction forces (Elokely and Doerksen, 2013). The docking stimulation analysis revealed that the selected compound is highly responsible for fibrinolytic mechanism as it has strong interaction on the specific active site of the t-PA that converts circulatory plasminogen to plasmin. The plasmin induced the degradation of fibrin blood clots into fibrin fibril (Parry et al., 2000). Streptokinase has been used to activate t-PA previously and it triggered the activation of t-PA. Molecular modelling study revealed the interaction of lysine side chain with Asp-740 (Castellino, 1979). The skeleton of cardiac glycoside, steroid and flavonol from the medicinal plants interacted with t-PA binding sites which were characterized by molecular docking stimulation analysis. Drug likeness properties of the selected ligands were determined using computational biology tool and this study is an integral part of the discovery of lead molecules. Lipinski’s rule of five is used to determine the chemical characters of the lead molecule which influence membrane permeability and it decides the bioavailability of the lead molecule within the cells (Ursu et al., 2011). Topological polar surface area, polar surface area or molecular weight of the lead molecule influenced the permeability of the lead molecule through the biological membrane within the cell. Lipophilicity revealed the absorption of lead molecule and the LogS, high LogP value is associated with poor adsorption and the low LogP value indicated high absorption potential (Leeson and Springthorpe, 2007; Pollastri, 2010). In this experiment, all selected lead molecules followed Lipinski’s rule of five.
5 Conclusions
The present finding revealed that the compounds caesalpinine C, and vanillylamine could be used as the effective thrombolytic agent. These two compounds are predominant in various medicinal plants and could be formulated as the effective thrombolytic agents to treat cardiovascular diseases including, stroke. These thrombolytic agents from the natural sources have less or no side effects. Caesalpinine C showed good docking score among the tested natural compounds. Further in vivo trial need to test the fibrinolytic effect of these natural compounds and also critically require analyzing the thrombolytic activity.
Acknowledgements
The authors extend their appreciations to the deputyship forResearch & Innovation, Ministry of Education in Saudi Arabia forfunding this research work through the project number (lFP-2020-30).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A synthetic DNA encoding a modified human urokinase resistant to inhibition by serum plasminogen activator inhibitor. J. Biol. Chem.. 1991;266(13):8476-8482.
- [Google Scholar]
- Streptokinase—a clinically useful thrombolytic agent. Biotechnol. Adv.. 2004;22(4):287-307.
- [Google Scholar]
- Fruit and vegetable intake and risk of cardiovascular disease in US adults: the first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am. J. Clin. Nut.. 2002;76(1):93-99.
- [Google Scholar]
- Protein-based virtual screening of chemical databases. 1. Evaluation of different docking/scoring combinations. J. Med. Chem.. 2000;43(25):4759-4767.
- [Google Scholar]
- A unique enzyme—protein substrate modifier reaction: plasmin/streptokinase interaction. Trend. Biochem. Sci.. 1979;4(1):1-5.
- [Google Scholar]
- Inhibitory effects of total flavones of Hippophae Rhamnoides L on thrombosis in mouse femoral artery and in vitro platelet aggregation. Life Sci.. 2003;72(20):2263-2271.
- [Google Scholar]
- Traditional utilization of wild date palm (Phoenix sylvestris) in rural Bangladesh: an approach to sustainable biodiversity management. J. For. Res.. 2008;19(3):245-251.
- [Google Scholar]
- Cytotoxic & thrombolytic activity of methanolic extract of Macaranga denticulata Bark. Phar. Innov.. 2015;4:36.
- [Google Scholar]
- Coronary thrombolysis: streptokinase or recombinant tissue-type plasminogen activator? Ann. Int. Med.. 1990;112(7):529-538.
- [Google Scholar]
- Terminalia arjuna Wight & Arn.—a useful drug for cardiovascular disorders. J. Ethnopharmacol.. 2007;114(2):114-129.
- [Google Scholar]
- In silico pharmacology for drug discovery: applications to targets and beyond. British. J. Pharmacol.. 2007;152(1):21-37.
- [Google Scholar]
- Docking challenge: protein sampling and molecular docking performance. J. Chem. Inform. Model.. 2013;53(8):1934-1945.
- [Google Scholar]
- Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem.. 2004;47:1739-1749.
- [Google Scholar]
- Solving molecular docking problems with multi-objective metaheuristics. Molecules. 2015;20(6):10154-10183.
- [Google Scholar]
- Building a virtual ligand screening pipeline using free software: a survey. Brief Bioinform.. 2016;17(2):352-366.
- [Google Scholar]
- Anti-thrombosis effect of diosgenin extract from Dioscorea zingiberensis CH Wright in vitro and in vivo. Phytomedicine. 2011;18(6):458-463.
- [Google Scholar]
- iGEMDOCK: a graphical environment of enhancing GEMDOCK using pharmacological interactions and post-screening analysis. BMC Bioinformat.. 2011;12(1):1-11.
- [Google Scholar]
- The many roles of computation in drug discovery. Science. 2004;303(5665):1813-1818.
- [Google Scholar]
- The success of natural products in drug discovery. Pharmacol. Pharm.. 2013;04(03):17-31.
- [Google Scholar]
- The influence of drug-like concepts on decision-making in medicinal chemistry. Nat. Rev. Drug Dis.. 2007;6(11):881-890.
- [Google Scholar]
- On the mechanism of fibrin-specific plasminogen activation by staphylokinase. J. Biol. Chem.. 1991;266(18):11826-11832.
- [Google Scholar]
- Recombinant streptokinase: Opportunity for an improved agent. Blood Coagul. Fibrinolysis. 1993;4(6):1039-1040.
- [Google Scholar]
- Virtual screening strategies in drug discovery. Curr. Opin. Chem. Biol.. 2007;11(5):494-502.
- [Google Scholar]
- Contrast media: interactions with other drugs and clinical tests. Eur. Radiol.. 2005;15(7):1463-1468.
- [Google Scholar]
- Sustained reflow in dogs with coronary thrombosis with K2P, a novel mutant of tissue-plasminogen activator. J. Am. Coll. Cardiol.. 1992;20(1):228-235.
- [Google Scholar]
- Evaluation of chemical composition of medicinal plants belonging to Euphorbiaceae. Pak. Vet. J.. 2001;14:160-162.
- [Google Scholar]
- Molecular mechanisms of plasminogen activation: bacterial cofactors provide clues. Trend. Biochem. Sci.. 2000;25(2):53-59.
- [Google Scholar]
- Acoplamiento molecular para actividad trombólitica de algunos compuestos aislados de Clausena lansium. J. Negat. No. Posit. Results JONNPR. 2017;2:115-119.
- [Google Scholar]
- Understanding drug-likeness. Wiley Interdisciplin. Rev. Comput. Mol. Sci. 2011;1(5):760-781.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2021.101732.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







