Translate this page into:
Molecular identification and structural detection of anti-cancer compound from marine Streptomyces akiyoshiensis GRG 6 (KY457710) against MCF-7 breast cancer cells
⁎Corresponding author at: School of Life Sciences, Sun Yat-Sen University, Guangzhou 510275, PR China. liwenjun3@mail.sysu.edu.cn (Wen-Jun Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The current study was concentrated to detection of anti-cancer compound from marine Streptomyces akiyoshiensis GRG 6 (KY457710) against MCF-7 breast cancer cells. The partial purification of TLC was moved three different active spots. Among the spots, the third spot of the extract was shown excellent anti-cancer properties against MCF-7 human breast cancer cells. The available anti-cancer compounds present in the partially purified extract was confirmed by GC–MS data interpretation. Three fractions of the compounds were purified by preparative HPLC and their respective spectrum was confirmed by analytical HPLC. Active anti-cancer fraction was separately purified by preparative HPLC and the cytotoxicity result was shown with 100 µg/mL concentration. The anti-cancer compound of pyrrolo [1,2-a]pyrazine-1,4-dione, hexahydro-3 was detected by LC-MS analysis after interpretation with GC–MS data. Further, the morphological damages and increased proliferation with more death cells of pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 treated MCF-7 were observed by phase contrast and fluorescence microscope techniques. Finally, the nuclear condensation and increased necrotic cells of the pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 treated MCF-7 cells was clearly identified by fluorescence microscope techniques using Hoechst 33,342 stain. Therefore, our result was prove, the pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 rich Streptomyces akiyoshiensis GRG 6 (KY457710) as a potential anti-cancer agent against MCF-7 breast cancer cells.
Keywords
Actinomycetes
Thin layer chromatography
Preparative HPLC
Cytotoxicity assay
Morphological damages
Nuclear damages
1 Introduction
Marine has underexplored environments, which has more chance to harboring microorganisms with potential antibiotic producing ability (Parama Cita et al., 2017). It has unpredictable sources of organic nutrients, which helped to growth of microorganisms for produce new structural and chemical molecules. It has larger biodiversity, mutual relationship and more beneficial microbes with their unique chemical structure. Most advantages are, it exhibited high temperature, high pH, high salt and high nutrients to microbes to produce enormous new compounds production (Yara et al., 2020). Among the microbes, actinomycetes are gram positive, spore forming, GC rich mycelium like filamentous bacteria, and widely distribution in different environmental conditions (Priyanka et al., 2019). They are most documented classes of bacteria from various habitats including terrestrial, mangrove, marine, cave and desert due to the excellent bioactive metabolites production with broad spectrum biological activities (Rajivgandhi et al., 2018a). Approximately, 15, 000 bioactive compounds and 2,000 enzymes against various industrial, biomedical and pharmaceutical applications (Valan Arasu, et al., 2013).
Generally, actinomycetes populations are higher in soli environment compared with any other microorganisms. In particular, it has the ability to produce new secondary metabolites in all kinds of environment with greater geographical diversity. Among the actinomycetes, genus Streptomyces remains unchallenged group without effective competitor, known to synthesize more bioactive compound. Previously, it covered 65% of total identified actinomycetes compound having more anti-microbial, anti-cancer, anti-viral, anti-larvicidal and immunosuppressive properties (Odumosu et al., 2017). In addition, it showed more biological activity frequently from beginning onwards. Notably, the Streptomyces sp. synthesized antibiotics of penicillin, erythromycin, streptomycin and some other antibiotics, which have excellent broad spectrum activity (Govindarajan et al., 2014). Previously, the inhibition of cancer cells by chemical compounds is ineffective due to the resistant development. So, researchers are interested to discover novel antibiotics from new routes that effectively inhibit the cancer cells without causing any side effects. The human breast cancer cell of MCF-7 is most important, which cause severe infection worldwide. Based on these advantages, the current study was concentrated on isolation and identification of marine actinomycetes Streptomyces akiyoshiensis GRG 6 (KY457710) from marine soli and it anti-cancer property was evaluated against MCF-7 breast cancer cells.
2 Materials and methods
2.1 Extraction and identification of actinomycetes extract
After isolation of actinomycete strains form Gulf of Mannar region, Rameshwaram, Tamilnadu, India, the well grown actinomycetes strains were used to extract with various solvents ethyl acetate, alcohol, ethanol, petroleum ether and acetic acid by liquid-liquid extraction experiment (Kandula and Terli, 2013). Then, the extracted crude extract was partially purified by TLC method using DMSO as a solvent. Also, the active mobile phases of chloroform-methanol (10:1,v/v), chloroform-ethyl acetate (10:25.5,v/v), benzene-acetic acid-water (5:1:10, v/v), toluene-chloroform-methanol (10:16:25 v/v), and methanol-dichloromethane-water (2:2:2 v/v) were used to detect the active compounds based on the movement of the compounds by scrapping method. Then the scrapped material was mixed in methanol and analyzed by GC–MS after silica gel removal (Suresh et al., 2020).
2.2 Cytotoxicity assay of crude extract
The 96-well plate was filled by required volumes of medium, MCF-7 breast cancer cells and different concentration of crude extracts at 37 °C. The crude extract plus DMSO were performed for control. Then, MTT solution (50 µL) was added into all the 96-well wiht 4 h at 37 °C. Next, the production of formazan crystals in the treated wells were filled by DMSO and read at 600 nm O.D using micotitre plate after color intensity formation. Then, the cytotoxicity percentages were calculated based on the bellowed formula using control and test culture (Rajivgandhi et al., 2019). The 50% percentage of cell death (IC50) was noted for further experiments,
2.3 Molecular identification of actinomycetes
Actinomyecte pellet was mixed in universal primers of Forward 5-GCCTAAGACAATCGAAGTCCAG-3 and reverse 5-CAAGCCGTGTGCCAAGAAACCGGC-3 and amplified by thermal cycler PCR machine (Al-Ansari et al., 2019). The purified PCR product was used for sequences by automated sequencer in Rajivgandhi Centre for Biotechnology, Tiruvanandapuram, India. The received sequences were compared to with parallel NCBI sequence (www.ncbi.nlm.nih.gov/blast), and then submitted into GeneBank, and accession number was obtained. Then, the multiple sequences were arranged using CLUSTAL W program. The above 1000 replicates of bootstrap resembling was used with confidential metrics and drawn a phylogenetic tree by Niebuhr joining method using MEGA 7.0 software.
2.4 Purification of active cytotoxicity fraction
Based on the cytotoxicity effect, the active strain extract was further purified by preparative HPLC for separate the anti-cancer compounds (Ramachandran et al., 2019). The crude extract was diluted with methanol and purified by preparative HPLC method using acetonitrile:methanol:ammonium acetate:water (35:5:5:20) as a mobile phase. Then, purified extract was analyzed by analytical HPLC, and all the analytical HPLC was performed against MCF-7 breast cancer cells by MTT assay. Based on the highest cytotoxicity, the respective fractions were further purified by preparative HPLC to detect the pure compound identification. Then, the purified extract was analyzed by LC-MS to detect the active compound identification.
2.5 Morphological damage by phase contrast microscope
The morphological differentiation treated and untreated A549 cells morphology was identified by phase contrast microscope (Rajivgandhi et al., 2018b). Six well plate of cover slip containing staled MCF-7 breast cancer cells was treated with IC50 concentration of purified HPLC fraction, and maintained at room temperature one day. Next, the cells were fixed by 4% formaldehyde and allowed to incubate at 10 min. After incubation, the changed morphology of MCF-7 breast cancer cells was compared with untreated control cells by phase contrast microscope examination at 40× magnification.
2.6 Dual fluorescence staining assay
The cover slip of 6-well plate was used to grown the MCF-7 culture and treated with IC50 concentration of purified HPLC fraction at 37 °C for 24. The separated cover slip was washed with 1× PBS and followed by 10 µg/mL AO/EB staining treatment after 10 min. Then, the stained cover slip was maintained at 15 min in dark room condition. Finally, the excess stain was removed by what man No. 1 filter paper and apoptotic differentiation of treated and untreated cells were viewed by fluorescence microscope (Carl Zeiss, Germany) with 40x magnification (Kanipandian et al., 2019).
2.7 Intracellular nuclear damage by Hoechst 33342 staining assay
The IC50 concentration treated MCF-7 culture was grown on cover slip of 6-well plate at room temperature one day. Then, cells were used to centrifuge for receive pellet, and followed by PBS wash. Then, the attached cells of the pellet was stained by Hoechst 33,342 dye and allowed 15 min to bind into the intracellular nucleus. After 15 min, the cells were viewed by fluorescence microscope and result was compared with untreated control images at 40× magnifications (Fluoro Max™-4 Spectrometer, Horiba Scientific, Germany) (Naveen Kumar et al., 2018).
3 Result
3.1 Isolation and identification of actinomycetes
The pure, powdery growth with pale white color, mild powdery colonies was emerged 100 numbers on the starch casein agar surface. In addition, the exhibited colonies were shown with aerial mycelium, separate hyphae growth and spore producing ability. Based on the previous reports, the interpretation was indicated that the emerged colonies were actinomycetes and available in Fig. 1a, b. Marine is a rich nutrient harboring source for survive the actinomycetes effectively. The under explored environmental conditions such as undefined heat, undefined salt, excess nutrient, greater biodiversity nature is additional sources for improving the secondary metabolites in actinomyectes (Ozcengiz et al., 2010). Recently, Valan Arasu et al. (2013) reported that the marine environment has the capacity to activate the genes in actinomycetes, and it synthesized greater bioactive secondary metabolites. The statement was agreed by Ramachandran et al., 2019, marine is an excellent reservoir for produce antibiotics for some infections.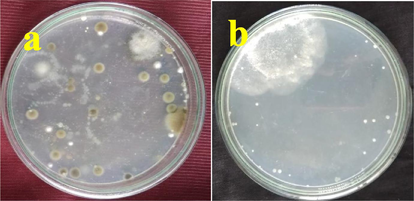
Isolation and identification of actinomycetes from marine soil samples by serial dilution method on starch casein agar.
3.2 Extraction and crude extract preparation
Among the various solvent, our resulted crude extract was given better yield and movement in methanol solution by TLC method. Among the 5 spot of the TLC movement, the spot 2, spot 3 and spot 5 has 0. 40, 0. 22 and 0. 30 mm (Fig. 2a). They were indicated that the active metabolites were present in these moved spots. Based on the previous report, all the moved spots were scrapped and merged together in one tube. Next, the sample was mixed into DMSO and stored at 4 °C for further use. Previously, TLC method of purification was important method to separate the active metabolites initially. It is used to purify the active metabolites partially to perform against various infections.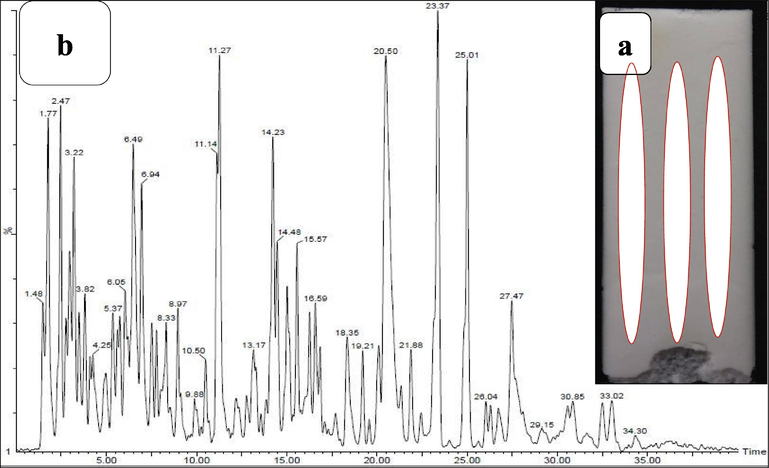
Partial purification of active compounds of actinomycetes strain GRG 6 (a) and GC–MS analysis of available chemical compounds from the partially purified extract (b).
3.3 GC–MS analysis
After careful interpretation with Sun Yat-Sen University Wiley library, we have received 15 bioactive compounds with various biological activities. Based on the interpretation, 5 major anti-cancer compounds were present in the partially purified GRG 6 crude extract. They are 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione, Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3, Phenol, 2,4-bis(1,1-dimethylethyl), BIS(2-Ethylhexyl) Phthalate, 5-Pyrrolidino-2-pyrrolidone. The retention time of 32.45, 30.60, 28.50, 24.20 and 25. 40, occupied area of 646098, 8123456, 723452, 7834251, 7056781 and 65876981, percentages of area 10.04, 16.65, 14.76, 9.08, 12.05 and 10.68 and height percentages of 1.63, 1.90, 09.02, 08.32 and 09.72 were observed respectively. All the results were suggested that the GRG 6 extract has anti-cancer compounds (Fig. 2b). It may influence the stimulation of apoptosis cells highly. Previously, the actinomycetes extract were alter the nucleus of cancer cells due to the necrotic cells (Chen et al., 2018). The result was most accordance with earlier report of Nong et al. (2020), and the anti-cancer properties of actinomycetes were isolated from marine environment. Finally, our result was indicated that the isolated actinomycete strain GRG 6 has anti-cancer properties.
3.4 Cytotoxicity assay
The increased formazan production of partially purified TLC of GRG 6 strain was observed excellent cytotoxicity against MCF-7 breast cancer cells. The excellent proliferation ability was observed against MCF-7 breast cancer cells were viewed in 96-well plate. At the concentration of 200 µg/mL, the partially purified GRG 6 crude extract was shown 52% inhibition when compared with untreated control (Fig. 3). The concentration was very low compared with previous report of actinomycetes crude extract against cancer cells (Davies-Bolorunduro et al., 2019). At 250 µg/mL, the more formazan was produced and culture was shown with confluence colonies. This result was agreed by English et al. (2017), actinomycetes extract has increased cytotoxicity effect against cancer cells. Therefore, our result was suggested that the partially purified GRG 6 extract has anti-cancer properties.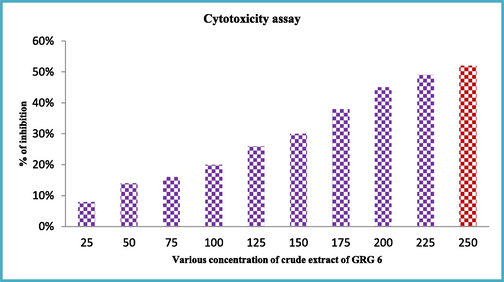
Cytotoxicity effect of thin layer chromatography purified GRG 6 extract against MCF-7 breast cancer cells.
3.5 Molecular characterization of GRG 6 strain
In our result, the similarity of identified sequences was closely associated with Streptomyces genus. The 80% GC content of the GRG 6 has yield of 569 bp with 97.33% similarity to Streptomyces akiyoshiensis GRG 6 by NBlast report. After submission with NCBI, we have received the accession number of KY457710. Finally, the identified GRG 6 strain was confirmed as Streptomyces akiyoshiensis GRG 6 (KY457710). The closely related species and their respective sequences were obtained from NCBI and make a phylogenetic tree by Neighbour-joining method using MEGA 7.0 software (Fig. 4).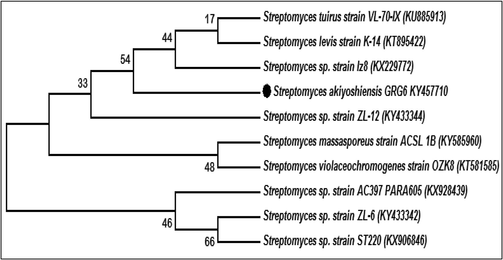
Molecular identification and Phylogenetic tree construction of marine Streptomyces akiyoshiensis GRG 6 (KY457710).
3.6 Purification of active anti-cancer compound by preparative HPLC
Based on the analytical HPLC, the result was shown with three different fractions (Fig. 5a). All the three fractions were performed against MCF-7 breast cancer cells by MTT assay, and result was confirmed that the 2nd and 3rd fractions were shown with anti-cancer activity (Fig. 5c). Among the two, fraction 3 has excellent cytotoxicity effect against tested MCF-7 cells compared with 3rd fraction. Whereas, the fraction 1 was shown no any activity against MCF-was observed. The cytotoxicity result exhibited with 35% and 52% inhibition range at 200 µg/mL and 100 µg/mL of 2nd and 3rd fractions were shown respectively. The result was indicated that the 3rd fraction was very effective against MCF-7 breast cancer cells at very lowest concentration of 150 µg/mL (Fig. 5b). Based on the result, the 3rd fraction was purified separately by preparative HPLC using same mobile phase and analyzed by preparative HPLC for confirmation (Fig. 5b). In result, the single sharp anti-cancer peak was shown in the fig (Fig. 5b), and this fraction concentration was chosen as an IC50 concentration for all other experiments.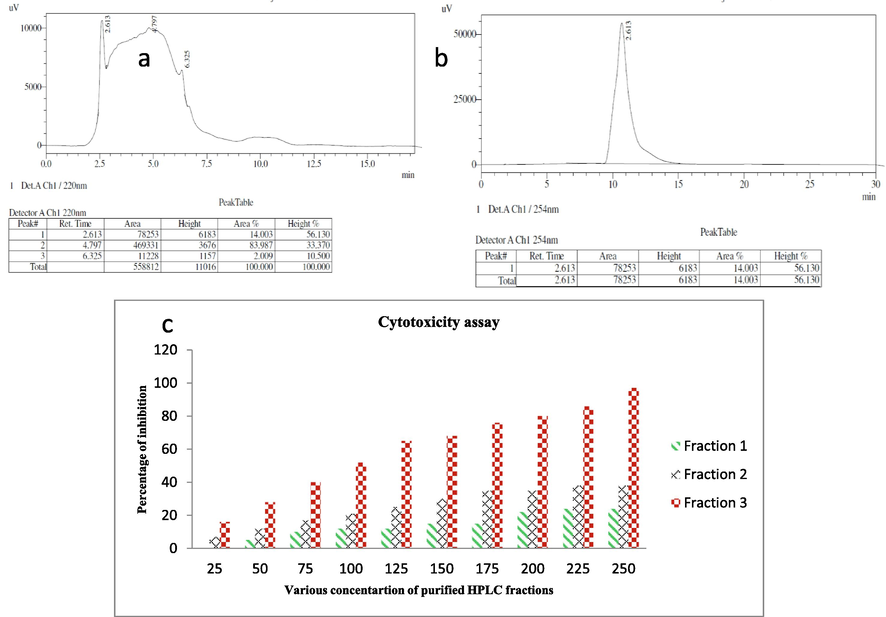
Analytical HPLC fractions of purified Streptomyces akiyoshiensis GRG 6 (KY457710) extract (a), separation of specific anti-cancer compound fraction using preparative HPLC (b) and evaluation of purified anti-cancer compound fractions against MCF-7 cancer cells (c).
3.7 LC-MS analysis
Based on the observation of HPLC peak, the compound pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 was present in the LC-MS peak. The same compound was available in the GC–MS peak, and this compound has excellent anti-cancer properties (English et al., 2017). In the LC-MS peak, the compound pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 was exhibited the molecular ion at m/z = 211 and molecular formula is C11H18N2 and the molecular mass is 210.27. Therefore, the result was suggest, the purified fraction 3 of Streptomyces akiyoshiensis GRG 6 (KY457710) has compound of pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 (Fig. 6). The similar result was reported by Lalitha et al. (2016), and the compound pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 has anti-cancer activity against A549 lung cancer cells. Therefore, after the interpretation of previous published articles, our result was confirmed after the identified compound was pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 and it has more anti-cancer property against MCF-7 breast cancer cells.![LC-MS detection of anti-cancer compounds pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 from purified Streptomyces akiyoshiensis GRG 6 (KY457710) extract by purified HPLC.](/content/185/2020/32/8/img/10.1016_j.jksus.2020.10.008-fig6.png)
LC-MS detection of anti-cancer compounds pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 from purified Streptomyces akiyoshiensis GRG 6 (KY457710) extract by purified HPLC.
3.8 Damaged cancer cell morphology by phase contrast microscope
The IC50 concentration of pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 treated MCF-7 breast cancer cell morphology was damaged when compared with control cells (Fig. 7a, b). The damaged cell membrane was showed with shrink and loosely attached morphology. Whereas, the tightly arranged bundle morphology of untreated cells was observed. In addition, the loosely associated morphology of the cells was indicated that the MCF-7 cells lost their antigenicity and pathogenicity in inside of the life cycle. The supportive evidence of Singh et al. (2019) reported that the actinomycetes mediated pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 has excellent anti-cancer properties against cancer cells and proved by phase contrast microscope images.![Morphological damages of pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 treated MCF-7 breast cancer cells (a, b).](/content/185/2020/32/8/img/10.1016_j.jksus.2020.10.008-fig7.png)
Morphological damages of pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 treated MCF-7 breast cancer cells (a, b).
3.9 Confirmation of morphological damage by AO/EB staining assay
After addition of IC50 concentration, the entire cells were damaged and confirmed by observation of AO/EB staining. The arrangement of the cells was showed with rough colonies in the treated result, whereas the smooth colonies were observed in untreated control cells (Fig. 8b). Here, the fluorescent dyes of AO/EB were played a major role in differentiation of damaged and undamaged membrane morphology. The dye AO has the ability to bind in live cells and emitted green color production, whereas the dye EB has the ability to bind damaged cells due to the intracellular leakages of cytosolic materials and it emitted red color. In our result, the treated cells were exhibited with condensed morphology and the condensation of the cells was showed with yellow to orange color (Fig. 8a). In mechanism, the intracellular damages of pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 will not allow the cell growth from initial G0 stage to G1 stage. The more number of apoptotic cells were formed due to the intracellular damages of cells (Fig. 8a, b). Recent report of Law et al. (2017) also supported to this evidence, and marine actinomycetes mediated pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 has excellent anti-cancer properties at increasing concentration. Therefore, the AO/EB staining assay result was proved that the pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 has the ability to stimulate the functional genes against MCF-7 lung cancer cells.![Live/dead cells variation of pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 treated MCF-7 breast cancer cells (a, b).](/content/185/2020/32/8/img/10.1016_j.jksus.2020.10.008-fig8.png)
Live/dead cells variation of pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 treated MCF-7 breast cancer cells (a, b).
3.10 Nuclear damage by Hoechst 33342 stain assay
After 24 h treatment of IC50 treated cells were showed drastic morphology after addition of 10 µg/mL of Hoechst 33342 stain. The structural arrangement of MCF-7 cells were collapsed due to the interfere of IC50 dose as confirmed by Hoechst 33,342 staining assay. After adding Hoechst 33342 stain in the treated cells, the white color appearance of condensed nucleus was exhibited in the treated cells (Fig. 8c). Whereas, the dark tightly closed nuclear molecules was observed in the untreated control cells (Fig. 8d). The specific role of Hoechst 33342 stain was enter and bind into the A:T rich region of DNA and exhibited decreased blue color to white color morphology was observed. After complete attachment, the cells were exhibited with bright white color morphology in condensed nucleus places. It revealed that the cells were changed to severe apoptosis and late apoptosis colonies. On the contrary, mild blue color of the untreated cells suggested that the cells continuously viable. The result clearly confirms that the pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 possess enhanced cell membrane disruption ability in MCF-7 cancer cells.
4 Conclusion
The molecular identification of Streptomyces akiyoshiensis GRG 6 (KY457710) was isolated from marine soil sample and confirmed NCBI submission. The partial purification of Streptomyces akiyoshiensis GRG 6 (KY457710) crude extract was shown with better cytotoxicity assay. Based on the GC–MS and LC-MS result, the anti-cancer compound of pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 was identified. Interestingly, the anti-cancer compound has excellent anti-cancer activity against MCF-7 human breast cancer cells at the concentration of 250 µg/mL. The morphological damages and intracellular damages of ROS and Hoechst 33,342 stain results were confirmed the anti-cancer potential of pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3. Finally, we have concluded that the marine Streptomyces akiyoshiensis GRG 6 (KY457710) compound pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 has more anti-cancer property against MCF-7 human breast cancer cells.
Acknowledgments
All the authors gratefully acknowledge the National Natural Science Foundation of China (Project Approval Numbers: 41950410573, 91951205, 31670009 and 31850410475) and Postdoctoral Science Foundation of China (Project Approval Number: 2019M663213) for financial support for this work. The authors extend their appreciation to the Researchers Supporting Project number (RSP-2020/70), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antimicrobial potential of Streptomyces sp. to the Gram positive and Gram negative pathogens. J. Infect. Public Health. 2019;12:861-866.
- [Google Scholar]
- Streptopyrazinones A−D, rare metabolites from marine-derived Streptomyces sp. ZZ446. Tetrahedron. 2018;74:2100-2106.
- [Google Scholar]
- Anticancer potential of metabolic compounds from marine actinomycetes isolated from Lagos Lagoon sediment. J. Pharm. Anal.. 2019;9:201-208.
- [Google Scholar]
- Evaluation of fermentation conditions triggering increased antibacterial activity from a near-shore marine intertidal environment-associated Streptomyces species. Synythesis Systematic Biotechnol.. 2017;2:28-38.
- [Google Scholar]
- Antimicrobial potential of phylogenetically unique actinomycete, Streptomyces sp. JRG-04 from marine origin. Biology. 2014;42:305-311.
- [Google Scholar]
- Production, purification and characterization of an antimicrobial compound from marine Streptomyces coeruleorubidus BTSS-301. J. Pharm. Res.. 2013;7:397-403.
- [Google Scholar]
- Induction of intrinsic apoptotic signaling pathway in A549 lung cancer cells using silver nanoparticles from Gossypium hirsutum and evaluation of in vivo toxicity. Biotechnol. Rep,. 2019;23:e00339
- [Google Scholar]
- Anticancer potential of pyrrole (1, 2, a) pyrazine 1, 4, dione, hexahydro 3-(2-methyl propyl) (PPDHMP) extracted from a new marine bacterium, Staphylococcus sp. strain MB30. Apoptosis. 2016;21:566-577.
- [Google Scholar]
- Streptomyces colonosanans sp. nov., a novel actinobacterium isolated from malaysia mangrove soil exhibiting antioxidative activity and cytotoxic potential against human colon cancer cell lines. Front. Microbiol.. 2017;8:877.
- [Google Scholar]
- Naveen Kumar, S., G. Rajivgandhi N. Manoharan, 2108. Cytotoxicity effect of marine Sponge Alkaloid, Fascaplysin on HepG2 Hepatocellular carcinoma cell. Frontiers in Labaratory Medicine, 2, 41-48.
- Ansamycin derivatives from the marine-derived Streptomyces sp. SCSGAA 0027 and their cytotoxic and antiviral activities. Bioorg. Med. Chem. Lett.. 2020;30:127168
- [Google Scholar]
- Antimicrobial activities of the Streptomyces ceolicolor strain AOB KF977550 isolated from a tropical estuary. J. Taibah Univ. Sci.. 2017;11:836-841.
- [Google Scholar]
- Homologous expression of aspartokinase (ask) gene in Streptomyces clavuligerus and its hom-deleted mutant: effects on cephamycin C production. Bioengineered Bugs. 2010;3:191-197.
- [Google Scholar]
- Parama Cita, Y., A. Suhermanto, O., Karna Radjasa and P. Sudharmono, 2017. Antibacterial activity of marine bacteria isolated from sponge Xestospongia testudinaria from Sorong, Papua, Asian Pacific Journal Tropical Biomedicine, 7 (5), 450–454.
- Marine, Characterisation and identification of antibacterial compound frommarine actinobacteria: in vitro and in silico analysis. J. Infect. Public Health. 2019;12:83-89.
- [Google Scholar]
- Graphene/nickel oxide nanocomposite against isolated ESBL producing bacteria and A549 cancer cells. Mater. Sci. Eng., C. 2019;102:829-843.
- [Google Scholar]
- Antibiofilm effect of Nocardiopsis sp. GRG 1 (KT235640) compound against biofilm forming Gram negative bacteria on UTIs. Microb. Pathog.. 2018;118:190-198.
- [Google Scholar]
- Antibacterial and anticancer potential of marine endophytic actinomycetes Streptomyces coeruleorubidus GRG 4 (KY457708) compound against colistin resistant uropathogens and A549 lung cancer cells. Microb. Pathog.. 2018;125:325-335.
- [Google Scholar]
- Extraction and partial purification of secondary metabolites from endophytic actinomycetes of marine green algae Caulerpa racemosa against multi drug resistant uropathogens. Biocatal. Agric. Biotechnol.. 2019;17:750757
- [Google Scholar]
- 3-Benzyl-Hexahydropyrrolo[1,2-a]Pyrazine-1,4-dione extracted from exiguobacterium indicum showed anti-biofilm activity against pseudomonas aeruginosa by attenuating quorum sensing. Front. Microbiol.. 2019;10:1269.
- [Google Scholar]
- Isolation and molecular characterization of novel Streptomyces sp. ACT2 from marine mangrove sediments with antidermatophytic potentials. J. King Saud Univ. - Sci.. 2020;32:1902-1909.
- [Google Scholar]
- Antibacterial and antifungal activities of polyketide metabolite from marine Streptomyces sp. AP-123 and its cytotoxic effect. Chemosphere. 2013;90:479-487.
- [Google Scholar]
- Actinomycetes from the Red Sea Sponge Coscinoderma mathewsi: isolation, diversity, and potential for bioactive compounds discovery. Microorganisms. 2020;8:783.
- [Google Scholar]







