Translate this page into:
Molecular docking and in vivo immunomodulatory activity of Albizia procera bark on doxorubicin induced immunosuppressive rats
⁎Corresponding author at: Dept of Pharmacology, Santhiram College of Pharmacy, Nandyal, Andhra Pradesh, India. praveenpharmaco@gmail.com (Praveen Kumar Pasala),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
To study the immunomodulatory potential of Albizia procera (AP) bark using in vivo models and by in silico approach.
Methods
In silico models involved to study binding affinity of AP bioactive molecules on immune modified proteins such as Human NF-kappa B p52 (NFkB P52), human tumor necrosis factor-alpha (TNF-α). In vivo studies to evaluated immunomodulatory activity of ethanolic extract AP bark (EEAP) Doxorubicin (DOX) induced immunosuppressive rats.
Results
Docking results showed AP bioactive molecules 3-O-[α-L-arabinopyranosyl-(1 2)-β- → D fucopyranosyl - (16) - 2 - acetamido - 2 - deoxy- β - → Dglucopyranosyl] echinocystic acid (Compound 1), 3-O-[α-L-arabinopyranosyl-(12)-β- → D fucopyranosyl - (16) - 2 - acetamido - 2 - deoxy- β - → Dglucopyranosyl] acacic acid lactone (Compound 2), Catechin, Quercetin, Isoquercetin were showed immune modulatory activity due to high binding affinity and H bonding interaction with active sites of NFkB P52, TNF-α, without H bonding on anti-inflammatory cytokines IL 10. Based on docking Compound 1, Compound 2, Catechin, Quercetin, Isoquercetin were concluded as immunomodulatory potential candidate. EEAP exhibited a dose related incline in cell count of total leukocyte, neutrophils, and lymphocytes. The suppressive outcome of DOX on these cells was not reflected in EEAP treated rats. It enhanced the rate of clearance of the carbon particles in dose dependent manner from the blood circulation in both normal rats and in the immunosuppressive rats. Delayed type of hypersensitivity test (DTH) results showed an increase in footpad thickness of paw significantly in response to antigen, as an impact of EEAP treatment stimulatory response is observed on lymphocytes along with other essential cells of reaction and thus increased the cell mediated immunity.
Conclusion
AP improves the immune function in DOX induced immunosuppressive rats.
Keywords
In silico
Doxorubicin
Immunomodulatory
Cell mediated immunity
Albizia procera bark
Ethanolic extract
1 Introduction
Protection mechanism that is active in defending the host from destructive foreign microorganisms is the vertebrate immune system, which has very essential surveillance control to track the integrity of host tissues (Delves et al., 2017). The characteristic feature of immunomodulator to regulate diseases is to elicit the excitation or suppression of immune responses. Literature suggests that several conventional medicines play a pivotal role in increase and decrease of intensity of hosts immune response. Natural substances extracted from plants may act as immunomodulators to regulate some immune mediated responses (Saravanan et al., 2012). AP is a Fabaceae tree known as white siris, safed siris, and forest siris (Yoshikawa et al., 1998). It is abundantly available in India, Myanmar, New Guinea, northern Australia (Khatoon et al., 2014). AP areal plant extracts showed antioxidant (Sivakrishnan and Kottaimuthu, 2014). AP leaves extract reported Analgesic, Antibacterial, and CNS Depressant Activities (Khatoon et al., 2014) antidiarrheal activity (Hossan et al., 2018), bark ethanolic extract showed anti-HIV (Panthong et al., 2015) anti hyperglycemic activity (Anand et al., 2018; Praveen Kumar et al., 2014), Spermicidal activity (Shaik et al., 2017), anti-inflammatory (Sangeetha et al., 2020) effects were proved on rodent and cellular models. Carbohydrates, saponins, flavonoids, steroids, alkaloids, glycosides, terpenoids, and proteins were reported in methanolic extracts of AP bark and leaves (Srivastava et al., 2020) Protocatechuic acid, Julibroside, Quercetin, Budmunchiamines-A, Machaerinic acid, Isoquercetin, Catechin (Kokila et al., 2013) isolated from Albizia species, Triterpene glycosides (Miyase et al., 2010; Zhang et al., 2018) Triterpenoid saponins with N-acetyl sugar (Melek et al., 2007) (+)-catechin protocatechuic acid (Panthong et al., 2015) were isolated from the bark, total phenolic contents investigated in AP leaves (khatoon et al., 2014), pentacyclic triterpenic acid (Duke, 1983) Saponins and sapogenins (Chandel and Rastogi, 1980) was extracted from the seed, α-Spinasterol and oleanolic acid extracted from roots (Rastogi and Mehrotra, 1993) Four Acylated Triterpenoid Saponins from AP (Yoshikawa et al., 1998). Sapogenins, a non-saccharide portion isolated from seed and root of AP (Shaik et al., 2017). Previous reports showed that AP is having potent antioxidant activity and even phytochemistry of these plants reveals presence of saponins which are having immunomodulating potential hence the present research focused to elicit the immunomodulator intensity of the AP and molecular docking studies were involved to estimate the binding affinity on immunomodulatory proteins.
2 Materials and methods
Drugs: DOX, Camlin black ink and Alsevier’s solution, carbon ink suspension, Leishman reagent, WBC diluting fluid, all chemicals used were analytical grade. Software: BIOVIA Discovery studio2017 R2 tool. CASTp, PyRx tool.
2.1 In silico analysis
2.1.1 Binding score evaluation using PyRx tool
The binding affinity score of AP bioactive molecules on immunomodulatory receptors such as, human NF-kappa B p52 (PDB: 1A3Q), tumor necrosis factor (TNF-alpha) (PDB: 2AZ5), Viral Interleukin 10 (PDB: 1VLK) (Maurya et al., 2012) using PyRx tools. 3D chemical structures (Rudrapal et al., 2021b) of bioactive molecules such as Budmunchiamine, Catechin, Protocatechuic acid, Quercetin, Isoquercetin, Machaerinic acid, were retrieved from Pub Chem and 3-O-[α-L-arabinopyranosyl-(12)-β- → D fucopyranosyl - (16) - 2 - acetamido - 2 - deoxy- β - → Dglucopyranosyl] echinocystic acid (Compound 1), 3-O-[α-L-arabinopyranosyl-(12)-β- → D fucopyranosyl - (16) - 2 - acetamido - 2 - deoxy- β - → Dglucopyranosyl] acacic acid lactone (Compound 2) were draw using Chem Draw tool (Figs. 1 and 2). PyRx software using load molecule and import tools bars, then minimized protein, ligand converted to PDBQT (Othman et al., 2021), then maximized GRID parameter (Rudrapal et al., 2021a) then performed docking study (Trott and Olson, 2009). Protein and ligand complex and distance (Rudrapal et al., 2022) visualized using BIOVIA Discovery studio 2017 R2 tool (Kumar et al., 2021). Levamisole used as standard immunomodulatory agents and preformed compared analysis. Computed Atlas of Surface Topography of Protein (CAST p) tool was used to identified binding sites (Junejo et al., 2021) of the proteins (Binkowski, 2003) (Fig. 3).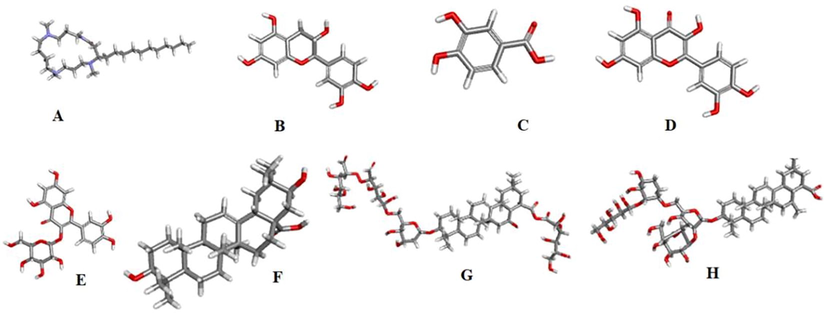
3D structure of A. Budmunchiamine B. Catechin C. Protocatechuic acid D. Quercetin E. Isoquercetin F. Machaerinic acid G. Compound 1 H. Compound 2.
3D Visualization of a) interleukin 10 b) TNF alfa c) NF-kappaB p52.
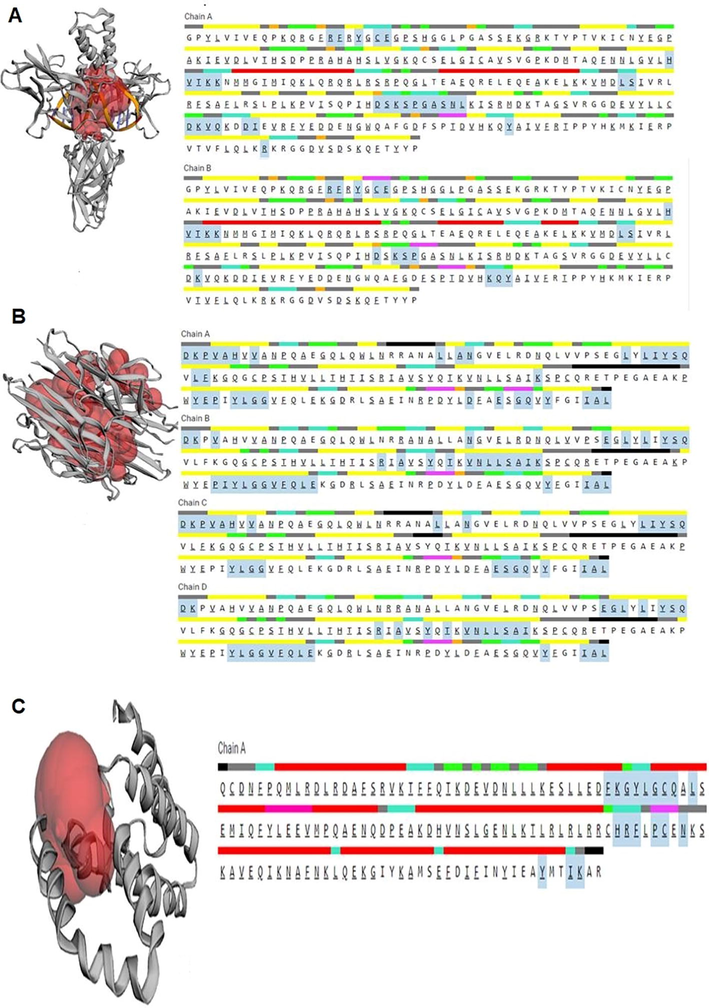
Binding pocket (red color) obtained from CASTp tool along with the sequence which shows the highlighted residues forming the binding pocket. A) IL 10 B) TNF Alfa c) NF-kappa B p52.
2.2 Preparation of extract
AP bark was obtained from forest department in Tirupati, Andhra Pradesh (voucher specimen (APB/ singh-2014-15). Dried bark was converted into coarse particles by a mechanical grinder and passed to the sieve with a mesh size of 40.
Soxhlet ethanol extraction procedure was used for ethanolic extraction of AP bark (Redfern et al., 2014) and resulting extracts were concentrated with the aid of rotary evaporator and dried.
2.3 Animals
The complete study of these experiments on animals were approved and supervised by ethical committee (CPCSEA) vides approved number Regd No. 439/01/a/CPCSEA. Animals were maintained as per CPCESA guidelines (CPCSEA, 2003).
2.4 Acute toxicity studies
Acute oral toxicity studies extract were performed according to the OECD guidelines (OECD, 2002). Rats were safe up to 2000 mg/kg Bw.t.
2.5 Immunomodulatory activity of EEAP
Wistar albino rats of both gender were equally divided into 6 groups, with 6 in each (Nugroho et al., 2012). Group-I: Normal control. Group-II: EEAP (200 mg/kg B.wt.) Group-III: EEAP (400 mg/kg B.wt.) Group-IV: DOX (4.67 mg/kg B.wt. i.p on 1st and 4th day). Group-V: EEAP (200 mg/kg B.wt.) + DOX (4.67 mg/kg B.wt. i.p on 1st and 4th day) Group-VI: EEAP (400 mg/kg B.wt.) + DOX (4.67 mg/kg B.wt. i.p on 1st and 4th day). The rats were treated with respect to the above group treatment daily for 14 days. On the 15th day, total leucocyte counts (TLC), differential leucocyte count (DLC), Neutrophil adhesion (Tripathi et al., 2012), T-Cell population (Patel and Asdaq, 2010), phagocytic activity (Lubega, 2013), delayed type hypersensitivity (Okoli et al., 2008), humoral immune response were determined (Pravansha et al., 2012).
3 Results
3.1 TLC and DLC count
The count of WBC (p < 0.0001; 45.2%), neutrophils (p < 0.0001; 59%), basophils (p < 0.01; 21%) monocytes (p < 0.001, 25%), lymphocytes (p < 0.0001; 42.8%) significantly decreased in DOX treated rats while EEAP treated rats WBC (p < 0.0001), neutrophils (p < 0.0001), lymphocytes (p < 0.0001) were significantly increased in DOX induced immune suppressed rats (Table 3).
3.2 Neutrophil adhesion
Neutrophil adhesion prominent decrease (p < 0.01**) in DOX treated rats while EEAP 200 and 400 mg/kg treatment showed an significant incline in B.wt by 33.50%, 44.15% in neutrophil adhesion in DOX induced immune suppressed rats (Table 3; Fig. 7A).
3.3 Lymphocytes and lymphocyte rosette.
EEAP 200 and 400 mg/kg B.wt treated rats significantly decreased % rosette formation 15.58%, 26.55% respectively, lymphocytes proliferation 14.26%, 41.59% respectively, in in DOX induced immune suppressed rats (Table 3; Fig. 7B and C).
3.4 Delayed type hypersensitivity
DOX treated rats foot pad thickness was significantly (p < 0.001***) decreased compared to normal rats. Foot pad thickness was significantly (p < 0.001***) restored by the EEAP treatment in DOX induced immune suppressed rats (Table 3; Fig. 7D).
3.5 Hemagglutination antibody titer
DOX treated rats showed significantly decreased (p < 0.001***) Hemagglutination antibody titer compared to normal rats. However, EEAP treatment regain in antibody titer values significantly increased (p < 0.001***) in DOX treated rats and normal rats (Table 3; Fig. 7E).
3.6 Phagocytic index
DOX treated rats phagocytic index significantly decreased (p < 0.001***) compared to untreated rats. However phagocytic index was significantly (p < 0.001***) reversed with EEAP of 200 and 400 mg/kg B. wt respectively, treatment in DOX rats (Table 3; Fig. 7F).
4 Discussion
Immunomodulatory agents are mainly for the continuous improvement of immunity through immune activation for a long period of time to prevent various diseases. Herbal plants and their derivatives were reported to exhibit the immunomodulation in recent years (Wagner, 1990).
It plays a pivotal role in the generation of inflammatory mediators and regulation of innate and adaptive immune responses mainly increase transcription and expression of pro-inflammatory cytokines mediated by NF-κB p65, TNF-α (Wang et al., 2021) and IL-10 (Abd-Elhakim et al., 2020; El-Gendy et al., 2020). Hence Molecular docking studies were involved between bioactive molecule of AP and NF-κB p65, TNF-α and IL-10. Molecular docking studies results showed that the interaction analysis depicted that reactive groups of Compound 1 form of hydrogen bonds with active sites of ARG A: 52, SER A: 226, LYS A: 221, LYS A: 252, TYR A: 285, SER B:227 in Human NF-kappa B p52 with binding score −7.1 K/Cal, Catechin form H Bonding with active sites of ARG D: 82, GLN D:125, LEU B: 93, with binding score −11.5 K/Cal, Quercetin form H Bonding with GLN B: 125, with binding score −6.7 K/Cal, Isoquercetin showed H Bonding with active sites of TYR C: 151, SER D: 60 with binding score −8.3 K/Cal, compound 1 form H Bonding with active sites of GLN B: 125,LEU B: 126, SER C: 60, GLN C: 61, with binding score −8.2 K/Cal, compound 2 showed H Bonding with actives sites of TYR B:119, PRO B: 117, GLY B:121, GLU D: 53, LYS D:1 with binding score −8.3 K/Cal of TNF α. AP phytochemicals were not form H Bonding on active sites of IL 10, it indicates phytochemicals showed inhibitory effect on inflammatory proteins only (Tables 1, 2, Figs. 4–6). Docking results were compared to standard immunomodulatory agent Levamisole. ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05, ns = not significant ‘*’ represents the comparison of groups with control group I, ‘#’ represents the comparison of groups with negative control group IV.
Sl. No.
Name of the compound
Binding affinity (-K/Cal)
IL 10
TNF α
NF-kappa B p52
1
Budmunchiamine
−7
−7.3
−4.9
2
Catechin
−9.1
−11.5
−9.7
3
Protocatechuic acid
−4.7
−5.6
−5.7
4
Quercetin
−7.2
−7.7
−6.7
5
Isoquercetin
−7.4
−8.3
−7.8
6
Machaerinic acid
−8.9
−9.7
−7.6
7
Compound 1
−7.3
−8.2
−7.1
8
Compound 2
−8.9
−8.3
−7.5
9
levamisole
−5.9
−4.5
−5.0
Sl. No.
Protein name
Interaction with IL 10
Interaction with TNF α
Interaction with NF-kappa B p52
1
Budmunchiamine
Pi Alkyl- LEU A: 26, LEU A: 23, LEU A: 105, LEU A: 101, PHE A: 15
Pi Alkyl -TYR C: 119, TYR C: 59
Pi Alkyl- LYS A: 221, 252, GLY A: 50
2
Catechin
H Bonding-LYS A: 40, LYS A: 34, THR A: 39; Pi-Pi PHE A:30, PHE A: 37
H Bonding- ARG D: 82, GLN D:125, LEU B: 93; Van der Waals GLN B: 125
H -Bonding - LEU B: 117, GLN B: 157; P- Alkyl ALA B: 104, ILE B: 119, ARG B: 160
3
Protocatechuic acid
H Bonding-ASN A: 97, HIS A: 90, ALA A: 29; Pi Alkyl ARG A: 32
Van der Waals ASN B: 92, Pi Alkyl-LEU B: 93
H -Bonding - LEU B: 117, LYS B: 153; Pi Alkyl ALA B: 104, ARG B: 103
4
Quercetin
H Bonding-LYS A: 40, HIS A: 90, ALA A:29; Pi Alkyl- ARG A: 32
H Bonding- GLN B: 125, ARG D: 82; Pi Alkyl- AAL B: 91, VAL D: 91
H Bonding- ARG A: 193, SER A: 195, ALA A:104; Pi Alkyl- LYS A: 153, ARG A:103
5
Isoquercetin
H Bonding LEU A: 48, Pi Pi PHE A 37, 30; Vander Waals-LEU A:47
H Bonding- TYR C: 151, SER D: 60, Pi-Pi-TYR C: 119, TYR D:119
H Bonding- ARG A: 193, SER A: 195, ARG A: 103, Van der Waals - LYS A: 153, SER A: 195, SER A: 161, ARG A: 156, ARG A: 160
6
Machaerinic acid
H Bonding- PHE A:15; Pi Pi PHE A: 111, PHE A: 56: LEU A: 105, 101, 69, 98,52,26,65,23, TYR AA: 72, ASP A:13
Pi Alkyl -TYR C: 119, TYR D: 119, LEU C: 57; Pi Alkyl-TYR D:59, LEU D: 57
Pi Alkyl- LYS A: 143, HIS A: 140, LEU A 187
7
Compound 1
Pi Alky-PHE A:56, LEU A: 65: ILE A: 69; H Bonding- PHE A: 15; THR A: 39, LYS A: 40, PHR A: 72
H Bonding- GLN B: 125,LEU B: 126, SER C: 60, GLN C: 61; Pi Alkyl- TYR D: 59, TYR C: 119, TYR D: 119, LEU D:57
H Bonding- ARG A: 52, SER A: 226, LYS A: 221, LYS A: 252, TYR A: 285, LYS B: 283, SER A: 231, ARG A: 232, THR B:278, ARG B:290, SER B:227, Pi Alkyl- LYS A: 229, HIS B: 282
8
Compound 2
H Bonding- ARG A: 72, TYR A: 72:Pi Alkyl- PHE A: 30; LEU A: 23,26,69,65,101,105
H Bonding -TYR B:119, PRO B: 117, GLY B:121, GLU D: 53, LYS D:11, Pi Alkyl-TYR A: 119, LEU D: 55, TYR B:151,ILE B: 118: TYR B:59
H Bonding- SER A: 206, ARG A: 160, ARG A: 103, ASP A:94,Pi Alkyl - LYS A: 210, PRO A: 208, PRO A: 211, ILE A: 119
Group
WBC
Neutrophils
Basophils
Eosinophils
Monocytes
Lymphocytes
I
12.27 ± 0.31
2.72 ± 0.10
0.32 ± 0.02
0.18 ± 0.03
0.33 ± 0.04
8.70 ± 0.28
II
ns13.14 ± 0.49
****3.76 ± 0.29
ns0.21 ± 0.02
ns0.23 ± 0.03
ns0.28 ± 0.05
ns9.66 ± 0.4
III
****20.55 ± 0.42
(67.48%)
****4.41 ± 0.14
(38.3%)
ns 0.37 ± 0.06
* 0.34 ± 0.06
* 0.47 ± 0.03
**** 15.66 ± 0.41
(44.4%)
IV
****6.72 ± 0.09 (45.2%)
****1.37 ± 0.03 (49.6%)
** 0.11 ± 0.02 (21%)
ns 0.15 ± 0.01
***0.08 ± 0.01 (25%)
****4.97 ± 0.12 (42.8%)
V
**** 8.46 ± 0.17
* 1.73 ± 0.07
ns 0.13 ± 0.03
ns 0.19 ± 0.01
ns 0.11 ± 0.01
**** 6.26 ± 0.09
(20.6%)
VI
**** 10.48 ± 0.21
**** 2.20 ± 0.11 (37 %)
ns 0.17 ± 0.04
* 0.24 ± 0.02
ns 0.13 ± 0.01 (5%)
**** 7.63 ± 0.16 (34.8%)
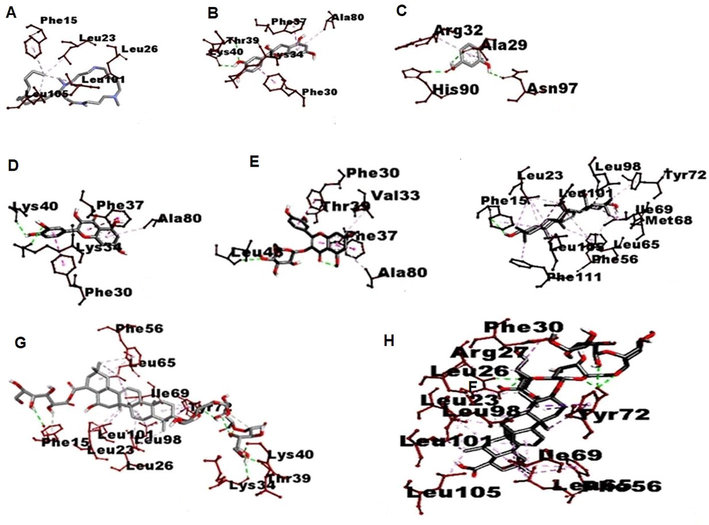
3D visualization of type of Interaction on interleukin 10. A. Budmunchiamine B. Catechin C. Protocatechuic acid D. Quercetin E. Isoquercetin F. Machaerinic acid G. Compound 1 H. Compound 2 I. Levamisole.
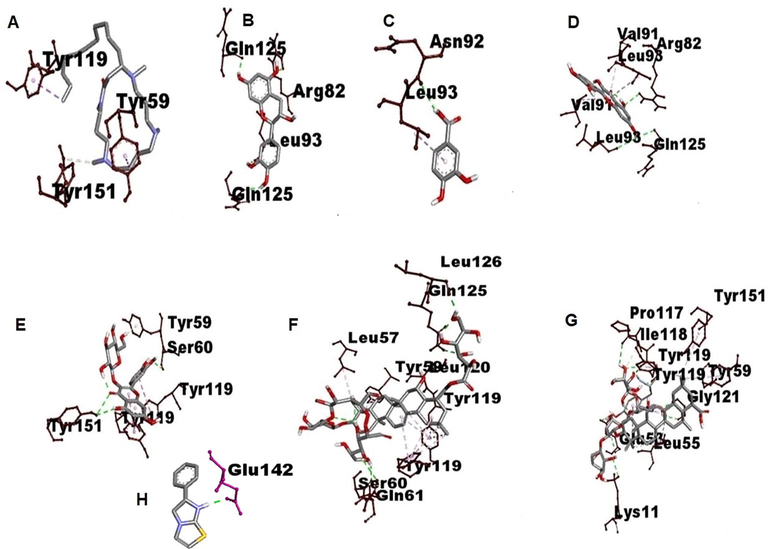
3D visualization of type of Interaction on TNF alfa. A. Budmunchiamine B. Catechin C. Protocatechuic acid D. Quercetin E. Isoquercetin F. Machaerinic acid G. Compound 1 H. Compound 2.
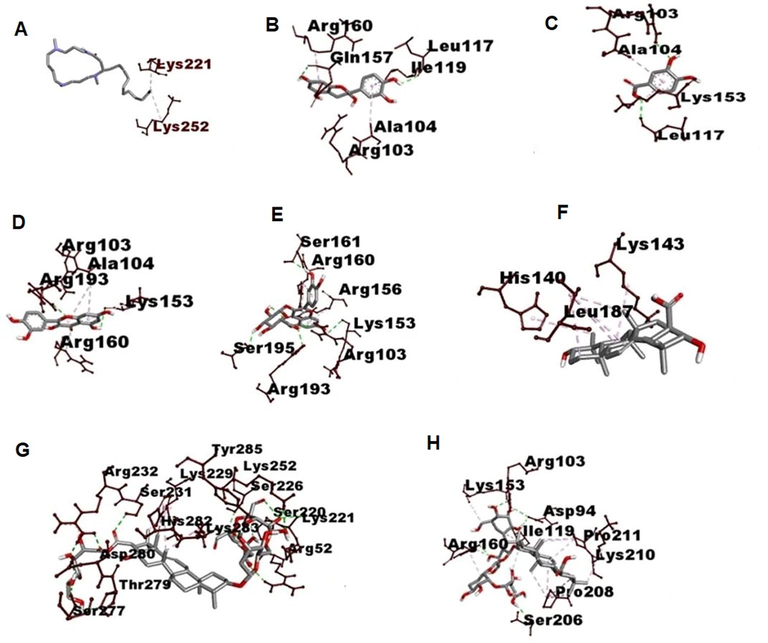
3D visualization of type of Interaction on NfKB. A. Budmunchiamine B. Catechin C. Protocatechuic acid D. Quercetin E. Isoquercetin F. Machaerinic acid G. Compound 1 H. Compound 2 I. levamisole.
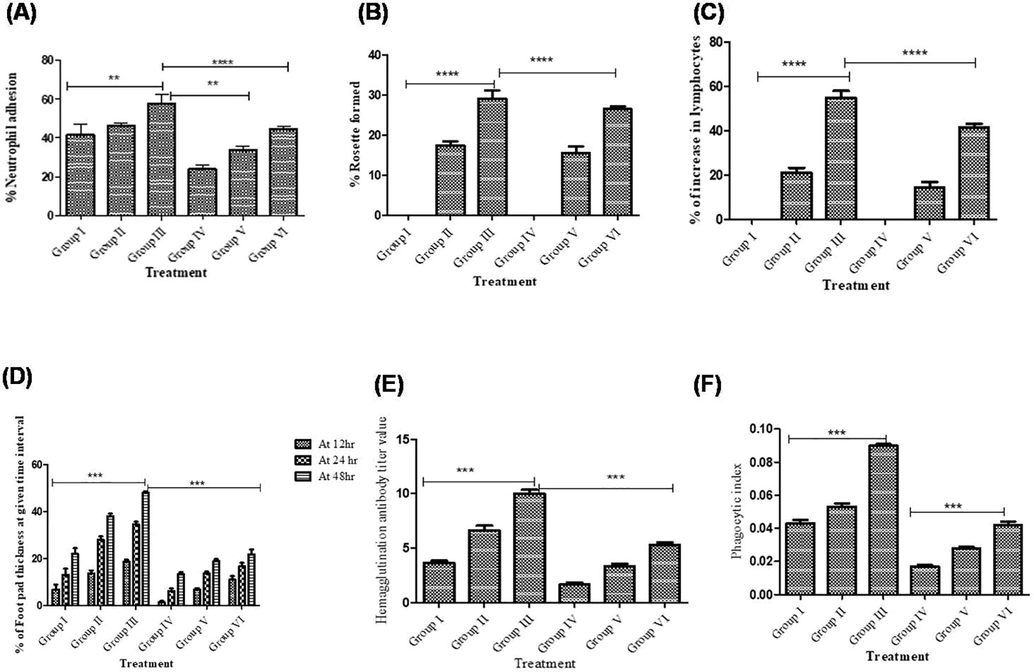
(A) EEAP effect on % Neutrophil adhesion; (B & C) EEAP on lymphocytes and lymphocyte rosette; (D) EEAP on footpad thickness (delayed type hypersensitivity). (E) EEAP effect on Hemagglutination antibody titer (F) EEAP effect on Phagocytic index.
DOX lead to DNA damage of bone marrow cells (Shaldoum et al., 2021), also induced a programmed cell death of lymphocytes by reducing spleen, lymph node and thymus T cells and B cells (Abdelaziz et al., 2019). Treatment of DOX inhibits the proliferation of lymphocyte on cancerous mouse model (Bhinge et al., 2012). Due to the stimulatory impact of plant extract on hematopoietic lymphocytes and bone marrow and hematopoietic cells, EEAP therapy has declined the total cell count of leukocytes, including lymphocytes, monocytes and basophils. The EEAP effect was tested on DOX-induced immunosuppressive rats with a reduced TLC count due to stimulation of hematopoietic stem cells (Sonneveld et al., 1981). Treatment improved the titer of the hemagglutination antibody that represents the increase in humoral response to SRBC and also indicates the increased responsiveness of subsets of T and B lymphocytes involved in the synthesis of antibodies (Benacerraf, 1978; Shukla et al., 2009). Dose dependent increment of hemagglutination value was observed in the current study, which signifies the immunomodulation activity via humoral immunity. Large influxes of non-specific inflammatory cells are characterized by delayed type hypersensitivity reaction, where the sensitized T-lymphocytes are transformed into lymphoblasts (Descotes, 1999). When colloidal carbon particles are injected directly into the systemic circulation in the form of ink, the rate of macrophage clearance of carbon from the blood is controlled by an exponential equation. (Gokhale et al., 2003). EEAP treated rats displayed a noticeable rise in the phagocytic index, and it is suspected that this could be due to an inclination of reticuloendothelial system activity due to previous treatment of animals.
However, the neutrophil, a non-dividable end cell with minimal protein synthesis ability, is capable of a wide range of responses, including chemotaxis, phagocytosis, exocytosis, and both intracellular and extracellular killing. Marginalization of blood stream neutrophils requires significant adhesion, initiated along with the b2 integrins interactions present on the neutrophils (Sun et al., 1996). The adherence of nylon fibers to neutrophils indicated the movement of blood vessel cells and the cell count of neutrophils that enter the inflammation site (Kasote et al., 2012). In the current research, EEAP exhibited a significant surge in percent neutrophils in both normal and in DOX induced immunosuppressive rats. various chemical constituents such as Triterpene glycosides (Aminin et al., 2014; Pislyagin et al., 2014), protocatechuic acid (Sobhani et al., 2021), polyphenols (Mileo et al., 2019) and phytochemicals such as flavonoids (Kim et al., 1999), triterpenoids (Szuster-Ciesielska et al., 2011), saponins (Rao Chavali et al., 1987) alkaloids (Bachhav and Sambathkumar, 2016) from various plant origin were proved for their immunomodulatory and anti-inflammatory properties. The presence of Triterpene glycosides and polyphenols, saponins and alkaloids are responsible for its potent immunomodulatory activity. According to the molecular docking results AP Triterpenoids saponins like compound 1 and 2, flavonoids Catechin, Quercetin, Isoquercetin were showed H Bonding with active sites of immunomodulatory receptors and good binding affinity score, may be these are potential lead molecules of immunomodulatory activity.
5 Conclusion
The ancient use of AP has been scientifically verified for its immunomodulatory effects. It suggests that the plant can be utilized as immunomodulatory agent as confirmed by in silico and in vivo studies.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Involvement of tumor necrosis factor-α, interferon gamma-γ, and interleukins 1β, 6, and 10 in immunosuppression due to long-term exposure to five common food preservatives in rats. Gene. 2020;742:144590.
- [CrossRef] [Google Scholar]
- The impact of mesenchymal stem cells on doxorubicin-induced testicular toxicity and progeny outcome of male prepubertal rats. Birth Defects Res.. 2019;111:906-919.
- [CrossRef] [Google Scholar]
- Aminin, D.L., Pislyagin, E.A., Menchinskaya, E.S., Silchenko, A.S., Avilov, S.A., Kalinin, V.I., 2014. Immunomodulatory and anticancer activity of sea cucumber triterpene glycosides. pp. 75–94. https://doi.org/10.1016/B978-0-444-63294-4.00003-6.
- In vitro α-amylase and Α-glucosidase inhibitor activities of albizia procera stem bark. Asian J. Pharm. Clin. Res.. 2018;11:344-347.
- [CrossRef] [Google Scholar]
- Evaluation of immunomodulatory activity of the alkaloid fraction of Trichopus zeylanicus gaertn on experimental animals. Indian J. Pharm. Sci.. 2016;78:161.
- [CrossRef] [Google Scholar]
- A hypothesis to relate the specificity of T lymphocytes and the activity of I region-specific Ir genes in macrophages and B lymphocytes. J. Immunol.. 1978;120:1809-1812.
- [Google Scholar]
- The opposite effects of doxorubicin on bone marrow stem cells versus breast cancer stem cells depend on glucosylceramide synthase. Int. J. Biochem. Cell Biol.. 2012;44:1770-1778.
- [CrossRef] [Google Scholar]
- CASTp: Computed Atlas of Surface Topography of proteins. Nucleic Acids Res.. 2003;31:3352-3355.
- [CrossRef] [Google Scholar]
- Triterpenoid saponins and sapogenins: 1973–1978. Phytochemistry. 1980;19:1889-1908.
- [CrossRef] [Google Scholar]
- CPCSEA. Guidelines for laboratory animal facility – committee for the purpose of control and supervision of experiments on animals. Indian J. Pharmacol.. 2003;35:257-274.
- [Google Scholar]
- Roitt’s Essential Immunology. John Wiley & Sons; 2017.
- An Introduction to Immunology. London: Taylor and Francis; 1999.
- Albizia Procera (Roxb.) Benth. Handbook of Energy Crops. Centre for New Crops and Plants Products; 1983.
- Comparative study between human mesenchymal stem cells and etanercept as immunomodulatory agents in rat model of rheumatoid arthritis. Immunol. Res.. 2020;68:255-268.
- [CrossRef] [Google Scholar]
- Investigations into the immunomodulatory activity of Argyreia speciosa. J. Ethnopharmacol.. 2003;84:109-114.
- [CrossRef] [Google Scholar]
- Hossan, M.F., Hossain, J., Faruq, K.O., Azam, S., Rahman, S., Jainul, M.A., Azad, A.K., 2018. Diarrhea preventive potency in methanolic leaves extract of albizia procera (Roxb.) Benth. in mice. Department of Pharmaceutical Technology, Faculty of Pharmacy, International Islamic University Abstract This project aimed to investigate the potency, 1, 45–52.
- Antidiabetic bioactive compounds from Tetrastigma angustifolia (Roxb.) Deb and Oxalis debilis Kunth.: Validation of ethnomedicinal claim by in vitro and in silico studies. South African J. Bot.. 2021;132:164-175.
- [Google Scholar]
- Immunomodulatory activity of ether insoluble phenolic components of n-butanol fraction (EPC-BF) of flaxseed in rat. Asian Pac. J. Trop. Biomed.. 2012;2:S623-S626.
- [CrossRef] [Google Scholar]
- Analgesic, antibacterial and central nervous system depressant activities of Albizia procera leaves. Asian Pac. J. Trop. Biomed.. 2014;4:279-284.
- [CrossRef] [Google Scholar]
- Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure–activity relationships. Biochem. Pharmacol.. 1999;58:759-765.
- [CrossRef] [Google Scholar]
- Phytopharmacological properties of Albizia species: A Review. Int. J. Pharm. Pharm. Sci.. 2013;5:70-73.
- [Google Scholar]
- Cerebroprotective Effect of Aloe Emodin: In Silico and In Vivo Studies. Saudi J. Biol. Sci. 2021
- [CrossRef] [Google Scholar]
- Effect of the total crude extracts of Hibiscus sabdariffa on the immune system in the Wistar albino rats. Afr. J. Pharm. Pharmacol.. 2013;7:1942-1949.
- [Google Scholar]
- QSAR, docking and in vivo studies for immunomodulatory activity of isolated triterpenoids from Eucalyptus tereticornis and Gentiana kurroo. Eur. J. Pharm. Sci.. 2012;47:152-161.
- [CrossRef] [Google Scholar]
- Triterpenoid saponins with N-acetyl sugar from the bark of Albizia procera. Phytochemistry. 2007;68:1261-1266.
- [CrossRef] [Google Scholar]
- Polyphenols: immunomodulatory and therapeutic implication in colorectal cancer. Front. Immunol.. 2019;10
- [CrossRef] [Google Scholar]
- Echinocystic acid 3,16-O-bisglycosides from Albizia procera. Phytochemistry. 2010;71:1375-1380.
- [CrossRef] [Google Scholar]
- Immunomodulatory effects of hexane insoluble fraction of Ficus septica Burm. F. in doxorubicin-treated rats. Asian Pacific J. Cancer Prev.. 2012;13:5785-5790.
- [CrossRef] [Google Scholar]
- OECD, 2002. The Organization of Economic Co-operation and Development Guidelines Test No. 423: Acute Oral toxicity - Acute Toxic Class Method, OECD Guidelines for the Testing of Chemicals, Section 4. Oecd 1–14.
- Acanthus montanus: An experimental evaluation of the antimicrobial, anti-inflammatory and immunological properties of a traditional remedy for furuncles. BMC Complement. Altern. Med.. 2008;8:1-11.
- [CrossRef] [Google Scholar]
- Toward a treatment of antibacterial and antifungal infections: Design, synthesis and in vitro activity of novel arylhydrazothiaz olylsulfonamides analogues and their insight of DFT, docking and molecular dynamic simulations. J. Mol. Struct.. 2021;130862
- [Google Scholar]
- Anti-HIV-1 integrase activity and molecular docking of compounds from Albizia procera bark. Pharm. Biol.. 2015;53:1861-1866.
- [CrossRef] [Google Scholar]
- Immunomodulatory activity of methanolic fruit extract of Aegle marmelos in experimental animals. Saudi Pharm. J.. 2010;18:161-165.
- [CrossRef] [Google Scholar]
- Pislyagin, E.A., Aminin, D.L., Silchenko, A.S., Avilov, S.A., Andryjashchenko, P. V., Kalinin, V.I., Padmakumar, K., 2014. Immunomodulatory action of triterpene glycosides isolated from the sea cucumber Actinocucumis typica. Structure-activity relationships. Nat. Prod. Commun. 9, 1934578X1400900. https://doi.org/10.1177/1934578X1400900610.
- Immunomodulatory and antioxidant effect of Leptadenia reticulata leaf extract in rodents: possible modulation of cell and humoral immune response. Immunopharmacol. Immunotoxicol.. 2012;34:1010-1019.
- [CrossRef] [Google Scholar]
- Assessment of anti hyperglycemic fractions isolated from Albizia procera stem bark chloroform extract using STZ induced diabetic albino rats. Pharmacogn. J.. 2014;6:29-35.
- [CrossRef] [Google Scholar]
- An in vitro study of immunomodulatory effects of some saponins. Int. J. Immunopharmacol.. 1987;9:675-683.
- [CrossRef] [Google Scholar]
- Compendium Indian Medicinal Plants. Lucknow: Central Drug Research Institute and Publications & Information Directorate, New Delhi; 1993.
- Using Soxhlet ethanol extraction to produce and test plant material (essential oils) for their antimicrobial properties. J. Microbiol. Biol. Educ.. 2014;15:45-46.
- [CrossRef] [Google Scholar]
- Identification of bioactive molecules from Triphala (Ayurvedic herbal formulation) as potential inhibitors of SARS-CoV-2 main protease (Mpro) through computational investigations. J. King Saud Univ. Sci. 2022
- [CrossRef] [Google Scholar]
- Repurposing of phytomedicinederived bioactive compounds with promising anti-SARS-CoV-2 potential: Molecular docking, MD simulation and drug-likeness/ADMET studies. Saudi J. Biol. Sci. 2021
- [CrossRef] [Google Scholar]
- In silico screening of phytopolyphenolics for the identification of bioactive compounds as novel protease inhibitors effective against SARS-CoV-2. J. Biomol. Struct. Dyn 2021:1-17.
- [CrossRef] [Google Scholar]
- Attenuation of oxidative stress in arthritic rats by ethanolic extract of Albizia procera benth bark through modulation of the expression of inflammatory cytokines. J. Ethnopharmacol.. 2020;250:112435.
- [CrossRef] [Google Scholar]
- Immunomodulatory potential of Enicostema axillare (Lam.) A. Raynal, a traditional medicinal plant. J. Ethnopharmacol.. 2012;140:239-246.
- [CrossRef] [Google Scholar]
- Role of anti-fertility medicinal plants on male & female reproduction. J. Complement. Altern. Med. Res.. 2017;3:1-22.
- [CrossRef] [Google Scholar]
- Immunomodulatory effects of bee pollen on doxorubicin-induced bone marrow/spleen immunosuppression in rat. J. Food Biochem.. 2021;45
- [CrossRef] [Google Scholar]
- Immunomodulatory activities of the ethanolic extract of Caesalpinia bonducella seeds. J. Ethnopharmacol.. 2009;125:252-256.
- [CrossRef] [Google Scholar]
- Hepatoprotective activity of ethanolic extract of aerial parts of Albizia procera Roxb (Benth.) against paracetamol induced liver toxicity on wistar rats. Int. J. Pharm. Pharm. Sci.. 2014;6:233-238.
- [Google Scholar]
- Immunomodulatory; anti-inflammatory/antioxidant effects of polyphenols: A comparative review on the parental compounds and their metabolites. Food Rev. Int.. 2021;37:759-811.
- [CrossRef] [Google Scholar]
- Cytotoxicity of doxorubicin for normal hematopoietic and acute myeloid leukemia cells of the rat. Cancer Chemother. Pharmacol.. 1981;5:167-173.
- [CrossRef] [Google Scholar]
- A brief review on phytopharmacological reports on Albizia procera. Asian J. Pharm. Pharmacol.. 2020;6:144-149.
- [Google Scholar]
- Role of leukocyte beta 2-integrin in PAF-induced shock and intestinal injury. Am. J. Physiol. Liver Physiol.. 1996;270:G184-G190.
- [CrossRef] [Google Scholar]
- Betulin and betulinic acid attenuate ethanol-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS), cytokine (TNF-α, TGF-β) production and by influencing intracellular signaling. Toxicology. 2011;280:152-163.
- [CrossRef] [Google Scholar]
- Immunomodulatory property of ethanolic extract of Trigonella foenum-Graeceum leaves on mice. Der Pharm. Lett.. 2012;4:708-713.
- [Google Scholar]
- AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009
- [CrossRef] [Google Scholar]
- Search for plant derived natural products with immunostimulatory activity: recent advances. Pure Appl. Chem.. 1990;62:1217-1222.
- [CrossRef] [Google Scholar]
- Artesunate protects immunosuppression mice induced by glucocorticoids via enhancing pro-inflammatory cytokines release and bacterial clearance. Eur. J. Pharmacol.. 2021;890:173630.
- [CrossRef] [Google Scholar]
- Four acylated triterpenoid saponins from Albizia procera. J. Nat. Prod.. 1998;61:440-445.
- [CrossRef] [Google Scholar]
- Melanogenesis-inhibitory and cytotoxic activities of triterpene glycoside constituents from the bark of Albizia procera. J. Nat. Prod.. 2018;81:2612-2620.
- [CrossRef] [Google Scholar]







