Translate this page into:
Mode of application of acaricides against the ectoparasitic mite (Varroa destructor) infesting honeybee colonies, determines their efficiencies and residues in honey and beeswax
⁎Corresponding author at: Zoology Department, Faculty of Science, Tanta University, Tanta 31527, Egypt. yehia.elnagar@science.tanta.edu.eg (Yahya Al Naggar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives: Ensuring adequate treatments for acaricide efficacy to combat mite infestation is a pre-requisite for healthy honeybees and a good yield of hive products. Therefore, this study aimed to assess the effect of the mode of application on efficiency of two acaricides; Vapcozin-20 (amitraz) and Mavrik 2F (fluvalinate) against Varroa mite infesting brood and adult honeybees. Methods: To do so, we used 21 honeybee colonies (3 colony per treatment) between December 2018 and March 2019. Varroacides were applied by three different methods: cotton strips (impregnated in each tested acaricide solution for 24 h) or direct spraying (5 mL for each colony) or using carton paper (impregnated in each tested acaricide solution for 5 min) then put directly on the top of brood combs. All treatments were applied twice at a month interval. We also quantified acaricides residues in honey and beeswax after 1, 15, 30, 60 and 90 days of acaricides treatment. Results: The different application methods of both acaricides against V. destructor showed relatively similar efficiencies in both brood and adult honeybees that ranged from 93.8 to 100% for Mavrik 2F and from 83.75 to 96.37% for Vapcozin with no significant differences between different application methods. Fluvalinate residues detected in both honey and wax collected from colonies treated with the strips method, exceeded the maximum residue limits (0.05 ppm for both honey and wax) according to EU Pesticides database. While amitraz residues were not detected in any colonies treated with Vapcozin-20 after 3 months of treatment, regardless of the application method. Conclusions: The three different methods of application of Mavrik 2F and Vapcozin-20 showed great efficiency for the control of Varroa mite, however the application of both miticides using carton paper method was the most appropriate because it showed very minimal acaricides residues.
Keywords
Honeybees
Varroa mite
Honey
Beeswax
Mavrik 2F
Vapcozin-20
Acaricides residues
1 Introduction
Honeybees provides highly valued pollination services for a wide variety of agricultural crops (Al Naggar et al., 2018). However, over the last few years, a lot of studies have been published about the losses of honeybee colonies and the declining population of native and wild bees (Goulson et al., 2015; Jacques et al., 2017). Several factors are implicated behind these losses including pathogens and parasites, poor nutrition, beekeeping management, climate change, and pesticides (Kerr et al., 2015; Al Naggar and Baer, 2019). However, a combination of Varroa and viruses are now frequently implicated in collapsing colonies (Martin et al., 2012).
The parasitic mite, Varroa destructor, became the single greatest threat to honey bee health, the apiculture and pollination industries, since it spread from its native host, the Asian honey bee (Apis cerana) to the naive European honey bee (A. mellifera). (reviewed in, Traynor et al., 2020). Varroa infestation occur mainly during winter and causes significant impacts on honeybee colony health as a consequence of its transmission of a cocktail of viruses while feeding on honeybee haemolymph and fat bodies (Martin et al., 2012; Wilfert et al., 2016; Ramsey et al., 2019). Highly infested weak colonies facilitate mite dispersal and disease transmission to stronger and healthier colonies. In untreated honeybee colonies, heavy Varroa infestation causes 100% mortality in a few weeks (Kanga et al., 2010; Rosenkranz et al., 2010).
Chemical treatments against V. destructor are almost inevitable for commercial beekeeping. Synthetic acaricides such as fluvalinate, flumethrin, amitraz, coumaphos, and cymiazole have been successfully used to control V. destructor (Al Naggar et al., 2016; Gracia et al., 2017). However, the use of acaricides inside beehives implies a risk of contamination of honey and other hive products (beeswax for example) (Wallner, 1999; Martel et al., 2007; Calatayud-Vernich et al., 2018). Acaricides mostly contaminate beeswax due to its non-polar nature, while honey remains relatively free of contaminants (Calatayud-Vernich et al., 2018).
Honeybees colonies require regular Varroa control during the whole year which means repeated application of varroacides that led to contamination of many hive products (Abou-Shaara, 2014; Pohorecka et al., 2018) Moreover, the effect of using these acaricides to control Varroa mites has long been a concern to the beekeeping industry due to unintended negative impacts on honeybee health (reviewed in Tihelka, 2018). Thus, selecting the best acaricide application or delivery method became a necessity not only for controlling Varroa mite but also to safe bees from unintended negative impacts and minimizing the residues accumulated in honey and other bee matrices. Moreover, beekeepers all over the world pay a great attention to novel treatments and application methods of acaricides due to the developed resistance of honeybees against these inhive chemicals (Tihelka, 2018).
Here we assessed the effect of the different mode of application on efficiency of two acaricides; Mavrik 2F (fluvalinate) and Vapcozin-20 (amitraz) against Varroa mite in brood and adult honeybees. To do so, we applied these varroacides using strips or direct spraying or carton paper methods. We also quantified acaricides residues in honey and beeswax at different time points to investigate the effect of the delivery method on acaricides levels.
2 Materials and methods
2.1 Acaricides
We used two commercial acaricides: Mavrik 2F (22% w/w of fluvalinate as active ingredient), produced by AAKO B.V.- Holland and Vapcozin-20 (20% amitraz as active ingredient), produced by Veterinary and Agricultural products Mfg. Co. Ltd – Jordan. The two chemical acaricides were used in concentration 2% (adding 20 mL/litter of water) for controlling Varroa mites. Treatments were applied in three forms: 1) as stripes of cotton (2 × 20 cm) which impregnated for 24 h in each tested acaricide solution and held in one comb between the brood nests of each tested colonies; 2) with direct spraying (5 mL for each colony) on the honey bee combs including mature and immature stages of bees and 3) using carton paper (20 × 20 cm) which impregnated for 5 min in each tested acaricide solution and put directly on the top of brood combs (Fig. 1). All treatments were applied twice only at month interval.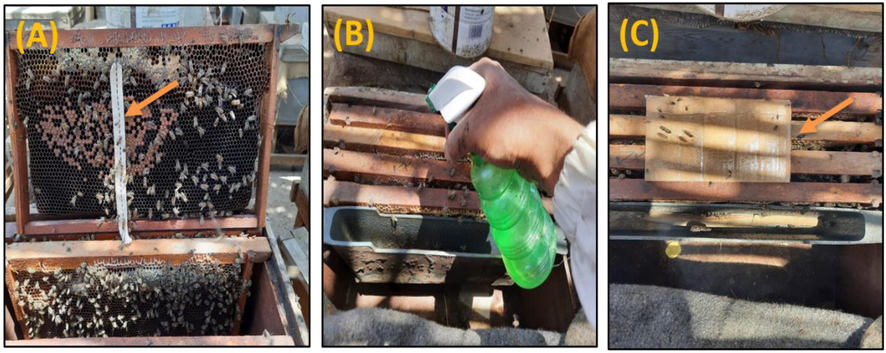
Application methods of acaricides against Varroa mite. A) Cotton strips; (2 × 20 cm) which impregnated for 24 h in each tested acaricide solution and held in one comb between the brood nests. B) Spraying (5 mL of each acaricide solution for each colony) on the honey bee combs including mature and immature stages of bees and C) Cartoon paper: (20 × 20 cm) which impregnated for 5 min in each tested acaricide solution and put directly on the top of brood combs.
2.2 Experimental design
The experimental design for the study utilized a total of 21 colonies of local hybrid Carniolan honeybees (A. mellifera carnica) maintained at a private apiary at Dirut region Assiut governorate, Egypt, between December 2018 and March 2019. Colonies were normalized for strength, based on the number of standards, deep Langstroth 5 frames covered with bees (one frame of honey, one frame of pollen and three brood frames). By 1st of December 2018, colonies were randomly assigned into three groups: Group1: Mavrik 2F (cotton strips or spraying or carton paper), Group 2: Vapcozin-20 (Cotton strips or spraying or carton paper); Group 3: no treatment (Control). Three colonies were used per each treatment. All treatments were applied twice at a month interval.
2.3 Data and sample collection
An initial set of measurements and samples were taken from colonies prior to treatments. Infestation with Varroa destructor in all tested colonies during experimental period was determined in worker sealed brood (pupae with pigmented eyes) (Mites per Fifty pupae, MPFP) and in adult workers (Mites per Hundred Bees, MPHB). For pupae, 50 individual cells for each tested colony were inspected as illustrated in Fig. S1. In adult workers (collected from the brood nest), the percentage of infestation (%) was determined in approximately 100 living adult bee workers picked directly from the combs (De Jong, 1988). Infestation percentage was determined before and after acaricides treatments. Honey and beeswax samples were also sampled for acaricides residue analysis. Honey samples were collected directly from each colony about (50 g from random locations near and far from the application form of the acaricide. Wax samples (about 50 g each) were randomly cut 2.5 cm2 at different locations including the area of strip application. One composite sample (three colonies) from each application form was made. Samples were taken at 5 periods during the experiment procedures from 4th of December 2018 to 6th of March 2019 after 1, 15, 30 days of the first treatment after 30 and 60 days of the second treatment.
2.4 Efficiency of acaricides against Varroa destructor
Efficacy of the applied miticides against infection with the mite V. destructor was calculated by use of the Henderson-Tilton formula (Henderson and Tilton, 1955). (Eq. (1)).
2.5 Acaricides residue analysis
Mavrik 2F (fluvalinate) residues were extracted from honey and beeswax samples according to the methods of Bogdanov et al. (1997) and Zimmermann et al. (1993), respectively with slight modifications using gas liquid chromatography (GLC: Agilent 6890 gas chromatograph equipped with a Ni63-electron capture detector, ECD, USA) for detection. Vapcozin-20 (amitraz) residues were extracted from honey by using the method of Tseng and Chang (1999) and Pass and Mogg (1991), with slight modification. While in bee wax, Vapcozin-20 (amitraz) residues were extracted according to the method of Zimmermann et al. (1993) that modified by Bogdanov et al. (1998) using high performance liquid chromatography (HPLC: Thermo Finnigan Model, Germany) for detection. Detection limits of amitraz was 0.0163 and 0.0213 ppm in honey and wax, while it was 0.002 and 0.011 ppm for tau-fluvalinate in honey and wax, respectively.
2.6 Statistical analysis
Normality was confirmed by the Kolmogorov–Smirnov test and homogeneity of variance was confirmed by use of Levine’s test. Transformation of data was done when required to meet these assumptions of parametric statistics. Differences among rates (MPHB) of infestation with Varroa destructor in different treatment groups before after treatment were firstly assessed by Two-way analysis of variance (ANOVA) (treatment × time) however there was no interaction, so One-way analysis of variance (ANOVA) followed by a Tukey’s post hoc test were used. For all analyses the level of Type I error was set as p < 0.05.
3 Results
3.1 Efficiency of tested acaricides against Varroa infestation
At the beginning of the experiment, mean rates of infestation with phoretic Varroa destructor of adult and pupae (brood) of honeybee workers of all experimental colonies were 29.52 MPHB and 29.47 MPFP, respectively. There were no significant differences (p > 0.05) in rates of infestation with V. destructor among replicates assigned to experimental groups prior to treatment. The infestation rates with Varroa mite decreased significantly (P < 0.05) in brood and adult honeybee workers of colonies treated with Mavrik 2F as compared to only non-treated (control) colonies (Fig. 2a, c). The different application methods of Mavrik 2F against V. destructor showed however, relatively similar efficiencies in both brood and adult honeybees that range from 93.8 to 100 (Table 1).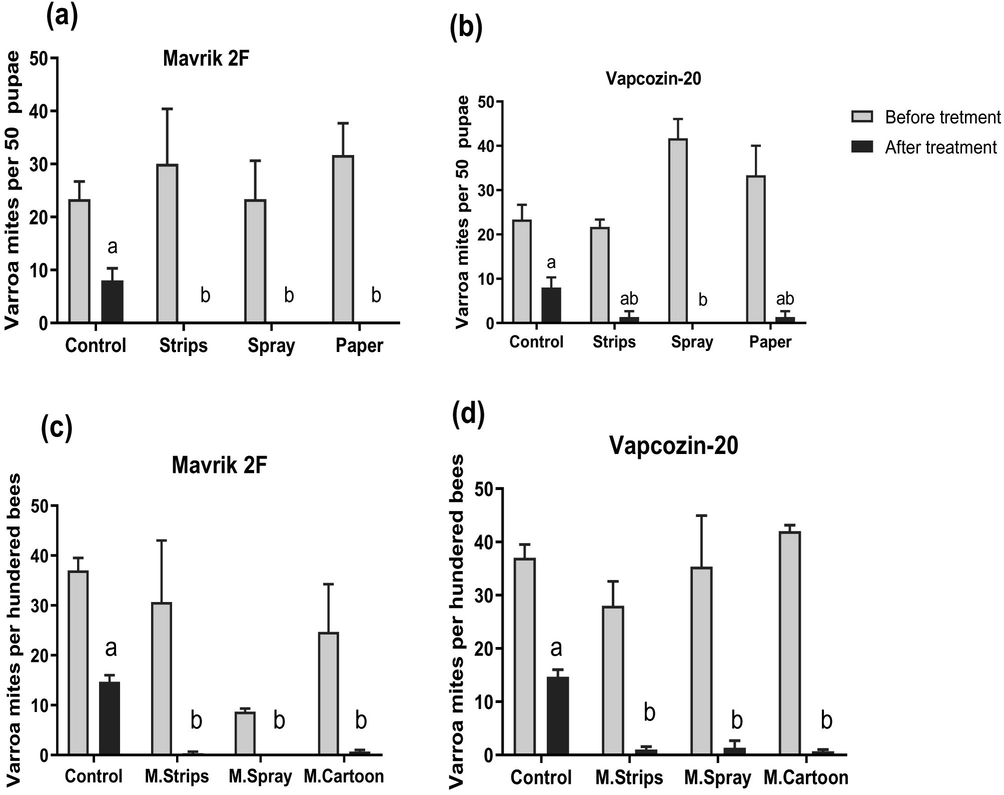
Rate of infestation with V. destructor (mean ± SD; n = 3 colonies) of honey bee brood and adult before and after treatment with Mavrik 2F and Vapcozin-20 acaricides. Means with different letters are significantly different (one-way ANOVA with tukey post hoc test, p < 0.05).
Acaricide/M. of Application
Mavrik 2F
Vapcozin-20
Brood
Adult
Brood
Adult
C. Strips
100
97.51
83.75
90.94
Spraying
100
100
100
90.66
C. Paper
100
93.81
88.7
96.37
Similarly, the mean rates of infestation with Varroa mite decreased significantly (P < 0.05) in both brood and adult honey bee workers of treated colonies with Vapcozin-20 using the three different application methods compared to non- treated control colonies, with efficiencies ranged from 83.75 to 96.37 (Table 1; Fig. 2b, d). In the other hand, the direct spraying of Vapcozin-20 on honeybee colonies was the only method that significantly suppressed the level of Varroa infestation in honeybee brood with 100% efficiency (Table 1; Fig. 2b). There were no significant differences in terms of efficiency against Varroa mites between the different application methods of either Mavrik 2F or Vapcozin-20 acaricides (Table 1).
3.2 Residues of acaricides in honey and beeswax
The residues of fluvalinate (Mavrik 2F) and amitraz (Vapcozin-20) were detected in greater concentrations after 24 h of treatment in honey samples (Table 2). Controlling Varroa mite with the spraying application method of both acaricides showed the greatest concentration in honey samples after 24 h of the first treatment (18.74 and 5.57 ppm, respectively) while, the least residues were detected in honey samples collected from colonies treated with carton paper method (0.61 and 0.32 ppm) for Mavrik 2F and Vapcozin-20, respectively. After two months of second treatment with Mavrik 2F, fluvalinate residues were only detected in honey samples (0.13 ppm) collected from colonies treated with the cotton strip-application method that exceeded the maximum residue limit (0.05 ppm) according to EU Pesticides database (Anonymous, 2019). Residues of amitraz were not however, detected in any honey samples collected from colonies treated with Vapcozin-20 after two months of the second treatment regardless of the application method (Table 2). MRL = Maximum residue limit according to EU Pesticides database (Anonymous, 2019). ND, non-detected.
Time of sampling (day)
Fluvalinate (mavrik 2F)
Amitraz (vapcozin-20)
Strip
Spray
Paper
Strip
Spray
Paper
First treatment
1
2.34
18.74
0.61
1.13
5.57
0.32
15
4.67
4.77
0.64
2.76
2.87
0.7
30
2.4
1.98
0.3
1.5
0.93
0.12
Second treatment
60
1.9
1.03
0.08
0.87
ND
0.04
90
0.13
ND
ND
ND
ND
ND
MRL*
0.05
0.2
Acaricides residues of fluvalinate and amitraz were detected in beeswax samples after 24 h, 15 day, 30 and 60 days after treatment of Varroa mites (Table 3). Residues of fluvalinate and amitraz were higher in honey and beeswax samples collected from colonies treated with either Mavrik 2F and Vapcozin-20 using the strip application method, followed by direct spraying method, while the least residues were detected in samples collected from colonies treated with carton paper method (Table 2). MRL = Maximum residue limit according to EU Pesticides database (Anonymous, 2019).
Time of sampling (day)
Fluvalinate (mavrik 2F)
Amitraz (vapcozin-20)
Strip
Spray
Paper
Strip
Spray
Paper
First treatment
1
4.17
30.6
0.88
2.68
17.8
0.7
15
7.8
11.4
1.7
5.35
7.7
1.4
30
5.12
4.6
1
3.7
2.2
0.4
Second treatment
60
3
2.76
0.76
2.6
0.92
0.26
90
0.12
0.01
ND
0.08
ND
ND
MRL*
0.05
0.2
After 24 h of spray treatment method, the residues of both fluvalinate and amitraz in beeswax were greatest (30.6 and 17.8 ppm, respectively) while, the least residues detected for both acaricides were in samples collected from colonies treated using carton paper; 0.88 and 0.7 ppm, respectively. Interestingly, residues of fluvalinate were detected in only colonies treated with the cotton strips (0.12 ppm) and spray (0.01 ppm) application methods after two months of the second Mavrik 2F treatment, while, residues of amitraz were detected only in wax samples collected from colonies treated with Vapcozin-20 using the strip application method (0.08 ppm) (Table 3). Importantly, residues of both fluvalinate and amitraz in beeswax samples collected from colonies treated with the cotton strip method after two months of the second treatment, exceeded the maximum residue limit (0.05 and 0.2 ppm, respectively) according to EU Pesticides database (Anonymous, 2019) (Table 3). Collectively, the detected residues of both acaricides were highest in beeswax samples compared with honey samples regardless of the different application methods.
4 Discussion
Varroa mite (Varroa destructor) represents the major challenge for beekeeping worldwide. Various methods and materials have been therefore suggested and tested for its control that included plant extracts, essential oils, biological agents, mechanical methods, and some chemicals (Abou-Shaara, 2014; Abou-Shaara et al., 2016; Gajger et al., 2020; Masry et al., 2020). Here, we tested the efficiency of two acaricides Mavrik 2f (fluvalinate) and Vapcozin-20 (amitraz) against Varroa mite using three different application methods (direct spraying, cotton strips and carton paper) and quantified their residues in honey and beeswax. Both acaricides showed great efficacy against Varroa mites regardless of the application method. However, the use of carton papers was the most appropriate application method for these compounds because it showed very minimal acaricides residues in honey and beeswax.
Our results showed clearly that Mavrik 2F and Vapcozin-20 are highly effective against Varroa mites over the winter in Egypt. Although the different application methods of both acaricides showed relatively similar efficiencies against Varroa mite, the direct spraying method demonstrated the best efficiency. Our results are in agreement with Sajid et al. (2020) who evaluates the effectiveness of five miticides (fluvalinate, flumethrin, amitraz, formic acid, and oxalic acid) on Apis mellifera colonies and reported similar findings. The use of thymol as dust achieved the best reduction % of Varroa infestation followed by vermiculite blocks and dilution in sugar syrup (Emsen and Kelly, 2007). In the same context, three oxalic acid application methods (trickling, spraying, and sublimation) at three or four (sublimation) doses, using 110 broodless colonies in early January 2013 have been compared against Varroa mite (Al Toufailia et al., 2015). The authors found that applying oxalic acid via sublimation in broodless honeybee colonies in winter is a highly successful way to manage Varroa destructor and does not cause any damage to the colonies. Both the dose of acaricide and application method have been showed to affect colony performance and Varroa mortality and these effects were also correlated with different climate conditions (Al Toufailia et al., 2015).
Recently, Gajger et al. (2020) also evaluated seven products for controlling Varroa mite, six were treated during Summer and one was treated during Autumn. The authors found that, treating colonies with CheckMite+ (coumaphos) as strips achieved the best Varroa reduction % while the least reduction % was in colonies treated with Apiguard (thymol) that was applied in an aluminum tray and coated sheet placed on the top bar of frames. Therefore, it could be concluded that, the overall efficacy of an acaricide could be affected by numerous factors, including the concentration of the compound involved, treatment period, and the colony and apiary environment (Gracia et al., 2017). Moreover, the efficacy of some compounds depends on the evaporation pressure within the colony; therefore, the time of year or the ambient temperature during treatment application can influence the effectiveness of the treatment (Calderone and Spivak, 1995). Consequently, the use of appropriate application method or/and the chemical state or form of an acaricide (solid, liquid or gas) is very important for better Varroa management and to avoid any hazard on honeybee health.
Veterinary drugs of varying chemical nature are used worldwide (except Australia) to regulate V. destructor. If these varroacids are fat-soluble and non-volatile, the risk of contamination of bee products increases with repeated applications over the years (Wallner, 1999). In the current study, residues of fluvalinate detected in honey and beeswax were higher than amitraz residues in the three different application methods. This might be related to the instability of amitraz in in honey and beeswax (Bogdanov, 1989; Korta et al., 2001; Pohorecka et al., 2018). Amitraz residues were completely degraded after 10 days of treatment in honey and after one day in beeswax (Korta et al., 2001). However, the different application methods could affect the acaricides residues levels in honey and other hive matrices. For example, fumigation of beehives with amitraz results in contamination of honey stored in combs (Pohorecka et al., 2018). In the present study, samples of honey and beeswax collected from colonies treated with Vapcozin-20 (amitraz) using the direct spraying method, contained the highest residues compared to the levels found in colonies treated with other application methods. This might be due to the direct exposure of honey and beeswax with the chemical as reported earlier (Martel et al., 2007). On the other hand, previous studies showed that the solubility of fluvalinate in wax is very high, and it was detected in wax at concentrations reaching 8000 times greater than honey and these residues were stable for more than a year in beeswax (Bogdanov et al., 1998; Mullin et al., 2010).
The use of acaricides is likely to increase due to resistance developed by mites which requires increased treatments in the future. This undoubtedly will increase the levels of acaricides residues in honey and other bee products. Therefore, beekeepers should apply miticides by away that achieve good efficiency against the Varroa mites and of minimal residues. In the current study, residues of fluvalinate and amitraz have been detected in only wax samples collected from colonies treated with the strip method after three months of acaricides treatment. Moreover, fluvalinate residues detected in both honey and wax collected from colonies treated by this method, exceeded the maximum residue limits (0.05 ppm for both honey and wax) according to EU Pesticides database. This acaricide is applied by beekeepers through pesticide-impregnated plastic strips and is subsequently distributed throughout a colony by nestmate interaction and trophallaxis. Therefore, it could adversely impact honeybee health because fluvalinate has already been reported as impacting queen and drone performance and competitiveness (Sokol, 1996; Rinderer et al., 1999) and increasing susceptibility to viral pathogens(Locke et al., 2012).
On the other hand, treating colonies with either fluvalinate or amitraz using cartoon paper as a delivery method led to very minimal or non-detectable residues in both honey and wax in the current study. This new delivery method, looks promising, efficient, very simple and cheap. Further studies are however still required to test this method with other acaricides and under different apiary and environmental conditions.
5 Conclusion
The efficiency of two acaricides Mavrik 2f (fluvalinate) and Vapcozin-20 (amitraz) against Varroa mites have been tested using three different application or delivery methods and their residues in honey and beeswax have been quantified. Both acaricides showed great efficacy against Varroa mites regardless of the application method. However, the use of carton papers was the most appropriate delivery method for these compounds because it showed very minimal acaricides residues in honey and beeswax. Beekeepers should avoid treating their colonies using fluvalinate strips, because it can easily accumulate in colonies to reach unsafe levels.
Acknowledgement
This research has been funded by Cooperative association for development of bees industry in Riyadh (NAHHAL), Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Continuous management of Varroa mite in honey bee, Apis mellifera, colonies. Acarina. 2014;22(2):149-156.
- [Google Scholar]
- Impacts of intensive dusting or spraying with Varroa control materials on honey bee workers and drones. J. Apic. Res.. 2016;31(2):113-119.
- [CrossRef] [Google Scholar]
- Consequences of a short time exposure to a sublethal dose of Flupyradifurone (Sivanto) pesticide early in life on survival and immunity in the honeybee (Apis mellifera) Sci. Rep.. 2019;9(1)
- [CrossRef] [Google Scholar]
- Effects of treatments with Apivar® and Thymovar® on V. destructor populations, virus infections and indoor winter survival of Canadian honey bee (Apis mellifera L.) colonies. J. Apic. Res.. 2016;54(5):548-554.
- [CrossRef] [Google Scholar]
- Beekeeping and the need for pollination from an agricultural perspective in Egypt. Bee World.. 2018;95(4):107-112.
- [Google Scholar]
- Towards integrated control of varroa: 2) comparing application methods and doses of oxalic acid on the mortality of phoretic Varroa destructor mites and their honey bee hosts. J. Apicult. Res.. 2015;54(2):108-120.
- [CrossRef] [Google Scholar]
- Anonymous (2019). EU Pesticides Database. <http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=pesticide.residue.CurrentMRL&language=EN>.
- Bestimmung von Amitraz und seine Metaboliten in Honig durch HPLC. Mitt. Sekt. Bienen FAM1. 1989;3:1-9.
- [Google Scholar]
- Bogdanov, S., P. Martin, Lullmann, C. 1997. Harmonised method of the European honey commission Apidologie (extra issue) pp. 1–59.
- Acaricide residues in some bee products. J. Apic. Res.. 1998;37(2):57-67.
- [CrossRef] [Google Scholar]
- Pesticide residues in honey bees, pollen and beeswax: assessing beehive exposure. Environ. Pollut.. 2018;241:106-114.
- [CrossRef] [Google Scholar]
- Plant extracts for control of the parasitic mite Varroa jacobsoni (Acari: Varroidae) in colonies of the western honey bee (Hymenoptera: Apidae) J. Econ. Entomol.. 1995;88(5):1211-1215.
- [Google Scholar]
- Varroa jacobsoni does reproduce in worker cells of Apis cerana in South Korea. Apidolo. 1988;19:241-244.
- [Google Scholar]
- The effect of three methods of application on the efficacy of thymol and oxalic acid for the fall control of the honey bee parasitic mite Varroa destructor in a northern climate. Am. Bee J.. 2007;147(6):535-539.
- [Google Scholar]
- Control of Varroa destructor mite infestations at experimental apiaries situated in Croatia. Diversity. 2020;12(1):12.
- [CrossRef] [Google Scholar]
- Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 2015;347:1255957.
- [CrossRef] [Google Scholar]
- Field efficacy of acaricides against Varroa destructor. PLoS ONE. 2017;12(2)
- [CrossRef] [Google Scholar]
- Tests with acaricides against the brown wheat mite. J. Econ. Entomol.. 1955;48:157-161.
- [Google Scholar]
- A pan-European epidemiological study reveals honey bee colony survival depends on beekeeper education and disease control. PLoS One. 2017;12 e0172591
- [CrossRef] [Google Scholar]
- Monitoring for resistance to organophosphorous and pyrethroid insecticides in Varroa mite populations. J. Econ. Entomol.. 2010;103:1797-1802.
- [CrossRef] [Google Scholar]
- Climate change impacts on bumblebees converge across continents. Science. 2015;349:177-180.
- [Google Scholar]
- Study of acaricide stability in honey. Characterization of amitraz degradation products in honey and beeswax. J. Agric. Food Chem.. 2001;49:5835-5842.
- [CrossRef] [Google Scholar]
- Host adaptations reduce the reproductive success of Varroa destructor in two distinct European honey bee populations. Ecol. Evol.. 2012;2(6):1144-1150.
- [Google Scholar]
- Acaricide residues in honey and wax after treatment of honey bee colonies with Apivar ® or Asuntol ® 50. Apidologie. 2007;38(6):534-544.
- [CrossRef] [Google Scholar]
- Global honey bee viral landscape altered by a parasitic mite. Science. 2012;336(6086):1304-1306.
- [CrossRef] [Google Scholar]
- Evaluating the efficacy of Jatropha oil extract against Varroa mites infested honey bee colonies, Egypt. J. Biol. Pest Co.. 2020;30:91.
- [CrossRef] [Google Scholar]
- High levels of miticides and agrochemicals in North American Apiaries: implications for Honey Bee health. PLoS ONE.. 2010;5(3)
- [CrossRef] [Google Scholar]
- Effect of amitraz and its metobolites on intestinal motility. Comp. Biochem. Phystol.. 1991;99(12):169-172.
- [CrossRef] [Google Scholar]
- Amitraz marker residues in honey from honeybee colonies treated with Apiwarol. J. Vet. Res.. 2018;62:297-301.
- [CrossRef] [Google Scholar]
- Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. PNAS. 2019;116(5):1792-1801.
- [CrossRef] [Google Scholar]
- Varroa in the mating yard: the effects of Varroa jacobsoni and Apistan® on drone honey bees. Am. Bee J.. 1999;139(2):134-139.
- [Google Scholar]
- Biology and control of Varroa destructor. J. Invertebr. Pathol.. 2010;103:S96-S119.
- [CrossRef] [Google Scholar]
- Efficacy assessment of soft and hard acaricides against Varroa destructor mite infesting honey bee (Apis mellifera) colonies, through sugar roll method. Saudi J. Biol. Sci.. 2020;27(1):53-59.
- [CrossRef] [Google Scholar]
- The influence of a multi-month persistence of Fluwarol in a hive of a honey bee colony. Med. Wete.. 1996;52:718-720.
- [Google Scholar]
- Effects of synthetic and organic acaricides on honey bee health: a review. Slov. Vet. Res.. 2018;55(3)
- [CrossRef] [Google Scholar]
- Varroa destructor: a complex parasite, crippling Honey Bees Worldwide. Trends Parasitol. 2020:2020020374. Preprints 2020
- [CrossRef] [Google Scholar]
- Determination of amitraz residue in fruit by high performance liquid chromatography. J. Food Drug Anal.. 1999;7(3):225-232.
- [Google Scholar]
- Varroacides and their residues in bee products. Apidologie. 1999;30(2-3):235-248.
- [CrossRef] [Google Scholar]
- Deformed wing virus is a recent global epidemic in honeybees driven by Varroa mites. Science. 2016;351(6273):594-597.
- [CrossRef] [Google Scholar]
- Bestimmung von Brompropylat, 4,4-Dibrombenzophenon, Coumaphos und Fluvalinate in Bienenwachs. Deut. Lebensm. Rundsch.. 1993;89:341-343.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.101236.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







