Translate this page into:
Mitigating effects of Passiflora incarnata on oxidative stress and neuroinflammation in case of pilocarpine-Induced status epilepticus model
⁎Corresponding author. habdrabou@ksu.edu.sa (Hossam Ebaid)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Virulent epileptogenic insult is still one of the most life-threatening deficits during the clinical consequences and neurological emergencies of epilepsy. Thus, this study is designed to determine the changes in the expression of neurotransmitters, imbalance in blood electrolytes; oxidative stress, levels of pro-/anti-inflammatory cytokines, after treatment with pasipay (Passiflora incarnata). The effect of oral administration of 200 mg/kg body weight of P. incarnata on pilocarpine-induced seizures was assessed. The correlation between seizure activity and levels of proinflammatory cytokines; anti-inflammatory cytokines; oxidative stress biomarkers, monoamines, neurotransmitters; electrolytes and Th1/Th2 activities was quantified. The present study revealed a highly significant amelioration in amino acids, neurotransmitters, blood electrolytes, antioxidants and inflammatory cytokines in epileptic-treated rats with pasipay (P. incarnata). In conclusion, the present study supports further attempts to abrogate the neural dysfunction via antioxidant and anti-inflammatory cascade activities using P. incarnata.
Keywords
Pilocarpine
Epilepsy
Passiflora
Inflammation
CD4+ and CD8+-cytokines
Oxidative stress
Neurotransmitters
- AEDs
-
Antiepileptic drugs
- BBB
-
Blood-brain barrier
- CAT
-
Catalase
- DPPH
-
2,2-diphenyl-1-picrylhydrazyl
- GABA
-
gamma-aminobutyric acid
- IL4
-
Interleukin 4
- GSH
-
glutathione
- GR
-
glutathione reductase
- GPx
-
glutathione peroxidase
- GAPDH
-
glyceraldehyde-3-phosphate dehydrogenase
- MDA
-
malondialdehyde
- IkB
-
inhibitor of kB kinase
- FAS
-
Cell surface death receptor
- SOD
-
Superoxide dismutase
- Th1/Th2
-
T helper1/T helper2
- TNF-α
-
Tumor necrosis factor-α
- PILO
-
Pilocarpine hydrochloride
- L-DOPA
-
L-dihydroxyphenyalanine
Abbreviations
1 Introduction
An epileptic seizure is a kind of brain dysfunction caused by over discharge. In the injured brain, endothelial cells release cytokines, activating T cells and triggering acquired immune disorders, thus leading to the pathogenesis of epilepsy (Huang and Zhu, 2018). Damage of blood–brain barriers (BBB) causes entry of inflammatory immune cells. Activation and neutrophil recruitment by kinin lead to mitochondrial alterations, microglial activation and generation of reactive oxygen species (ROS). ROS, in turn, cause oxidative damage, which alters the cellular tight junctions. In addition, other animal studies revealed that breakdown of BBB leads to transcriptional changes in the neurovascular connections and eventual neurodegeneration (Shlosberg et al., 2010). The inflammatory processes represent key contributors to acute and chronic neurological disorders, such as epilepsy (Poole et al., 2000). IL-1β plays a role in the initiation and progression of neuronal dysfunction during epilepsy.
The antiepileptic drugs (AEDs) of epilepsy is associated with dose-related and chronic toxicity, and teratogenic effects (Poole et al., 2000; Ropper, 2005). Drugs based on natural products and folk remedies can offer safe and efficient alternative for AEDs (Raza et al., 2003).
Passiflora species have gained worldwide prominence because of their varied content of phyto-constituents of high therapeutic value that represent potential raw material for the development of new drugs. Amongst the 500 species of the genus Passiflora, Passiflora incarnata (P. incarnate) is the one that has extensive clinical applications throughout the world. P. incarnata Linneaus possesses significant CNS depressant properties (Dhawan et al., 2004). The drug is highly effective in convulsions when given prior to an approaching attack. P. incarnata is praised for its control over meningeal inflammations and their consequent spasms of childhood (Dhawan et al., 2004).
Various phytoconstituents were reported to be present in P. incarnata including carbohydrates)Dhawan et al., 2004), amino acids, and a cyanogenic glycoside gyanocardin. In addition, monoflavonoid of Passiflora sp. was reported to reduce morphine withdrawal in vitro (Capasso et al., 1998). Chrysin (5,7-dihydroxyflavone), one of the monoflavonoid found in Passiflora sp., has been used as an anxiolytic anticonvulsant)Zanoli et al., 2000). Chrysin, a natural product found in a variety of flowers and fruits, has received attention because of its potent antioxidant and anti-inflammatory properties (Ahad et al., 2014; Hajimohammadi and Nosrati, 2018).
Despite the well-documented phytochemical reports on P. incarnata, the exact mode of its anti-inflammatory and antioxidant effects and the phyto-constituents responsible for its much-acclaimed effects have never been described clearly. Moreover, only few studies focusing on the potential role of P. incarnata as an anti-inflammatory and antioxidant herbal food supplement in epileptic models are available. The study aims at assessing the ameliorative changes after treatment with P. incarnata in case of pilocarpine-induced epilepsy in male albino rats.
2 Materials and methods
2.1 Animals and ethics committee approval
White thirty-two Sprague Dawley adult male rats (150–180 g) were obtained from the animal house of the National Research Institute, Eldoki, Giza, Egypt. They were housed in individual cages (23± 1 °C; humidity, 55± 5%, 12-h light/dark cycle). Food and water were available ad libitum. All animal procedures were in accordance with the guidelines for the use of experimental animals by the AEC of Beni-Suef University under an approval number is BSU/FS/020/102.
2.2 Preparation of Passiflora incarnata
An extract of passionflower Passiflora incarnata L was as herbal medicine. It was obtained from the dietary supplement manufacturer, Naturactive Co. (Elusanes Passiflore; Lot# PAS K00018; Pierre Fabre Medicament Prod., 45, Place Abel Gance, 92100 Boulogne). According to the entire pharmacopeia leaflet, the active substance for each capsule is Passiflora incarnata L. Each capsule contains the dry extract of the aerial parts of Passiflora incarnata L. The dose of each capsule was calculated as 200 mg on maltodextrin and colloidal silica hydrate. Extraction Solvent: 70% Ethanol (V/V).
2.3 Induction of epilepsy in the rats using pilocarpine and animal groups
Epilepsy was induced as previously performed (Turski et al. 1989; Abdel-Reheim 2009). Prior to treatment with 300 mg/kg of pilocarpine (New Jersey, USA) hydrochloride injection, the experimental rats were intraperitoneally injected with methylscopolamine (Nanjing, China) (1mg/kg) for 30 min. Then, animal’s behavior was evaluated as indicative of seizure activity was assessed. These criteria for successful modeling: epileptic seizure symptoms (sluggishness, salivation, tremors, convulsions, etc.). Such behaviors were observed in model rats for 120 min in comparison to the normal rats. Rats were considered suffer from seizure episodes when generalized seizure activity was continuously observed without normal behavior during each episode. Thus, the attacks occurred every 2–5 min. Seizures were terminated with diazepam (4 mg/kg, i.p.) delivered every 20 min as needed. Control rats were treated with PBS (pH 7.4; 0.2 ml/rat) was injected instead of pilocarpine, followed, 1 h later, by diazepam.
Rats were divide into four groups (n = 8) as follows: (1) Control group (C) received the standard diet, free access to water and orally fed with PBS (pH 7.4; 0.2 ml/rat) using intragastric intubation at intervals parallel to the treated groups. (2) P. incarnata group (PI) was orally fed with Passiflora extract using intragastric intubation at a dose of 200 mg/kg b.wt/day, continued for four consecutive weeks; (3) the positive epileptic group, epileptic control “E” was treated with 300 mg/kg of pilocarpine hydrochloride injection as described previously; and (4) Epileptic treated with P. incarnate (E-PI) (300 mg/kg of pilocarpine hydrochloride injection, then orally fed with Passiflora extract at a dose of 200 mg/kg b.wt/day; continued for four weeks).
2.4 Blood, tissue sampling and biochemical measurements
Blood samples were collected in two parts, EDTA-anticoagulated and EDTA-free samples. Plasma were stored at −80 °C for subsequent measurement of cytokines by ELISA in duplicates. Sera samples were separated for electrolytes. Brain tissues were cleaned from the fatty parts and homogenized in ice-cold 50 mM sodium phosphate buffer (pH 7.4). The supernatant was separated and lipid peroxidation level (MDA) was estimated according to the methods of Preuss et al. (1998), and glutathione reductase (GR) was estimated as previously mentioned (Kar and Mishra 1976).
On the other hand, MyBioSource Co. assay kits were used for quantitative detection of L-dihydroxyphenyalanine (L-DOPA; µg/ml); serotonin (ng/ml); epinephrine (ng/ml); Norepinephrine (pg/ml); Na+/K+ATPase (mmol/ml); glutamate (µg/ml); aspartic acid (ng/ml); gamma-aminobutyric acid (GABA, pg/ml) and glycine (μg/ml); using kits with catalogue numbers (MBS9357024, MBS775307, MBS031232, MBS269993, MBS8243226, MBS047402, MBS7254596, MBS269152, MBS095285, respectively). Creatine kinase (CPK, U/L) activity was estimated using EnzyChrom™ Assay Kit (Cat. No: ECPK-100). Acetylcholinesterase (U/L) was performed according to the manual of QuantiChrom™ Assay Kit (Cat. No: DACE-100).
According to the instruction manual of MyBioSource; sera concentration of Sodium (Na+, mmol/L) and Potassium (K+, mmol/L) were estimated using kits with Cat. No. MBS2540574 and MBS2540590, respectively. On the other hand, Calcium (Ca+2, mg/dL) and Magnesium (Mg+2, mg/dL) levels were quantitatively determined as the manual of QuantiChrom™ Assay (Cat. No: DICA-500 and DIMG-250, respectively).
Level of cytokines were estimated in plasma, according to ELISA quantitative instruction manuals e.g. IL-1β and TNF-α (RayBiotech™; Cat. No: ELR-IL1β & ELR-TNFα, respectively), IL-6 and IL-10 (Quantikine®; Cat. No: R6000B & R1000, respectively), IL-13 and TGF-β (using Picokine™ Cat. No: MBS175932 & MBS175833) and IL-17 (using CUSABIO Cat. No: CSB-E07451r).
Regarding antibodies for Flowy cytometry, cells were stained with mAbs and analyzed using a FACSCalibur (BD, Franklin Lakes, NJ) according to Neu et al. (2013) and Abuelsaad (2014). Briefly, purified lymphocytes from brain tissues (1 x 106 cells/50 ml PBS) were washed once with washing buffer (3% (v/v), FBS and 0.1% (w/v) NaN3 in PBS. Cells were resuspended in blocking buffer (3% (v/v) FBS; 5% (v/v) normal human AB serum (Cat# C11-020; PAA Laboratories, Germany and 0.1% NaN3 (w/v) in PBS) with purified CD16/CD32 FccII/III mAb; AbD Serotec Co, USA) to prevent non-specific binding. Cells were incubated with mAb Fluor-conjugated FITC mouse anti-rat antibody purchased from AbD Serotec Co, USA as follows: CD4-FITC (MCA153F, respectively), and PE-conjugated anti-CD8 (CAT# MCA48PE). Subsequently, cells were washed, fixed in paraformaldehyde (PFA; 4% (v/v) in PBS; Sigma Aldrich, Germany) and stored at 4 ○C in washing buffer until further use. A FACS Calibur flow cytometer was used for data acquisition, with Diva software (BD Biosciences) for data analysis. After gating on viable cells, 10,000 events per sample were analyzed. For each marker, the threshold of positivity was defined beyond the nonspecific binding observed in the presence of a relevant control mAb.
2.5 Statistical analysis
The data were analyzed using Tukey-Kramer method for post-hoc analysis to compare various groups with each other. The results were expressed as mean ± standard deviation (SD). Statistical significance interval is considered as P < 0.05 for all data. All results were analyzed using Statistical Package for Social Science (SPSS) version 20 software (IBM Corp., 2011).
3 Results
3.1 Oxidative stress
Table 1 reveals an altered oxidative/antioxidant status and shows a significant increase (P < 0.001) in MDA level in the epileptic non-treated group (E) after pilocarpine injection. MDA recorded the highest level in epilepsy group of 579.631 ± 66.56 nmol/g tissue, compared to the control (C), PI, and E-PI groups (324.77 ± 54.21, 252.82 ± 30.05 and 467.66 ± 32.26 nmol/g tissue, respectively). Therefore, treatment with Passiflora extract (200 mg/kg) showed ameroliative effects on brain lipid peroxidation and modified the altered level of MDA.
Groups
MDA
(nmol/g tissue)Glutathione reductase
(nm/mg tissue)SOD
(U/g tissue)Catalase
(U/g tissue)
C
324.765 ± 54.208a
47.914 ± 1.380bc
10.286 ± 1.419bc
490.053 ± 20.993b
E
579.631 ± 66.562b
25.543 ± 4.284a
4.599 ± 0.610a
358.342 ± 28.354a
PI
252.822 ± 30.055a
36.800 ± 3.637ab
13.134 ± 2.642c
477.962 ± 50.196b
EP_PI
467.661 ± 32.265b
57.867 ± 9.843c
8.741 ± 1.442b
431.595 ± 11.027b
F value
29.824
16.654
13.733
12.204
P <
0.000
0.001
0.001
0.002
In addition, treatment with P. incarnata caused a significant increase (P < 0.001) in the antioxidant enzymatic levels of glutathione reductase, GR (57.867 ± 9.84 nm/mg tissue), SOD (8.74 ± 1.44 U/g tissue) and CAT (431.60 ± 11.03 U/g tissue). These values were compared to the dramatic decrease in the epileptic non-treated group which recorded 36.800 ± 3.64 nm/mg tissue, 13.134 ± 2.64 and 477.962 ± 50.20 U/g tissue for GR, SOD and CAT, respectively.
3.2 Changes in brain electrolytes
Regarding the changes in brain electrolytes, pilocarpine injection caused dramatic decrease in K+, Mg++ and Ca++ levels in brain tissues, recording 3.05 ± 0.08 mmol/L; 0.29 ± 0.08 mg/dL and 8.00 ± 0.25 mg/dL, respectively (Fig. 1). In addition, Na+ recorded the highest level in the epileptic non-treated group (189.32 ± 7.62 mmol/L). These imbalanced levels were corrected after treatment with Passiflora incarnata extract (200 mg/kg); and recorded 164.66 ± 5.27 mmol/L; 4.07 ± 0.47 mmol/L; 0.698 ± 0.04 mg/dL and 8.63 ± 0.21 mg/dL for Na+; K+, Mg++ and Ca++, respectively.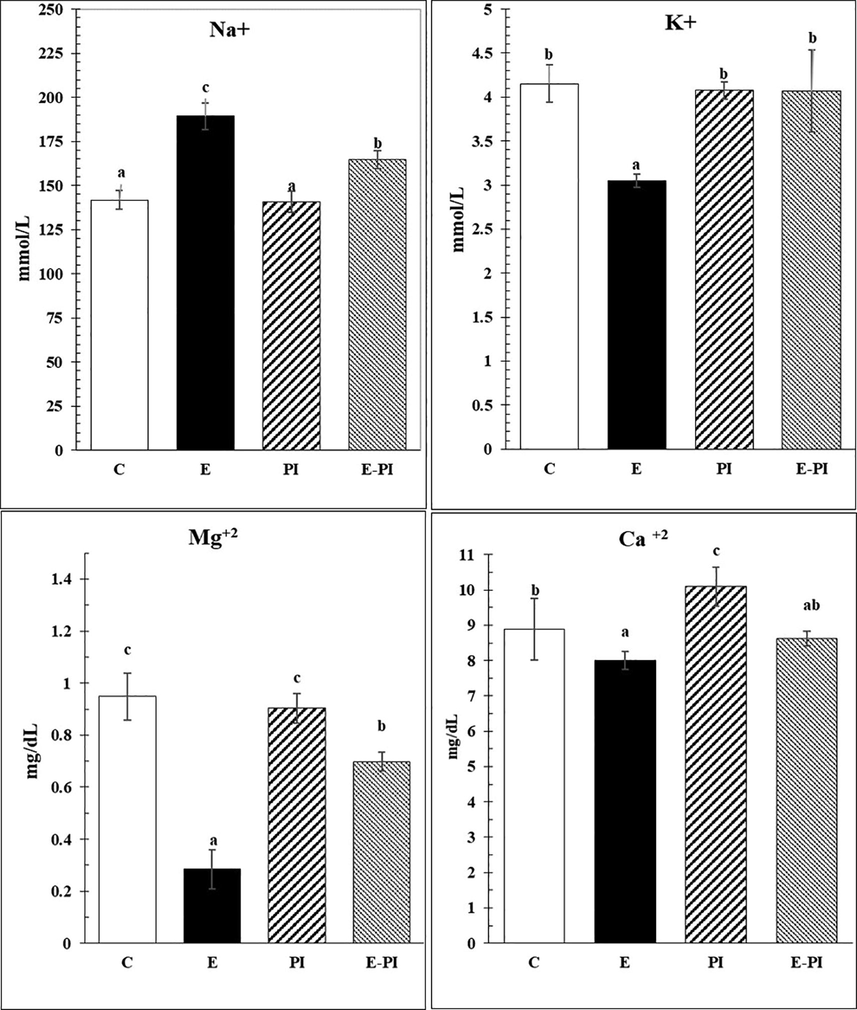
Changes in sera electrolytes of different groups: Control (C); Epileptic-non treated group (E); Passiflora incarnata group (PI) and Epileptic-treated with P. incarnata; (E-PI) (200 mg/Kg. b.wt). Groups not sharing common superscripts denote significant differences (*P < 0.05, **P < 0.01 and ***P < 0.001). Values were represented as Mean ± SD; n = 8 rats.
In addition, Fig. 2 shows a significant decrease (P < 0.001) in the level of Na+/K+-ATPase in brain tissues (an electrogenic transmembrane ATPase) after induction of status epilepsy, which recorded 2.11 ± 0.17 mmol/ml. The treatment with P. incarnata remodified Na+/K+-ATPase to the normal level 4.57 ± 0.34 mmol/ml as recorded in the control group (4.44 ± 0.38 mmol/ml). Moreover, creatine kinase (CK) showed a highly significant increase of 294.35 ± 12.53U/L in the second group (E) and returned to 183.72 ± 5.34 U/L after treatment with P. incarnata, though still significantly higher than the control group (111.23 ± 5.92 U/L).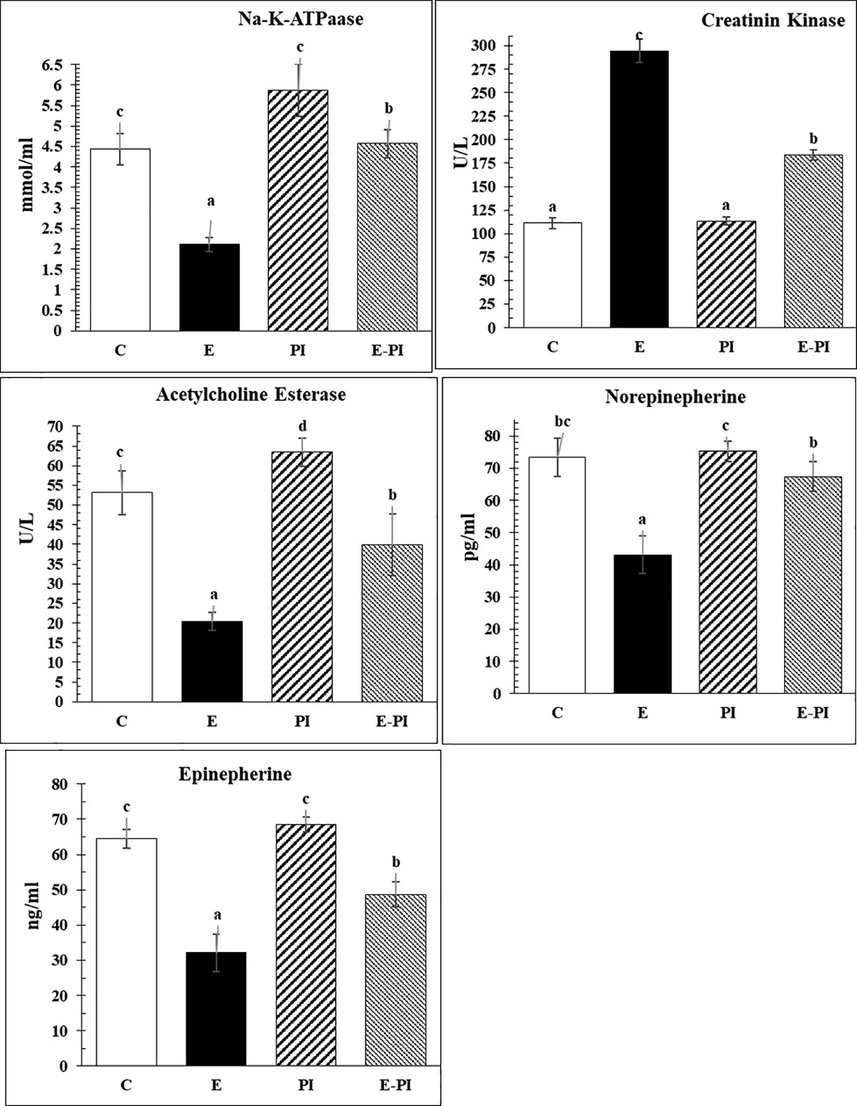
Changes in brain energy and neurotransmitters of different groups: Control (C); Epileptic-non treated group (E); Passiflora incarnata group (PI) and Epileptic-treated with P. incarnata; (E-PI) (200 mg/Kg. b.wt). Groups not sharing common superscripts denote significant differences (*P < 0.05, **P < 0.01 and ***P < 0.001). Values were represented as Mean ± SD; n = 8 rats.
3.3 Changes in neurotransmitters
In addition, the present data revealed significant decrease (P < 0.001) in different neurotransmitters and classical monoamines, e.g. norepinephrine (NE) and epinephrine (EP), whereas they decreased in the epileptic non-treated group, recording levels of 43.17 ± 5.75 and 32.10 ± 5.37 Pg/ml, respectively. Treatment with P. incarnata mitigated and upregulated them again to the healthy levels of 67.37 ± 4.62 and 48.65 ± 3.59 for norepinephrine and epinephrine, respectively (Fig. 2).
Regarding changes in other monoamines, e.g. dopamine (DOPA) and serotonin (5-HT), the epileptic non-treated group demonstrated a significant increase (P < 0.001) in DOPA (57.70 ± 3.77 µg/ml) and a significant decrease (P < 0.001) in serotonin level (39.78 ± 2.56 ng/ml), as compared to the control group, which recorded 24.60 ± 2.36 µg/ml and 77.07 ± 7.90 ng/ml for DOPA and serotonin, respectively (Fig. 3). Moreover, the treatment with P. incarnata modified both DOPA and serotonin levels, approximating their normal values of 37.43 ± 3.53 µg/ml and 67.72 ± 4.05 ng/ml, respectively (Fig. 3).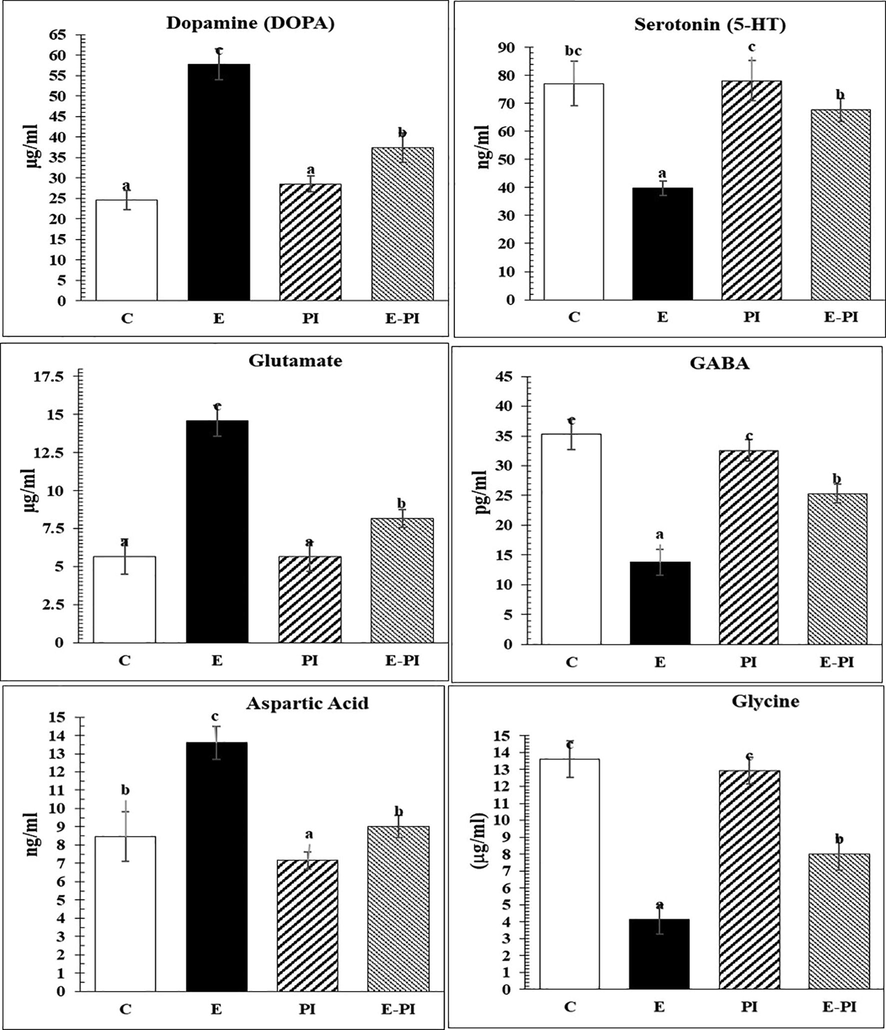
Changes in neurotransmitters (monoamines) and some amino acids of different groups: Control (C); Epileptic-non treated group (E); Passiflora incarnata group (PI) and Epileptic-treated with P. incarnata; (E-PI) (200 mg/Kg. b.wt). Groups not sharing common superscripts denote significant differences (*P < 0.05, **P < 0.01 and ***P < 0.001). Values were represented as Mean ± SD; n = 8 rats.
The effects of status epilepticus on the brain concentrations of glutamate, gamma-aminobutyric acid (GABA), aspartate and glycine were measured, as illustrated in Fig. 3. As mentioned in the epileptic non-treated group, GABA and glycine showed significant decrease level (13.82 ± 2.17 pg/ml and 4.13 ± 0.88 μg/ml, respectively), whereas glutamate and aspartic acid levels showed significant increase (14.58 ± 1.02 μg/ml and 13.60 ± 0.90 ng/ml, respectively). Treatment with P. incarnata modified the imbalanced levels of glutamate, γ-aminobutyric acid (GABA), aspartate and glycine to normal values of 8.165 ± 0.613 μg/ml; 25.338 ± 1.601 pg/ml; 9.022 ± 0.621 ng/ml and 8.000 ± 0.947 μg/ml, respectively.
4 Phenotyping of CD4+ and CD8+ brain infiltrative cells
Meanwhile, phenotyping of CD4+ and CD8+ brain infiltrative cells is illustrated in Table 2 and Fig. 4 by FACS-analysis. Changes in CD4+ and CD8+ markers in brain-infiltrating cells showed a highly significant (P < 0.001) elevation in both CD4+ and CD8+ expressed cells in the second group (E) (P < 0.001), recording values of 54.13 ± 4.54% and 36.52 ± 3.08%, respectively. However, treatment with P. incarnata caused a diminution in the subpopulation of CD4 + and CD8 lymphocytes and corrected the above-mentioned activities, recording 27.89 ± 2.31% and 17.31 ± 1.20% for CD4+ and CD8+, respectively. The modified activity was compared to the control group (C) which recorded 17.02 ± 2.63% and 11.55 ± 1.29% for CD4+ and CD8+, respectively.
Groups
CD4+ cells
CD8+ cells
C
17.017 ± 2.626a
11.550 ± 1.290a
E
54.133 ± 4.539c
36.517 ± 3.076c
PI
15.167 ± 1.595a
11.800 ± 1.203a
EP_PI
27.885 ± 2.314b
17.312 ± 1.198b
F value
218.563
237.969
P <
0.000
0.000
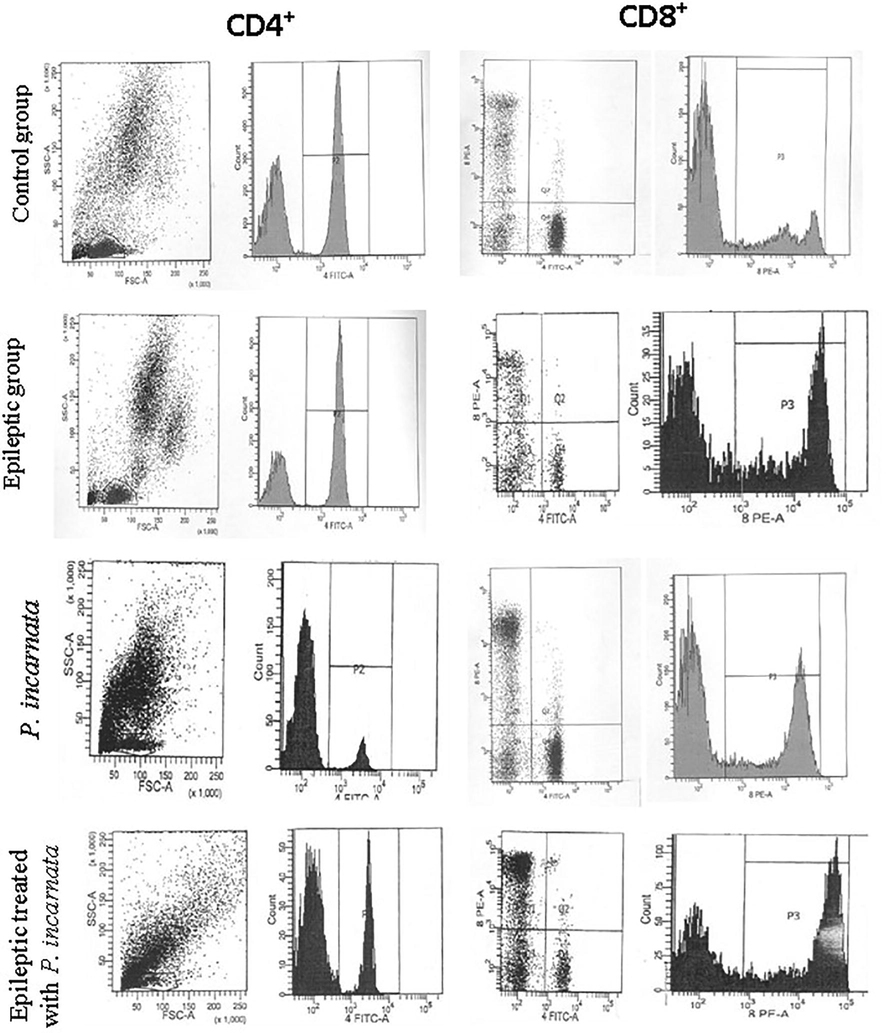
Representative dot plots of FACS-analysis showing changes in CD4 + and CD8 + brain infiltrating lymphocytes in different animal groups.
5 Assessment of the proinflammatory cytokines
Concerning assessment of the proinflammatory cytokines’ level, the ameroliative levels of IL-1β, IL-6, IL-17A, TNF-α, and TGF-β were significantly (P < 0.001) reduced to 30.03 ± 5.02; 59.72 ± 7.42; 69.42 ± 2.66; 53.37 ± 1.74 and 74.76 ± 5.30 pg/mL, respectively, after treatment with P. incarnata (Fig. 5). These ameroliative changes were compared to their level in the epileptic non-treated group (92.40 ± 7.34; 114.68 ± 2.40; 94.03 ± 4.27; 108.00 ± 6.27 and 124.70 ± 3.10 pg/mL, respectively). In addition, the treatment with Passiflora extract (200 mg/kg) showed an ameroliative increase in anti-inflammatory cytokines, e.g. IL-10 and IL-13, which re-increased significantly (P < 0.001) in the epileptic-treated group with P. incarnata, recording 67.17 ± 4.43 and 93.88 ± 9.27 pg/mL, respectively.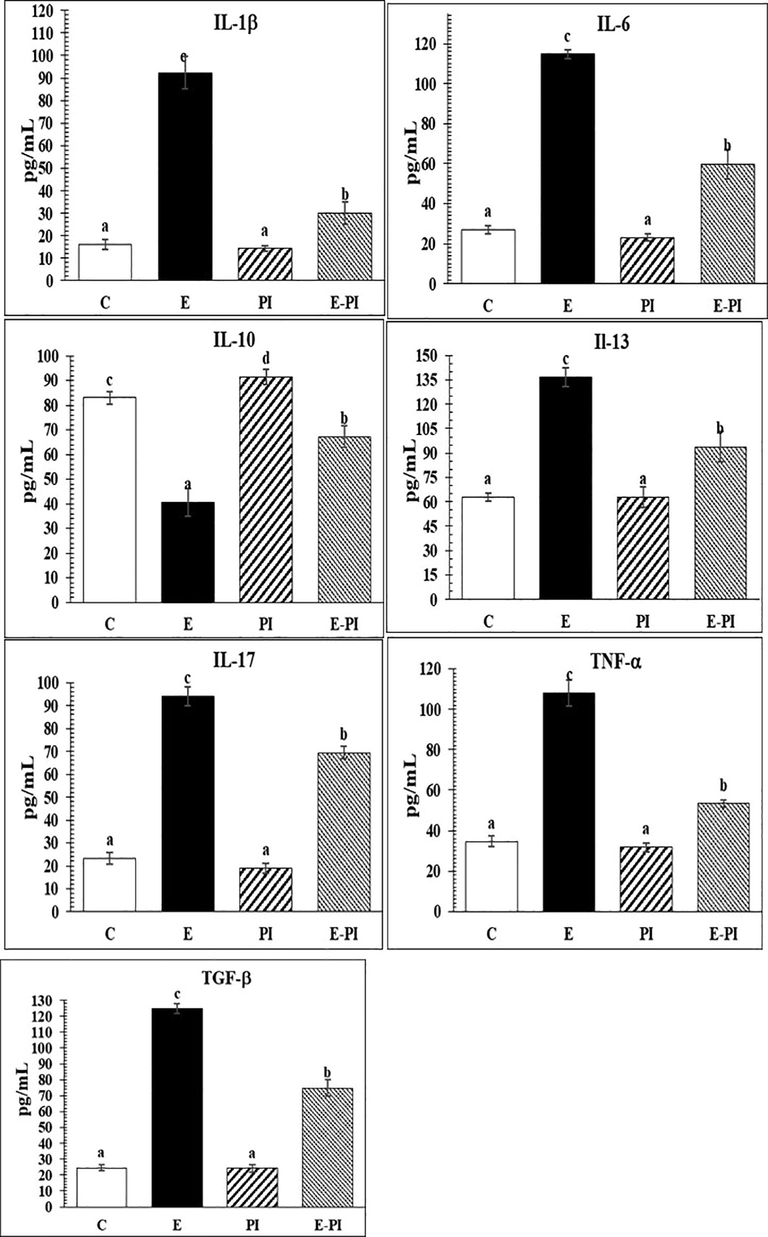
Changes in plasma pro and anti-inflammatory cytokines of different groups: Control (C); Epileptic-non treated group (E); Passiflora incarnata group (PI) and Epileptic-treated with P. incarnata; (E-PI) (200 mg/Kg. b.wt). Groups not sharing common superscripts denote significant differences (*P < 0.05, **P < 0.01 and ***P < 0.001). Values were represented as Mean ± SD; n = 8 rats.
6 Discussion
The present results showed that the antioxidant effect of P. incarnata treatment came in agreement with many reports (de Carvalho et al., 2011; Elsas et al., 2010; Sena et al., 2009). It is noteworthy that the antioxidant activities of Passiflora spp. can be traced to its nutritional components of flavonoids and phenols, as well as cysteine, glutathione, ascorbic acid, tocopherol, tannins, and aromatic amines (Saravanan et al., 2014). Generally, flavonoids can be oxidized by radicals, resulting in more stable, less-reactive radicals. Some flavonoids can scavenge superoxides, while others can scavenge the highly reactive oxygen-derived radical peroxynitrite (Esposito et al., 2002; Nabavi et al., 2015). In addition, Ingale and Kasture (2017) referred to the antioxidant potential of Passiflora via significant DPPH (2,2-diphenyl-1-picrylhydrazyl) and H2O2 scavenging ability due to the content of phenolic compounds, mainly flavonoids.
Moreover, Sasikala et al. (2014) observed a highly antioxidant scavenging capacity of Passiflora extracts spp. towards free radicals. These properties can reduce and donate hydrogen ions and mitigate side effects of free radicals released during lipid peroxidation and cell damage. In addition, Saravanan et al. (2014) confirmed that extracts of Passiflora spp. could donate electrons to the released free radicals in biological tissues and reduce its inflammatory stress. Also, Nabavi et al. (2015) mentioned that chrysin, as one of the flavonoids component of P. incranata, can reduce nerve cell damage via preventing the release of nitric oxide and inflammatory cytokines from activated microglia, hence preventing neurodegeneration events.
Furthermore, Colomeu et al. (2017) reported that phenolic compounds are ubiquitously present in plants with antioxidant properties. These activities may be attributed to their redox properties, e.g. hydrogen donors, reducing agents, singlet oxygen quenchers and metal chelators.
The significant increment (P < 0.001) in the levels of electrolytes recorded throughout the present data could be explained by Liu et al. (2008) who reported significant content of electrolytes and minerals in pasipay (P. incarnata), e.g. Na+, K+, Mg+2 and Ca+2. Therefore, they suggested such herbal drug as supplement food source in pharmaceutical and industrial fields.
In accordance with our data, during epileptic episodes, a massive increase of amino acids and neurotransmitters is observed (Bramlett and Dietrich 2004). These molecules lead to increased Ca2+, Na+ and K+ fluxes via stimulation of glutamate receptors. As a result, catabolic processes will be activated, resulting in breakdown of the blood–brain barriers (BBB) (Werner and Engelhard 2007). Furthermore, chrysin, as the main plant extract and bioactive component content of P. incarnata, can modify Na+, K+-ATPase activity in the prefrontal cortex and hippocampus of mice (Borges et al., 2016).
Previously, Singh et al. (2012) demonstrated that rats treated with extracts of P. incarnata showed a significant treatment of seizure severity and immobility period in a dose- and time-dependent manner. These effects may be traced to the retained levels of serotonin and noradrenaline in the brain.
The recorded changes in brain amino acid and neurotransmitters levels are strikingly similar to those observed in other studies. The current anticonvulsant effects of pasipay (P. incarnata) may be related to its activation for benzodiazepine receptor (BZD), GABAergic and opioid systems (Fernández et al., 2006). Nassiri-Asl et al. (2007) revealed that a 100% protection for seizure episodes and mortality occurs after treatment with a dose of 0.4 mg/kg of P. incarnata.
Because GABA is a major inhibitory neurotransmitter, the present data revealed that P. incarnata increased the level of GABA. This is in accordance with the findings of Appel et al. (2011) and Guerrero and Medina (2017) who noticed that P. incarnata bioactivities may be mediated by GABA modulation by activation of the kappa opioid receptor (KOPr) that leads to support of GABAergic activity or attenuation of glutamatergic activity. This may confirm that P. incarnata could activate KOPr and result in protective effects against pentylentetrazole (PTZ)-induced seizure (Nassiri-Asl et al., 2007). The presence of GABA has been reported in P. incarnata and has also been suggested to elicit GABA currents in hippocampal neurons (Elsas et al., 2010).
The flavonoids content of P. incarnate exerted the modulation of the GABA system, e.g. chrysin and/or homoorientin, orientin, vitexin, and isovitexin (Mani and Natesan 2018; Mark Jr et al., 2008; Ropper 2005). In addition, Borges et al. (2016) and Souza et al. (2015) confirmed that chrysin flavonoids can improve the brain (hippocampal) dysfunction and reduce depressive behavior via elevation of brain-derived neurotrophic factor (BDNF). Furthermore, apigenin, the main flavonoid of P. quadragularis extract, can enhance GABAergic system, leading to a sedative activity (Gazola et al., 2015). In addition, Gazola et al. (2018) results showed that the GABAA/benzodiazepine receptors are involved in the sedative activity of vitexin-2″-O-xyloside (V2OX), the main flavonoid of P. quadrangularis’ extract.
Our results revealed a significant decrease in noradrenaline and serotonin in the epileptic brain, which came in parallel with the findings of other studies, e.g. Madhyastha et al. (2005). In addition, Singh et al. (2012) recorded a retention of serotonin and noradrenaline levels of the brain after treatment with P. incarnata. They referred such retention to the presence of alkaloids contents, e.g. harmala alkaloids. These alkaloids are reversible monoamine oxidase (MAO)-A inhibitor, thereby prevent the degradation of serotonin and noradrenaline (Abourashed et al., 2003). Therefore, the recorded ameliorative increased levels of serotonin and noradrenaline in the present study might be due to MAO-A inhibitory action of harmala alkaloids content in P. incarnata. In conclusion, the bioactive metabolites of P. incarnata might ameliorate the different consequences of epilepsy.
The inflammatory response that occurs in brain tissues during epilepsy involves MHC class I and II proteins, and macrophages activation, and CD4+ and CD8+ lymphocytes (Atkinson and Eisenbarth, 2001). The cytokine is a biomarker for inflammation and predict the next seizure episodes (Wang et al., 2015). The present data revealed a significant elevation for different proinflammatory cytokines e.g. IL-1, Il-6, TNF-α and IL-17 in epileptic non-treated group. These findings came in parallel with Wang et al. (2015) and Huang and Zhu (2018). Some studies reported that the level of serum IL-1β, IL-6 and IL-17 were significantly elevated in CSF patients with epilepsy (Mao et al., 2013; Peltola et al., 2000; Pernhorst et al., 2013; Sinha et al., 2008; Uludag et al., 2013). Other studies reported that after injury and breakdown of blood–brain barriers (BBB), elevated TGF-β expression resulted from the activated astrocytes and microglia. These events came in correlation with the neural dysfunction resulting in elevation of IL-1, IL-6, IL-8, IL-10, G-CSF, TNFα and MCP-1 (Ajmo Jr et al., 2008; Das et al., 2012; Morganti-Kossmann et al., 2010; Walker et al., 2010).
Regarding IL-6, it was elevated after focal and generalized seizures in epileptic patients (Alapirtti et al., 2009; Bauer et al., 2009; Lehtimäki et al., 2004; Lehtimäki et al., 2007). It was similarly increased in many neurological diseases such as trauma, Alzheimer’s disease and meningitis (Bauer et al., 2009; Frei et al., 1988; Woodroofe et al., 1991). Wang et al. (2015) reported that IL-6 was a unique cytokine associated with seizure severity. Therefore, IL-17 (Mao et al., 2013) and IL-6 (Wang et al., 2015) may be proposed as candidates to indicate seizure severity in general.
The present data came in parallel with others studies, e.g. Dinarello (2011); Choi et al. (2011); Zurolo et al. (2011) and Wang et al. (2015). The maximal increase in proinflammatory cytokines was reaching a high peak after six hours; IL-1 (445%), IL-6 (405%), and TNF- α (264%). They confirmed such high fold increment in glial cells within areas of intense microglia activation. The elevated proinflammatory cytokines may be due to the activated microglia cells, astrocytes, and cytokine production in late phases after seizures.
Strongly correlated with the recorded level of GABA, Prud'homme et al. (2013) reported that GABA suppresses both T cells and macrophages; suppresses production of the inflammatory cytokines; and increases TGF-beta production and regulatory T cells (Treg; IL-17). The recorded decreased level of GABA in epileptic group came in parallel with the significant high levels of IL-1β, IL-6, TNF-α and Il-17, released from the activated astrocytes and microglia (Su et al., 2015; Wang et al., 2000). Taken together, these events might contribute to the neuronal excitability via inhibition of the GABA system during epileptogenesis.
The present data revealed a strong correlation between neurotransmitters and oxidative stress imbalance and the pro-inflammatory cytokines levels in epilepsy group. The present data demonstrated that treatment with P. incarnata significantly mitigates the neuroinflammation events. In this context, chrysin as a monoflavonide of P. incranata is known as a potent antioxidant and anti-inflammatory agent Lee and Park 2015). In addition, chrysin could be a potential prophylactic agent for cerebral ischemia/reperfusion injury (Yao et al., 2014). Mani and Natesan’s (2018) study confirmed that chrysin prevents cellular membrane damage, protein damage, and the imbalance of cellular functions, via limiting the lipid peroxidation. They added that chrysin could bind to COX-2 and limit inflammatory cytokines via blocking NF-kB activation. Chrysin also significantly suppresses the serum levels of pro-inflammatory cytokines, IL-1β, IL-6, TNF-α and TGF-β expression and inhibits NF-кB activation (Ahad et al., 2014). Moreover, chrysin is reported as an inhibitory agent for TNF-α and IL-1β (Bai et al., 2013). The anti-inflammatory activity of chrysin was reported via blocking histamine release and pro-inflammatory cytokine expression (Bae et al., 2011). Therefore, Bae et al. (2011) confirmed a molecular basis for the neuroprotective effects of chrysin in ischemia/reperfusion injury.
Furthermore, the present results recorded a significantly high level of TNF-α in sera of epileptic untreated rats. TNF-α induces the recruitment of inflammatory cells and promotes ROS generation. Therefore, chrysin flavonoid contents may significantly reduce the expression of TNF-α in epileptic treated rats. This finding demonstrates the previously-reported efficacy of chrysin as a potent inhibitor of the TNF-α pathway (Lee and Park, 2015).
However, the present data showed more efficiency of P. incarnata to decrease CD4+ and CD8+ activities. These differences on T lymphocytes proliferation may be related to the different types of the phenolic compounds content (Atoui et al., 2005; Colomeu et al., 2017; Zucolotto et al., 2012). In addition, the anti-inflammatory activities of P. incarnata may be related to their polyphenols contents, e.g. Rutin, Isoorientin, Vitexin, and Catechin; due to their capability to decrease the inflammatory cells by anti-proliferative action (Colomeu et al., 2017; Tang et al., 2014).
In conclusion, the anticonvulsant effects of P. incarnata may be due to its antioxidant and anti-inflammatory actions, as well as reduction in IL-1β; Il-6, IL-17, TNF-α and TGF-β levels and inhibition in amino acids and GABAergic. However, unlike other natural products, the therapeutic benefits of P. incarnata remain nascent in current literature. Taken together, the present work tries to support further attempts to modulate seizures and epilepsy manifestation by means of modulating the inflammatory cascades using P. incarnata.
7 Availability of data and materials
The data used to support the findings of this study are included in the article.
Acknowledgements
This study is supported by the Deanship of Scientific Research at King Saud University through the Research group Project No. RG1436-004. The authors also thank the Deanship of Scientific Research and RSSU at King Saud University for their technical support.
Compliance with ethical standards
Ethical approval and consent to participate
All animal procedures were conducted in accordance with the standards set in the guidelines for the care and use of experimental animals by the Animal Ethics Committee of Beni-Suef University under an approval number is BSU/FS/020/102.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Physiological and biochemical studies on the melatonin effect on the fertility of epileptic rats. J. Egypt Ger. Soc. Zool.. 2009;58:1-25.
- [Google Scholar]
- High-speed extraction and HPLC fingerprinting of medicinal plants–II. Application to harman alkaloids of genus Passiflora. Pharm. Biol.. 2003;41:100-106.
- [Google Scholar]
- Supplementation with Astragalus polysaccharides alters Aeromonas-induced tissue-specific cellular immune response. Microb. Pathog.. 2014;66:48-56.
- [Google Scholar]
- Chrysin, an anti-inflammatory molecule, abrogates renal dysfunction in type 2 diabetic rats. Toxicol. Appl. Pharmacol.. 2014;279(1):1-7.
- [Google Scholar]
- The spleen contributes to stroke-induced neurodegeneration. J. Neurosci. Res.. 2008;86(10):2227-2234.
- [Google Scholar]
- Interleukin-6, interleukin-1 receptor antagonist and interleukin-1beta production in patients with focal epilepsy: a video–EEG study. J. Neurol. Sci.. 2009;280:94-97.
- [Google Scholar]
- Modulation of the γ-aminobutyric acid (GABA) system by Passiflora incarnata L. Phytother. Res.. 2011;25:838-843.
- [Google Scholar]
- Type 1 diabetes: new perspectives on disease pathogenesis and treatment. The Lancet. 2001;358:221-229.
- [Google Scholar]
- Tea and herbal infusions: their antioxidant activity and phenolic profile. Food Chem.. 2005;89:27-36.
- [Google Scholar]
- Chrysin suppresses mast cell-mediated allergic inflammation: involvement of calcium, caspase-1 and nuclear factor-κB. Toxicol. Appl. Pharmacol.. 2011;254(1):56-64.
- [Google Scholar]
- Effects and the mechanisms of chrysin on sepsis-associated acute lung injury of rats chrysin inhibits acute lung injury. Life Sci. J.. 2013;10:1052-1058.
- [Google Scholar]
- Etiology and site of temporal lobe epilepsy influence postictal cytokine release. Epilepsy Res.. 2009;86(1):82-88.
- [Google Scholar]
- Chrysin promotes attenuation of depressive-like behavior and hippocampal dysfunction resulting from olfactory bulbectomy in mice. Chem. Biol. Interact.. 2016;260:154-162.
- [Google Scholar]
- Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J. Cereb. Blood Flow Metab.. 2004;24(2):133-150.
- [Google Scholar]
- Capasso, A., Piacente, S., Pizza, C., Sorrentino, L. (1998). Flavonoids reduce morphine withdrawal in‐vitro J. Pharm. Pharmacol. 50:561-564.
- Increased levels of HMGB1 and pro-inflammatory cytokines in children with febrile seizures. J. Neuroinflammation. 2011;8(1):135.
- [Google Scholar]
- Comparison of antioxidant and ant proliferative effect among four Passiflora spp. J. Agric. Life Sci.. 2017;4:1-8.
- [Google Scholar]
- New perspectives on central and peripheral immune responses to acute traumatic brain injury. J. Neuroinflammation. 2012;9:236.
- [Google Scholar]
- Involvement of GABAergic non-benzodiazepine sites in the anxiolytic-like and sedative effects of the flavonoid baicalein in mice. Behav. Brain Res.. 2011;221(1):75-82.
- [Google Scholar]
- Dinarello, C.A. (2011). Interleukin-1 in the pathogenesis and treatment of inflammatory diseases Blood:blood-2010-2007-273417.
- Passiflora incarnata L. (Passionflower) extracts elicit GABA currents in hippocampal neurons in vitro, and show anxiogenic and anticonvulsant effects in vivo, varying with extraction method. Phytomedicine. 2010;17:940-949.
- [Google Scholar]
- A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol. Aging. 2002;23(5):719-735.
- [Google Scholar]
- Central nervous system depressant action of flavonoid glycosides. Eur. J. Pharmacol.. 2006;539(3):168-176.
- [Google Scholar]
- Production of B cell stimulatory factor-2 and interferon gamma in the central nervous system during viral meningitis and encephalitis. Evaluation in a murine model infection and in patients. J. Exp. Med.. 1988;168:449-453.
- [Google Scholar]
- Involvement of GABAergic pathway in the sedative activity of apigenin, the main flavonoid from Passiflora quadrangularis pericarp. Rev. Bras. Farmacogn.. 2015;25(2):158-163.
- [Google Scholar]
- The sedative activity of flavonoids from Passiflora quadrangularis is mediated through the GABAergic pathway. Biomed. Pharmacother.. 2018;100:388-393.
- [Google Scholar]
- Guerrero, F.A., Medina, G.M. (2017). Effect of a medicinal plant (Passiflora incarnata L) on sleep. Sleep Sci. 10:96.
- Scavenging effect of pasipay (passiflora incarnate L.) on singlet oxygen generation and fatty acid photooxygenation. Food Sci. Nutr.. 2018;6:1670-1675.
- [Google Scholar]
- MiR-134 expression and changes in inflammatory cytokines of rats with epileptic seizures. Eur. Rev. Med. Pharmacol. Sci.. 2018;22:3479-3484.
- [Google Scholar]
- Protective effect of standardized extract of Passiflora incarnata flower in parkinson and alzheimer's disease Ancient Science of. Life. 2017;36:200-206.
- [CrossRef] [Google Scholar]
- Catalase, peroxidase and polyphenol oxidase activities during rice leaf senescence. Plant Physiol.. 1976;57(2):315-319.
- [Google Scholar]
- Anti-inflammatory effect of chrysin on RAW 264.7 mouse macrophages induced with polyinosinic-polycytidylic acid. Biotechnol. Bioprocess Eng.. 2015;20:1026-1034.
- [Google Scholar]
- Regulation of IL-6 system in cerebrospinal fluid and serum compartments by seizures: the effect of seizure type and duration. J. Neuroimmunol.. 2004;152(1-2):121-125.
- [Google Scholar]
- Increased plasma levels of cytokines after seizures in localization-related epilepsy. Acta Neurol. Scand.. 2007;116:226-230.
- [Google Scholar]
- Physical and chemical analysis of Passiflora seeds and seed oil from China. Int. J. Food Sci. Nutr.. 2008;59:706-715.
- [Google Scholar]
- Effect of intracerebroventricular methotrexate on brain amines. Indian J. Physiol. Pharmacol.. 2005;49:427.
- [Google Scholar]
- Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry. 2018;145:187-196.
- [Google Scholar]
- Mao, L.Y., Ding, J., Peng, W.F., Ma, Y., Zhang, Y.H., Fan, W., Wang, X. (2013). Interictal interleukin-17A levels are elevated and correlate with seizure severity of epilepsy patients Epilepsia 54:e142-145 doi:10.1111/epi.12337.
- Mark Jr, T.M., Reginald Hills, B., Dixon, P. (2008). The effects of chrysin, a Passiflora incarnata extract, on natural killer cell activity in male Sprague-Dawley rats undergoing abdominal surgery. AANA J. 76:113.
- Morganti-Kossmann, C., Semple, B., Ziebell, J., Yan, E., Bye, N., Kossmann, T. (2010). Modulation of immune response by head injury. In: New Insights to Neuroimmune Biology. Elsevier, pp 193-220.
- Neuroprotective effects of chrysin: From chemistry to medicine. Neurochem. Int.. 2015;90:224-231.
- [CrossRef] [Google Scholar]
- Anticonvulsant effects of aerial parts of Passiflora incarnata extract in mice: involvement of benzodiazepine and opioid receptors. BMC Complement. Altern. Med.. 2007;7:26.
- [Google Scholar]
- CD14-dependent monocyte isolation enhances phagocytosis of listeria monocytogenes by proinflammatory, GM-CSF-derived macrophages. PLoS One. 2013;8:e66898
- [Google Scholar]
- Interleukin-6 and interleukin-1 receptor antagonist in cerebrospinal fluid from patients with recent tonic–clonic seizures. Epilepsy Res.. 2000;41:205-211.
- [Google Scholar]
- TLR4, ATF-3 and IL8 inflammation mediator expression correlates with seizure frequency in human epileptic brain tissue. Seizure. 2013;22:675-678.
- [Google Scholar]
- Patients’ perspectives on services for epilepsy: a survey of patient satisfaction, preferences and information provision in 2394 people with epilepsy. Seizure. 2000;9:551-558.
- [Google Scholar]
- Comparative effects of chromium vanadium and Gymnema sylvestre on sugar-induced blood pressure elevations in SHR. J. Am. Coll. Nutr.. 1998;17(2):116-123.
- [Google Scholar]
- Prud'homme, G., Glinka, Y., Wang, Q. (2013) .GABA exerts anti-inflammatory and immunosuppressive effects (P5175). Am. Assoc. Immnol.
- Anticonvulsant effect of FS-1 subfraction isolated from roots of Delphinim denudatum on hippocampal pyramidal neurons. Phytother. Res.. 2003;17:38-43.
- [Google Scholar]
- Adams and Victor's Principles of Neurology. Vol vol 179. Division New York: McGraw-Hill Medical Pub; 2005.
- Antioxidant, analgesic, anti-inflammatory and antipyretic effects of polyphenols from Passiflora subpeltata leaves–A promising species of Passiflora. Ind. Crops Prod.. 2014;54:272-280.
- [Google Scholar]
- Sasikala, V., Saravana, S., Parimelazhagan, T. (2014). Evaluation of antioxidant potential of different parts of wild edible plant Passiflora foetida L.
- Neuropharmacological activity of the pericarp of Passiflora edulis flavicarpa degener: putative involvement of C-glycosylflavonoids. Exp. Biol. Med.. 2009;234:967-975.
- [Google Scholar]
- Shlosberg, D., Benifla, M., Kaufer, D., Friedman, A. (2010). Blood–brain barrier breakdown as a therapeutic target in traumatic brain injury. Nature Rev. Neurol. 6:393.
- Dual protective effect of Passiflora incarnata in epilepsy and associated post-ictal depression. J. Ethnopharmacol.. 2012;139:273-279.
- [CrossRef] [Google Scholar]
- Flavonoid Chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharmacol. Biochem. Behav.. 2015;134:22-30.
- [Google Scholar]
- Role for pro-inflammatory cytokines in regulating expression of GABA transporter type 1 and 3 in specific brain regions of kainic acid-induced status epilepticus. Neurochem. Res.. 2015;40:621-627.
- [Google Scholar]
- Tea catechins inhibit cell proliferation through hydrogen peroxide-dependent and-independent pathways in human T lymphocytic leukemia Jurkat cells. Food Sci. Technol. Res.. 2014;20:1245-1249.
- [Google Scholar]
- Cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: a novel experimental model of intractable epilepsy. Synapse. 1989;3:154-171.
- [Google Scholar]
- Interleukin-6, interleukin-1 beta and interleukin-1 receptor antagonist levels in epileptic seizures. Seizure. 2013;22:457-461.
- [Google Scholar]
- Progenitor cell therapy for traumatic brain injury: effect of serum osmolarity on cell viability and cytokine production. Regen. Med.. 2010;5:65-71.
- [Google Scholar]
- Interleukin-1beta inhibits gamma-aminobutyric acid type A (GABA(A)) receptor current in cultured hippocampal neurons. J. Pharmacol. Exp. Ther.. 2000;292:497-504.
- [Google Scholar]
- Wang, Y., Wang, D., Guo, D. (2015). Interictal cytokine levels were correlated to seizure severity of epileptic patients: a retrospective study on 1218 epileptic patients. J. Transl. Med. 13:378 doi:10.1186/s12967-015-0742-3.
- Pathophysiology of traumatic brain injury. Br. J. Anaesth.. 2007;99:4-9.
- [CrossRef] [Google Scholar]
- Detection of interleukin-1 and interleukin-6 in adult rat brain, following mechanical injury, by in vivo microdialysis: evidence of a role for microglia in cytokine production. J. Neuroimmunol.. 1991;33:227-236.
- [Google Scholar]
- Chrysin protects against focal cerebral ischemia/reperfusion injury in mice through attenuation of oxidative stress and inflammation. Int. J. Mol. Sci.. 2014;15:20913-20926.
- [Google Scholar]
- Behavioral characterisation of the flavonoids apigenin and chrysin. Fitoterapia. 2000;71:S117-S123.
- [Google Scholar]
- Analysis of C-glycosyl flavonoids from South American Passiflora species by HPLC-DAD and HPLC-MS. Phytochem. Anal.. 2012;23:232-239.
- [Google Scholar]
- Activation of Toll-like receptor, RAGE and HMGB1 signalling in malformations of cortical development. Brain. 2011;134:1015-1032.
- [Google Scholar]







