Translate this page into:
Microwave-assisted green synthesis of silver nanoparticles by extracts of fig fruits and myrrh oleogum resin and their role in antibacterial activity
⁎Corresponding authors at: Microbiology Department, Howard University, 2400 Sixth Street, N.W, Washington, DC 20059, USA (Jamilah A. Alsulaimi) and Department of Botany & Microbiology, College of Science, P.O. Box-22452, King Saud University, Riyadh-11495, Saudi Arabia (Kahkashan Perveen). Jamilah.Alsulami@bison.howard.edu (Jamilah A. Alsulami), kperveen@ksu.edu.sa (Kahkashan Perveen),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Multidrug-resistant bacteria hindering disease management have become a matter of serious concern. Nanotechnology has evolved as a fresh promise for managing infectious diseases. The intended purpose of this study was to synthesize silver nanoparticles using fig fruit (Ficus carica) and myrrh oleogum resin (Commiphora sp.) extracts and microwave irradiation. UV–visible, FTIR, TEM, DLS and Zeta-potential analysis were deployed to characterise silver nanoparticles. The synthesized nanoparticles were assessed for antibacterial activity against four pathogenic bacteria, and their effects on the bacteria's cells were examined as well through SEM. Fig fruit and myrrh’s extract reaction solution changed color (reddish brown) after 90 and 120 s of microwave operation, respectively. UV–Vis-validated the synthesis of silver nanoparticles with fig fruit extract (FAgNPs) and myrrh extract (MAgNPs), showing absorption bands between 434.41 nm and 434.95 nm. TEM revealed that the FAgNPs and MAgNPs were predominantly spherical and of various sizes. The average diameter of FAgNPs and MAgNPs was 33.79 nm and 31.63 nm, respectively, and both of them were moderately polydispersed and relatively stable colloids. The antibacterial evaluation of FAgNPs shows that the highest level of inhibition was against S. aureus and it was higher than that of Augmentin, and next to it were E. coli and S. pyogenes. While in case of MAgNPs the most sensitive pathogen was S. aureus, followed by E. coli and S. pyogenes, respectively. FAgNPs and MAgNPs had MIC values of 15 µg/ml against S. aureus, compared to 30 µg/ml against E. coli. SEM images showed that treatment with FAgNPs and MAgNPs caused S. aureus cells to become malformed. Similar cell damage was also observed in E. coli cells treated with FAgNPs and MAgNPs. This study for the first time report the synthesis of silver nanoparticles utilizing fig fruit and myrrh extracts and microwave irradiation.
Keywords
Green Silver nanoparticles
Microwave irradiation
Antimicrobial action
Environmental pollution
Medicine
Infectious diseases
- AgNO3
-
silver nitrate
- AgNPs
-
Silver nanoparticles
- AMR
-
antimicrobial resistance
- CFU
-
Colony-forming unit
- DLS
-
Dynamic light scattering
- FAgNPs
-
Fig extract mediated synthesised silver nanoparticles
- FTIR
-
Fourier transform infrared
- MAgNPs
-
Myrrh extract mediated synthesised silver nanoparticles
- MIC
-
Minimum inhibitory concentration
- MRSA
-
methicillin-resistant S. aureus
- PdI
-
Polydispersity index
- TEM
-
Transmission electron microscopy
- UV–Vis
-
Ultraviolet–visible
Abbreviations
1 Introduction
Nano-medicine, an interdisciplinary field that uses nanotechnology in medicine, might improve the management of many ailments. Human health and microbes are intricately linked. Some help, some hurt. Tropical and subtropical nations get severe diseases from microorganisms, including bacteria, fungus, and viruses. Due to the indiscriminate use of commercial antimicrobial medications to treat such infections, human pathogenic bacteria have acquired diverse antibiotic resistance in recent years. Emerging infectious diseases and the rapid medication resistance of harmful bacteria are concerning. Despite modern therapies, microbial infections are still common and deadly (Maxson & Mitchell 2016). Due to the ongoing growth of pathogens resistant to traditional antimicrobials, pharmaceutical firms are pushed to create novel antimicrobials. Modifying antimicrobial substances or creating new compounds to boost antibacterial activity for treatment, antisepsis, or disinfection is important. Most of the bacteria are harmless to humans; several are beneficial, while many are categorized as pathogenic and cause infectious diseases. Streptococcus and Pseudomonas may cause pneumonia, while E. coli, gastroenteritis, urinary tract infection, meningitis, and S. aureus are mainly associated with skin and respiratory diseases. With that, these two may be associated with hospital-acquired infections as well. With time, many have developed antimicrobial resistance (AMR); a common example is methicillin-resistant S. aureus (MRSA); another known group is carbapenem-resistant gram-negative bacteria. Thus, there is an urge for the development of new antimicrobial agents and drugs to tackle AMR.
Nanotechnology alters essential material characteristics, including those of metal nanoparticles (Debnath et al. 2022). Silver may treat burns, urinary tract infections, central venous catheter infections, and acute and chronic bone inflammation (Vishwanath et al., 2022). These findings are corroborated with silver-based antimicrobials. Nanotechnology in personalised medicine provides a once-in-a-generation possibility to improve disease detection and therapy (Bhardwaj & Rasool 2023). Nanomaterials' design adaptability, minuscule sizes, massive surface-to-volume ratio, and ease of surface modification using multivalent ligands to increase target molecule avidity make them ideal therapeutic and diagnostic tools. Nanomaterials can interact with many biological systems, enabling them to benefit from tailored therapeutic insights. Nanoscale silver (less than 100 nm) has different catalytic properties than bulk silver, such as surface plasmon resonance, a large effective scattering cross section, and a high toxicity to many microbes (Abbasi et al. 2016). Thus, metal-based nanoparticles have promising biological and physiochemical properties as antimicrobials and therapeutic agents. It can solve nano medicine problems and may also harm cells and sub-cellular conditions. Thus, after cytotoxicity and clinical studies, nanoparticles can be widely used as antimicrobials in consumer and industrial products.
Eco-friendly and reliable metallic nanoparticle production is an essential step in nanotechnology applications. Biosynthesizing nanoparticles from plants or their products has great potential. Bacteria, fungi, and plant leaf extract can be used to synthesise silver nanoparticles without toxic chemicals, making them eco-friendly and compatible for pharmaceutical and biomedical applications (Shanmugapriya et al. 2021). Toxic chemical species adsorbed on the surface during chemical synthesis may harm medical applications. Bioinspired nanoparticle synthesis is cheaper and greener than chemical and physical methods. The bio- or green synthesis of nanoparticles requires fewer components and chemicals, and they can be synthesized in one go. In this type of synthesis, natural products such as plant extracts or microorganism cultures are used with different concentrations of silver nitrate (AgNO3). This nanoparticle synthesis process depends on various factors, such as the type of material used, concentrations of the components used, temperature, and duration of incubation. To expedite the reaction process, thermal, photo, and microwave irradiation are also explored (Abbasi et al. 2016; Ashraf et al., 2020; Shanmugapriya et al. 2021; Perveen et al., 2021; Miranda et al 2022; Satheesh et al., 2021; Kaur et al., 2023). There are reports on the synthesis of silver nanoparticles mediated by extracts of F. carica (Fig) and Cammiphora sp. (myrrh) (Patil 2020; Nadaf et al. 2022). However, none have reported microwave-assisted synthesis of nanoparticles using fig fruit or myrrh oleo gum resin extract. This study describes for the first time the quick, synthesis of silver nanoparticles utilizing fig fruit and myrrh extracts and microwave irradiation. With that, the green-synthesized silver nanoparticles were evaluated for their antimicrobial potential.
2 Materials and Methods
2.1 Preparation of extracts and reaction solution for the synthesis of nanoparticles
Protocols mentioned earlier were used for the preparation of plant extracts, silver nitrate solution, and the reaction solution of AgNO3 and plant extract (Nagarajan et al., 2021, Al-Otibi et al. 2021). First and foremost, the extracts of fig fruit and myrrh oleogum resin were prepared separately. Surface-sterilized (0.1 % sodium hypochlorite) fig fruits were dried for one week in a 50 °C hot air oven. Using a mixer grinder, the dry specimens were ground into a fine powder. Myrrh oleo gum resins were rinsed with deionized water, air-dried, and ground into fine powder. The prepared sample powder (20 g) was mixed with 100 mL of triple deionized water. The mixture was heated on a hot plate at 100 °C for 15 min. The mixture was filtered after being cooled at room temperature. Then 1 mL of AgNO3 (5 mM) with 9 mL of filtrate was mixed. The prepared solution was microwaved at high frequency (Samsung, 1300 W, 2450 MHz) till the colour of the solution changed to reddish brown. The fig-mediated silver nanoparticles (FAgNPs) and myrrh-mediated synthesized silver nanoparticles (MAgNPs) were analyzed by Ultraviolet–visible (UV–Vis) spectroscopy, Fourier transform infrared (FTIR) spectroscopy, dynamic light scattering (DLS), zeta potential, and Transmission electron microscopy (TEM) to examine their features and characteristics.
2.2 Characterization of silver nanoparticles
FAgNPs and MAgNPs were analysed by UV–Vis spectroscopy. The absorbance was recorded from 300 nm to 600 nm (Silambarasan & Jayanthi 2013). FTIR analysis was done to identify the functional groups present in the colloidal solution (Perveen et al. 2021). For further characterization of the AgNPs’ shape and size, DLS measurement, Zeta potential and TEM analyses were carried out (Al-Otibi et al., 2021).
2.3 Evaluation of the antibacterial activity of FAgNPs and MAgNPs
Four bacterial strains (ATCC), standard bacterial culture) were used; all the tested strains were acquired from King Khalid University Hospital, K.S.U. Saudi Arabia (Table 1). All cultures were grown in nutrient broth at 37 °C and maintained on nutrient agar slants at 4 °C.
Name
Reference
Staphylococcus aureus (MRSA)
ATCC 29213
Pseudomonas aeruginosa
ATCC 27853
Streptococcus pyogenes
ATCC 12384
Escherichia coli
ATCC 25922
Antimicrobial activity of the FAgNPs and MAgNPs was evaluated by employing agar well diffusion assay as described earlier by Zahin et al. (2021). Bacterial culture in a nutrient broth incubated for 24 h at 37 °C was adjusted to a turbidity of 0.5 MacFarland standards (108 CFU/mL) for the assay. Four wells per plate were made in each nutrient agar plate. FAgNPs and MAgNPs (50 µl @ 30 µg/mL) were poured into respective wells with the help of a micropipette. Antibiotic-Augmentin was included as positive control (10 µg/mL). With that, fig extract and myrrh extract were also analysed. The plates were incubated for 24 h at 37 °C. The antibacterial activity was interpreted from the size of the diameter of zone of inhibition measured to the nearest (mm) as observed from the clear zone surrounding the well.
The minimum inhibitory concentration (MIC) of FAgNPs and MAgNPs was determined against pathogenic bacteria using the standard micro-broth dilution method in 96-well flat-bottom plates, as described previously by Husain et al. (2019). Different concentrations of FAgNPs and MAgNPs were prepared by two-fold dilutions in 96-well plates. In this experiment, a positive control (Augmentin) was also included, while DIW was used as a negative control. Bacterial cell suspension (1.5 × 108 CFU/mL) was prepared in nutrient broth, and 100 µl of this was added to all wells except the negative control. All plates were then incubated at 36 °C for 18–20 h. The lowest concentration at which visible microbial growth inhibition is achieved was considered the MIC.
2.4 Investigation of morphological changes in the pathogenic bacteria caused by FAgNPs and MAgNPs
In order to investigate the effect of biosynthesized AgNPs on bacterial cell morphology, bacteria which were found sensitive to silver nanoparticles were processed further to observe under scanning electron microscope after getting treatment with sub-inhibitory concentrations of FAgNPs and MAgNPs (Alshaikh et al., 2023).
3 Results
The fig reaction solution (fig fruit extract and 5 mM AgNO3) changed colour in 120 s, while the myrrh reaction solution (myrrh extract and 5 mM AgNO3) turned reddish brown in 180 s (Fig. 1). The time taken by fig and myrrh reaction solutions to change their colour when incubated at room temperature and heated at 90 °C on a heating plate was also analysed. It was found that at room temperature, both reaction mixtures changed colour after 24 h. On the other hand, when the reaction solutions were heated on the heating plate, they changed their colour to dark brown in 3 h. The solution's transformation to a reddish-brown colour was supposed to be the first sign that silver nanoparticles were formed.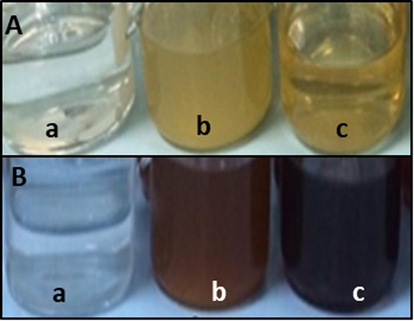
Reaction solution before (A) and after synthesis of silver nanoparticles (B). AgNO3, 5 mM (a), Fig fruit reaction solution (b), Myrrh reaction solution (c).
3.1 Characterization of synthesized AgNPs
In this study, FAgNPs and MAgNPs formation was initially confirmed using UV–visible spectroscopy due to surface plasmon resonance. The UV spectrum of FAgNPs and MAgNPs’ reaction solution was recorded the absorption peak between 320 and 600 nm. Fig. 2a and b clearly show a maximum surface Plasmon peak for FAgNPs and MAgNPs at 434.41 nm and 434.95 nm, respectively.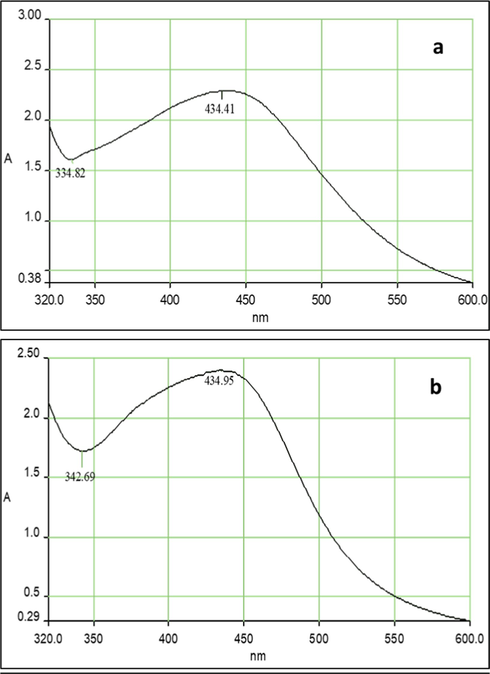
UV–visible spectral of FAgNPs (a) and MAgNPs (b) colloidal solution.
FTIR measurement was recorded to identify the possible biomolecules in fig fruit and myrrh extracts responsible for capping and leading to efficient stabilization of the silver nanoparticles (Fig. 3a and b). The FTIR spectra of FAgNPs and MAgNPs colloidal solutions show absorption bands in regions ranging from 3295.24 to 430.12 cm−1, and 3286.36 to 440.15, respectively. Therefore, there was a possibility of the stabilization of silver nanoparticles by proteins. These absorption peaks are a result of O–H, C–H, C = C, and S-S stretching.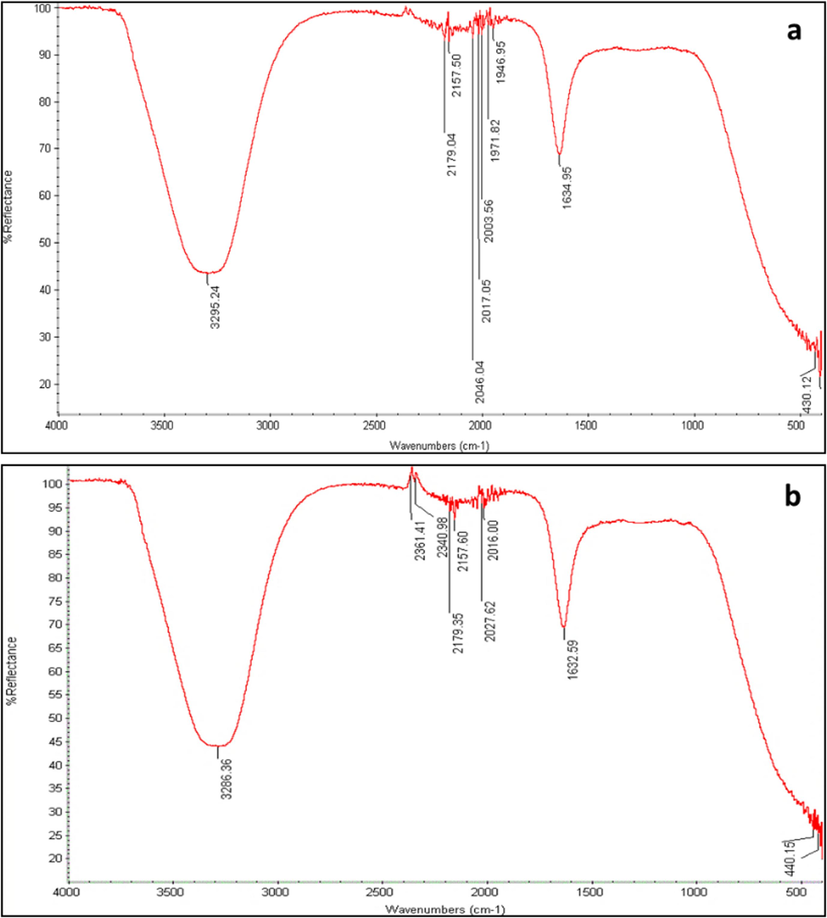
FTIR spectral of FAgNPs (a) and MAgNPs (b) colloidal solution.
The nanoparticles diameter as assessed by DLS indicated that the Z-average (d.mm) size for FAgNPs was 33.79 nm with a PdI of 0.480 (Fig. 4a). The Z-average (d.mm) size for MAgNPs was 31.63 nm with a PdI of 0.523 (Fig. 4b). The PdI data shows monodisperse to moderately polydisperse characteristics of FAgNPs and MAgNPs, respectively (Fig. 4a and b). The zeta potential of FAgNPs was −15 mV, while it was −16.1 mV for MAgNPs (Fig. 5a and b).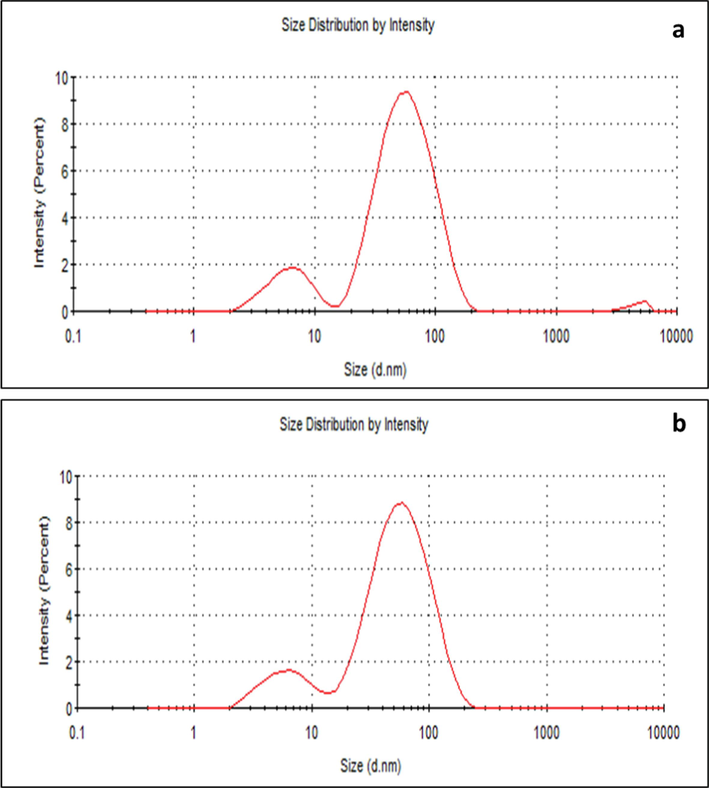
Size distribution by intensity of FAgNPs (a) and MAgNPs (b) measured by DLS.
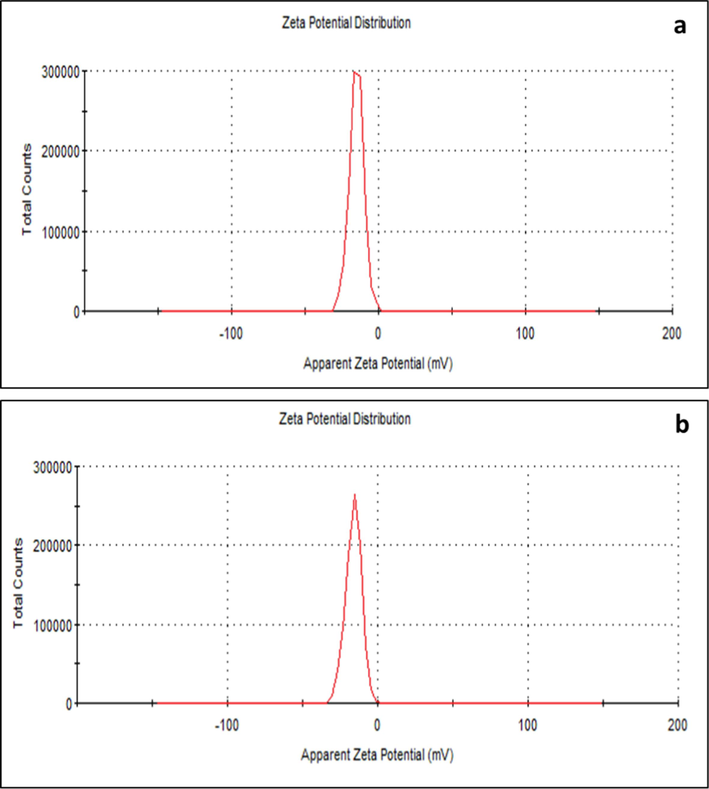
Apparent zeta potential of biosynthesised FAgNPs (a) and MAgNPs (b).
The micrographs of FAgNPs and MAgNPs obtained by TEM are presented in Fig. 6. TEM analysis reveals that the FAgNPs and MAgNPs were mostly spherical. Rarely, agglomeration was observed. FAgNPs of various sizes were seen in the micrographs, ranging from 4 nm to 36 nm (Fig. 6a). whereas in the case of MAgNPs, sizes ranged from 6 nm to 30 nm (Fig. 6b).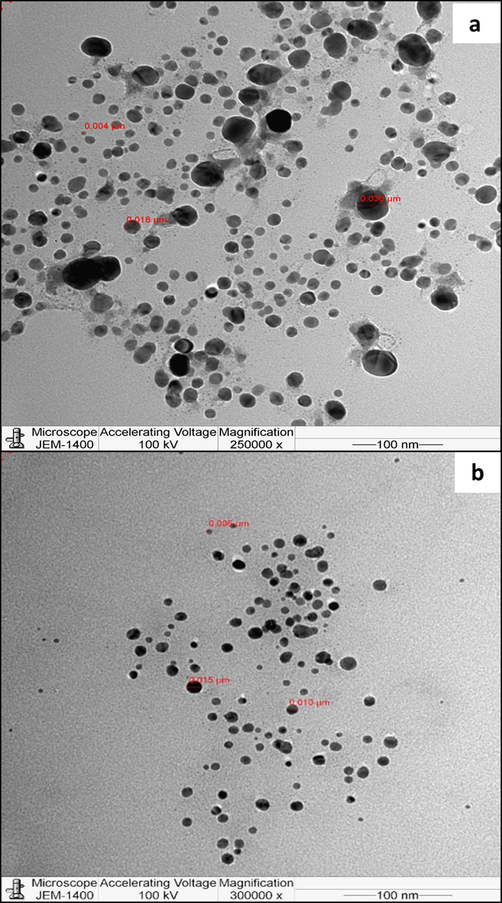
TEM micrographs of biosynthesised FAgNPs (a) and MAgNPs (b).
3.2 Assessment of the antibacterial action of biosynthesized AgNPs by agar well diffusion assay
The antimicrobial activity of FAgNPs and MAgNPs was investigated against four pathogenic bacteria (S. aureus, E. coli, S. pyogenes, and P. aeruginosa). The antibacterial activity of FAgNPs and MAgNPs demonstrated that they inhibited both gram-positive and gram-negative bacteria to different extents, whereas the plant extracts did not inhibit the growth of any bacteria tested. The antibacterial activity of FAgNPs and MAgNPs is represented in Fig. 7. FAgNPs and MAgNPs demonstrated the ability to limit the growth of three of the four human pathogenic bacteria studied. In general, FAgNPs were more effective than MAgNPs in inhibiting bacterial growth. FAgNPs showed that the highest level of inhibition against S. aureus (24.67 mm), and next to it were E. coli (14.67 mm) and S. pyogenes (8.0 mm). FAgNPs showed the strongest antibacterial activity against S. aureus (MRSA), and the inhibition was higher than that of Augmentin. Whereas, the antibacterial activity of MAgNPs shows that the most sensitive pathogen was S. aureus (23.0 mm), followed by E. coli (14.67 mm) and S. pyogenes (8.0 mm), respectively. While the results of the antibiotic Augmentin's (10 g/mL) antibacterial activity demonstrate that it was most effective against S. aureus (23.3 mm), E. coli (18 mm), and S. pyogenes (9.0 mm), respectively. P. aeruginosa was resistant to Augmentin, MAgNPs, and FAgNPs. The FAgNPs and MAgNPs greater reduction in E. coli growth than that caused by Augmentin. Whereas, all three treatments showed a very weak inhibition against S. pyogenes.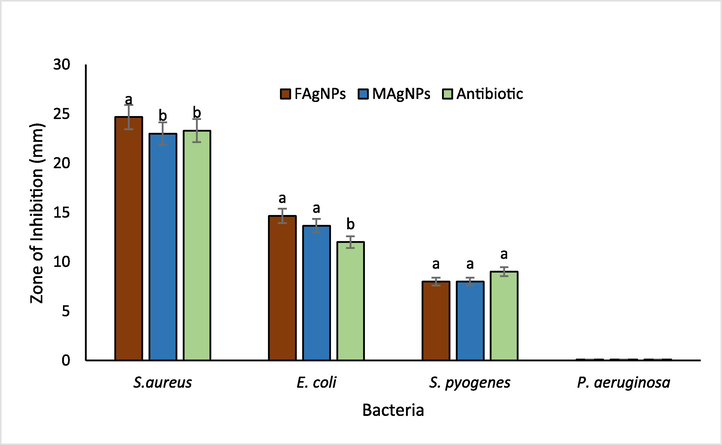
Antibacterial action of biosynthesised FAgNPs, MAgNPs (30 µg/ml) and Antibiotic-Augmentin (10 µg/ml) evaluated by agar well diffusion assay. The vertical bars represent means ± S.D (n = 3). Bars with different letters are significantly different (P ≤ 0.05) according to Tukey (HSD) test.
Since FAgNPs and MAgNPs exhibited strong to moderate antibacterial activity against S. aureus and E. coli, the MIC values of FAgNPs and MAgNPs was determined against these two pathogens only. The MIC value of both FAgNPs and MAgNPs against S. aureus was 15 µg/ml, whereas, against E. coli it was 30 µg/mL (Fig. 8).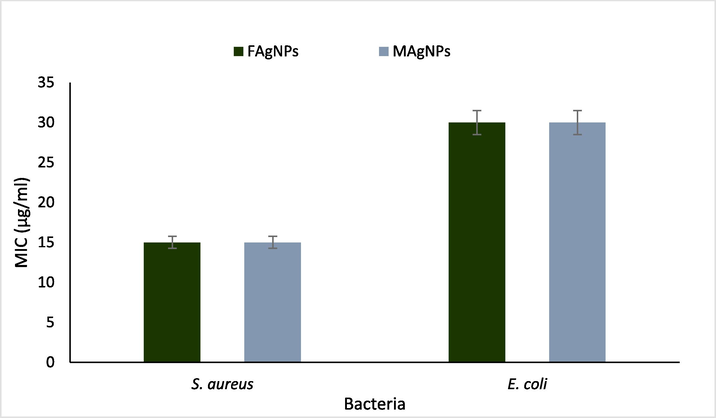
MIC values of FAgNPs and MAgNPs against S. aureus and E. coli.
3.3 Investigation of morphological changes in the pathogenic bacteria caused by FAgNPs and MAgNPs
S. aureus and E. coli were treated with sub-inhibitory concentrations of FAgNPs and MAgNPs and examined by SEM to determine the impact of synthesized nanoparticles on bacterial cell morphology. Figs. 9a1-3 and 9b1-3 depict the SEM images demonstrating morphological alterations in the bacterial cells of S. aureus and E. coli. The SEM images unmistakably demonstrate that the treatment with FAgNPs and MAgNPs caused changes in the bacterial cells. FAgNPs treatment caused S. aureus cells to conglomerate and some of the cells to become malformed (Fig. 9a2). The MAgNPs-treated S. aureus cells were tiny and damaged cells, and some ruptured cells were clearly visible (Fig. 9a3). Untreated S. aureus cells had a smooth surface and a well-defined shape (Fig. 9a1). E. coli cells treated with FAgNPs were severally damaged and distorted (Fig. 9b2). Similarly, E. coli cells exposed to MAgNPs were distorted and injured. Untreated E. coli cells showed a smooth morphology, and all cells shared the same attributes and morphology (Fig. 9b1).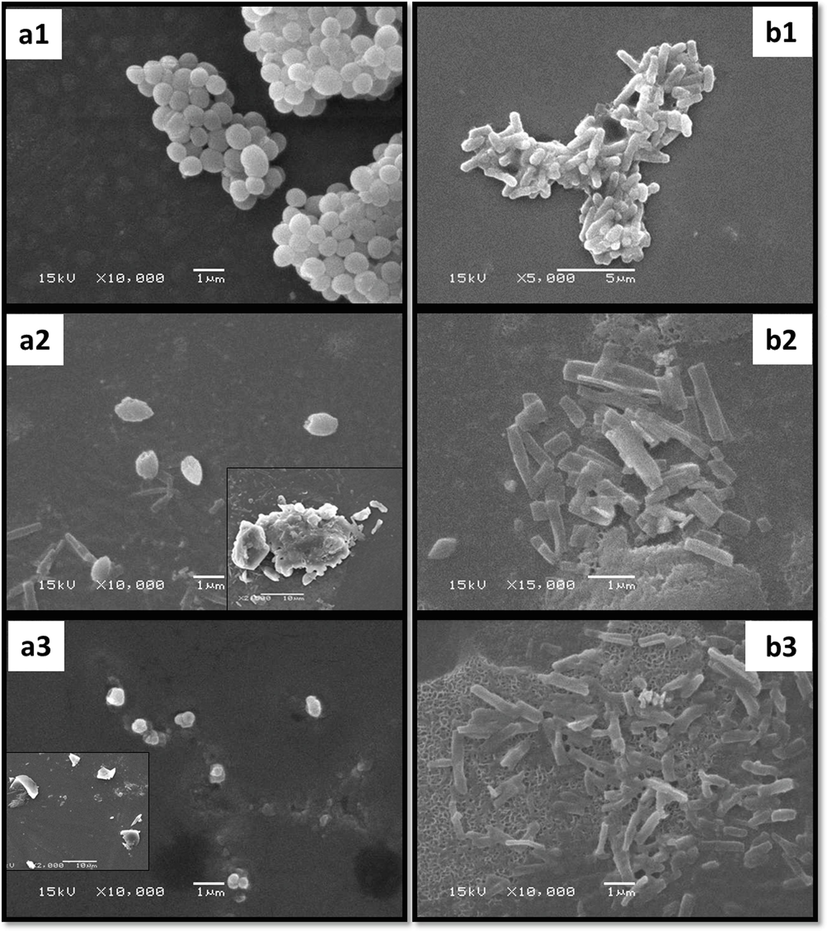
a-b: SEM micrographs of S. aureus (a1) and E. coli (b1) untreated cells; S. aureus (a2) and E. coli (b2) cells treated with sub inhibitory concentration of FAgNPs; S. aureus (a3) and E. coli (b3) cells treated with MAgNPs.
4 Discussions
The antimicrobial properties of silver extend back centuries. The physical and chemical characteristics of nanoparticles of silver are favourable. This study aims to synthesise silver nanaoparticles with distinct physicochemical properties using green synthesis with microwave irradiation technique and to examine their response to human pathogenic bacteria. Antibacterial activity, stability, specificity, biosafety, and biocompatibility may be enhanced by modifying silver nanoparticles (Dakal et al., 2016). However, there are some reports on the negative impact of nanoparticles on human health; the shape, size, and composition are some factors that may affect humans negatively. The smaller nanoparticles may enter the lungs and cross the cell membrane (Bhardwaj and Rasool, 2023).
In the present study, silver nanoparticles were synthesized from two different extracts, fig fruit and myrrh oleo gum resins. Microwave irradiation was utilized to excite and provide heat to the reaction solution that results in the synthesis of the silver nanoparticles. The whole process of the synthesis of FAgNPs and MAgNPs was rapid and completed in 120–180 s. Previously, fig leaves and myrrh extracts were reported to be used in the synthesis of silver nanoparticles; however, none have used microwave irradiation (Ulug et al., 2015; Ahmed et al., 2016; Patil 2020). Peng et al. (2013) reported a successful synthesis of AgNPs in 120 s when they used microwave irradiation, bamboo hemicelluloses, and glucose as stabilizing and capping agents. Whereas, another study reported microwave-assisted green synthesis of AgNPs in 15 min using orange peel extract (Kahrilas et al. 2014). It has been suggested that under a pressure-controlled atmosphere, microwaves quickly reach extremely high temperatures that facilitate the initial nucleation of nanoparticles (Tsuji et al., 2005; Kahrilas et al., 2014). The duration for the completion of nanoparticle synthesis varies due to reducing agent used and the incubation conditions (Ulug et al., 2015; Ahmed et al., 2016). Ashraf et al. (2020) reported the microwave-assisted synthesis of AgNPs in 30 s with Melia azedarach extract. There are reports of the synthesis of other metal nanoparticles with the use of plant extract and microwave irradiation (Tsuji et al., 2005; Ulug et al., 2015,). Earlier, Perveen et al. (2021) reported a successful synthesis of gold nanoparticles by utilizing microwave irradiation and the seed extract of T. ammi.
The UV–vis analysis of the FAgNPs and MAgNPs showed that the peak was between 400 and 500 nm; because of the characteristics of silver nanaoparticles, the highest absorption is generally observed in this range (Francis et al., 2018). To determine the phyto-chemicals of fig fruit and myrrh extracts that served as capping and stabilizing agent, FTIR analysis was carried out. Prominent broad peaks of 3295.24, 3286.36 recorded in the FAgNPs and MAgNPs colloidal solution, respectively, representing vibrations of the hydroxyl (–OH) group. Whereas variable stretching vibrations of alkene (C = C) with aromatic ring are represented by the peaks of 1634.95 and 1632.59 in the FAgNPs and MAgNPs, respectively. The FTIR spectrum shows absorption bands of C = H, -O–H, -S-H, -N = C = N, -C = O, and -S = O stretching vibrations that prove the presence of important functional groups such as flavonoids, alkaloids, and polyphenols. These functional groups encapsulate nanoparticles and prevent their agglomeration (Kahrilas et al. 2014). In addition, the presence of peptides and amino acids may have facilitated the capping of the silver nanoparticles.
DLS showed that the average sizes of the FAgNPs and MAgNPs were 33.79 nm and 31.63 nm, respectively, while zeta potentials of −15 mV, and −16.1 mV were noted for the FAgNPs and MAgNPs, respectively. The zeta potentials between ± 10 mV to ± 20 mV are considered relatively stable colloidal potentials (Patel and Agrawal, 2011). The PDI for FAgNPs and MAgNPs was 0.480 and 0.523, respectively, which shows polydispersed nanoparticles. However, further investigation is needed to understand how to reduce the polydispersion of the nanoparticles. The complexity of the extracts that mediated the formation of the nanoparticles could be a reason other than that other physical factors may have influenced the variation in size distribution of these nanoparticles. Interestingly, the FAgNPs and MAgNPs synthesized from two different plant extracts have shown very similar properties. The FAgNPs and MAgNPs may have been synthesized with comparable characteristics because of the likely presence of similar functional groups in the phytochemicals of the both extracts, as indicated by the FTIR. However, this aspect needs to be studied further to find out the most viable reasons that are important for the formation of silver nanoparticles.
The shape and size of FAgNPs and MAgNPs were further analysed by the TEM analysis. The FAgNPs and MAgNPs were almost spherical and of various sizes. These findings are similar to the results obtained earlier by Kahrilas et al. (2014) while utilizing microwave irradiation for the synthesis of sliver nanoparticles with orange peel extract. They synthesized AgNPs of various sizes with average size of 7.36 ± 8.06 nm. Spherical-shaped AgNPs of 12 to 46 nm were synthesized using M. azedarach leaf extract and microwave irradiation for 30 s (Ashraf et al. 2020). While Elephantopus scaber extracts mediated the synthesis of spherical AgNPs at 37.86 nm by microwave irradiation (Francis et al., 2018). Spherical AgNPs ranging from 25 to 40 nm were synthesized using leaf extracts of Fraxinus excelsior exposed for 30 s to microwave irradiation (Parveen et al. 2016).
FAgNPs and MAgNPs manifest the ability to repress the bacterial growth of three human pathogenic bacteria out of the four tested. The inhibition against S. aureus and E. coli was found to be significant. In general, FAgNPs were more effective than MAgNPs in inhibiting bacterial growth. It was noticed that Augmentin and MAgNPs were equally efficient against S. aureus, while FAgNPs had a considerable advantage over both. The results showed that both FAgNPs and MAgNPs had MIC values of 15 µg/ml against S. aureus, compared to 30 µg/ml against E. coli. These results agreed with previous work reported by many researchers (Dakal et al., 2016; Nadaf et al., 2022; Kaur et al., 2023). Nanoparticle size influences antibacterial activity. Silver nanoparticles' bioactive properties mainly depend on their size and are impacted by their various other characteristics, which affect the bacterial cells in different ways (Raza et al. 2016; Zhang et al. 2018; Miranda et al. 2022). Size and shape may impact the antibacterial effect of AgNPs.
The bacterial cells treated with sub-inhibitory MIC concentrations of FAgNPs and MAgNPs reveal cell alterations as noticed in SEM images of S. aureus and E. coli. Treatment with FAgNPs caused S. aureus cells to conglomerate and become malformed; MAgNPs-treated S. aureus cells were also damaged and ruptured. While E. coli cells treated with FAgNPs and MAgNPs were severally damaged and distorted. The large surface area of silver nanoparticles makes them antimicrobial (Priyadarshni et al. 2022). Nanoparticles destroy cells in the respiratory chain. Nanoparticles enhance the bactericidal effect of silver ions on bacterial cells (Rao et al. 2022). Although several mechanisms for the successful hindrance of microbial growth by silver nanoparticles have been speculated, this matter is still under investigation as no fully convincing theory has been proposed yet. Dakal et al. (2016) summarized the recognized mechanisms reported so far. That includes the damaging of the cell wall, cell membrane, intracellular structure and various biomolecules. The cellular toxicity, oxidative stress and impact on signal transduction pathways. The green chemistry technique for the formation of bio-nanoparticles offers several benefits, including scalability, economics, viability, sustainability, and environmental advantages. Due to their great selectivity, specificity, and sensitivity, application such eco-friendly nanoparticles for antibacterial, wound healing, and other medical applications makes these nano-biomaterials more acceptable. However, this study took into consideration only one concentration of plant extract and AgNO3. Therefore, there is a need to assess the effect of different concentrations of this content on the synthesis of silver nanoparticles; it may be possible that variations in concentrations may bring out different results. In addition, the impact of these nanoparticles on biofilm formation and cell toxicity has yet to be analyzed.
5 Conclusion
This study describes the speedy synthesis of silver nanoparticles utilizing fig fruit and myrrh extracts and microwave irradiation. Spherical silver nanoparticles were synthesized within 120 and 180 s of exposure of the fig fruit and the myrrh reaction solution to microwave irradiation, respectively. The characterization of synthesized silver nanoparticles by UV–Vis, FTIR, DLS, zeta potential, and TEM reveals that the FAgNPs and MAgNPs were spherical with an average size of 33.79 nm and 31.63 nm in diameter, respectively. Both FAgNPs and MAgNPs were polydispersed, negatively charged, and relatively stable colloids. The FAgNPs and MAgNPs have potent antibacterial action against pathogenic microorganisms, including MRSA. It was validated further by the SEM of S. aureus and E. coli treated with nanoparticles. The study provides a protocol for the synthesis of silver nanoparticles within a very short time by utilizing fig fruit and myrrh extract and microwave irradiation. Due to their great selectivity, specificity, and sensitivity, the utilisation of such eco-friendly nanoparticles for antibacterial, wound healing, and other medical applications makes these nano-biomaterials more acceptable.
Acknowledgement
The authors would like to acknowledge the support provided by Researchers Supporting Project Number RSP2023R358, King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Silver nanoparticles: synthesis methods, bio-applications and properties. Crit. Rev. Microbiol.. 2016;42(2):173-180.
- [Google Scholar]
- A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J. Adv. Res.. 2016;7(1):17-28.
- [Google Scholar]
- Biosynthesis of silver nanoparticles using Malva parviflora and their antifungal activity. Saudi Journal of Biological Sciences. 2021;28(4):2229-2235.
- [Google Scholar]
- Effect of silver nanoparticles alone and in combination with fluconazole on Candida albicans. Journal of King Saud University-Science. 2023;35(1):102399
- [Google Scholar]
- Microwave-assisted green synthesis and characterization of silver nanoparticles using Melia azedarach for the management of Fusarium wilt in tomato. Front. Microbiol.. 2020;11:238.
- [Google Scholar]
- Nanotechnology for the treatment of cancer: progress and challenges. Nanotechnology and Human Health 2023:285-307.
- [Google Scholar]
- Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol.. 2016;7:1831.
- [Google Scholar]
- A comprehensive review and meta-analysis of recent advances in biotechnology for plant virus research and significant accomplishments in human health and the pharmaceutical industry. Biotechnol. Genet. Eng. Rev. 2022:1-33.
- [Google Scholar]
- Microwave assisted green synthesis of silver nanoparticles using leaf extract of elephantopus scaber and its environmental and biological applications. Artif. Cells Nanomed. Biotechnol.. 2018;46(4):795-804.
- [Google Scholar]
- Mitigation of acyl-homoserine lactone (AHL) based bacterial quorum sensing, virulence functions, and biofilm formation by yttrium oxide core/shell nanospheres: Novel approach to combat drug resistance. Sci. Rep.. 2019;2019(9):18476.
- [Google Scholar]
- Microwave-assisted green synthesis of silver nanoparticles using orange peel extract. ACS Sustain. Chem. Eng.. 2014;2(3):367-376.
- [Google Scholar]
- Microwave assisted green synthesis of silver nanoparticles and its application: A review. J. Inorg. Organomet. Polym Mater.. 2023;33(3):663-672.
- [Google Scholar]
- Targeted treatment for bacterial infections: prospects for pathogen-specific antibiotics coupled with rapid diagnostics. Tetrahedron. 2016;72(25):3609-3624.
- [Google Scholar]
- pH Alteration in Plant-Mediated Green Synthesis and Its Resultant Impact on Antimicrobial Properties of Silver Nanoparticles (AgNPs) Antibiotics. 2022;11(11):1592.
- [Google Scholar]
- Green synthesis of gold and silver nanoparticles: Updates on research, patents, and future prospects. OpenNano. 2022;8:100076
- [Google Scholar]
- A comprehensive review on cellulose nanocrystals and cellulose nanofibers: Pretreatment, preparation, and characterization. Polym. Compos.. 2021;42(4):1588-1630.
- [Google Scholar]
- Microwave-assisted green synthesis of silver nanoparticles from Fraxinus excelsior leaf extract and its antioxidant assay. Appl. Nanosci.. 2016;6:267-276.
- [Google Scholar]
- Nanosuspension: an approach to enhancesolubility of drugs. J. Adv. Pharm. Technol. Res.. 2011;2:81-87.
- [Google Scholar]
- Ficus carica assisted green synthesis of metal nanoparticles: A mini review. Biotechnol. Rep,. 2020;28:e00569.
- [Google Scholar]
- Green, microwave-assisted synthesis of silver nanoparticles using bamboo hemicelluloses and glucose in an aqueous medium. Carbohydr. Polym.. 2013;91(1):348-355.
- [Google Scholar]
- Microwave-assisted rapid green synthesis of gold nanoparticles using seed extract of Trachyspermum ammi: ROS mediated biofilm inhibition and anticancer activity. Biomolecules. 2021;11(2):197.
- [Google Scholar]
- Biochemical analysis of cultivated mushroom, Pleurotus florida and synthesis of silver nanoparticles for enhanced antimicrobial effects on clinically important human pathogens. Inorg. Chem. Commun.. 2022;142:109673
- [Google Scholar]
- Nanoarchitectonics for enhanced antibacterial activity with Lactobacillus buchneri S-layer proteins-coated silver nanoparticles. J. Hazard. Mater.. 2022;426:128029
- [Google Scholar]
- Size-and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials. 2016;6(4):74.
- [Google Scholar]
- Satheesh, V., Mohamed, J.M.M., El-Sherbiny, M. et al. 2021. Sunlight-assisted green synthesis of silver nanoparticles using Zizania latifolia extract: toward antimicrobial applications. Biomass Conv. Bioref. https://doi.org/10.1007/s13399-022-03363-7.
- An eco-friendly Gnaphalium polycaulon mediated silver nanoparticles: Synthesis, characterization, antimicrobial, wound healing and drug release studies. J. Drug Delivery Sci. Technol.. 2021;61:102202
- [Google Scholar]
- Biosynthesis of silver nanoparticles using Pseudomonas fluorescens. Res J Biotechnol. 2013;8(3):71-74.
- [Google Scholar]
- Microwave-assisted synthesis of metallic nanostructures in solution. Chemistry–A. European Journal. 2005;11(2):440-452.
- [Google Scholar]
- Role of irradiation in the green synthesis of silver nanoparticles mediated by fig (Ficus carica) leaf extract. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2015;135:153-161.
- [Google Scholar]
- Silver as an antibiotic-independent antimicrobial: review of current formulations and clinical relevance. Surg. Infect. (Larchmt.). 2022;23(9):769-780.
- [Google Scholar]
- Antioxidant, antibacterial, and antimutagenic activity of Piper nigrum seeds extracts. Saudi Journal of Biological Sciences. 2021;28(9):5094-5105.
- [Google Scholar]
- Size-dependent cytotoxicity of silver nanoparticles to Azotobacter vinelandii: Growth inhibition, cell injury, oxidative stress and internalization. PLoS One. 2018;13(12):e0209020
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102959.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







