Translate this page into:
Microscopic characterization of bioaccumulated aluminium nanoparticles in simplified food chain of aquatic ecosystem
⁎Corresponding author. fuad.zi@mail.ru (Fuad H. Rzayev)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In the present context, the bioaccumulation of aluminium nanoparticles (AlNPs) in Elodea canadensis (a perennial aquatic plant), Melanopsis praemorsa (freshwater snail), and cercaria and sporocyst of some molluscs (infected with Cercaria agstaphensis 25 larvae) was studied using light microscope and transmission electron microscope. Further, the bioaccumulation of AlNPs in the simplified food chain components (plant, mollusc, and trematoda) was observed in electronograms using linear scanning of the level of gray intensity (grey value) of unstained ultra-thin sections. Results depicted that AlNPs were accumulated in the cell wall and intercellular spaces of E. canadensis leaf, cytoplasm of M. praemorsa's digestive glands (passing through by microvilli of epithelium cells), and parenchyma (wall tissue) as well as internal organs of the cercaria. In addition, the pathomorphological changes due to AlNPs in plant, mollusk, and parasite were also studied at the histological and ultrastructural level which showed thinning of the tunica propria of the digestive glands, irregularity of digestive glands, and formation of edema. Ultra-thin incisions made from the digestive glands infected with sporocysts containing cercariae showed that AlNPs passed through the wall of the sporocyst (capsule) into the parenchyma of the cercaria (wall tissue), and then accumulated in the internal organs of the cercaria. In a nutshell, this study revealed the adverse effect of AlNPs accumulation in plant, mollusk, and trematoda which emphasized to minimize the leakage of toxic nanoparticles contaminants in freshwater and marine environments.
Keywords
Aluminium nanoparticles
Aquatic ecosystem
Microscopy
Pathomorphological changes
1 Introduction
Recently, there has been an increase in the production and use of various types of nanomaterials. This leads to an increase in the disposal of toxic wastes in the environment. Nanoparticles contaminate the soil, pass into surface and groundwater, and affect microorganisms, fauna, and flora. Nanoparticles involved in waste materials, wastewater, and accidental leakage enter into the aquatic ecosystem through wind and rainwater (Klaine et al., 2008). The unique properties of nanomaterials vary in freshwater and marine environments (Handy et al., 2008). Some properties such as chemical composition, mass, concentration, surface concentration, surface load, surface contamination, nature, stability, and solubility of nanoparticles affect the aquatic environment.
In aquatic ecosystems, organisms are more exposed to nanoparticles effect and cause significant complications in human health in the final stages of the food chain. Therefore, the study of the ecotoxicological effects of nanomaterials in aquatic ecosystems is of particular importance. For the safe development of nanotechnology, it is necessary to understand the toxic effects of nanoparticles (Baun et al., 2008; Blaise et al., 2008; Tiede et al., 2009). Both marine and freshwater molluscs are used as model organisms. Bivalve molluscs have the ability to filter large volumes of water, clean micro-algae, bacteria, particles, nanoparticles, as well as the accumulation of various chemicals in their tissues (Dagnino et al., 2007). Nanoparticles are adsorbed on the surface of phytoplankton (algae, higher aquatic plants, etc.) in aquatic ecosystems and even penetrate into them and accumulate in their organs. The main organs in which nanoparticles accumulate in aquatic organisms are the digestive and secretory cells. Their main targets are the endosomal-lysosomal system and mitochondria. Nanoparticles cause immunotoxicity, oxidative stress, and damage to the cell proteins, biological membranes and nucleic acids (Crane et al., 2008; Rocha et al., 2015). Generally, nanoparticle enters into the aquatic environment, accumulate, and cause cyto- and ecotoxicity (Moore, 2006; Gagne et al., 2008; Ward and Kach, 2009; Canesi et al., 2012; Werlin et al., 2011).
In view of this, the major aim of this investigation was to study the accumulation of aluminium nanoparticles (AlNPs) as well as pathomorphological changes in Elodea canadensis (a perennial aquatic plant), Melanopsis praemorsa (freshwater snail), and cercaria and sporocyst of some molluscs (infected with Cercaria agstaphensis 25 larvae) using light microscope and transmission electron microscope (TEM).
2 Materials and methods
2.1 Food chain design
In this study, E. canadensis (Hydrocharitaceae) was used as primary constituent of the food chain. This plant not only plays a pivotal role in formation and stability of ecosystems in both saltless and marine water basins but also induces the nutritional flow in the food chain.
Freshwater snail (M. praemorsa; Melanopsidae) was used as the secondary constituent of the food chain in this context due to its active movement in the aquarium. The aquatic ecosystem should be enriched with oxygen for the survival and normal growth of the snail. Hence, the snail grows well and generates in the phytoplankton-rich water. M. praemorsa is fed with the extract of green algae.
Molluscs are considered intermediate hosts for the larval stages of trematodes.
2.2 Duration of the investigation
This study was conducted from January 2018 to April 2019 at the Institute of Zoology (Baku city), Republic of Azerbaijan, Azerbaijan. All the experiments were performed after the approval from the Ethics Committee of Azerbaijan Medical University (Ministry of Health of Azerbaijan Republic), Azerbaijan (No: EP 0023).
2.3 Nanoparticles used
Aluminium nanoparticles (purity − 99.9%) of 18 nm in size were obtained from Skyspring Nanomaterials Inc., USA. Stock solution (0.1% w/v) of AlNPs was prepared by mixing it with sterile double distilled water.
2.4 Nanoparticles exposure
E. canadensis was transferred in 0.1% (w/v) aqueous solution of AlNPs for 72 h in order to adsorb on the surface of the plant and to accumulate in the stem, leaves, and other organs. E. canadensis containing AlNPs was also fed to M. praemorsa. M. praemorsa molluscs, the second component of the food chain, were collected from the territory of Gazakh region, which is included in the Middle Kura basin (the area where Agstafachay and Jogaz rivers meet), brought alive to the Parasitology Laboratory of the Institute of Zoology of ANAS in a special containers, and distributed in glass containers with volume of 25 cm3. After 12–24 h, molluscs infected with trematode larvae were identified by SMZ 1270 (Nikon, China) stereoscopic microscope and trematode larvae were identified as C. agstaphensis 25 (Manafov, 2012). Fifteen molluscs infected with larvae of C. agstaphensis 25 were allocated for the experiment. Sterile molluscs spontaneously infected with the parasite and grown in the laboratory were fed with AlNPs adsorbed E. canadensis. The digestive glands of both sterile and infected molluscs were examined for 7 days after feeding with the plant.
2.5 Microscopic analysis of AlNPs bioaccumulated tissues
All components of simplified food chain (plant, mollusc, and parasites) from the control and the test groups were sacrificed and the abdominal area was dissected using a sterile scalpel. Araldite-Epon blocks were made from materials using general methods (Kuo, 2007). Leaves of plants and digestive glands of molluscs were fixed in 0.1 M phosphate buffer (pH − 7.4) constituting 2.5% glutaraldehyde, 2% paraformalaldehyde, 4% sucrose, and 0.1% picric acid and observed under TEM. Samples were post-fixed in 1% osmium tetraoxide solution within 2 h after being left in the same fixer for 24 h. The semi-thin (1–2 mm) section from the blocks was transferred to EM UC7 (Leica, Germany) ultra-microtome and stained with methylene blue, azure II, and toluidine blue. Samples were observed under light microscope (Model - Primo Star; Zeiss, Germany) and images of required portions were shot with digital camera (Model - EOS D650; Canon, China) (D’Amico, 2005). TEM (Model - JEM-1400; JEOL, Japan) was used to observe prepared and unstained sections of 50–70 nm thickness from the same blocks at 80–120 kV.
Electronograms were recorded for studying the morphometric analyses of the images. Soft Imaging Solutions Gmbh (Olympus, Germany) was used for developing TEM images. The histograms obtained during the study of electronograms were taken from ultra-thin sections made of non-stained preparations by ‘Intensity Profile’ computer software. The length (nm) of structures drawn in horizontal direction and the digits showing gray color patterns in the vertical direction (grey value) were provided. The intensity of the images in the electrograms depends on the degree of gray color. Black colour represents the weakest intensity while white colour indicates the strongest intensity; thereby allowing the accurate position of the bioaccumulation of nanoparticles in the cells.
3 Results
The ultrastructure of control E. canadensis and plant stored in the solution of AlNPs are shown in Fig. 1. Fig. 1A-D shows both semi-thin (Fig. 1A) and ultrathin (Fig. 1B-C) structure of control E. canadensis plants and found that the degree of transparency in the cell wall, cytoplasm, and cell organelles fluctuates between 5950 and 6300 (Fig. 1D). Due to the exposure of nanoparticles, the AlNPs were found to accumulate in the cell wall and intercellular spaces of the leaves of the plant (Fig. 1E-H). Fig. 1E is a semi-thin section of the plant, and Fig. 1F and G are ultrathin sections. Fig. 1E and F clearly show the structural changes in cell wall, cytoplasm, mitochondria, and chloroplasts that make up the plant's leaf. Fig. 1G shows AlNPs accumulation in the cell wall and shows degree of transparency (5300) (Fig. 1H).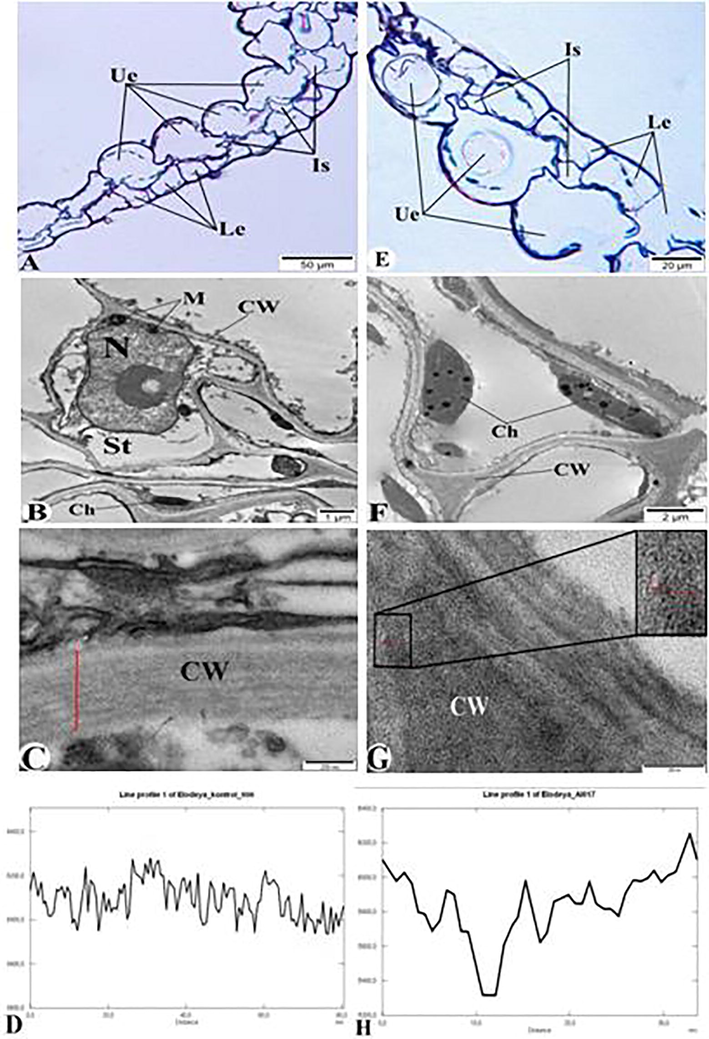
Control group (A-D) and AlNPs-exposed E. canadensis (E-H). A and E- semithin section (1 μm) of E. canadensis, B, C, F, and G – Electron microscopic image of E. canadensis (ultrathin cross section – 50–70 nm); D – Diagram of Fig. C; H – Diagram of Fig. G. Ue – upper epidermis; Is – intracellular spaces; Le – lower epidermis; St – cytoplasm, CW- cell wall, M- mitochondria, Ch – chloroplast, and N – nucleus.
During our research, structural changes were observed in the basal membranes of cells, chloroplasts, and mitochondria in the plant after the exposure of AlNPs. These changes were observed in both semithin (Fig. 2A) and ultrathin sections (Fig. 2B and C). Thus, the integrity of the walls of the cells that make up the upper epidermis and lower epidermis of E. canadensis leaf is disrupted, and the cytoplasm is destroyed (Fig. 2A). In those where the cell wall does not collapse, mitochondrias were swellen. Organelles were not observed in most of the cells (Fig. 2B and C).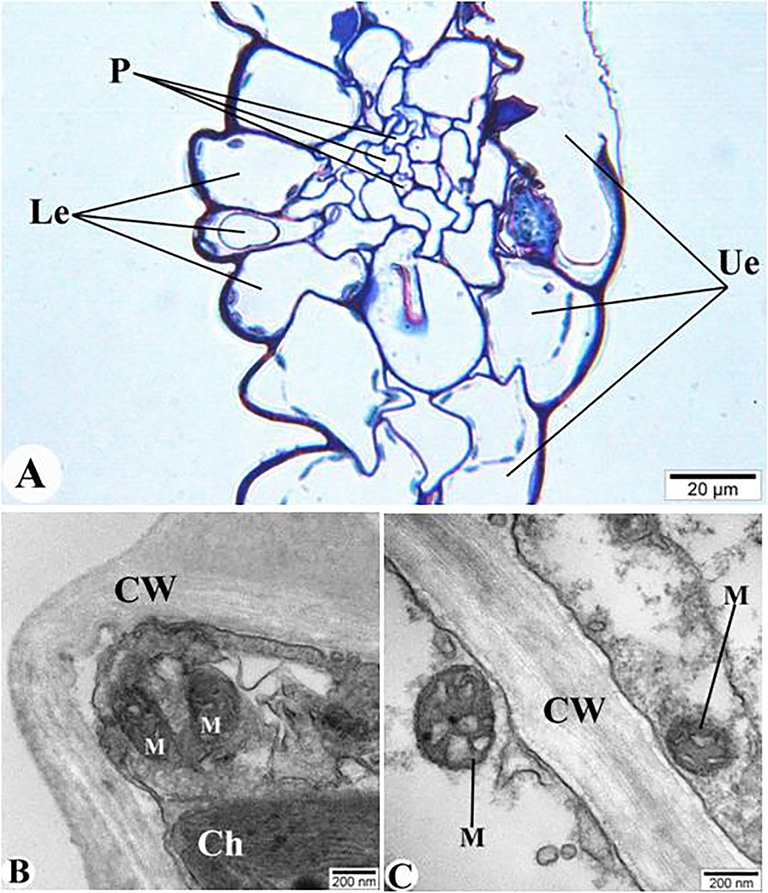
Structural changes due to the exposure of AlNPs to E. canadensis. A – semithin section (1 μm) of E. canadensis; B and C – TEM images (ultrathin section – 50–70 nm). Ue – upper epidermis; Le – lower epidermis; P– phloem, CW- cell wall, M- mitochondria, and Ch – chloroplast.
The digestive glands of M. praemorsa mollusc fed with E. canadensis were removed and examined under both light microscope and TEM. The snails' digestive glands were first studied in the control M. praemorsa (Fig. 3A-E). Electronograms of the digestive glands and epithelial cells from ultrathin sections showed that the degree of transparency varied from 6000 to 6400 (Fig. 3B-E).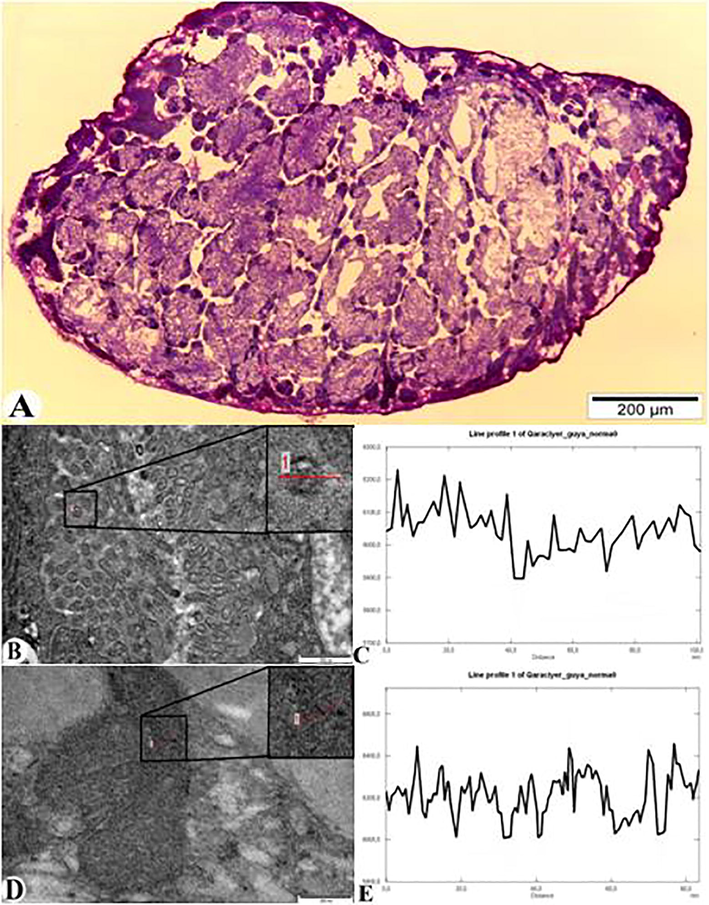
Light microscope (A) and TEM images (B and D) of the control group of M. praemorsa mollusc in the microvilli and cytoplasm of the epithelial cells of the digestive glands. A – general view of the digestive glands (semithin section – 1 μm); B – microvilli of the epithelium of the digestive gland, and its diagram (C); D – cytoplasm of the epithelial cells of the digestive gland and its diagram (E) (ultrathin section – 50–70 nm).
The entry of nanoparticles in the microvilli of epithelial cells of digestive glands and its localization in the cytoplasm of the cell is shown in Fig. 4. Fig. 4A shows a general view of the digestive glands under light microscope. Pathomorphological changes in the structure of the digestive glands affected by AlNPs exposure are also observed. Thinning of the tunica propria of the digestive glands was observed (indicated by black arrows), the distance between the glands was widened, and edema was formed (indicated by black snowflakes). The digestive glands were irregularly spaced and spaced far apart. The connective tissue between the glands was compressed and filled with edematous fluid. In general, the connection between the digestive glands was broken. The longitudinal epithelial and secretory cells that make up the gland were irregularly arranged and changes in the cytoplasm were also observed. Fig. 4B shows the entry of nanoparticles through microvilli of epithelial cells. Accordingly, size and transparency degree of these nanoparticles (5500) are shown in Fig. 4C. Fig. 4D and E show the bioaccumulation of AlNPs in the cytoplasm of epithelial cells of the digestive glands as well as the degree of transparency (5000).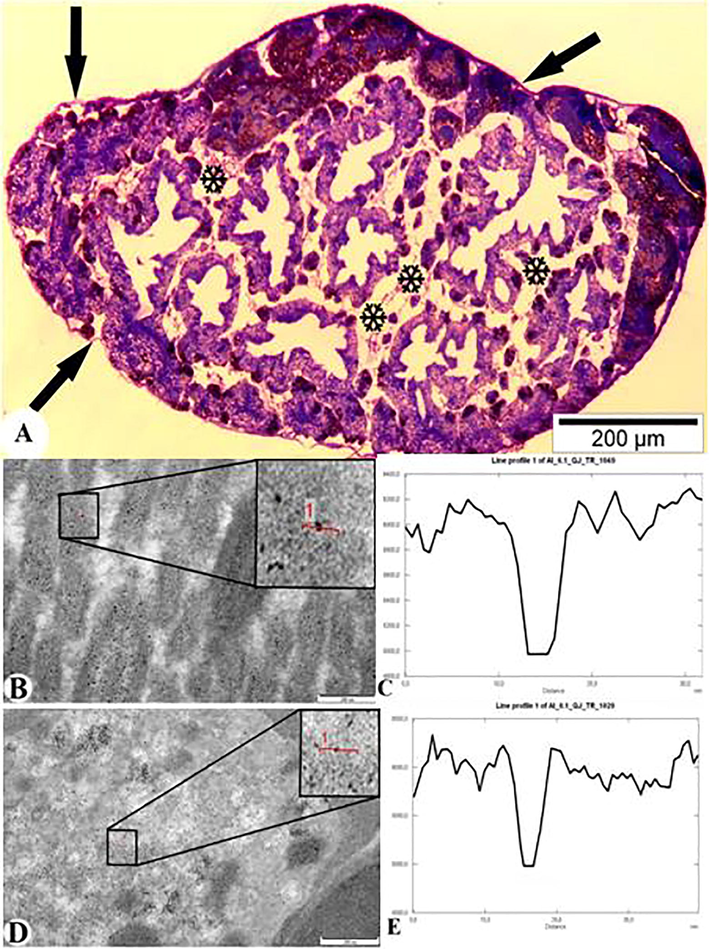
Light microscope (A) and TEM description (B and D) of the location of AlNPs in M. praemorsa mollusc in the microvilli and cytoplasm of the epithelial cells of the digestive glands. A – general view of the digestive glands (semithin section – 1 μm); B – microvilli of the epithelium of the digestive gland, the localization of AlNPs and its diagram (C); D – accumulation of AlNPs in the cytoplasm of the epithelial cells of the digestive gland and its diagram (E) (ultrathin section – 50–70 nm).
The digestive glands of infected molluscs were taken and blocks were made, and the semithin sections were first observed under light microscope. The images clearly showed the sporocysts in the digestive glands of the mollusc. These parasitic larvae caused severe deformation of the digestive glands. Some lumens of digestive glands were expanded and destroyed. The distance between the lumen increased and in some cases the connection was completely cut off. The covering membrane surrounding the glands was broken in some places, thereby violating its integrity. Tissue necrosis was observed. This is due to the fact that the tissue filled with parasites does not withstand mechanical expansion (Fig. 5A and B).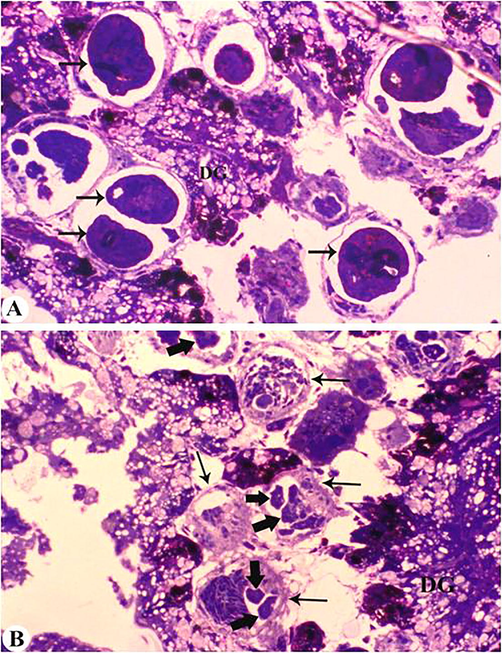
Semithin sections of the digestive glands of M. praemorsa mollusc infected with trematode larvae C. agstaphensis 25. A – digestive glands (sercaria indicated by an arrow) formed inside the sporocysts; B – sporocysts (indicated by a thin black arrow) and embryonic sercaria (indicated by a thick arrow); DG – digestive glands (semithin section – 1 μm).
As a result of TEM analysis of the digestive glands of parasite-infected molluscs, the entry and accumulation of AlNPs in helminths was also observed (Fig. 6). Fig. 6A shows the cercaria inside the sporocyst with an arrow. Electronograms of ultrathin incisions made from the digestive glands infected with sporocysts filled with cercariae showed that nanoparticles passed through the wall of the sporocyst (capsule) and parenchyma of the cercaria (wall tissue) into the internal organs of the cercaria (Fig. 6B-G). The TEM images show the AlNPs accumulation in the wall of the sporocyst capsule (Fig. 6B and C; Gray value 5400), parenchyma of the cercaria (Fig. 6D and E; Gray value 5000), and internal organs of the trematode (Fig. 6F and G Gray value 5000).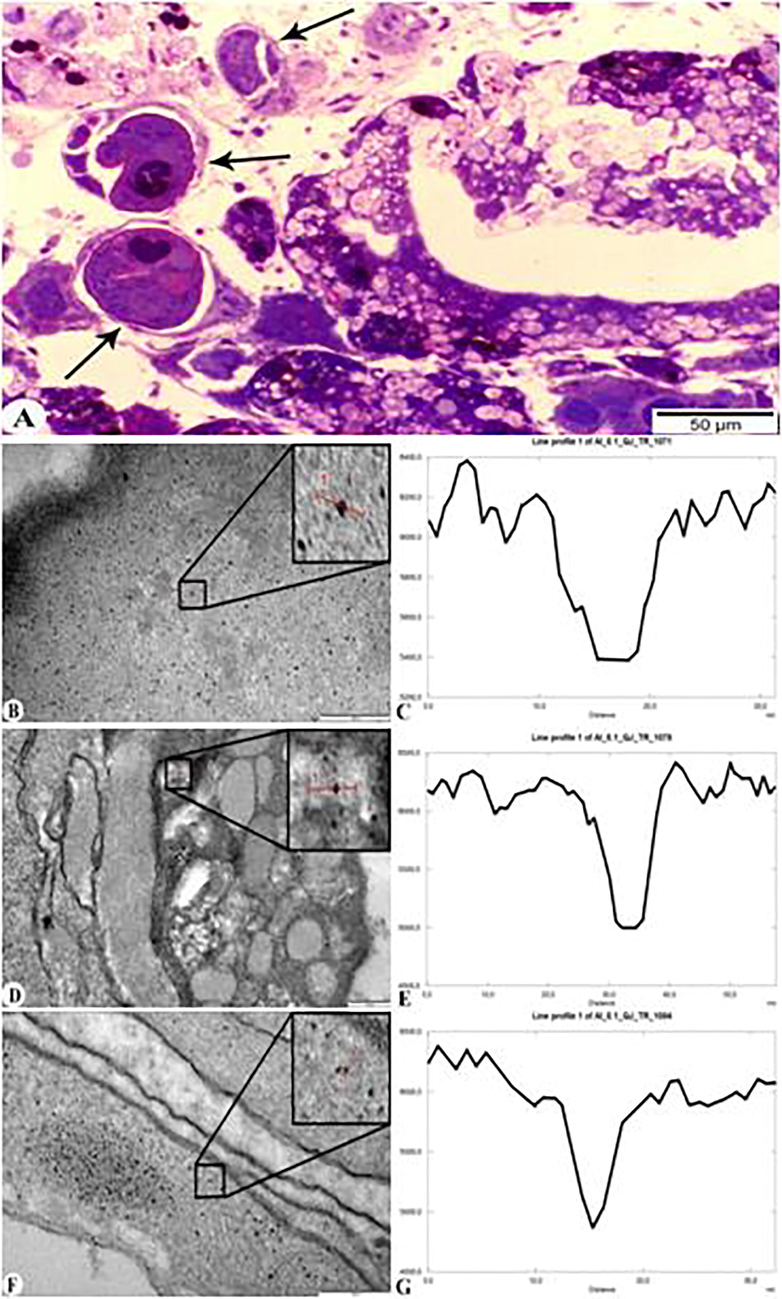
Digestive glands of M. praemorsa mollusc fed with AlNPs infected with trematode larvae C. agstaphensis 25. A – Parasite-infected digestive gland of mollusc (semithin sections − 1 μm); B and C – TEM images and diagram of the wall of the sporocyst (capsule) with AlNPs; D and E – TEM images and diagram of the cercarian parenchyma with AlNPs; F and G – TEM images and diagram of the internal organs of the parasite with AlNPs (ultrathin sections – 50–70 nm).
4 Discussion
Recently, the effect of nanoparticles on various components of the food chain, including different types of plants, has been studied by many researchers (Ahmadov et al., 2018, 2020; Rajput et al. 2018a; Agayeva, 2019; Fedorenko et al., 2021). The accumulation of Fe3O4 and AlNPs in a simple food chain (plant-mollusk-fish) was studied by feeding E. canadensis to Lymnaea auricalaria and M. praemorsa molluscs, which in turn fed Oncorhynchus mykiss fish (Ahmadov et al., 2018, Agayeva, 2019; Agayeva et al., 2020). The penetration of silver nanoparticles and gold nanoparticles through the root, movement, and accumulation in the stem and leaves of the pea plant has also been studied by TEM method. The accumulation of nanoparticles in cell organelles was observed during TEM analysis of epidermal, xylem, and phloem cells of the samples (Ahmadov et al., 2020).
In this context, the accumulation of AlNPs in the cell wall was observed. In addition, pathomorphological changes in all components of the food chain have been studied using light microscope and TEM. Pathomorphological changes in the plant under the exposure of different nanoparticles have been studied in the past (Rocha et al., 2015; Rajput et al., 2018a; Rajput et al., 2018b). Results showed damage to the cell wall, membrane, chloroplast structure, and thylakoid membranes, followed by abnormal size of plastoglobules and starch granules and swollen mitochondrial cristae. Similar changes in the organelles were observed in the present context too due to the exposure of AlNPs.
Previously, Aluminium (Al) was considered unavailable to the freshwater biota due to its lack of solubility trait at pH 6–8 (Driscoll and Schecher, 1989). Study showed that Al is accumulated in the freshwater snail (Lymnaea stagnalis) at neutral pH (Elangovan et al., 2000) and had lower impact on behaviour (Truscott et al., 1995) following exposure to environmentally relevant concentrations of the metal (Dixon and Gardner, 1998). Study exhibited that disparate nanoparticles accumulated in the digestive glands (digestive and excretory cells - yellow, green, and small granules). Further, authors demonstrated that these organs act as a ‘sink’ for Al, thereby regulating exposure of other soft tissues to this metal (Elangovan et al., 2000). In the present investigation, AlNPs were observed to accumulate in the digestive glands of M. praemorsa. As per the microscopic studies, Elangovan et al. (2000) observed a larger number of granules than the control group in the snail’s digestive gland. According to Mohammadein et al. (2013), the accumulation of metals in the mollusc Eobania vermiculata caused hypertrophy of the digestive tract, enlargement and increase in the number of secretory glands, and destruction of cytoplasm. Similar changes were also observed in M. praemorsa snails after the use of AlNPs in this study.
5 Conclusions
The AlNPs exposure was found to accumulate in the cell wall and intercellular spaces of the plant leaves. The nanoparticles caused structural changes in cell wall, cytoplasm, mitochondria, and chloroplasts. M. praemorsa fed with AlNPs-exposed E. canadensis showed thinning of the tunica propria of the digestive glands, widening of the glands, and formation of edema. In addition, the connective tissue between the glands was compressed and filled with edematous fluid. Electronograms of ultrathin incisions made from the digestive glands infected with sporocysts filled with cercariae showed that nanoparticles passed through the wall of the sporocyst and parenchyma of the cercaria into the internal organs of the cercaria. In a nutshell, the exposure of AlNPs in the aquatic ecosystem caused certain pathomorphological changes in the components that make up the food chain.
Acknowledgements
Authors would like to acknowledge Baku State University and Azerbaijan Medical University for the support. Authors extend their appreciation to the Researchers Supporting Project number (RSP-2021/20), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Transition of nanoparticles Fe3O4 and Al in a simplified aquatic food chain. Bull. Sci. Practice. 2019;5:54-63.
- [Google Scholar]
- Exposure of rainbow trout (Oncorhynchus mykiss) to magnetite (Fe3O4) nanoparticles insimplified food chain: Study on ultrastructural characterization. Saudi J. Biol. Sci.. 2020;27:3258-3266.
- [Google Scholar]
- Transfer of nanoparticles in a simplified aquatic food chain: from water plant Elodea canadensis to molluscs Lymnaea auricularia. J. Low Dimens. Syst.. 2018;2:41-45.
- [Google Scholar]
- The migration study of nanoparticles from soil to the leaves of plants. Biointerface Res. Appl. Chem.. 2020;10:6101-6111.
- [Google Scholar]
- Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing. Ecotoxicology. 2008;17(5):387-395.
- [Google Scholar]
- Ecotoxicity of selected nanomaterials to aquatic organisms. Environ. Toxicol.. 2008;223:591-598.
- [Google Scholar]
- Bivalve molluscs as a unique target group for nanoparticle toxicity. Marine Environ. Res.. 2012;76:16-21.
- [Google Scholar]
- Ecotoxicity test methods and environmental hazard assessment for engineered nanoparticles. Ecotoxicology. 2008;17(5):421-437.
- [Google Scholar]
- Development of an expert system for the integration of biomarker responses in mussels into an animal health index. Biomarkers. 2007;12(2):155-172.
- [Google Scholar]
- A Polychromatic staining method for epoxy embedded tissue: a new combination of methylene blue and basic fuchsine for light microscopy. Biotech. Histochem.. 2005;80(5-6):207-210.
- [Google Scholar]
- Reactive aluminium in UK surface waters. Chem. Speciation Bioavailab.. 1998;10(1):11-17.
- [Google Scholar]
- Aqueous chemistry of aluminium. In: Gileman H.J., ed. Aluminium and Health-A Critical Review. New York.: Arcel Dekker; 1989. p. :27-65.
- [Google Scholar]
- Localization and fate of aluminium in the digestive gland of the freshwater snail Lymnaea stagnalis. Tissue Cell. 2000;32(1):79-87.
- [Google Scholar]
- The toxic effect of CuO of different dispersion degrees on the structure and ultrastructure of spring barley cells (Hordeum sativum distichum) Environ. Geochem. Health. 2021;43:1673-1687.
- [Google Scholar]
- Ecotoxisity of CdTe quantom dots to freshwater mussel: impacts on immune system, oxidative stress and genotoxisity. Aquatic Toxicol.. 2008;86:333-340.
- [Google Scholar]
- The ecotoxicology of nanoparticles and nanomaterials: current status, knowledge gaps, challenges, and future needs. Ecotoxicology. 2008;17(5):315-325.
- [Google Scholar]
- Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem.. 2008;27:1825-1851.
- [Google Scholar]
- Electron microscopy: Methods and Protocols. Totowa: Humana Press; 2007. p. :625.
- Cercaria from trematodes in freshwater mollusc Melanopsis praemorsa from Azerbaijan. 1. Morphology and chaetotaxy of three new species of stylet cercaria (Trematoda, Plagiorchiida, Lecithodendroidea) Zoolojiskiy Zhurnal.. 2012;91:1443-1456.
- [Google Scholar]
- Bioaccumulation and histopathological changes of the digestive gland of the land snail Eobania vermiculata (Mollusca: Gastropoda), as biomarkers of terrestrial heavy metal pollution in Taif city, Italian. J. Zool.. 2013;80(3):345-357.
- [Google Scholar]
- Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ. İnt.. 2006;32(8):967-976.
- [Google Scholar]
- Toxicity of copper oxide nanoparticles on spring barley (Hordeum sativum distichum) Sci. Total Environ.. 2018;15:1103-1113.
- [Google Scholar]
- Effects of copper nanoparticles (CuO NPs) on crop plants: a mini review. BioNanoScience. 2018;8(1):36-42.
- [Google Scholar]
- Ecotoxicological impact of engineered nanomaterials in bivalve molluscs: an overview. Marine Environ. Res.. 2015;111:74-88.
- [Google Scholar]
- Considerations for environmental fate and ecotoxicity testing to support environmental risk assessment for engineered nanoparticles. J. Chromat.. 2009;A1216:503-509.
- [Google Scholar]
- Effect of aluminium and lead on activity of the freshwater snail Lymnaea stagnalis. Can. J. Fish. Aquat. Sci.. 1995;52:1623-1629.
- [Google Scholar]
- Marine aggregates facilitate ingestion of nanoparticles by suspension feeding bivalves. Marine Environ. Res.. 2009;68(3):137-142.
- [Google Scholar]
- Biomagnification of cadmium selenide quantum dots in a simple experimental microbial food chain. Natural Nanotechnol.. 2011;6:65-71.
- [Google Scholar]







