Translate this page into:
Methanolic extract of coconut (Cocos nucifera L.) haustorium mitigates pro-oxidant-mediated apoptotic cell death via Nrf-2 pathway and lipopolysaccharide-induced cytokine release in cells
⁎Corresponding authors. hak3962@sch.ac (Hak-Jae Kim), arunaksharan1990@gmail.com (Arunaksharan Narayanankutty)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cocos nucifera L is well-known for its edible and medicinal uses; multiple products from the different parts of the plant are being used by various communities in India. A less explored product is the coconut haustorium, the solid endosperm portion, which is highly nutritious. The present study first time evaluated the antioxidant, anti-inflammatory and cytoprotective properties of methanolic extract of Coconut haustorium (CHE). The cytoprotective effect of CHE was evaluated in hydrogen peroxide/malondialdehyde-induced toxicity IEC-6 (normal intestinal cell). Initially the biologically safe concentration of CHE and IC50 value of hydrogen peroxide and malondialdehyde was estimated by MTT assay. Treatment with H2O2 and MDA led to glutathione depletion and reduction in the catalase and glutathione reductase activity; which resulted in the increased oxidative stress and thereby cell death. Pretreatment with the different concentrations (10, 20 and 40 µg/mL) of CHE offered significant and dose-dependent protection against the toxicity induced by hydrogen peroxide and malondialdehyde. It is observed that the CHE pretreatment restored the antioxidant enzyme activities and GSH levels back to normal; the improvement in antioxidant status may be through the upregulation of Nrf2/HO1 pathway as observed in CHE pretreated cells. The anti-inflammatory potential was evaluated in the lipopolysaccharide (LPS-1 µg/mL) induced model in macrophages (Raw 264.7 cells). CHE pretreatment reduced the LPS- induced release of cytokines such as IL-1β, IL-6, and tumor necrosis factor-alpha (TNF-α). The results indicated the antioxidant, anti-inflammatory and cytoprotective effect of less explored haustorium from Coconut.
Keywords
Antioxidant activity
Coconut haustorium
Cytokine release
Cytoprotective effect
Anti-inflammatory activity
- CHE
-
Methanolic Extract of Coconut Haustorium
- Nrf2
-
Nuclear factor erythroid 2-related factor 2
- CD
-
Conjugated dienes
- TBARS
-
Thiobarbituric acid reactive substance
- GSH
-
Reduced glutathione
1 Introduction
Coconut is a common plant in South Asian countries including the Indian subcontinent, which has been known for its edible products and their pharmaceutical effects. This includes different forms of edible oils extracted from dried coconut kernel, and fresh kernel milk. Edible oils isolated from coconut kernels are well-known for numerous health beneficial effects; the virgin coconut oil extracted from fresh coconut milk has been reported to have antioxidant, anti-inflammatory (Narayanankutty et al., 2018), cytoprotective (Illam et al., 2017), hypoglycemic and anticancer (Narayanankutty et al., 2020b) activities. Coconut water and coconut inflorescence sap, obtained from coconut, are also known for numerous biological benefits (Joseph et al., 2021). Similarly, a protein isolated from the coconut kernel and coconut milk is another important health beneficial product (Li et al., 2018). In our preliminary study, the protective efficacy of coconut haustorium against fluoride-induced toxicity was also observed (Job et al., 2021b).
The antioxidant activities are usually estimated in animal models especially by intoxicating the animals using various chemicals or drugs (Li et al., 2019). Similarly, the anti-inflammatory activity is conducted using paw edema or ear edema models induced by lipopolysaccharide, carrageenan, formalin or dextran (Narayanankutty et al., 2020a). However, the use of animals is to be reduced, especially when the test compounds are lacking preliminary information regarding their biological effects. Therefore, several in vitro models are used as preliminary models for the evaluation of various biological properties including pharmacological and toxicological effects. Hence, it is expected that the coconut haustorium extract may also have similar biological properties and due to which it may protect against oxidative and inflammatory insults in cultured intestinal epithelial cells. Emphasis on the redox status in the cytoprotection and cytokine release in anti-inflammatory activity has been made in the present study.
2 Methodology
2.1 Reagents used in the experiment and cell lines
Normal colon epithelial cells from rats (IEC-6) and murine macrophages (Raw 264.7) were procured from the cell culture collection center of the National Centre for Cell Science-DBT, Pune. The cells were maintained under standard conditions using complete growth media (RPMI-1640) with 10% FBS, 5% CO2 at 37 °C.
2.2 Collection, extract preparation, and characterization of coconut haustorium
The sprouted coconuts (12–13 month old West coast tall variety) were collected from Kozhikode and de-husked, dried in an incubator at 50 °C and then powdered. The powder was extracted with 100% methanol and dried under vacuum concentration. The methanolic extract of coconut haustorium (CHE) was prepared by Soxhlet extraction (Borosil, Mumbai, India). The dried coconut haustorium powder was extracted using methanol (100%) for 6 h continuously. Total phenol contents (Ahmed and Tavaszi-Sarosi, 2019) and flavonoid contents (Wang et al., 2020) were determined by standard procedures. The phytochemical composition was analyzed by using LC/MS analysis (Malayil et al., 2020).
2.3 Detection of biologically safe concentration of CHE and cytoprotective effect
The nontoxic concentration of haustorium extract of Coconut (CHE) and cytotoxic activity of H2O2 and malondialdehyde (MDA) was determined by MTT assay (Nawaz et al., 2021). The cytoprotective effect was studied according to the protocols described by (Malayil et al., 2020). The percentage of cell death was calculated by comparing it with a control cell. The percentage inhibition of cell death with respect to the free radical control group was determined as follows;
2.4 Analysis of intracellular antioxidants and indicators of oxidative insults
The intra-cellular reduced glutathione (GSH) level in µmoles/mg protein was determined by the reaction of Ellman’s reagent (DTNB) (Nair et al., 2016). The activity of antioxidant enzyme catalase (CAT) was determined as the degradation of hydrogen peroxide per unit time as previously described (Illam et al., 2017). The lipid peroxidation indicator, thiobarbituric acid reactive substances (TBARS) and level of conjugated dienes (CD) were spectrophotometrically quantified according to the methods described by Narayanankutty et al. (2017). All these antioxidant parameters and oxidative stress status were finally expressed as nanomoles or IU per milligram of protein (Malayil et al., 2020).
2.5 Gene expression analysis by qPCR
The cells were harvested and cDNA was synthesized using CellAmp™ kits (Takara Bio, India) (Job et al., 2021a). The thermal cycling and sequences of primers used in the study and their sequences are given as supplementary material 1. The expression of each gene in different treatments was calculated by the 2-ΔΔCT method against the internal standard as beta-actin (Rao et al., 2013).
2.6 Inhibitory effect of CHE on Lipopolysaccharide-induced cytokine production from Raw 264.7 cells
The murine macrophage cell line Raw 264.7 was initially exposed to different doses of CHE (10, 20, and 40 µg/mL) for 24 h and later stimulated to secrete pro-inflammatory cytokines by the treatment with lipopolysaccharide (1 mg/mL). The levels of proinflammatory cytokines being released by the macrophages (IL-1β, IL-6, and TNF-α) were estimated using PeproTech ELISA kits (Rocky Hill, USA) according to the instruction manual. The quantity of nitric oxide in the media was quantified as described previously (House et al., 2020).
2.7 Statistical analysis
The data was presented in the manuscript as mean ± SD for different concentrations, each carried out in triplicate assays. Levels of significance and statistical operations were performed using one-way analysis of variance (ANOVA) followed by Tukey Kramer post hoc test (Graph pad Prism 7.0 version, La Jolla, USA).
3 Results
3.1 Total phenolic and flavonoid content as well as phenolic composition
The total phenol content of CHE was 44.32 ± 2.29 mg gallic acid equivalent (GAE) and the flavonoids content in the methanol extract of coconut haustorium was 7.48 ± 0.42 mg quercetin equivalent (QE). Further, the chemical characterization of the extract using LC-MS identified the presence of various simple phenolic acids and flavonoid compounds (Supplementary material 2).
3.2 Determination of cytotoxicity and biologically safe concentrations
The cytotoxicity of CHE was negligible up to a concentration of 50 µg/mL in both IEC-6 as well as macrophages (Raw 264.67) cells. The cell death was found to be<2% at the dose below 40 µg/mL and hence, the 10, 20, and 40 µg/mL were chosen for cytoprotective study. The cytotoxicity analysis by MTT determined the half-maximal cell death values for malondialdehyde as 352.18 ± 9.4 µM and hydrogen peroxide as 448.10 ± 6.8 µM. Thus, the concentrations of MDA and hydrogen peroxide were set as 450 and 350 µM (Fig. 1).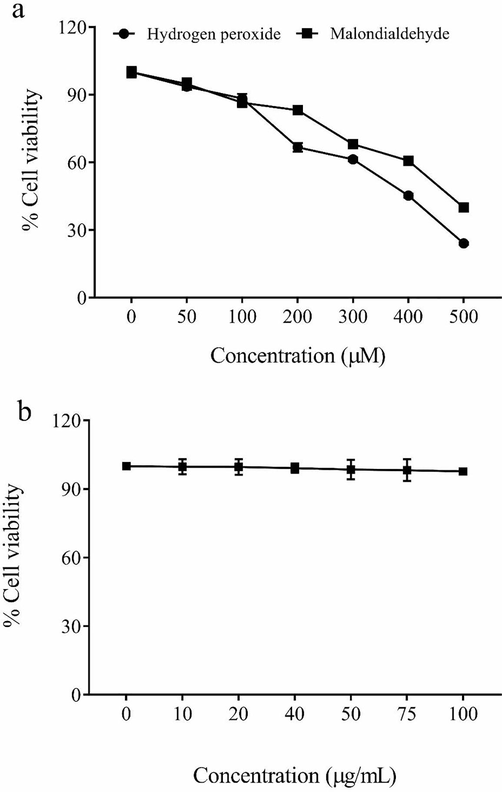
The cytotoxicity of hydrogen peroxide and malondialdehyde (a) methanolic extract of Coconut haustorium (b) determined using MTT assay for 48-hour period.
3.3 Cytoprotective effect of coconut haustorium
Treatment with H2O2-induced significant cell death (cell viability 49.1 ± 1.4%) in immortalized intestinal cells (IEC-6), at a concentration of 350 µM compared to the untreated cells. Further, pre-treatment with the CHE at the respective doses 10, 20, and 40 µg/mL protected the cell death induced by H2O2; the cell viability at these doses was estimated to be 55.8, 68.4, and 86.9% (Fig. 2a).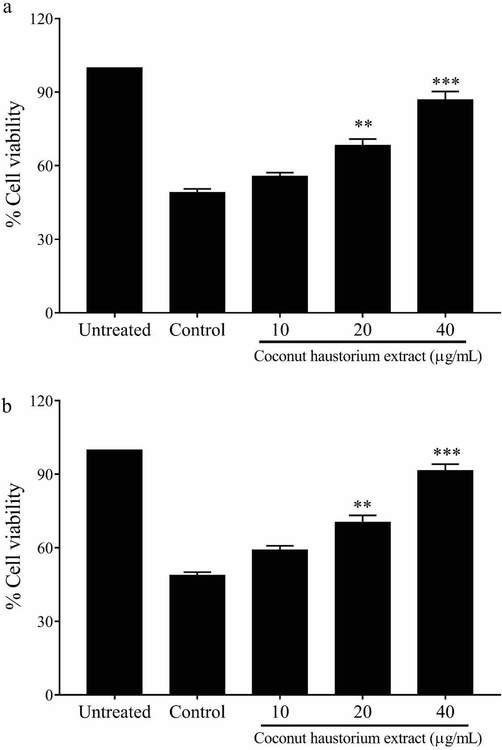
Protective effect of the CHE against the toxicity induced by hydrogen peroxide (a) and malondialdehyde (b) in immortalized intestinal cells (IEC-6).
Similarly, malondialdehyde (450 µM) treatment reduced the cell viability of IEC-6 cells to 48.8 ± 1.3%. The dose-dependent protective effect of CHE was observed in these cells upon pre-treatment with doses 10, 20, and 40 µg/mL. The cell viability during the respective treatment doses were 59.2, 70.4, and 91.5% (Fig. 2b).
3.4 Effect on cellular (IEC-6) antioxidant defence
3.4.1 Redox status in H2O2-treated cells
In the study, the H2O2-treated cells had a significantly higher level of lipid peroxidation products in terms of conjugated dienes and TBARS (Table 1). However, the cells upon treatment with 20 and 40 µg/mL of CHE reduced the CD and TBARS level significantly (p < 0.01). Further, the predominant cellular antioxidant tripeptide- glutathione was significantly depleted on treatment with H2O2. The pre-treatment with CHE reduced the glutathione depletion induced by H2O2 (Table 1). Similarly, the activity of catalase was increased by the treatment with H2O2; probably the rise is a cellular adaptation to counteract the increased H2O2 concentration. However, CHE pretreatment reduced the H2O2-induced elevation in catalase activity (p < 0.01). * p < 0.05; ** p < 0.01 and *** p < 0.001.
CAT
GSH
TBARS
CD
Normal
44.3 ± 3.5
4.28 ± 0.23
2.86 ± 0.32
35.67 ± 3.9
H2O2 (350 µM)
93.12 ± 4.7
1.87 ± 0.33
7.32 ± 0.34
105.1 ± 5.43
CHE 20 µg/mL
77.25 ± 4.8**
2.46 ± 0.28*
5.57 ± 0.52*
74.65 ± 6.46**
CHE 40 µg/mL
49.22 ± 5.2***
3.14 ± 0.25**
4.01 ± 0.47**
47.81 ± 5.18***
MDA (450 µM)
72.16 ± 5.2
2.03 ± 0.17
8.36 ± 0.38
92.10 ± 4.54
CHE 20 µg/mL
52.33 ± 4.8*
2.78 ± 0.34*
6.64 ± 0.23*
78.14 ± 5.82*
CHE 40 µg/mL
41.96 ± 5.4**
3.72 ± 0.36**
5.93 ± 0.37**
59.02 ± 3.11**
3.4.2 Redox status in MDA treated cells
MDA treatment resulted in a significant increase (p < 0.05) in the CD and TBARS levels (Table 1). Corroborating with these increases, the cellular glutathione level was reduced and catalase activity was increased (p < 0.05). However, pretreatment with the CHE-treatment at doses 20 (p < 0.05) and 40 µg/mL (p < 0.01) significantly reduced the MDA-induced alterations in the cellular redox status and which may have protected the cells from oxidative injury.
3.5 Changes in the expression pattern by qPCR
The treatment of IEC-6 cells with H2O2 (350 µM) or MDA (450 µM)-induced a significant elevation in the RNA expression of apaf-1, caspase 3, and caspase 7 genes (p < 0.001). Compared to the MDA models, the apoptosis induction in terms of fold change in these genes were more prominent in the hydrogen peroxide alone treated groups. However, pre-treatment with the different doses of CHE dose-dependently reduced the gene expression pattern of genes such as caspase-3, caspase-7 and apaf-1 (p < 0.01). On contrary to the expression of apoptotic genes, the Nrf-2 and heme oxygenase-1 expression was lowered in the H2O2 or MDA-treated cells. However, treatment with CHE has significantly induced the expression of these two genes (p < 0.001) and the effect was found to be dose-dependentFigure 3.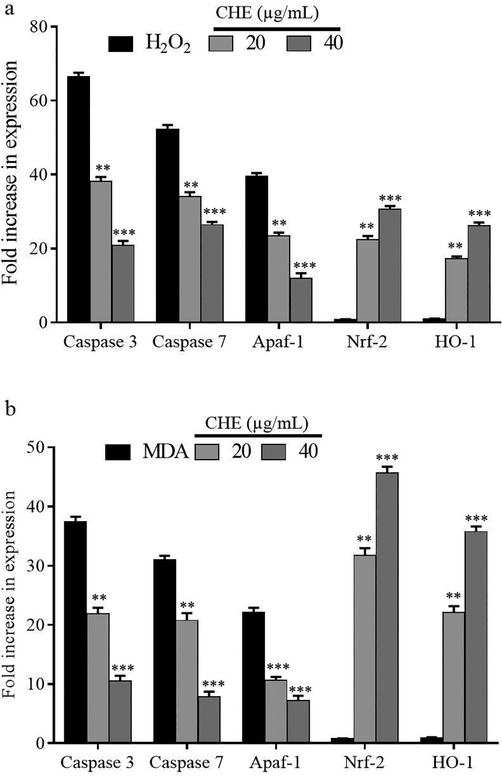
The effect of pre-treatment with CHE on the expression of apoptotic genes and antioxidant genes in cells treated with hydrogen peroxide (a) and malondialdehyde (b).
3.6 Anti-inflammatory activity in LPS-stimulated macrophages
Lipopolysaccharide was used to stimulate Raw 264.7 cells to induce the secretion of cytokines and nitric oxide radicals. In LPS alone treated macrophages, the release of cytokines like interleukins (IL-1β, IL-6) and TNF-α were significantly elevated (p < 0.001). Concomitantly, there observed a significant elevation in nitric oxide radicals in these cells. Pre-treatment with the polyphenol-rich CHE for 24 h at doses 20 and 40 µg/mL significantly reduced the secretion of IL-1β, IL-6 (p < 0.01) as well as TNF-α (p < 0.05). Further, the nitric oxide radicals were significantly reduced by the pre-treatment with CHE dose 20 and 40 µg/mL (Table 2). * p < 0.05; ** p < 0.01 and *** p < 0.001. IL-1β, IL-6 and TNF-α were represented as pg/mg protein whereas NO as µM/mg protein.
IL-1β
IL-6
TNF-α
NO
Untreated
79.5 ± 6.9
102.4 ± 14.3
211.7 ± 11.3
15.3 ± 3.2
LPS
468.3 ± 35.6
1223.6 ± 93.5
2026.7 ± 44.5
144.2 ± 7.3
CHE 10 µg/mL
322.5 ± 41.3*
1157.3 ± 58.2 ns
1838.2 ± 68.5*
101.2 ± 6.3 ns
CHE 20 µg/mL
258.4 ± 34.8**
955.4 ± 63.3**
1316.4 ± 64.9**
93.7 ± 3.7*
CHE 40 µg/mL
195.2 ± 21.5***
852.2 ± 52.1***
1085.2 ± 69.1**
59.5 ± 6.2**
4 Discussion
The study observed higher amounts of polyphenols and flavonoids in the CHE; LCMS analysis indicated the possible combination of various simple phenolic acids together with flavonoid compounds in it. The mitigating effect of CHE on intestinal epithelial cells (IEC-6) against the toxicities of H2O2 and malondialdehyde has been described in the study. Hydrogen peroxide is a common product formed during the normal metabolism of the cell as well as by the gut microflora (Lennicke et al., 2015; Singh et al., 2018). Likewise, malondialdehyde (MDA) is also known to be formed in intestinal epithelial cells and plays a crucial role in the various pathological conditions of the colon (Kruidenier et al., 2003). Therefore, these molecules are perfect models of intestinal oxidative damage and pathology and are supported by previous literature (Cheng et al., 2011).
Results indicated increased oxidative damage and cell death in IEC-6 cells treated with either hydrogen peroxide or MDA; further, a significant increase was noted in the gene expression of various caspases and apaf-1. Previous reports of Cheng et al. (2011) and indicated that hydrogen peroxide and MDA are potent inducers of apoptosis in cells; hence, the increased expression of apoptotic genes in the free radical treatments possibly indicate the formation of apoptosome complex formation and mediating the apoptotic cell death (Bratton and Salvesen, 2010). Further, the pre-treatment with CHE at their respective low and high concentrations prevented the hydrogen peroxide/MDA-induced expression of caspases and apaf-1; this indicates the protective role of CHE.
The redox imbalance developed in the cells is evident from the reduction in reduced glutathione content of the cells. Being an intracellular antioxidant (Narayanankutty et al., 2019), the reduction in the intracellular GSH pool may have resulted in the subsequent diminution in the activities of glutathione peroxidase and glutathione-s-transferase. Glutathione peroxidase (GPx) is responsible for the cleaving and neutralization of peroxide radicals formed in the cellular milieu. Thus, the reduced activity of GPx may often be counteracted by the increased activity of catalase, which explains the observed hike in the activity of catalase in hydrogen peroxide or MDA-treated cells.
Previous studies by Martins and English (2014) has also observed a similar increase in catalase activity in cells exposed to peroxide radicals. Further, the increased peroxide stress often increase the cellular levels of early lipid peroxidation products like conjugated and TBARS (Al-Sheddi et al., 2016). The pre-treatment with CHE increased the intracellular glutathione levels subsequently reducing the catalase activity. The reduction in catalase activity may be due to the activation of GPx-mediated detoxification of peroxide radicals in these cells. The enhancement in enzymatic and non-enzymatic antioxidants is also reflected in the reduced TBARS and CD levels in CHE-treated cells. The increased antioxidant system such as GSH levels is well corroborated with the increased expression of genes Nrf-2 and heme oxygenase 1 during CHE pre-treatment. The Nrf2/HO1 signalling is proven to govern the intra-cellular antioxidant defence; the nuclear translocation of Nrf2 from the cytosolic compartment and subsequent binding with the specific sequence of antioxidant response element (ARE) regulates the de novo glutathione biosynthesis (Loboda et al., 2016). Besides, independent reports have previously indicated that individual phenolic and flavonoid compounds identified in the CHE are capable of inducing nrf2-mediated glutathione biosynthesis (Zhou et al., 2019). As hydrogen peroxide and MDA are involved in various intestinal disorders (Carini et al., 2017), the cytoprotective effect of CHE may also be helpful in the prevention of various oxidative stress-induced intestinal pathology.
Besides, the anti-inflammatory properties of CHE are evaluated in lipopolysaccharide-stimulated murine macrophages (Nguyen et al., 2020). The pre-treatment with CHE is shown to reduce the secretion of interleukins. Further, the LPS-mediated increase in the cellular nitric oxide content is also inhibited by CHE pre-treatment; the nitric oxide-inducible nitric oxide synthase (iNOS) is also an important regulator of inflammation in the body (Joo et al., 2014). The inflammatory cytokine and iNOS are involved in the onset and progression of inflammatory conditions in chronic diseases (McGeer and McGeer, 2004). It is therefore possible that the CHE may be beneficial in preventing chronic diseases by inhibiting cytokine secretion and nitric oxide activity.
5 Conclusion
The present study reports the cytoprotective effect of Coconut haustorium and its anti-inflammatory properties. Overall, by the antioxidant-mediated cytoprotective potential and inflammation reduction properties of the CHE may help to prevent the onset of various degenerative diseases; further, the possible use of coconut haustorium as a functional food is also evident.
Acknowledgement
The authors acknowledge King Saud University, Riyadh, Saudi Arabia for funding this research through Researchers Supporting Project No: RSP 2021/11. AN acknowledge the support of Kerala State Council for Science, Technology and Environment for the award of Student Project Scheme. Infrastructural development assistance from DBT-STAR scheme and RUSA, Govt. of India is duly acknowledged by AN.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Identification and quantification of essential oil content and composition, total polyphenols and antioxidant capacity of Perilla frutescens (L.) Britt. Food Chem.. 2019;275:730-738.
- [Google Scholar]
- Protective effect of Lepidium sativum seed extract against hydrogen peroxide-induced cytotoxicity and oxidative stress in human liver cells (HepG2) Pharm. Biol.. 2016;54(2):314-321.
- [Google Scholar]
- Carini, F., Mazzola, M., Rappa, F., Jurjus, A., Geagea, A.G., AL Kattar, S., Bou-AssI, T., Jurjus, R., Damiani, P., Leone, A., Tomasello, G., 2017. Colorectal Carcinogenesis: Role of Oxidative Stress and Antioxidants. Anticancer research 37, 4759-4766.
- The cytotoxic mechanism of malondialdehyde and protective effect of carnosine via protein cross-linking/mitochondrial dysfunction/reactive oxygen species/MAPK pathway in neurons. Eur. J. Pharmacol.. 2011;650(1):184-194.
- [Google Scholar]
- Variation in the polyphenol composition, antioxidant, and anticancer activity among different Amaranthus species. S. Afr. J. Bot.. 2020;135:408-412.
- [Google Scholar]
- Polyphenols of virgin coconut oil prevent pro-oxidant mediated cell death. Toxicol. Mech. Methods. 2017;27(6):442-450.
- [Google Scholar]
- Job, J.T., Rajagopal, R., Alfarhan, A., Narayanankutty, A., 2021a. Borassus flabellifer Linn haustorium methanol extract mitigates fluoride-induced apoptosis by enhancing Nrf2/Haeme oxygenase 1 –dependent glutathione metabolism in intestinal epithelial cells. Drug and chemical toxicology (Ahead of Print), 10.1080/01480545.01482021.01926476.
- Toxic effects of fluoride in intestinal epithelial cells and the mitigating effect of methanol extract of coconut haustorium by enhancing de novo glutathione biosynthesis. Environ. Res.. 2021;200:111717.
- [CrossRef] [Google Scholar]
- Inhibition of nitric oxide production in LPS-stimulated RAW 264.7 cells by stem bark of Ulmus pumila L. Saudi J. Biol. Sci.. 2014;21(5):427-435.
- [Google Scholar]
- Coconut inflorescence sap enhances exercise performance and plasma antioxidant status in young active men. NFS J.. 2021;23:37-43.
- [Google Scholar]
- Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J. Pathol.. 2003;201(1):28-36.
- [Google Scholar]
- Hydrogen peroxide – production, fate and role in redox signaling of tumor cells. Cell Commun. Signal.. 2015;13:39.
- [Google Scholar]
- Protective Effects of Fucoidan against Hydrogen Peroxide-Induced Oxidative Damage in Porcine Intestinal Epithelial Cells. Animals (Basel). 2019;9(12):1108.
- [CrossRef] [Google Scholar]
- Antioxidant Activity of Coconut (Cocos nucifera L.) Protein Fractions. 2018;23:707.
- Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell. Mol. Life Sci.. 2016;73(17):3221-3247.
- [Google Scholar]
- Borassus flabellifer haustorium extract prevents pro-oxidant mediated cell death and LPS-induced inflammation. Drug Chem. Toxicol. 2020:1-7.
- [Google Scholar]
- Catalase activity is stimulated by H(2)O(2) in rich culture medium and is required for H(2)O(2) resistance and adaptation in yeast. Redox Biol.. 2014;2:308-313.
- [Google Scholar]
- Inflammation and the degenerative diseases of aging. Ann. N. Y. Acad. Sci.. 2004;007
- [Google Scholar]
- Virgin coconut oil supplementation ameliorates cyclophosphamide-induced systemic toxicity in mice. Hum. Exp. Toxicol.. 2016;35(2):205-212.
- [Google Scholar]
- Narayanankutty, A., Aswathi, S., Joice Tom, J., 2020a. Targeting Toll like Receptors in Cancer: Role of TLR Natural and Synthetic Modulators. Current pharmaceutical design (E-pub Ahead of Print), 10.2174/1381612826666200720235058.
- Health impacts of different edible oils prepared from coconut (Cocos nucifera): A comprehensive review. Trends Food Sci. Technol.. 2018;80:1-7.
- [Google Scholar]
- Glutathione, an Antioxidant Tripeptide: Dual Roles in Carcinogenesis and Chemoprevention. Curr. Protein Pept. Sci.. 2019;20(9):907-917.
- [Google Scholar]
- Vitamin E supplementation modulates the biological effects of omega-3 fatty acids in naturally aged rats. Toxicol. Mech. Methods. 2017;27(3):207-214.
- [Google Scholar]
- Curcumin enriched VCO protects against 7, 12-Dimethyl benz [a] anthracene-induced skin papilloma in mice. Nutr. Cancer. 2021;73(5):809-816.
- [Google Scholar]
- In vitro cytotoxic potential of Solanum nigrum against human cancer cell lines. Saudi J. Biol. Sci.. 2021;28(8):4786-4792.
- [Google Scholar]
- Nguyen, T.Q.C., Binh, T.D., Kusunoki, R., Pham, T.L.A., Nguyen, Y.D.H., Nguyen, T.T., Kanaori, K., Kamei, K., 2020. Effects of Launaea sarmentosa Extract on Lipopolysaccharide-Induced Inflammation via Suppression of NF-κB/MAPK Signaling and Nrf2 Activation. Nutrients 12.
- An improvement of the 2̂(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath. 2013;3:71-85.
- [Google Scholar]
- Hydrogen peroxide production by lactobacilli promotes epithelial restitution during colitis. Redox Biol.. 2018;16:11-20.
- [Google Scholar]
- Quantitative assessment of phenolic acids, flavonoids and antioxidant activities of sixteen jujube cultivars from China. Plant Foods Hum. Nutr.. 2020;75(2):154-160.
- [Google Scholar]
- Recent Advances of Natural Polyphenols Activators for Keap1-Nrf2 Signaling Pathway. Chem. Biodivers.. 2019;16(11)
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2021.101715.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







