Translate this page into:
Methanol extract of Artemisia brevifolia as a curative agent against CCl4 induced nephrotoxicity in albino rats
⁎Corresponding authors. asmabinm@gmail.com (Asma Ashraf), mushahid@ksu.edu.sa (Shahid Mahboob)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Carbon tetrachloride (CCl4) is a Potential nephrotoxin that causes severe and prolonged chemical toxicity. Investigations have been accomplished on numerous species of genus Artemisia, but the plant Artemisia brevifolia has hardly been used in this respect. The present study was considered to ascertain the effects of “Artemisia brevifolia plant extract” against CCl4 prompted nephrotoxicity in male albino rats. Forty-eight male albino rats were allocated into eight groups containing 6 rats in each. Group I was the control group. Group II was given dimethyl sulfoxide (DMSO) (1 ml/kg) orally. Group III was treated with dissolved CCl4 in olive oil (1 ml/kg). Group IV was given CCl4 + Silymarine. Group V and VI were administered with Artemisia brevifolia extract (150 and 300 mg/kg respectively) + CCl4. Group VII and VIII were provided with only 150 and 300 mg/kg extract of Artemisia brevifolia respectively. Results displayed that CCl4 administration significantly (p < 0.05) reduced the activity of antioxidant enzymes like catalase (CAT), peroxidase (POD), Superoxide dismutase (SOD), glutathione (GSH), glutathione reductase (GSR), glutathione-S-transferase (GST), albumin, creatinine clearance and Comet parameters (head length, % in head) while increased the level of thiobarbituric acid reactive substances (TBARS), hydrogen peroxide (H2O2), urea, creatinine, urobilinogen, urinary proteins and comet parameters (number of comets, tail length, comet length, tail moment, % in tail). The administration of Artemisia brevifolia significantly (p < 0.05) improved the histopathology of renal tissues. The results revealed that Artemisia brevifolia has protective consequences against CCl4 induced kidney damage.
Keywords
Carbon tetrachloride
Nephrotoxicity
Artemisia brevifolia
Protective effects
Antioxidant enzymes
1 Introduction
CCl4 is one of the most potent toxins, which is widely used in scientific research to produce an experimental model that mimics the oxidative stress in many pathophysiological situations (Yoshioka et al., 2016; Samad et al., 2020). Several previous studies have indicated that CCl4 is the best model for the generation of reactive oxygen species (ROS) in many tissues (Kamisan et al., 2014). The toxicity produced by CCl4 depends on the production of trichloromethyl radical (CCl3•), which further converts trichloromethyl into trichloromethyl peroxyl radical (CCl3O2•) in the presence of oxygen that is more toxic than trichloromethyl radicals (Ali and Abdelaziz, 2014). Chlorine and trichloromethyl radicals are formed due to the metabolic regeneration of CCl4 by cytochrome P-450 enzymes, which increases the oxidant-antioxidant instability. These Free radicals trigger lipid peroxidation and initiate a prolonged chain reaction (Naz et al., 2014). Excessive free radical production causes massive damage in lipids, proteins and DNA. Extensive disruption of DNA strands may trigger compensatory cell transformation and cell death due to the effect of CCl4-induced toxicity. It is revealed that CCl4-induced oxidative stress leads to renal toxicity inciting several uncontrolled disorders by producing acute and chronic kidney failure (Khan and Zehra, 2013; Naz et al., 2014).
There are various types of plants having efficient medicinal and therapeutics values. Herbal medicines are considered healthier, which have been proved a blessing for the treatment of several diseases (Bora and Sharma, 2011; Bari et al., 2020). The increasing tendency for the effective treatment of oxidative stress based diseases has inspired investigators to assess the antioxidant potential of therapeutic plants and plant-based active constituents (Wang et al., 2019). The demand for remedial plants has been increased worldwide due to their potential therapeutic role (Afsar et al., 2015). In the current situation, pieces of evidence are available to illustrate the immense capability of medical plants used in several curative techniques (Zhao et al., 2020). Various plants are the source of bioactive compounds that are effective ROS scavengers (Alkreathy et al., 2014; Sahreen et al., 2015). The naturally occurring antioxidants act as a protective agent to counter the effects of free-radicals that may instigate various permanent damages. Natural antioxidants containing plant extracts have shown useful curative abilities against chemically persuaded damages (Bakr e al., 2019). Secondary metabolites of plants are the prime reason for their medicinal properties. Scavenging of free radicals via plant-based products can provide another viable approach to cure tissue injury caused by oxidative stress (Khan et al., 2009).
The Artemisia genus is one of the extensively distributed genera of Asteraceae family containing over 500 different species, and this genus is distributed primarily in North America, Asia and Europe (Mehrdad et al., 2007). It has antibacterial, anticholesterolemic, insecticidal, antiseptic, cholagogue, antipyretic, purgative, diuretic, vasodilatory functions and is also used for jaundice treatment and inflammation of gall bladder (Gruenwald, 2000). Artemisia brevifolia, locally named as ‘Afsanteen’ is widely used in ethno-veterinary medicine system of Pakistan as an anthelmintic plant (Iqbal et al., 2004). It is found in the temperate Himalayas on dry stony slopes and open areas. It is one of the major species of cold desert Himalaya especially in Ladakh and Kashmir areas. Its flowering shoots and leaves have been used as a local medicine since years. It has been used as a carminative and digestive, hence mixed with local meat recipes. It has been known to be an antiseptic, anthelmintic, laxative, aphrodisiac, blood purifier, febrifuge, stomachic and antidote to scorpion stings (Watts, 1971; Chinese Academy of Sciences, 2013; Himalayan Voices, 2020: web). But the protective role of Artemisia brevifolia on the kidneys has not yet been investigated on the basis of scientific evidences. By keeping this in view, recent study is planned to investigate the curative capability of methanolic extract of the A. brevifolia on CCl4 persuaded nephrotoxicity in albino male rats.
2 Material and methods
2.1 Chemical
CCl4 was obtained from Sigma Aldrich, USA. The dose was prepared through dilution in olive oil.
2.2 Plant collection and preparation of extract
A. brevifolia plant was collected at the end of September 2018 from the area of Noori Top (Naran valley, KPK), recognized by a botanist ensuing the preserved samples in the Herbarium, Department of Botany, UAF, Pakistan. The A. brevifolia plant’s leaves were dried in the air beneath shady place to eradicate moistness. For extraction, dried leaves were minced to powder form and immersed in methanol for 72 h to obtain the hydro-alcoholic crude extract using Erlenmeyer flask at room temperature. This extraction process was repeated twice. For the filtration process, the Whatman number 1 filter was utilized. Methanol was vaporized through a rotary-evaporator at 40 °C under low pressure to collect the sticky content. For quality maintenance, the extract was kept at −20 °C.
2.3 Experimental design
Mature male albino rats (160–250 g) were used for this experiment and resided in the animal rearing house of UAF. Rats were provided with tap water and regular food with maintained 12 h light/dark cycle at 25 ± 3 °C. This study was authorized by the ethics commission of UAF. Doses of the plant extract were selected by following Waris et al., 2018. Forty-eight albino male rats were distributed into 8 groups comprising 6 of each. Group I was named as control group. Group II was given with dimethyl sulfoxide (DMSO:5%) with 10% of olive oil (1 ml/kg) orally and referred as vehicle control group. Group III was treated with CCl4 dissolved in olive oil (1 ml/kg) orally. Group IV was given CCl4 + Silymarine (50 mg/kg). Group V and VI were administered with A. brevifolia extract dissolved in DMSO (150 and 300 mg/kg respectively) orally with CCl4. Group VII and VIII were given orally with only 150 and 300 mg/kg extract of A. brevifolia respectively.
After 30 days, rats were given anesthesia and then decapitated. To separate plasma, trunk blood was drawn into heparinized syringes. Blood centrifugation was carried out for ten minutes at 3000 revolutions per minute (rpm). Plasma was stored at −20 °C for further analysis. Urine samples were collected before dissection through the method of Watts (1971). After dissection, the left kidney was fixed in 10% formalin buffer for histopathological evaluation and right kidney was kept at −80 °C to assess the activities of antioxidant enzymes. Kidney tissues were homogenized in Na3PO4 buffer at 12,000 rpm for 15 min and the temperature was maintained at 4 °C. This supernatant was ultimately used to assess multiple parameters.
2.4 Acute toxicity test
Three male rats were treated intragastrically with 150 mg/kg body weight dose (prepared in 5% DMSO) and were monitored for 2 weeks to check the mortality rate. No toxicity progression was observed in the rats, but the methodology was followed with augmented order of doses including 150, 200, 250 and 300 mg/kg of body weight as the maximum dose. Three rats were used per treatment. Mortality was not observed even in case of the highest dose tested during the study, so therefore for the assessment of nephrotoxicity experiment, 150 mg/kg and 300 mg/kg body weight doses were selected.
2.5 Analysis of antioxidant enzyme activity
CAT and POD activity was evaluated by following the process developed by Chance and Maehly (1955). SOD activity was assessed through the methodology of Kakkar et al. (1984). GSR activity was evaluated by the method of Carlberg and Mannervik (1975), whereas GST action was evaluated through Habig et al. (1974) procedure. The activity of GSH was assessed by using the protocol of Jollow et al. (1974).
2.6 Estimation of TBARS and hydrogen peroxide (H2O2)
TBARS level was assessed through the process of Ohkawa et al. (1979). The level of H2O2 was determined by using the methodology of Pick and Keisari (1981).
2.7 Biochemical analysis of serum and urine profile
AMP diagnostic kits (AMEDA labordiagnostic-Gmbh, Austria) were used for the assessment of creatinine, creatinine clearance, albumin, urinary protein, urea and urobilinogen level. Creatinine clearance, albumins, urinary proteins and urobilinogen were measured from urine, while serum creatinine and urea levels were measured from the blood.
2.8 Analysis of DNA damage
The procedure of Dhawan et al. (2009) was used to evaluate DNA impairment. In normal melting point agarose, sterilized slides were dipped and then let them to set at normal temperature. A slight portion of the kidney was put in 1 ml cold lysing-solution then dipped in 75 μl of low-melting point agarose mixture. Then carefully coated this mixture on precoated slides and gently covered with a coverslip. For 8–10 min, slides were placed on icepacks. A low melting point agarose mixture was coated again on slides after removal of the coverslip and permitted to solidify on icepacks again. After three coatings of low melting-point agarose, slides were dipped in lysing solution for at least 10 min and shifted to the refrigerator for two hours. 1% ethidium bromide was used for staining purposes. After that, electrophoresis was performed, and the slide was analyzed under a fluorescent microscope. For the assessment of DNA impairment CASP 1.2.3. b Image analysis software was used. 50–100 renal cell’s nuclei were analyzed to observe the DNA damage.
2.9 Histopathology
A fixative solution consisting glacial acetic acid (5 ml), formaldehyde (5 ml) and pure alcohol (85 ml) was utilized for the fixation of renal tissues. Thin sections (approximately 4–5 µm) of renal tissue were sliced and used for slides preparation. Stain (Hematoxylin/eosin) was applied for staining purposes. Slides were studied under light microscope (Leica LB, Germany) at 40X.
2.10 Statistical assessment
The complete data was presented as Mean ± SEM. The variations between control and experimental groups were evaluated via one-way ANOVA, followed by Tukey’s test through Minitab software. The p < 0.05 was contemplated as a level of significance.
3 Results
3.1 Effect of A. brevifolia on antioxidant enzymes
CCl4 administration showed significantly (p < 0.05) reduced activity of the antioxidant-enzymes, including catalase (CAT), peroxidase (POD), Superoxide dismutase (SOD), glutathione (GSH), glutathione reductase (GSR), glutathione-S-transferase (GST) when matched with control and vehicle-control group. The rats of CCl4 + Silymarin group displayed a remarkable (p < 0.05) increase in the enzyme activity in contrast to CCl4 administered group. Similarly, co-treated (CCl4 + A. brevifolia) groups showed considerable (p < 0.05) increase (dose-dependent) in antioxidant enzyme activity when compared to the CCl4 administered group. Rats administered with A. brevifolia alone showed significant (p < 0.05) increase in the activity of CAT, POD, SOD, GST, GSR and GSH content in comparison to CCl4 administered group (Table 1). 1. Values in a same column having dissimilar superscripts are substantially different from other values (p < 0.05). 2. CCl4 treated group was compared with control and vehicle control while all remaining groups were compared with CCl4 treated group. 3. CAT; Catalase, POD; Peroxidase, SOD; Superoxide dismutase, GSH; Glutathione, GSR; Glutathione reductase, GST; Glutathione-S-transferase.
Groups
CAT (U/mg protein)
POD (U/mg protein)
SOD (nanomole)
GST (mg/dl)
GSH (nM/min/mg/protein)
GSR (Nm NADPH oxidized/min/mg tissues)
Control
8.62 ± 0.19 a
6.88 ± 0.08 a
5.88 ± 0.06 a
24.9 ± 0.38 a
12.1 ± 0.63 a
4.35 ± 0.04 a
Vehicle control
8.66 ± 0.20 a
6.81 ± 0.06 a
5.82 ± 0.04 a
25.0 ± 0.20 a
12.6 ± 0.41 a
4.26 ± 0.04 a
CCl4
4.24 ± 0.10b
2.89 ± 0.03b
1.69 ± 0.06b
11.4 ± 0.37b
4.66 ± 0.29b
0.68 ± 0.05b
CCl4 + silymarin
8.24 ± 0.05 a
6.63 ± 0.07 a
5.16 ± 0.04 ac
22.0 ± 0.40c
11.3 ± 0.24 ac
3.93 ± 0.02c
A. brevifolia (150 mg/kg) + CCl4
7.06 ± 0.07c
5.97 ± 0.12c
4.97 ± 0.05c
20.7 ± 0.38 d
10.7 ± 0.29c
3.53 ± 0.05c
A. brevifolia (300 mg/kg) + CCl4
7.77 ± 0.05c
6.65 ± 0.03 a
5.19 ± 0.03 ac
22.0 ± 0.32c
11.3 ± 0.16 ac
3.86 ± 0.04c
A. brevifolia (150 mg/kg)
8.33 ± 0.06 a
6.83 ± 0.05 a
5.41 ± 0.04 a
23.5 ± 0.25 ac
11.8 ± 0.08 ac
4.04 ± 0.03 ac
A. brevifolia (300 mg/kg)
8.73 ± 0.08 a
6.92 ± 0.04 a
5.83 ± 0.04 a
24.3 ± 0.36 a
12.0 ± 0.06 a
4.36 ± 0.05 a
3.2 Effect of A. brevifolia on TBARS and H2O2
Rats administered with CCl4 displayed remarkable (p < 0.05) rise in the hydrogen peroxide (H2O2) and thiobarbituric acid reactive substances (TBARS) levels in contrast to rats of control and vehicle-control groups. In the co-treatment of CCl4 + silymarin, levels of H2O2 and TBARS were decreased substantially (p < 0.05) as matched with the CCl4 group. Similarly, Artemisia + CCl4 groups exhibited a considerable decline in H2O2 and TBARS levels (dose-dependently) in contrast to CCl4 administered group. Rats doctored with A. brevifolia only displayed significant (p < 0.05) reduction in H2O2 and TBARS levels when matched with CCl4 and cotreated groups almost near to the normal range (Table 2). 1. Values in a same column having dissimilar superscripts are substantially different from other values (p < 0.05). 2. CCl4 treated group was compared with control and vehicle control while all remaining groups were compared with CCl4 treated group. 3· H2O2; hydrogen peroxide, TBARS; thiobarbituric acid reactive substances.
Groups
TBARS (nM/min/mg protein)
H2O2 (µM/min/mg protein)
Control
15.9 ± 0.76 a
1.76 ± 0.07 a
Vehicle control
16.5 ± 0.55 ac
1.82 ± 0.07 a
CCl4
29.7 ± 1.22b
6.71 ± 0.09b
CCl4 + silymarin
17.2 ± 0.16c
2.04 ± 0.03c
A. brevifolia (150 mg/kg) + CCl4
18.6 ± 0.18 d
2.49 ± 0.08c
A. brevifolia (300 mg/kg) + CCl4
17.5 ± 0.22 cd
2.13 ± 0.05c
A. brevifolia (150 mg/kg)
16.8 ± 0.24 ac
1.99 ± 0.05 a
A. brevifolia (300 mg/kg)
16.6 ± 0.19 ac
1.86 ± 0.05 a
3.3 Effect of A. brevifolia on serum and urine profile
CCl4 administration exhibited significant (p < 0.05) escalation in the urea, creatinine, urobilinogen and urinary proteins concentrations while creatinine clearance and albumin concentration were reduced remarkably (p < 0.05) in urine as matched with control or vehicle control group. In CCl4 + silymarin administered group, the level of urea, creatinine, urobilinogen and urinary proteins decreased significantly (p < 0.05) when compared to CCl4 treated group. In contrast, creatinine clearance and albumin concentrations were elevated significantly (p < 0.05) as matched with CCl4 group. Cotreatment of A. brevifolia + CCl4 displayed significantly (p < 0.05) reduced level of urea, creatinine, urobilinogen and urinary protein concentrations while creatinine clearance and albumin concentrations were increased in comparison to the CCl4 administered rats. Rats treated with A. brevifolia alone displayed substantial (p < 0.05) decrease in urea, creatinine, urobilinogen and urinary proteins concentrations. In contrast, creatinine clearance and albumin concentrations were increase when matched with CCl4 and cotreated groups (Table 3). 1. Values in a same column having dissimilar superscripts are substantially different from other values (p < 0.05). 2. CCl4 treated group was compared with control and vehicle control while all remaining groups were compared with CCl4 treated group.
Groups
Urea (mg/dl)
Creatinine (mg/dl)
Creatinine clearance (ml/min)
Albumin (mg/dl)
Urobilinogen (mg/dl)
Urinary Proteins (mg/dl)
Control
19.1 ± 0.35 a
1.85 ± 0.03 a
1.47 ± 0.04 a
7.74 ± 0.12 a
3.40 ± 0.10 a
13.8 ± 0.50 a
Vehicle control
19.2 ± 0.36 a
1.87 ± 0.04 a
1.44 ± 0.02 a
7.72 ± 0.11 a
3.42 ± 0.09 a
15.1 ± 0.57c
CCl4
61.8 ± 1.76b
4.92 ± 0.09b
0.63 ± 0.03b
3.18 ± 0.09b
9.64 ± 0.21b
34.1 ± 1.73b
CCl4 + silymarin
23.4 ± 0.70c
1.99 ± 0.05 a
1.32 ± 0.01 a
7.38 ± 0.05 a
4.17 ± 0.16c
16.9 ± 0.19 cd
A. brevifolia (150 mg/kg) + CCl4
24.5 ± 0.59c
2.25 ± 0.04c
1.16 ± 0.02c
7.07 ± 0.04 a
4.82 ± 0.05c
18.9 ± 0.22 d
A. brevifolia (300 mg/kg) + CCl4
24.1 ± 0.41c
2.07 ± 0.04c
1.28 ± 0.02 a
7.24 ± 0.05 a
4.56 ± 0.05c
17.3 ± 0.30 cd
A. brevifolia (150 mg/kg)
21.2 ± 0.24 ac
2.04 ± 0.04c
1.31 ± 0.02 a
7.45 ± 0.06 a
4.08 ± 0.06c
16.4 ± 0.07 cd
A. brevifolia (300 mg/kg)
19.5 ± 0.28 a
1.94 ± 0.03 a
1.41 ± 0.05 a
7.52 ± 0.09 a
3.77 ± 0.06 ac
15.5 ± 0.35c
3.4 Effect of A. brevifolia on DNA damage
CCl4 administration showed substantial (p < 0.05) rise in the number of comets, comet length, tail length, tail moment, % DNA in tail and olive moment while a considerable decline was noted in % DNA in head and head length when matched with control and vehicle control. CCl4 + silymarin showed remarkable (p < 0.05) decrease in the number of comets, tail length, comet length, tail moment % DNA in tail and olive moment and a significant (p < 0.05) elevation in % in head and head length in contrast to CCl4 administered rats. Similarly, A. brevifolia + CCl4 administered groups (dose-dependent) revealed remarkable (p < 0.05) fall in number of comets, tail length, comet length, % DNA in tail, olive moment, tail moment and a remarkable (p < 0.05) rise in % DNA in head and head length in contrast to CCl4 administered rats. Rats treated with A. brevifolia alone displayed significant decrease in the number of comets, comet length, tail length, tail moment, % in DNA in tail and olive moment and a significant (p < 0.05) escalation was noticed in head length and % DNA in head in comparison to CCl4 and co-treated rats (Table 4). 1. Values in a same column having dissimilar superscripts are substantially different from other values (p < 0.05). 2. CCl4 treated group was compared with control and vehicle control while all remaining groups were compared with CCl4 treated group.
Groups
Number of comets
Comet length
Tail length
Head length
% in head
% in tail
Tail Moment
Olive tail moment
Control
24.0 ± 1.53 a
31.8 ± 1.46 a
4.79 ± 0.23 a
43.5 ± 1.38 a
96.1 ± 0.20 a
3.81 ± 0.20 a
0.56 ± 0.04 a
2.42 ± 0.05 a
Vehicle control
25.6 ± 1.18 a
31.9 ± 1.81 a
4.80 ± 0.25 a
42.6 ± 1.40 a
95.9 ± 0.19 a
4.05 ± 0.16 a
0.63 ± 0.06 a
2.45 ± 0.04 a
CCl4
44.3 ± 1.87b
69.1 ± 1.56b
16.4 ± 0.29b
26.5 ± 1.09b
88.0 ± 0.42b
11.9 ± 0.42b
1.92 ± 0.09b
4.33 ± 0.05b
CCl4 + silymarin
29.9 ± 0.67c
34.7 ± 1.36c
5.80 ± 0.13 ac
40.6 ± 0.89c
95.2 ± 0.16 a
4.78 ± 0.16 a
0.94 ± 0.06c
2.57 ± 0.04 a
A. brevifolia (150 mg/kg) + CCl4
34.4 ± 0.51 d
39.0 ± 1.36 d
6.01 ± 0.07c
39.6 ± 0.81c
94.1 ± 0.14c
5.87 ± 0.14c
0.95 ± 0.02c
2.71 ± 0.02c
A. brevifolia (300 mg/kg) + CCl4
31.3 ± 0.69 cd
38.1 ± 0.44 d
5.81 ± 0.14 ac
40.9 ± 1.02c
94.5 ± 0.09c
5.48 ± 0.09c
0.87 ± 0.06 ac
2.49 ± 0.03 a
A. brevifolia (150 mg/kg)
29.6 ± 0.80c
33.1 ± 1.76c
5.53 ± 0.07 ac
41.6 ± 0.83 ac
94.6 ± 0.05c
5.34 ± 0.05c
0.65 ± 0.03 a
2.41 ± 0.01 a
A. brevifolia (300 mg/kg)
28.0 ± 0.99 ac
31.9 ± 1.47 a
5.28 ± 0.11 ac
44.3 ± 0.90 a
95.0 ± 0.14 a
4.93 ± 0.14 ac
0.55 ± 0.03 a
2.39 ± 0.02 a
3.5 Curative effects of A. brevifolia on tissue histology of kidney
Normal histology of renal tissues was detected in control and vehicle control group (A-B). In CCl4 treated group of rats; renal tissues exhibited fibrosis in renal interstitium, glomerular collapse, tubular damage, tubular dilation, devastation of Bowman’s capsule in nephrons and obstruction in capillaries (C). CCl4 + silymarin treated group decreased the level of destruction in kidney tissues in contrast to impairment caused by CCl4 treatment (D). The effect of co-treatment with CCl4 and low dose of A. brevifolia (150 mg/kg) was observed as it eliminated chronic damages (E). Treatment with CCl4 and high dose of A. brevifolia (300 mg/kg) was resulted in maintaining normal renal morphology, showed normal tubular and glomerular anatomy, reduced capillary blockage and interstitial edema (F). The group of rats administered with A. brevifolia alone showed normal histology of renal tissues as in control group (G-H) (Fig. 1).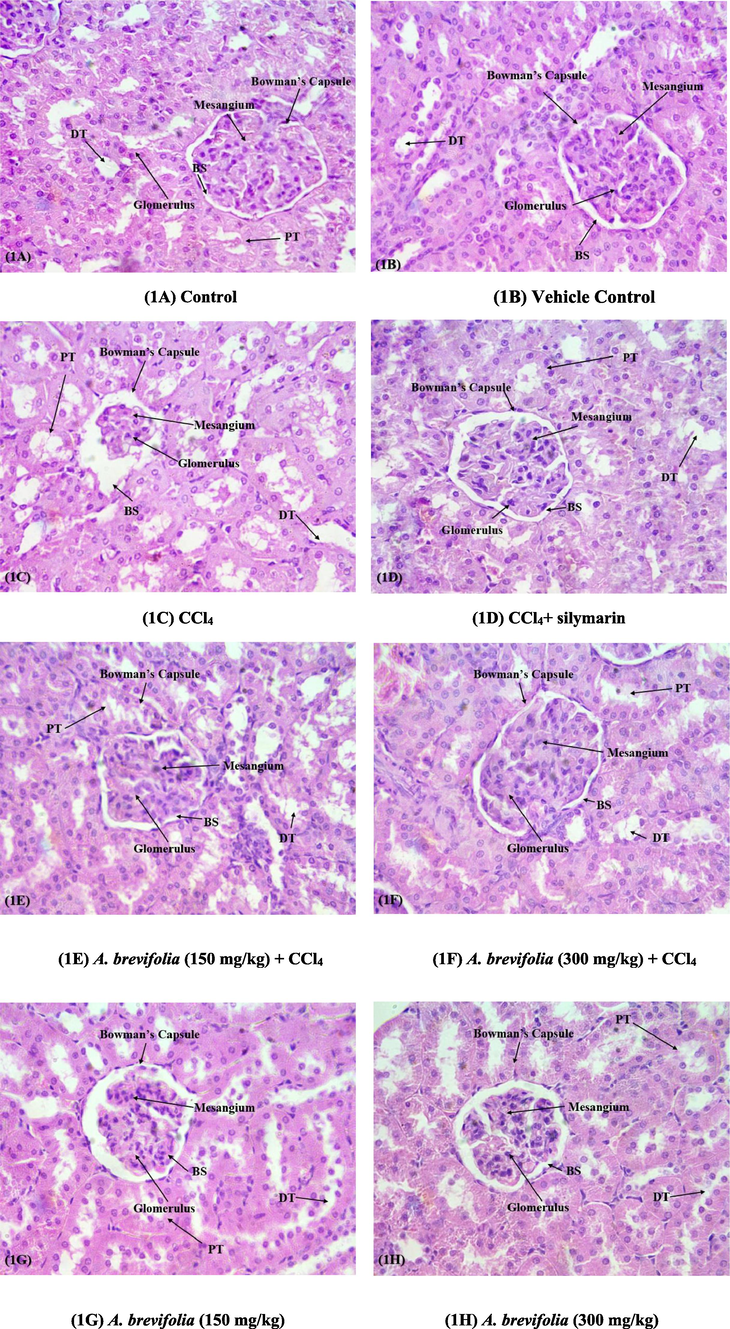
Analysis of nephro-histology. H&E stain; 40X (A) Control Group; normal histology (B) Vehicle Control Group; normal histology (C) CCl4 treated group; degeneration of renal tissues and tubule, hemorrhage and glomerular atrophy (D) CCl4 + silymarine treated group; recovery of degenerated tissues (E) A. brevifolia (150 mg/kg) + CCl4; Minor recoveries of tubular degeneration towards normal (F) A. brevifolia (300 mg/kg) + CCl4; effective recovery of renal tubule and resurgence of damaged kidney tissues (G) A. brevifolia (150 mg/kg); normal histology (H) A. brevifolia (150 mg/kg); normal and improved renal histology. BS; Bowman’s space, DT; Distal tubules, PT; Proximal tubules.
4 Discussion
CCl4 is broadly used as a common toxin to produce damage in different body organs. It has been revealed that CCl4 releases free radicals, which in turn damages the various organs of the body (Weber et al., 2003). Hepatic fibrosis (Abraham and Wilfred, 2000), kidney damage (Khan et al., 2012), testicular injury (Shyu et al., 2008) and heart muscle destruction (Mohamed, 2010; Khan, 2012) have been reported due to chronic CCl4 exposure. It affects lipid bilayer structure of membrane through peroxidation process and production of trichloromethyl radical which leads to cellular damage (Goodla et al., 2019). The abundance of naturally occurring herbal and medicinal plants have been used for hundreds of years by man (Afsar et al., 2016). Artemisia genus is extensively dispersed in temperate areas of Asia, Europe and North America consisting of perennial, biennial and annual herbs or small shrubs. Phytochemical analysis of Artemisia displays the presence of phenolics and flavonoids including rutin, cholorogenic acid, caffeic acid, artemisinin, rutin, kaempferol and quercetin (Khan et al., 2015). A. brevifolia is one of the largest, commonly distributed plant having significant herbal and medicinal importance (Mehrdad et al., 2007).
In this research, a considerable decrease in antioxidant enzymes activity such as CAT, SOD, POD, GST, GSR and GSH was observed, in the meantime a substantial increase in the TBARS and H2O2 levels in the renal cells was noticed due to CCl4 exposure. Many research studies have demonstrated that CCl4 intoxication is the major source of free radical generation in many tissues such as the kidney, lungs, liver and blood (Hamed et al., 2012). CAT plays a significant role in the alteration of hydrogen peroxide to water (Aitken and Roman, 2008). SOD assists in the conversion of O−2 into hydrogen peroxide and O2 (Liochev and Fridovich, 2010). GSR oxidizes the glutathione disulfide by transforming it into reduced glutathione. This glutathione is crucial for sperms production as the Sertoli cells alter glutathione into the amino acids, which are mandatory for male gametogenesis (Fafula et al., 2019). GSH shows a vital role in stopping the injury to cellular components caused by free radicals and peroxides (Khan et al., 2018). CCl4 treatment stimulated the intoxication by producing free radicals. This massive production of free radicals leads to the increased lipid peroxidation and H2O2 levels and reduced antioxidant enzyme activity (Sahreen et al., 2015; Sajid et al., 2016). Toxic manifestations of the reactive species can be improved by taking the diet rich in antioxidant metabolites. Aerial parts of A. brevifolia probably have diverse metabolites containing antioxidant abilities, as demonstrated by Singh et al., 2009 in another species of Artemisia genus. A. brevifolia administration alleviated the oxidative stress, which is evidenced by the increased activity of antioxidant enzymes and decreased levels of TBARS and H2O2. Bioactive compounds in A. brevifolia (as in A. scoparia, according to the Sajid et al., 2016) might be a primary reason in free radical’s reduction, which shows its antioxidant potential.
CCl4 administration caused a significant increase in the urea, creatinine, urobilinogen and urinary proteins concentrations while remarkably reduced creatinine clearance and albumin concentration in urine samples. Urobilinogen, under normal conditions, is not the component of urine; however, its high level in urine can be an indication of nephrotoxicity induced by CCl4. In addition, increased urea, creatinine, urinary proteins and a considerable decline in creatinine clearance and albumin are the markers of chronic renal oxidative damage (Khan et al., 2010). Decrease in urinary protein might occur as an effect of CCl4 on the synthesis of proteins from hepatocytes along with enhanced proteinuria (Sajid et al., 2016). A. brevifolia brought serum and urine parameters to average values and alleviated disturbance in serum and urine profile. Results recommended the potential curative capabilities of A. brevifolia.
Comet assay is a technique used to interpret damage in the double-helical structure of DNA (Azqueta and Collins, 2013). In a recent study, a considerable increase in the number of comets, tail length, % DNA in tail, comet length, olive moment and tail moment whereas a substantial decline in % DNA in head and head length was observed in renal cells of rats after CCl4 administration. Our findings showed prominent DNA damage with long tail length of the comets in renal cells of CCl4 treated rats. Oxidants and free radicals encourage the metal ions to produce further reactive species, which can cause DNA damage (Phaniendra et al., 2015) A. brevifolia treatment inverted the changes in comet parameters dose-dependently and displayed efficient protective potential against DNA damages in higher doses. Our results are in coherence with (Sajid et al., 2016) in which A. scoparia reduced the DNA damage induced by CCl4.
In CCl4 treated group, renal tissues exhibited fibrosis in renal interstitium, glomerular collapse, tubular damage, tubular dilation, the annihilation of Bowman’s capsule of nephrons and obstruction in capillaries. Administration with A. brevifolia (dose-dependent) resulted in maintaining normal renal morphology and showed normal tubular and glomerular anatomy and reduced capillary blockage and interstitial edema. These findings were consistent with Sahreen et al., 2015, who detected the histopathological abnormalities in renal tissues of rats administered with CCl4 and these abnormalities were diminished after Carissa opaca plant extract treatment.
5 Conclusion
In conclusion, our study illustrated that Artemisia brevifolia could diminish the renal damages induced by CCl4, which may restore antioxidant activities, lipid peroxidation and H2O2 levels, serum and urine profile, DNA damages and histological abnormalities of renal tissues. The curative effects of A. brevifolia may be associated with its antioxidant and curative potential. Consequently, it is suggested that A. brevifolia plant may be used in the future to enhance the antioxidant potential in mammals as well as in humans.
Acknowledgement
The author (MUI) gratefully acknowledge the University of Agriculture, Faisalabad, for the support and technical facilities to complete this research. The authors (SM, ZA and KAAG) express their sincere appreciation to the Deanship of Scientific Research at the King Saud University for its funding of this research through the Research Group Project No. RG-1435-012.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Lysosomal enzymes in the pathogenesis of carbon tetrachloride induced injury to the kidney and testis in the rat. Indian J. Pharmacol.. 2000;32:2501.
- [Google Scholar]
- Antipyretic, anti-inflammatory and analgesic activity of Acacia hydaspica R. Parker and its phytochemical analysis. BMC Complement Altern. Med.. 2015;15:136.
- [Google Scholar]
- Growth inhibition and apoptosis in cancer cells induced by polyphenolic compounds of Acacia hydaspica: involvement of multiple signal transduction pathways. Sci. Rep.. 2016;6(1)
- [Google Scholar]
- Antioxidant systems and oxidative stress in the testes. Oxid. Med. Cell. Longevity. 2008;1(1):15-24.
- [Google Scholar]
- The protective effect of date seeds on nephrotoxicity induced by carbon tetrachloride in rats. Int. J. Pharm. Sci. Rev. Res.. 2014;26(2):62-68.
- [Google Scholar]
- CCl4 induced genotoxicity and DNA oxidative damages in rats: hepatoprotective effect of Sonchus arvensis. BMC Complement Altern. Med.. 2014;14:452.
- [Google Scholar]
- The essential comet assay: a comprehensive guide to measuring DNA damage and repair. Arch. Toxicol.. 2013;87:949-968.
- [Google Scholar]
- Assessment of ginger extract and ginger nanoparticles protective activity against acetaminophen-induced hepatotoxicity and nephrotoxicity in RatsEnglish. PVJ. 2019;39(4):479-486.
- [Google Scholar]
- Anti-hyperglycemic efficacy of Derris ovalifolia in alloxan-induced diabetic wister rats. Pak. Vet. J.. 2020;40(1):108-112.
- [Google Scholar]
- Chinese Academy of Sciences. Catalogue of Life China, 2013 Annual Checklist (Biodiversity Committee, Chinese Academy of Sciences). Chinese Academy of Sciences (CAS). https://doi.org/10.15468/rgugm4.
- Comet assay: a reliable tool for the assessment of DNA damage in different models. Cell Biol. Toxicol.. 2009;25:5-32.
- [Google Scholar]
- Biological significance of glutathione S-transferases in human sperm cells. J. Hum. Reprod. Sci.. 2019;12(1):24-28.
- [Google Scholar]
- Protective effects of ammannia baccifera against CCl4-induced oxidative stress in rats. Int. J. Environ. Res. Public Health. 2019;16(8):1440.
- [Google Scholar]
- PDR for herbal medicines. NJ, USA: Montvale; 2000.
- Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem.. 1974;249:7130-7139.
- [Google Scholar]
- Therapeutic potential of ginger against renal injury induced by carbon tetrachloride in rats. Sci. World J.. 2012;2012:12. Article ID 840421
- [Google Scholar]
- Himalayan Voices. <http://himalayanvoices.org/?q=onlinelib/documentation/natural/ethnobotanic/display/256>. Ethnobotanic Database. (accessed July 25, 2020).
- Iqbal, Z., M. Lateef, M. Ashraf and A. Jabbar, 2004. Anthelmintic activity of Artemisia brevifolia in sheep. J. Ethnopharmacol. 93: 265-8.Khan, R. A., Khan, M. R., Sahreen, S., 2012. Protective effect of Sonchus asper extracts against experimentally-induced lung injuries in rats: a novel study. Exp. Toxicol. Pathol. 64:725-31.
- Bromobenzene-induced liver necrosis. protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151-169.
- [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys.. 1984;21:130-132.
- [Google Scholar]
- Effect of methanol extract of Dicranopteris linearis against carbon tetrachloride-induced acute liver injury in rats. BMC Complement Altern. Med.. 2014;14(1):123.
- [Google Scholar]
- Effects of Launaea procumbens on brain antioxidant enzymes and cognitive performance of rat. BMC Complement Altern. Med.. 2012;12:181.
- [Google Scholar]
- Phytochemical and in vitro biological evaluation of Artemisia scoparia Waldst. & Kit for enhanced extraction of commercially significant bioactive compounds. J. Appl. Res. Med. Aromat. Plants. 2015;2(3):77-86.
- [Google Scholar]
- Role of free radicals and certain antioxidants in the management of huntington’s disease: a review. J. Anal. Pharm. Res.. 2018;7:386-392.
- [Google Scholar]
- Prevention of CCl4-induced nephrotoxicity with Sonchus asper in rat. Food Chem. Toxicol.. 2010;48:2469-2476.
- [Google Scholar]
- Amelioration of CCl4-induced nephrotoxicity by Oxalis corniculata in rat. Exp. Toxicol. Pathol.. 2013;65:327-334.
- [Google Scholar]
- Carbon tetrachloride induced nephrotoxicity in rat: protective role of Digera muricata (L.) Mart. J. Ethnopharmacol.. 2009;122:91-99.
- [Google Scholar]
- Mechanism of the peroxidase activity of Cu, Zn superoxide dismutase. Free Radical Biol. Med.. 2010;48(12):1565-1569.
- [Google Scholar]
- Detection of sesquiterpene lactones in ten Artemisia species population of Khorasan provinces. Iran J. Basic Medi. Sci.. 2007;10:183-188.
- [Google Scholar]
- Prophylatic role of L-Carnitine and ubiquinone in combating the cardiotoxicity induced by carbon tetrachloride in rat. Int. J. Acad. Res.. 2010;2:529.
- [Google Scholar]
- Pistacia chinensis: a potent ameliorator of CCl 4 induced lung and thyroid toxicity in rat model. Biomed Res. Int.. 2014;2014 192906
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [Google Scholar]
- Free radicals: properties, sources, targets, and their implication in various diseases. Ind. J. Clin. Biochem.. 2015;30:11-26.
- [Google Scholar]
- Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages—induction by multiple nonphagocytic stimuli. Cell. Immunol.. 1981;59:301-318.
- [Google Scholar]
- Protective effects of Carissa opaca fruits against CCl4-induced oxidative kidney lipid peroxidation and trauma in rat. Food Nutri. Res.. 2015;59:28438.
- [Google Scholar]
- Proficiencies of Artemisia scoparia against CCl4 induced DNA damages and renal toxicity in rat. BMC Complement Altern. Med.. 2016;16:149.
- [Google Scholar]
- Methanolic extract of Nepeta paulsenii as an ameliorative agent against CCl4 induced testicular damage in male albino rats. J. King Saud Univ. – Sci.. 2020;32(1):1168-1174.
- [Google Scholar]
- Hsian-tsao (Mesona procumbens Heml.) prevents against rat liver fibrosis induced by CCl4 via inhibition of hepatic stellate cells activation. Food Chem. Toxicol.. 2008;46:3707-3713.
- [Google Scholar]
- Chemical composition and antioxidant activity of essential oil from residues of Artemisia scoparia. Food Chem.. 2009;114(2):642-645.
- [Google Scholar]
- Protective effects of baicalein against cadmium-induced oxidative stress in rat testes. Pak. Vet. J.. 2019;39(2):216-220.
- [Google Scholar]
- Hepatoprotective role of Artemisia scoparia Waldst. and kit against CCl4-induced toxicity in rats. Pol. J. Environ. Stud.. 2018;27(3):1307-1314.
- [Google Scholar]
- A simple capillary tube method for the determination of the specific gravity of 25 and 50 micro l quantities of urine. J. Clin. Pathol.. 1971;24:667-668.
- [Google Scholar]
- Hepatotoxicity and mechanism of action of halo-alkanes: carbon tetrachloride as a toxicological model. Crit. Rev. Toxicol.. 2003;33:10536.
- [Google Scholar]
- Carbon tetrachloride-induced nephrotoxicity in mice is prevented by pretreatment with zinc sulfate. Biol. Pharm. Bull.. 2016;39:1042-1046.
- [Google Scholar]
- regulating activity of polysaccharides from Portulaca oleracea L. on dendritic cells of mice immunized against foot-and-mouth disease. Pak. Vet. J.. 2020;40(1):7-12.
- [Google Scholar]







