Translate this page into:
Metal powder-assisted laser induced breakdown spectroscopy (LIBS) using pulse CO2 laser for liquid analysis
⁎Corresponding author. khumaeni@fisika.fsm.undip.ac.id (Ali Khumaeni)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Analysis of impurity and trace element in liquid is urgently needed in environment and industries. In this present work, new sampling technique of laser induced breakdown spectroscopy (LIBS) has been devised using metal coarse utilizing pulse CO2 laser. Experimentally, a liquid and polluted liquid was dropped into a metal coarse powder. A pulse CO2 laser (10.6 µm, 200 ns) beam with a laser energy from 0.75 to 1.5 J was bombarded on a metal coarse to induce a plasma. The plasma contains elemental analytical lines obtained from the liquid material; the metal coarse itself is never ablated and only works to induce a plasma. By this present technique, identification of major and minor elements in liquid material target such as commercial milk liquid and multi-vitamin liquid has successfully be made. Furthermore, a quantitative analysis of impurity of Cr has been demonstrated, resulting in good precision and good sensitivity with limit of detection of approximately 2 µg/ml. This present developed technique is possible to be employed for analysis of major, impurity, and trace elements in liquid and water without any tedious sample pretreatment.
Keywords
Liquid analysis
Metal coarse powder
LIBS
TEA CO2 laser
1 Introduction
Analysis of impurity and trace metal elements is required in various fields including industries and environment. In food and herbal products such as tea, dry fruits, moringa, and shilajit, periodic analysis of impurity and poisonous elements are very important to control the quality of the products (Aldakheel et al., 2021; Rehan et al., 2021; Aldakheel et al., 2021). In environment, analysis of impurity and trace metals elements as contaminants in water is urgently needed to identify and ensure the pollution of the metal in the water (Xiao et al., 2009; Siepak and Sojka, 2017). In drinking water, analysis of impurity is really necessary to ensure the quality of water from its impurity. In industries such as food and drinking industries, analysis of liquid is very important to control the stability of main ingredient in commercial products (Siepak and Sojka, 2017; Wang et al., 2017; Ekezie et al., 2017). In metal industries, analysis of metal liquid is made to ensure the quality of the metal product from the impurity, which may contain during the production process (DiGiovanni et al., 2019; Hudson et al., 2017).
Many various techniques have been employed to analysis of water and liquid including ICP-OES, AAS, and XRF spectroscopy (Duarte et al., 2016; Pinheiro et al., 2019; Virgilio et al., 2016; Zou et al., 2018). By using mentioned techniques, analysis of elements in liquid with high accuracy and responsiveness can be carried out. However, those methods require serious sample pretreatment, are labor intensive and therefore should be carried out off-site, and are high-cost instrument.
Laser-induced breakdown spectroscopy (LIBS) has recently become an increasingly popular technique for rapid and in-situ qualitative and quantitative elemental analysis of various samples in different forms such as solids, liquids, and gases (Girón et al., 2018; Tiwari et al., 2018; Yao et al., 2020; Moncayo et al., 2017). In this technique, a pulse Nd:YAG laser is commonly used as an energy source to induce a luminous plasma, which emits atomic emission from the material target. However, researchers most often apply LIBS for solid and gas targets rather than liquid samples. This is mainly due to the technical complication in the use of LIBS for analysis of liquid sample (Ripoll et al., 2021; Cama-Moncunill et al., 2020). To solve this issues, various sampling techniques have been developed such as liquid jets (Skočovská et al., 2016; Bhatt et al., 2021; Aguirre et al., 2015), using filters for liquid deposition (Wang et al., 2015), aerosol technique (Yang et al., 2016), and solid conversion (Díaz Pace et al., 2006; Lin et al., 2017). However, most developed techniques are still limited by low reproducibility and detection sensitivity. The disadvantages of LIBS for liquid analysis include fast plasma quenching due to the splashing and fluctuation of liquid (Yaroshchyk et al., 2005; Fichet et al., 2006; Tognoni et al., 2002). Furthermore, it has been found the limit of detection (LoD) of analyte is still quiet high in the order of tens part per million (ppm). To overcome this problem, some researchers used metal nanoparticles mixed with the liquid sample to enhance the emission sensitivity (Dell'Aglio et al., 2018). The other improved sampling techniques in LIBS for analysis of liquid were developed by Jin Yu et al. utilizing surface-assisted thin film (Tian et al., 2016; Xiu et al., 2014; Xiu et al., 2013). The results certified that the developed techniques can overcome the matrix effect from the sample targets and improve the limit of detection of the analytes.
In other direction, we have employed pulse transversely excited atmospheric (TEA) CO2 laser for specific applications of LIBS including analysis of organic powder material (Khumaeni et al., 2009) and aerosol or liquid analysis (Khumaeni et al., 2014). The results certified that LIBS using pulse TEA CO2 laser can improve the LoD of analyte to few ppm level. Pulse TEA CO2 laser is very suitable for analysis of organic material, liquid and soft solid material because the laser has longer wavelength (10.64 μm) and longer pulse duration (200 ns) compared to the Nd:YAG laser (1.064 μm and 10 ns in wavelength and pulse duration), which is commonly used in LIBS. Therefore, the TEA CO2 laser has high absorbance in those material targets. Furthermore, by assisting a metal subtarget (Khumaeni et al., 2011), plasma temperature can be enhanced increasing the effective dissociation and excitation of analyte in the sample, resulting higher detection limit. Recently we employed a new technique of pulse CO2 LIBS by assisting metal coarse powder for sensitive analysis of trace metal elements in soil material (Khumaeni et al., 2017). The method was used for the rapid determination of poisonous metal elements of Cr, Pb, and Hg in loam soil. The LoD for Cr, Pb, and Hg were approximately 0.8, 15, and 0.7 mg/kg, respectively.
In this present work, the technique of metal-coarse-powder-assisted CO2 LIBS is applied for the direct analysis of liquid material. To this end, few amounts of liquid was dropped and trapped into the coarse metal powder. The pulse CO2 laser was subsequently bombarded into the metal coarse. Some liquid materials were employed including liquid commercial milk, liquid vitamin, and contaminated water. The emission reproducibility of analytes was examined and finally semi-quantitative analysis of impurity in contaminated water was demonstrated.
2 Experimental procedure
The setup of experiment used in this present work is schematically shown in Fig. 1. The system consisted of high-energy pulse TEA CO2 laser (a wavelength of 10.6 µm, energy of 3 J, pulse duration of 200 ns), a high resolution monochromator (Jobin-Ybon HRS-2, focal length of 640 mm, 2400/mm, spectral resolution of 0.02 nm) with an optical multichannel analyzer system (Lambda Vision 1012), and optical system including optical fiber, ZnSe lens, sample holder.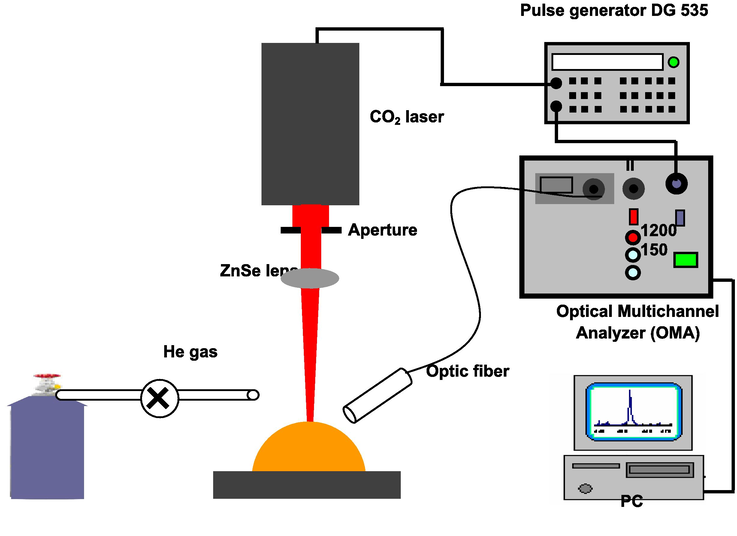
Experimental setup used in this work.
Experimentally, a pulse TEA CO2 laser beam was directed and focused by ZnSe lens with a focal length of 200 mm on liquid materials, which are trapped in coarse metal powders to induce a luminous plasma (Fig. 2). The laser beam was focused both on liquid and metal coarse. The focus point size of laser beam on the coarse metal powder was approximately 2 mm in diameter. The size of coarse metal powder varies from approximately 100–500 µm. The laser energy was varied from 0.75 to 1.5 J by an inserting a metal plate in front of ZnSe lens. The produced plasma emission was sent via optical fiber into a high resolution monochromator (Jobin-Ybon HRS-2, focal length of 640 mm, 2400/mm, spectral resolution of 0.02 nm) with an optical multichannel analyzer system (Lambda Vision 1012) to obtain the emission spectra. The gate width and gate delay of the optical multichannel analyzer were 10 µs and 100 µs, consecutively.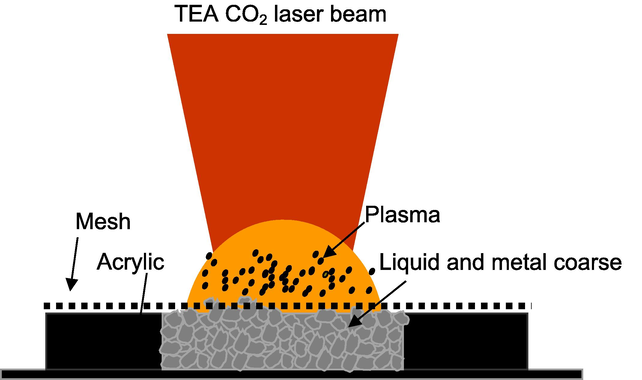
Illustration of metal coarse supported induced plasma.
The samples used in this study were liquid commercial milk, which were overwhelmingly available in the local market. The other samples included commercial Ca and Mg vitamins, phosphoric acid, sodium chloride solution, KNO3 solution and polluted water containing Cr at various concentrations of 10, 20, 40, and 200 µg/ml. During experiments, 350 mg coarse metal powders were placed in a hole (diameter of 10 mm) made in an acrylic plastic plate (thickness of 8 mm), on which the copper plate was tightly attached in the tight contact of the back of acrylic; the copper plate functions to keep the coarse metal powder from falling down during laser bombardment. Subsequently, 3 ml liquid sample was homogeneously dropped into the metal powder and finally covered by stainless steel mesh with a lattice constant of 0.4 mm and a diameter of 0.1 mm, which was placed in tight contact on the front side of the metal powder. The steel mesh functions to cover and keep the metal powder from the blow-off of coarse during laser bombardment. It should be mentioned that during data acquisition, the metal mesh was not damaged and ablated because the power density of laser beam on the mesh was lower than the ablation threshold of the mesh material due to the plasma shielding effect as reported in our previous work (Khumaeni et al., 2017). The samples were then settled in a metal chamber with a diameter of 120 mm and a height of 100 mm. Inside the chamber can be filled by with various surrounding gases including air, N2, and He. In this present work, high-purity He gas (99.999%) was flowed in the metal chamber during the data acquisition. The flow rate of the gas was 3 L per minute and the pressure inside the chamber was set at 1 atmosphere. It should be mentioned that the helium plasma provided lower background emission compared to the use of a nitrogen plasma.
3 Results and discussion
At initial, we examine that the metal coarse powder only functions to initiate a breakdown plasma and is not ablated by the laser bombardment. To this end, coarse metal powders without any samples were tested. Fig. 3 shows emission spectrum ranging at the wavelength of 640 nm to 680 nm obtained from the coarse metal powder target. The laser energy used was 0.75 J. Emission lines of H at 656.3 nm and He at 667.8 nm occur very clear with very sharp and high-intensities and low background emission. The H line is assumed originating from H2O, which is still deposited and trapped in the metal coarse powder, while the He line comes from He gas flowed in the metal chamber during experiments. It should be noticed that no Fe emission lines identified in the spectrum. In this region, the Fe line has a wavelength at around 657 nm as reported here (Mammosser et al., 2012). We also confirmed that even though the gate delay time of spectrometer was opened from 1 µs, it is still no Fe line was identified in this spectrum region. This indicated that no ablation happened from the coarse metal powder. The metal powder only functions to initiate and induce a breakdown plasma. It is assumed that the mechanism of plasma generation by assisting coarse metal powders is follows: once the pulse CO2 laser beam falls on coarse metal powder, only electrons escape from the coarse metal by multiphoton absorption process. Those free electrons accelerate in the electric field of the laser light, inducing a chain ionization in the gas and generating an initial breakdown plasma. The plasma then absorbs incident laser beam by inverse bremsstrahlung. The plasma absorption for the CO2 laser is much higher compared to the case of pulse Nd:YAG laser because the coefficient of plasma absorption is proportional to the inverse square of the frequency of laser light, thus most of the CO2 laser energy is absorbed by the breakdown plasma, resulting in large-volume breakdown plasma. Furthermore, no ablation of metal surface happens by the CO2 laser with a laser energy of 1.5 J and focusing point of lens of 200 mm. This result is similar with our previous work (Mammosser et al., 2012), namely when pulse TEA CO2 laser was directed and focused on a metal powder or metal plate, no ablation happens from the metal and only high-intensity breakdown plasma occurs. Therefore, identification of elements from the liquid sample by using coarse metal powders assisted LIBS can be made without any interference of emission lines from the metal powder itself. The technique of coarse metal powder is then applied to identification and analysis of elements in liquid sample.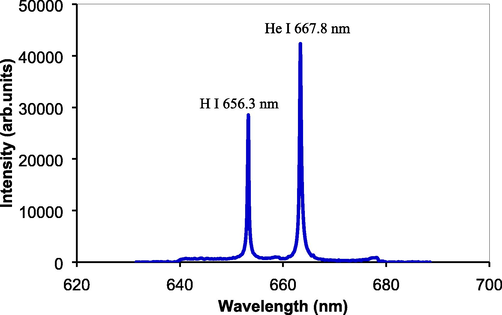
Emission spectrum obtained from the coarse metal powder only without any liquid sample.
Prior to liquid elemental analysis, we examined the plasma temperature generated by the present technique. For this purpose, we used Boltzmann distribution technique applying the ratio of two emission lines of neutral Cu at 510.5 nm and 521.8 nm obtained from the CuSO4 powder mixed with iron coarse powder; this techniques have been presented in our previous papers (Khumaeni et al., 2017). Fig. 4 displays how the plasma temperature changes with increasing time. The temperature has long lifetime and has a peak of approximately 7000 K at 1 µs and finally slightly decreases up to approximately 6500 K at 30 µs. This temperature profile is almost the same pattern with our previous work of metal-assisted laser-induced breakdown spectroscopy using pulse TEA CO2 laser (Khumaeni et al., 2011). Namely, the plasma emission has long lifetime and the temperature slightly decreases with the plasma lifetime. This sophisticated phenomenon is never happened in the case of LIBS using an Nd:YAG laser where the plasma lifetime is quiet short up to around 10 µs. Therefore, with long lifetime of plasma emission and quiet high plasma temperature, atoms can effectively be excited in the plasma region, resulting in sensitivity increment compared to the case of standard LIBS using an Nd:YAG laser.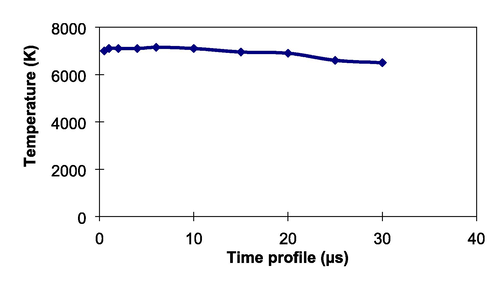
Plasma temperature at various plasma lifetime.
Analysis of major elements in commercial liquid product was then made using our present technique. First, we used liquid commercial milk containing Ca at a concentration of 1000 µg/ml. It is known that Ca is a primary source of nutrition and support the bone strength for human being and contains at high concentration in the milk liquid. Ionic emission lines of Ca at 393.3 nm and 396.8 nm clearly appear with high intensity and low background emission as shown in Fig. 5(a). Furthermore, Al lines at 394.1 nm and 396.1 nm are faintly detected near Ca lines. Using the ionic Ca II 393.3 nm, limit of detection of Ca was estimated to be 8 µg/ml. The other liquid samples used were two liquid vitamins contain Ca and Mg with concentrations of 6.8% and 3%, respectively. The emission spectra of Ca and Mg from the liquid vitamins are shown in Fig. 5(b) and (c), respectively. It can clearly be seen that for Ca emission spectrum, high emission intensities of ionic Ca at 393.3 nm and 396.8 nm occur clearly together with He emission line at 388.8 nm. For Mg spectrum shown in Fig. 5(c), Some Mg lines including neutral Mg at 285.3 nm and ionic Mg at 279.2 nm and 281.3 nm appear with quiet high intensities. The other liquid sample was phosphoric acid containing P at a concentration of 30 mg/kg. Phosphor is a macronutrient, which is used a plant to store and use energy including for the process of photosynthesis. Fig. 5(d) shows emission spectrum of P obtained from the phosphoric acid liquid. It can be seen that the neutral P lines clearly occur with high intensity and low background emission at the wavelengths of 253.6 nm and 255.3 nm. It should also be noticed that no emission lines of Fe appear in the wavelength of around 250 nm, whereas the Fe has many emission lines in this region. This certified that the iron coarse powder is not ablated during data acquisition of liquid sample. Based on the result, it confirmed that the technique of iron coarse metal powder-assisted CO2 laser-induced breakdown spectroscopy is successfully used for the analysis of major elements in liquid sample.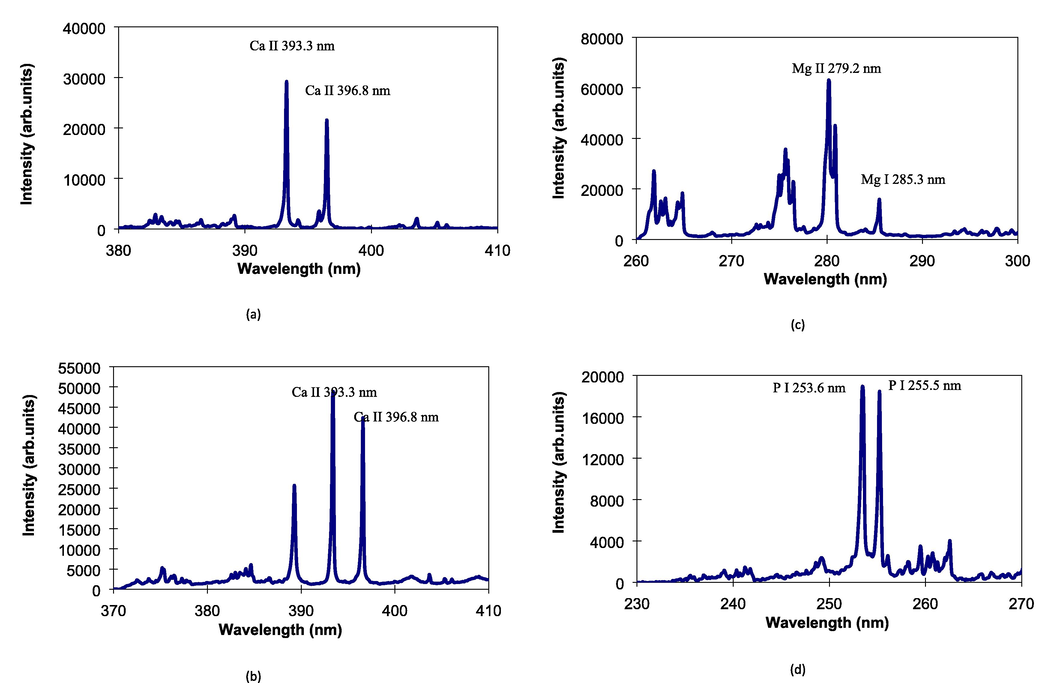
Emission spectra obtained from (a) the commercial liquid milk containing 1000 µg/ml Ca, (b) the commercial liquid vitamin containing 6.8% Mg, (c) the commercial liquid vitamin containing 3% Mg, and (d) the Phosphoric acid liquid.
The present technique was then examined to perform analysis of delicate elements in liquid. First, the technique is employed to identification of chlorine. It is known that the analysis of chlorine is very required in building construction to ensure durability of constructions and structural safety. However, Cl analysis is very delicate to be conducted using the standard LIBS technique because Cl has quiet high ionization energy, resulting in low detection sensitivity. Fig. 6 shows emission spectrum of Cl obtained from the sodium chloride solution with a Cl concentration of 30%. Three analytical lines of neutral Cl at 833.3 nm, 837.6 nm, and 842.8 nm clearly occur with low background emission. Identification was further made in nitrogen from the KNO3 solution. Total nitrogen analysis was important in environmental and agricultural fields. Nitrogen is one of essential elements needed for plant growth. Thus, periodical inspection of nitrogen in water and liquid is very required to control the content of nitrogen in water. Fig. 7 displays the emission spectrum of nitrogen obtained from the KNO3 solution. Neutral N emission lines at 742.4 nm, 744.2 nm, and 746.8 nm were clearly detected together with unknown emission lines.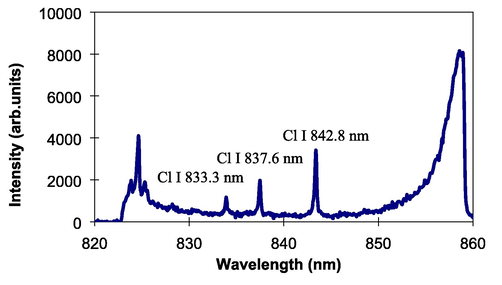
Emission spectrum obtained from the NaCl solution.
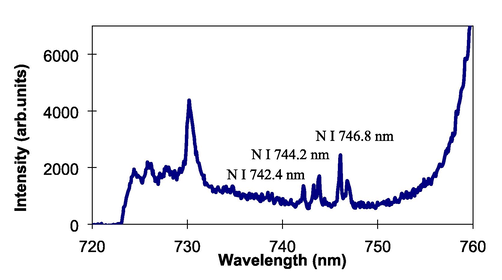
Emission spectrum obtained from the KNO3 solution.
Taking advantages of the successful identification of major and delicate elements in various liquid samples, the present technique was further used to quantitative analysis of Cr in water. The accurate and rapid analysis of Cr as impurity in water is necessary in environmental and industrial fields. Fig. 8 shows emission spectrum of Cr taken from the tap water containing 40 µg/ml Cr. Very clear and sharp neutral Cr lines at 425.4 nm, 427.4 nm, and 428.9 nm are obtained with high emission intensities and quiet low background emission. Also, Ca I 422.6 nm emission lines exhibits in the spectrum; the Ca emission comes from the tap water. Prior to quantitative analysis, the reproducibility of emission intensities of Cr with respect to the number of laser shots was examined. For this purpose, the emission spectra were repeatedly obtained every 20 laser shots using the same sample in Fig. 8. As shown in Fig. 9, the emission intensities of Cr have a good reproducibility with the laser dependence. The same tendency also occurs for Ca emission, namely its intensities almost the same with the laser shot dependence. This graph confirmed that the present technique can be used to perform analysis with a good precision proven by good reproducibility of emission intensity with laser shot dependence.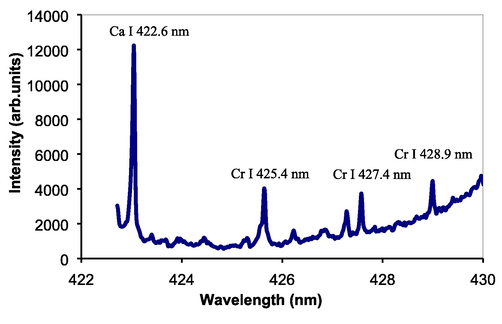
Emission spectrum of Cr taken from the tap water containing 40 µg/ml Cr.
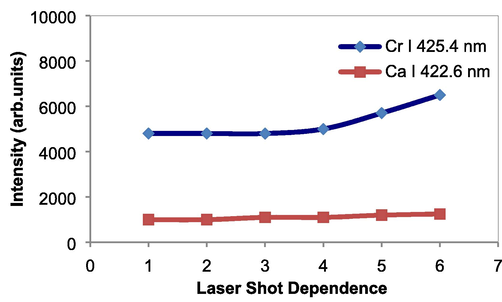
The reproducibility of emission intensities of Cr with respect to the number of laser shots.
Finally, the metal coarse powder-assisted LIBS technique further was employed to perform analysis of Cr in tap water containing various concentrations of Cr. Fig. 10 exhibits a calibration curve of Cr taken from the tap water containing various concentrations of Cr. Good linearity calibration curve with an intercept zero has been successfully produced. To perform high precision analysis, a standardization method was applied using the emission intensity of Ca. Each points in the calibration curve represents average of three spectra, which were taken by 20 shots of laser irradiation. It should be noticed that the standard deviation was quiet low of around 5%. Finally limit of detection of Cr in tap water was evaluated using Cr emission line at 425.4 nm. The LoD was estimated to be 2 µg/ml; the LoD was derived from the 3 times noise level divided detected emission intensity (Ingle and Crouch, 1988). This result demonstrated that the present technique can be applied to perform direct analysis of Cr in water containing Cr at few µg/ml level. As reported and reviewed by Wang et al., various sample preparation techniques have been devised for analysis of liquid including microdroplet, surface-assisted LIBS, and quick-freeze procedure (Wang et al., 2021). Those techniques generally attempt to overcome the chemical and physical matrix effect usually happens in LIBS experiment so that precision and high-sensitivity analysis can be realized. In this present work, we have successfully demonstrated that the unique and simple sample preparation of liquid analysis using the assist of coarse metal powder makes enable one to easier conduct analysis of liquid using LIBS with good precision and good sensitivity without tedious sample preparation.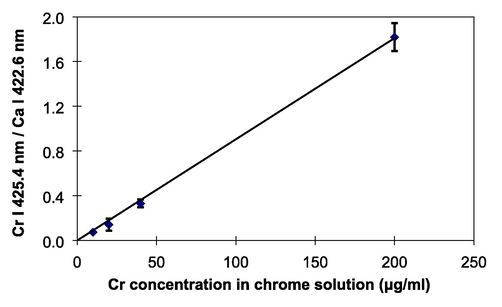
Calibration curve of Cr taken from the tap water containing various concentrations of Cr.
4 Conclusions
Developed technique utilizing metal coarse powder has been successfully demonstrated for an identification and analysis of Cr impurity in water. Besides, the present technique has also been employed to perform identification of major and delicate elements in liquid material target such as commercial milk liquid and multi-vitamin liquid. Impurity analysis of Cr has been demonstrated, resulting in good precision and good sensitivity with LoD of approximately 2 µg/ml. This present technique is possible to be employed for analysis of major, impurity, and trace elements in liquid and water without any tedious sample pretreatment.
Conflict of interest
None
Acknowledgement
This present work was financially supported by Institute for Research and Community Services Universitas Diponegoro under Research Grant of World Class Research Universitas Diponegoro, Contract No. 118-10/UN7.6.1/PP/2021.
References
- Dispersive liquid–liquid microextraction for metals enrichment: a useful strategy for improving sensitivity of laser-induced breakdown spectroscopy in liquid samples analysis. Talanta. 2015;131:348-353.
- [Google Scholar]
- Rapid determination and quantification of nutritional and poisonous metals in vastly consumed ayurverdic herbal medicine (Rejuvenator Shilajit) by humans using three advanced analytical techniques. Biol. Trace Elem. Res. 2021
- [CrossRef] [Google Scholar]
- Spectrochemical analysis using LIBS and ICP-OES techniques of herbal medicine (Tinnevelly Senna Leaves) and its anti-cancerous/antibacterial applications. Arabian J. Chem.. 2021;14(12):103451.
- [Google Scholar]
- Determination of As, Hg, S, and Se in liquid jets by laser-based optical diagnostic technique. Appl. Phys. B. 2021;127:8.
- [Google Scholar]
- Direct analysis of calcium in liquid infant formula via laser-induced breakdown spectroscopy (LIBS) Food Chem.. 2020;309:125754.
- [Google Scholar]
- Nanoparticle enhanced laser induced breakdown spectroscopy (NELIBS), a first review. Spectrochim. Acta, Part B. 2018;148:105-112.
- [Google Scholar]
- Analysis of heavy metals in liquids using Laser Induced Breakdown Spectroscopy by liquid-to-solid matrix conversion. Spectrochim. Acta, Part B. 2006;61:929-933.
- [Google Scholar]
- Experimental and numerical analysis of liquid metal embrittlement crack location. J. Mater. Eng. Perform.. 2019;28:2045-2052.
- [Google Scholar]
- Determination of lead in biomass and products of the pyrolysis process by direct solid or liquid sample analysis using HR-CS GF AAS. Talanta. 2016;146:166-174.
- [Google Scholar]
- A review on recent advances in cold plasma technology for the food industry: current applications and future trends. Trends Food Sci. Technol.. 2017;69:46-58.
- [Google Scholar]
- In-situ monitoring and characterization of airborne solid particles in the hostile environment of a steel industry using stand-off LIBS. Measurement. 2018;115:1-10.
- [Google Scholar]
- Applications of laser-induced breakdown spectroscopy (LIBS) in molten metal processing. Metal. Mater. Trans. B. 2017;48:2731-2742.
- [Google Scholar]
- Spectrochemical Analysis. New Jersey: Prentice Hall; 1988.
- Analysis of organic powder samples by using the metal-assisted subtarget effect in a Transversely-Excited Atmospheric (TEA) CO2 laser-induced he gas plasma at 1 atm. J. Korean Phys. Soc.. 2009;55:2441-2446.
- [Google Scholar]
- Direct analysis of powder samples using transversely excited atmospheric CO2 laser-induced gas plasma at 1 atm. Anal. Bioanal. Chem.. 2011;400:3279-3287.
- [Google Scholar]
- Analysis of sodium aerosol using transversely excited atmospheric CO2 laser-induced gas plasma spectroscopy. Curr. Appl Phys.. 2014;14:451-454.
- [Google Scholar]
- Coarse metal powder-assisted pulsed CO2 laser-induced breakdown spectroscopy for the direct determination of heavy metals in soil. Anal. Lett.. 2017;50:1992-1999.
- [Google Scholar]
- Highly concentrated, ring-shaped phase conversion laser-induced breakdown spectroscopy technology for liquid sample analysis. Appl. Opt.. 2017;56:5092-5098.
- [Google Scholar]
- J. Mammosser, S. Ahmed, K. Macha, Large-volume resonant microwave discharge for plasma cleaning of a Cebaf 5-Cell SRF cavity, Proceedings of IPA C2012, New Orleans, Louisiana, USA.
- Qualitative and quantitative analysis of milk for the detection of adulteration by Laser Induced Breakdown Spectroscopy (LIBS) Food Chem.. 2017;232:322-328.
- [Google Scholar]
- Evaluation of dilute-and-shoot procedure for determination of inorganic impurities in liquid pharmaceutical samples by ICP OES. Microchem. J.. 2019;146:948-956.
- [Google Scholar]
- Determination of nutritional and toxic metals in black tea leaves using calibration free LIBS and ICP: AES technique. Arab. J. Sci. Eng. 2021
- [CrossRef] [Google Scholar]
- Evaluation of thin film microextraction for trace elemental analysis of liquid samples using LIBS detection. Talanta. 2021;223:121736.
- [Google Scholar]
- Application of multivariate statistical approach to identify trace elements sources in surface waters: a case study of Kowalskie and Stare Miasto reservoirs, Poland. Environ. Monitor. Assessment. 2017;189(364):1-15.
- [Google Scholar]
- Optimization of liquid jet system for laser-induced breakdown spectroscopy analysis. Rev. Sci. Instrum.. 2016;87:043116.
- [Google Scholar]
- Elemental analysis of powders with surface-assisted thin film laser-induced breakdown spectroscopy. Spectrochim. Acta, Part B. 2016;124:16-24.
- [Google Scholar]
- Rapid analysis of pharmaceutical drugs using LIBS coupled with multivariate analysis. Laser Med. Sci.. 2018;33:263-270.
- [Google Scholar]
- Quantitative micro-analysis by laser-induced breakdown spectroscopy: a review of the experimental approaches. Spectrochim. Acta, Part B. 2002;57:1115-1130.
- [Google Scholar]
- Evaluation of standard dilution analysis (SDA) of beverages and foodstuffs by ICP OES. J. Anal. At. Spectrom.. 2016;31:1216-1222.
- [Google Scholar]
- Recent advances in laser-induced breakdown spectroscopy quantification: from fundamental understanding to data processing. TrAC Trends Anal. Chem.. 2021;143:116385.
- [Google Scholar]
- Quality analysis, classification, and authentication of liquid foods by near-infrared spectroscopy: a review of recent research developments. Crit. Rev. Food Sci. Nutr.. 2017;57:1524-1538.
- [Google Scholar]
- Simple, fast matrix conversion and membrane separation method for ultrasensitive metal detection in aqueous samples by laser-induced breakdown spectroscopy. Anal. Chem.. 2015;87:5577-5583.
- [Google Scholar]
- Characteristics, sources, water quality and health risk assessment of trace elements in river water and well water in the Chinese Loess Plateau. Sci. Total Environ.. 2009;650(2):2004-2012.
- [Google Scholar]
- Indirect laser-induced breakdown spectroscopy of transparent thin gel layer for sensitive trace element detection. Appl. Phys. Lett.. 2013;102:244101
- [Google Scholar]
- Feasibility of wear analysis in oils with ppm and sub-ppm sensitivity using laser-induced breakdown spectroscopy of thin oil layer on metallic target. Spectrochim. Acta, Part B. 2014;91:24-30.
- [Google Scholar]
- Laser-induced breakdown spectroscopy of liquid solutions: a comparative study on the forms of liquid surface and liquid aerosol. Appl. Opt.. 2016;55:7406-7411.
- [Google Scholar]
- Evaluation of heavy metal element detection in municipal solid waste incineration fly ash based on LIBS sensor. Waste Manage.. 2020;102:492-498.
- [Google Scholar]
- Quantitative determination of wear metals in engine oils using LIBS: the use of paper substrates and a comparison between single- and double-pulse LIBS. Spectrochim. Acta, Part B. 2005;60:1482-1485.
- [Google Scholar]
- Rapid in situ determination of heavy metal concentrations in polluted water via portable XRF: Using Cu and Pb as example. Environ. Pollut.. 2018;243:1325-1333.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.101901.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







