Translate this page into:
Metal organic framework-derived Ni-Cu bimetallic electrocatalyst for efficient oxygen evolution reaction
⁎Corresponding author. ykyusik@gachon.ac.kr (Kyusik Yun)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Discovering of advanced electrocatalyst for water splitting is importance of the improvement of metal air batteries, water electrolyzers. The approach have been developed to fabricate a novel electrocatalyst with more active sites, high surface area and high porous structure for improve the electrocatalytic activity. Metal organic frameworks have been emerging catalyst of higher crystalline, large surface area, has delivering greater potential for efficient OER electrocatalytic activity. Bimetallic metal organic framework (MOFs) is an excellent catalyst for energy storage and energy conversion system. In this work, we report the synthesis of bimetallic MOF by single step solvothermal method using Ni, Cu as metal sources and BDC as a linker. FE-SEM images indicated NiCu-MOF a narrow crystal formation. The electrocatalyst study of the synthesized catalyst was systematically investigated by linear sweep voltammetery, electrochemical impedance spectroscopy and Chronoamprometry under OER condition. The result demonstrated that NiCu-MOF exhibited better catalytic activity towards OER at onset potential and overpotential of 1.48 V and 250 mV with lower tafel slope of 169 mV/dec, indicating that 25% lower energy required for OER. Hence the proposed NiCu-MOF catalyst is an efficient catalyst for sustainable oxygen evolution reaction.
Keywords
Bimetallic MOF
Electro-catalysis
Linear sweep voltammetry
Onset potential
Overpotential
1 Introduction
The production of oxygen and hydrogen from renewable energy is the emerging technic of hydrogen based energy system. The best method for producing hydrogen gas in a perfect and sustainable way is the water electrolysis. (Yan et al., 2019; Tang et al., 2018; Zhang et al., 2017; Sapountzi et al., 2017) However, the worldwide energy emergency happens with remarkable population development and petroleum products exhaustion. In addition, the ignition of petroleum products has likewise caused a progression of ecological issues. Therefore, it is of indispensable significance to grow perfect, manageable and sustainable power options in contrast to non-renewable energy sources (Liang et al., 2020; Awodumi and Adewuyi, 2020). In past few years, various advancements have been made in saving clean energies. In spite of the fact that there are a few potential OER mechanism, all pathways include a few proton and electron move steps, which offer ascent to huge response boundaries and require the utilization of high overpotentials to drive the response at sensible rates. Till now, the noble catalyst such as Pt, RuO2, and IrO2 is the benchmark catalyst for efficient oxygen evaluation reaction. However, their scarcity on the earth and significant expense make enormous scope use unfeasible (Suen et al., 2017; Jiang et al., 2019; Song et al., 2018). Consequently, the development of alternative and nonprecious electrocatalyst is highly need for the oxygen evaluation reaction.
In recent years, the porous materials like metal-organic frameworks (MOFs) (Peng et al., 2014), porous organic polymers (POPs) (Gopi et al., 2020a, 2020b, 2020c), covalent triazene frameworks (CTFs) (Gopi and Kathiresan, 2017) has been received the great attention towards the energy related application and oxygen evaluation reaction due to their high adsorption capacity, large specific surface area, higher active sites and so on. Especially, MOF is the hybrid material which composed of different metal nodes and organic linkers and provided the superior electron transfer and rapid mass transport in various industries application (Rodenas et al., 2015; Suriyakumar et al., 2018; Tran et al., 2020). Several kind of catalyst have been reported to water oxidation (OER) under different condition (Du and Eisenberg, 2012; Gopi et al., 2020a, 2020b, 2020c). Among the porous materials, metal organic framework (Wei et al., 2020) as a significant class of materials, have been widely utilized as exceptionally proficient impetuses for OER (Shi et al., 2019; Budnikova, 2020). However the reported single metal doped MOF exhibited the poor the stability, low conductivity and activity in the OER. Recently, bimetallic MOF and multimetallic MOF has received great openings for the oxygen evaluation reaction due to the synergetic effect of high metallic active centers (Gopi et al., 2020b,c). The bimetallic MOF and their compositions have shown enhanced properties compared with monometallic MOF. Besides, it can be used as templates for different types of nanostructured materials containing carbon composite, metal doped MOF composites etc (Dang et al., 2017; Sun and Xu, 2014). Bimetallic based MOFs possess multi metal active centers, which enhance the higher stability, high surface adsorption capacity, high electrical conductivity etc (Chen et al., 2015). Resulting the better OER activity under harsh condition compare to single metal based MOF (Wang et al., 2020). It has been widely used in various field such as gas separation, water splitting, batteries, waste water treatment and fuel cells (Theerthagiri et al., 2018). Above mentioned different unique properties of bimetal based MOFs could provide new opportunities to prepare Ni, Mn, Co, Fe, Cu, etc., their oxides and nanocomposites electrocatalyst for water oxidation (Lu et al., 2017; Zhang et al., 2020). Inspiring of this emerging catalytic activity researchers focusing on bimetal based MOFs has better catalytic activity towards water oxidation reaction, compare to other single metal based MOFs. In particularly, bimetallic nickel and cobalt based catalyst displayed superior electrocatalytic activity (Li et al., 2016). The electronic structures of bimetallic MOF (NiCu-MOF) and their electrocatalytic activity towards OER still need to investigated.
In this work, we have synthesized bimetallic MOF by solvothermal method using Ni, Cu metal ions and BDC organic linker. The synthesized NiCu-MOF were characterized by different characterization FT-IR, SEM, PXRD and XPS etc. which confirmed the formation of metal complex. The synthesized bimetallic MOF catalyst was systematically investigated OER under standard condition. Among, NiCu-MOF catalyst displayed higher OER performance, with lower onset potential of 1.48 V vs RHE and tafel slope of 169 mV dec−1. The synergetic effect between two metals centers Ni and Cu enhanced the OER activity. In addition, the proposed bimetallic MOF (NiCu-MOF) showed excellent stability over 30000 s. Furthermore, no significant change was observed in the bimetallic MOF (NiCu-MOF) morphology properties after 30000 s water electrolysis. From these results well evidenced for that the NiCu-MOF is an excellent catalytic activity towards OER under basic condition. Hence the proposed NiCu bimetallic MOF is an efficient catalyst for sustainable oxygen evolution reaction.
2 Chemicals
All reagents and solvents were purchased from Sigma-Aldrich and Alfa Aesar and used without further purification. Nickel nitrate hexahydrate (Ni (NO3)2·6H2O, purity > 98%), Copper nitrate pentahydrate (Cu (NO3)2 5H2O, purity > 98%), Benzenedicarboxylic acid (BDC purity > 98%), ethanol and Potassium Hydroxide (KOH, purity > 85%).
2.1 Characterization
The microstructures morphology of the MOF were determined by Field Emission Microscopy JEOL at 15 kV acceleration and Energy Dispersion X-ray EDX. Functional group was analyzed by TENSOR 27 with RT DLaTGS (Varian detector). The crystal structure of MOF determined by powder X-ray diffraction PXRD using a Rigaku (model: SmartLab) instrument using Cu metal and Kα radiation with a wavelength of 1.5418 Å. X-ray photoelectron spectroscopy was analyzed by thermos fisher (Teta probe AR-XPS) UK. Cyclic voltammetry was carried out in Parstate, model 2273 with the three-electrode system, carbon paper as a working electrode, Pt foil as a counter electrode, and Ag/AgCl in 3 M KCl as a reference electrode.
2.2 Synthesis of NiCu-MOF
Bimetal-MOF was synthesized by solvothermal method, 9.02 mmol of Metal Salt Ni(NO3)2. 6 H2O and Cu(NO3)2 5H2O was dissolved in a mixture of solvent (water: ethanol), stirring for 15 min at room temperature. Separately, 12.1 mmol of NaOH solution was added in BDC (6.01 mmol) solution and mixed until become clear solution. Both the solution was mixed and transfer in to oil bath heated at 150 ℃ for 10 h. After completion, reaction mixture was cooled, formed precipitate filtered and washed with water, ethanol for removing unreacted starting materials and filtered. Filtered materials (Ni-MOF, Cu-MOF and NiCu-MOF) were dried at 60℃ for overnight.
2.3 Electrode preparation
1 × 1 cm2 carbon paper used as working electrode. 2 mg of synthesized catalyst to prepare the slurry by adding 1:1:1 mol ratio of prepared water: IPA: Nafion solution. The catalyst ink was manual grind for 10 min and coated on the carbon paper by brush coating. Modified electrode was dried at room temperature overnight.
3 Results and discussions
The surface morphology of the as-prepared catalyst was characterized by FE-SEM. The FE-SEM (Fig. 1) images shows different shape of morphology for different metal (Ni, Cu) with narrow crystal size distribution. Ni-MOF (Fig. 1a) and Cu-MOF (Fig. 1b) exhibits a layered pillar structure with size distributed from 0.2 to 0.5 µm. (Gao et al., 2018; Mollabagher et al., 2020) NiCu-MOF (Fig. 1c) shows homogenous size distribution over 2 mm and thickness around 20 nm. Elemental mapping (Fig. 1d) and EDX (Figs. S1–S3) of NiCu-MOF shows Ni and Cu metals are homogeneously distributed. Furthermore, detailed of crystallite nature and phase purity of the synthesized catalyst is determined by XRD (Fig. 2) the major diffraction peaks of Ni-MOF and Cu-MOF are good agreement with reported XRD pattern. (Shete et al., 2018; Saitou, 2014) XRD peaks of NiCu-MOF containing (1 1 1) and (2 0 0) planes at 45.2, 46.7° and 50.3, 51° indicate that the Ni and Cu has a polycrystalline structure. Which confirmed the interaction of the bimetal centers. In addition, FTIR was used to analyze the functional groups and metal bonding in NiCu-MOF. In bimetal-MOF, CH stretching band appears at 2892 and 1384 cm−1 (Fig. 3). The C-O band appears at 1672, 1570 and 1060 cm−1 indicating that the dicarboxylic acid coordinated with Ni and Cu. There is no peak absorbed at 3400 cm−1 indicating that carboxylic group completely react with metal. And then 928 cm−1 indicating that the presence of C-O-C stretching. And peak at 490 cm−1 indicating the formation metal complex with carboxylic group. The obtained XRD and FTIR results of NiCu-MOF are well correlated with each other.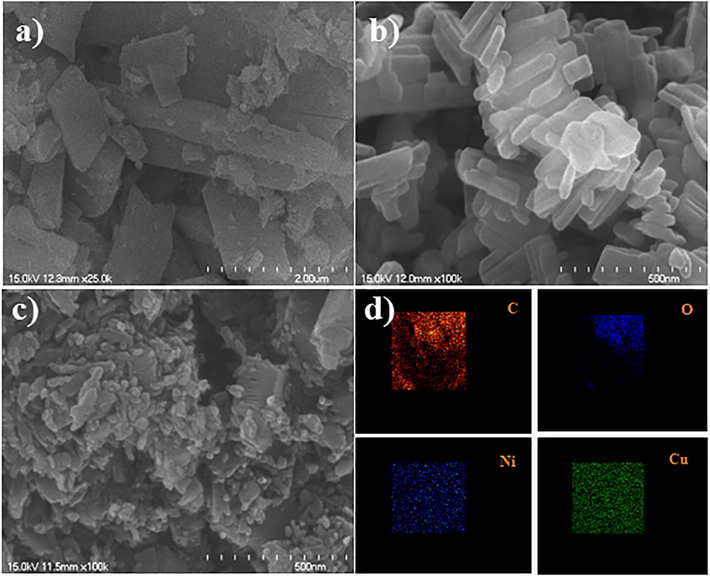
FE-SEM images of Ni and Cu single metal MOF and NiCu bimetal-MOF a) NiMOF, b) Cu-MOF, c) NiCu-MOF and d) elemental mapping of NiCu-MOF.
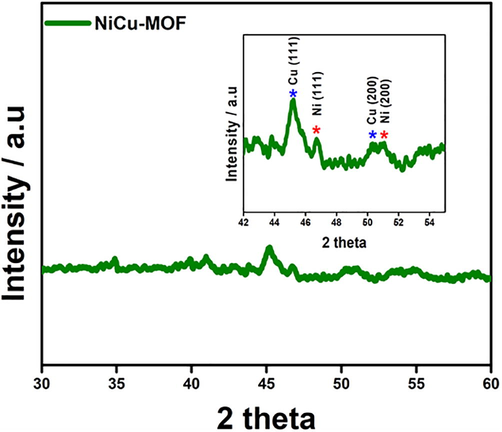
X-ray diffraction pattern of bimetallic NiCu-MOF.
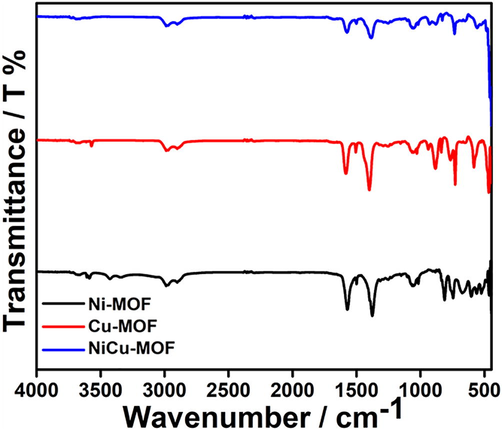
FT-IR spectrum of Ni and Cu single metal MOF and NiCu bimetal-MOF Ni-MOF (black), Cu-MOF (red), and NiCu-MOF (blue).
The more detailed elemental composition of as-synthesized sample NiCu-MOF were analyzed by XPS measurement. The elemental distribution of the sample shows in Fig. 4, core level indicates the presents of different elements C1s, O1s, Ni2p and Cu2p spectrum of NiCu-MOF (survey spectrum in Fig. S2). As shown in Fig. 4a the Ni2p spectrum two major peaks corresponding to the Ni 2p3/2 and Ni 2p1/2 located at 857.5 and 875.34 eV, then corresponding satellite peaks located at 863.18 and 881.19 eV (Zhu et al., 2017). (Fig. 4b) The Cu2p are present in two oxidation state Cu2+ and Cu+ in cupric oxide (CuO) and cuprous oxide (Cu2O). The peak at 935.1 and 955.15 eV corresponding to Cu 2p3/2 and Cu 2p1/2 in CuO. The peak at 945.45 and 964.5 eV corresponding to Cu 2p3/2 and Cu 2p1/2 in Cu2O (Arellano et al., 2015). The deconvoluted Fig. 4c C1s spectrum for MOF could be observed four different peaks at 282.9, 284.1, 285.14 and 288.7 eV associated with C = C, C–C, C-O and O-C = O as reported (Rabchinskii et al., 2018). Fig. 4d O1s spectrum demonstrated two main peaks at 531.6 and 532.67 eV indicates that the presence of CuO and Cu2O (Gao et al., 2019).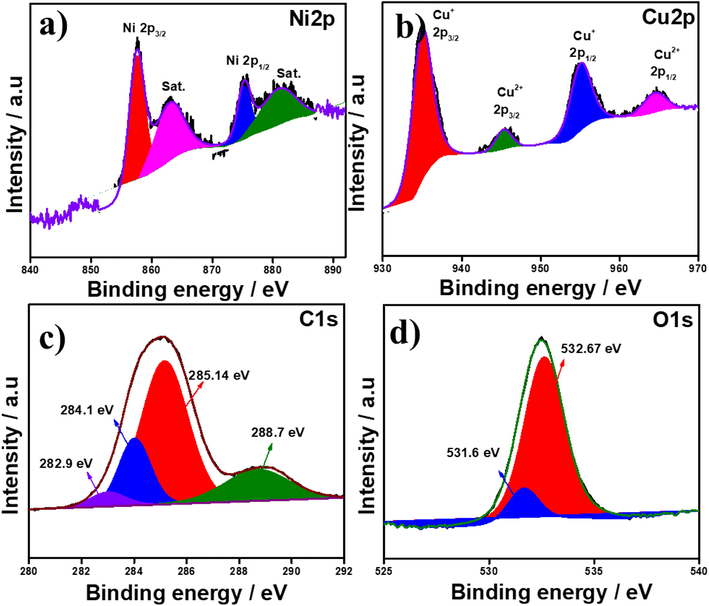
High resolution X-ray photoelectron spectra of NiCu-MOF a) Ni 2p, b) Cu 2p, c) C1s and d) O1s.
3.1 Oxygen evolution reaction
The synthesized NiCu-MOF assessed their electrochemical activity were studied in 1 M KOH solution and catalyst were coated on carbon paper electrode and used as working electrode. The linear sweep voltammograms were conducted at scan rate of 5 mV s−1. The current density of NiCu-MOF is higher as compared to other solo metal MOF (Ni-MOF, Cu-MOF). The peak around 1.39 V vs. RHE due to the nickel oxidation Ni(OH)2, which spontaneously formation of NiOOH on the working electrode (Hoang and Gewirth, 2016). The peak intensity of the Ni oxidation peak was reduced due to the formation Ni-Cu nanoclusters. The activity of the bimetal MOF depends on the ratio of the Ni and Cu metal present in the MOF and synergetic effect between both metal exhibited the better electrocatalytic reactivity towards oxygen radical intermediate. These radical also contribute to improve the formation of oxygen defects on the surface of the catalyst.
From the result (Fig. 5a) NiCu-MOF exhibits better OER activity compared with other catalyst, i.e., NiCu-MOF demonstrate lower onset potential 1.48 V and overpotential of 250 mV. The OER activity of other catalyst, 1.52 and 1.63 V with overpotential of 290 and 400 mV for Ni-MOF and Cu-MOF respectively. For comparison, RuO2 benchmark catalyst was done and showed in Fig. S3. Furthermore, the kinetic of the OER was calculated from LSV, i.e., plotting the logarithm of current density (log j) and overpotential (η). From Fig. 5b NiCu-MOF exhibits lower tafel slope of 169 mV dec−1 compare with other catalyst, 210 and 340 mV dec−1 for Ni-MOF and Cu-MOF respectively. From the tafel slope, evident that NiCu-MOF shows better catalytic activity towards among the other catalyst. Besides, electrochemical impedance spectroscopy (EIS) were carried out in the same electrolyte (1 M KOH), and it shows semi-circle like Nyquist plot was obtained. From (Fig. S4) the result, bimetallic NiCu-MOF catalyst showed remarkably high electrical conductivity and lower charge transfer resistance (Rct) about 47 Ω, whereas the Ni-MOF and Cu-MOF displayed slightly higher Rct values about 73.2 and 88.8 Ω respectively. Finally, the stability of the catalyst was evaluated by fixed onset overpotential η = 250 mV for 30000 s (Fig. S5). After the Chronoamprometry study FE-SEM analysis was performed and showed in Fig. S6.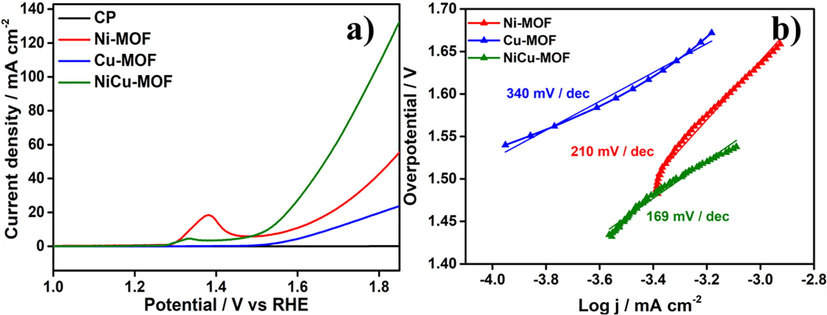
Electrochemical study a) LSV and b) tafel slope.
4 Conclusion
In summary, we have synthesized bimetallic MOF by solvothermal synthesis using Ni, Cu metal ions and BDC organic linker. The synthesized NiCu-MOF were characterized by FT-IR, SEM, PXRD and XPS etc. which confirmed the formation of metal complex. Among the synthesized catalyst was investigated OER under standard basic condition. NiCu-MOF catalyst displayed higher OER performance, with lower onset potential of 1.48 V vs RHE and tafel slope of 169 mV dec−1. The synergetic effect between two metals centers Ni and Cu enhanced the OER activity. In addition, the proposed bimetallic MOF (NiCu-MOF) showed excellent stability over 30000 s. Furthermore, no significant change was observed in the bimetallic MOF (NiCu-MOF) morphology properties after 30000 s water electrolysis. From these results well evidenced for that the NiCu-MOF is an excellent catalytic activity towards OER under basic condition.
Acknowledgement
This work was supported by the Gachon University research fund of 2019 (GCU-2019-0816) and the Gachon University research fund of 2020 (GCU-202002750001). The authors extend their appreciation to the Researchers supporting project number (RSP- 2021/247) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Dibenzothiophene oxidation in a model diesel fuel using CuO/GC catalysts and H2O2 in the presence of acetic acid under acidic condition. Fuel. 2015;149:15-25.
- [Google Scholar]
- The role of non-renewable energy consumption in economic growth and carbon emission: evidence from oil producing economies in Africa. Energy Strateg. Rev.. 2020;27:100434
- [Google Scholar]
- Recent advances in metal–organic frameworks for electrocatalytic hydrogen evolution and overall water splitting reactions. Dalton Trans.. 2020;49:12483-12502.
- [Google Scholar]
- From bimetallic metal-organic framework to porous carbon: high surface area and multicomponent active dopants for excellent electrocatalysis. Adv. Mater.. 2015;27:5010-5016.
- [Google Scholar]
- Nanomaterials derived from metal–organic frameworks. Nat. Rev. Mater.. 2017;3:1-14.
- [Google Scholar]
- Catalysts made of earth-abundant elements (Co, Ni, Fe) for water splitting: recent progress and future challenges. Energy Environ. Sci.. 2012;5:6012-6021.
- [Google Scholar]
- Facile synthesis of cuboid Ni-MOF for high-performance supercapacitors. J. Mater. Sci. Lett.. 2018;53:6807-6818.
- [Google Scholar]
- Three-dimensional porous Cu@ Cu 2 O aerogels for direct voltammetric sensing of glucose. Microchim. Acta. 2019;186:1-9.
- [Google Scholar]
- 1, 4-Phenylenediamine based covalent triazine framework as an electro catalyst. Polymer. 2017;109:315-320.
- [Google Scholar]
- Gopi, S., Giribabu, K., Kathiresan, M. and Yun, K., 2020. Cobalt (ii) ions and cobalt nanoparticle embedded porous organic polymers: an efficient electrocatalyst for water-splitting reactions. Sustain. Energ. Fuels. 4, 3797-3805.
- Cobalt-modified 2D porous organic polymer for highly efficient electrocatalytic removal of toxic urea and nitrophenol. Chemosphere. 2020;129052
- [Google Scholar]
- 2D Trimetal-organic framework derived metal carbon hybrid catalyst for urea electro-oxidation and 4-nitrophenol reduction. Chemosphere. 2020;267:129243
- [Google Scholar]
- High activity oxygen evolution reaction catalysts from additive-controlled electrodeposited Ni and NiFe films. ACS Catal.. 2016;6:1159-1164.
- [Google Scholar]
- Ru@ RuO2 core-shell nanorods: a highly active and stable bifunctional catalyst for oxygen evolution and hydrogen evolution reactions. Energy Environ. Mater.. 2019;2:201-208.
- [Google Scholar]
- Co–Ni-based nanotubes/nanosheets as efficient water splitting electrocatalysts. Adv. Energy Mater.. 2016;6:1501661.
- [Google Scholar]
- Transition metal-based metal-organic frameworks for oxygen evolution reaction. Coord. Chem. Rev.. 2020;424:213488
- [Google Scholar]
- First-row transition metal based catalysts for the oxygen evolution reaction under alkaline conditions: basic principles and recent advances. Small. 2017;13:1701931.
- [Google Scholar]
- Cu-metal organic frameworks (Cu-MOF) as an environment-friendly and economical catalyst for one pot synthesis of tacrine derivatives. RSC Adv.. 2020;10:1995-2003.
- [Google Scholar]
- Metal-organic framework nanosheets as building blocks for molecular sieving membranes. Science. 2014;346:1356-1359.
- [Google Scholar]
- Facile reduction of graphene oxide suspensions and films using glass wafers. Sci. Rep.. 2018;8:1-11.
- [Google Scholar]
- Metal–organic framework nanosheets in polymer composite materials for gas separation. Nat. Mater.. 2015;14:48-55.
- [Google Scholar]
- Characterization of Electrodeposited Ni and Ni? Mo Thin Films by X-ray Diffraction. Int. J. Electrochem. Sci.. 2014;9:6033-6042.
- [Google Scholar]
- Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Prog. Energy Combust. Sci.. 2017;58:1-35.
- [Google Scholar]
- On the direct synthesis of Cu (BDC) MOF nanosheets and their performance in mixed matrix membranes. J. Membr. Sci.. 2018;549:312-320.
- [Google Scholar]
- Metal–organic frameworks-based catalysts for electrochemical oxygen evolution. Mater. Horizons.. 2019;6:684-702.
- [Google Scholar]
- Transition metal oxides as electrocatalysts for the oxygen evolution reaction in alkaline solutions: an application-inspired renaissance. J. Am. Chem. Soc.. 2018;140:7748-7759.
- [Google Scholar]
- Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chem. Soc. Rev.. 2017;46:337-365.
- [Google Scholar]
- Functional materials derived from open framework templates/precursors: synthesis and applications. Energy Environ. Sci.. 2014;7:2071-2100.
- [Google Scholar]
- Metal organic framework laden poly (ethylene oxide) based composite electrolytes for all-solid-state Li-S and Li-metal polymer batteries. Electrochim. Acta.. 2018;285:355-364.
- [Google Scholar]
- Electrochemical oxidative cross-coupling with hydrogen evolution: a green and sustainable way for bond formation. Chem.. 2018;4:27-45.
- [Google Scholar]
- Recent advances in 2-D nanostructured metal nitrides, carbides, and phosphides electrodes for electrochemical supercapacitors–a brief review. J. Ind. Eng. Chem.. 2018;67:12-27.
- [Google Scholar]
- Metal–organic framework–derived Ni@ C and NiO@ C as anode catalysts for urea fuel cells. Sci. Rep.. 2020;10:1-10.
- [Google Scholar]
- MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem. Soc. Rev.. 2020;49:1414-1448.
- [Google Scholar]
- Metal-organic framework-based catalysts with single metal sites. Chem. Rev.. 2020;120:12089-12174.
- [Google Scholar]
- Sustainable and efficient hydrogen evolution over a noble metal-free WP double modified Zn x Cd 1–x S photocatalyst driven by visible-light. Dalton Trans.. 2019;48:11122-11135.
- [Google Scholar]
- New and efficient electrocatalyst for hydrogen production from water splitting: inexpensive, robust metallic glassy ribbons based on iron and cobalt. ACS Appl. Mater. Interfaces.. 2017;9:31340-31344.
- [Google Scholar]
- Catalysis of a single transition metal site for water oxidation: from mononuclear molecules to single atoms. Adv. Mater.. 2020;32:1904037.
- [Google Scholar]
- Two-dimensional metal–organic frameworks with high oxidation states for efficient electrocatalytic urea oxidation. Chem. Commun.. 2017;53:10906-10909.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2021.101379.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







