Translate this page into:
Melamine trisulfonic acid as an efficient catalyst for the synthesis of 2,6-dimethyl-4-substituted-1,4-dihydropyridine-3,5-diethyl/dimethylcarboxylate derivatives via Hantzsch reaction in solvent free condition
*Corresponding author. Tel.: +91 9944093020 smansoors2000@yahoo.co.in (S. Sheik Mansoor)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Available online 11 February 2013
Abstract
A facile and highly efficient one-pot synthesis of 1,4-dihydropyridine derivatives (1,4-DHPs) is reported via three component condensation reaction of aldehydes, ethyl acetoacetate or methyl acetoacetate and ammonium acetate using environmentally benign melamine trisulfonic acid (MTSA) as a catalyst in solvent free condition at 60 °C. The method presented here is applied to the tenets of green chemistry to the generation of biologically interesting products under solvent-free media that is less expensive and less toxic than those with organic solvents. Also, the catalyst is recyclable and could be reused without significant loss of activity. Even after three runs for the reaction, the catalytic activity of MTSA was almost the same as that of the freshly used catalyst.
The method also offers several advantages including high yields and simple work-up procedure.
Keywords
Melamine trisulfonic acid
1,4-Dihydropyridine
Hantzsch reaction
One-pot synthesis
Reusable catalyst
1 Introduction
In recent years, an increasing interest has been focused on the synthesis of Hantzsch 1,4-dihydropyridines, a class of model compounds of NADH coenzyme, due to the biological pertinence of these compounds to NADH redox process (Miri et al., 2006; Tewari et al., 2004). 1,4-dihydropyridines have been reported as anticancer (Tsuruo et al., 1983), neurotropic (Krauze et al., 1999), glycoprotein inhibitors (Zhou et al., 2005), anticoagulant (Kumar et al., 2011a), antioxidant (Vijesh et al., 2011), anti-inflammatory and anti-microbial agents (Kumar et al., 2011b). Calcium entry into the cytosol is mediated by multiple types of calcium channel, each with a distinct physiological role. Dihydropyridines are commercially used as calcium channel blockers for the treatment of cardiovascular diseases, including hypertension (Zamponi, 1998). Recently, the synthesis of dihydropyridines with respect to multidrug resistance (MDR) reversal in tumour cell gave a new dimension to their applications (Tanabe et al., 1998; Tasaka et al., 2001). Tuberculosis (TB) is a common and often deadly infectious disease caused by various strains of mycobacterium, usually Mycobacterium tuberculosis. Tuberculosis has been considered to be a disease of poverty for many years with quite rare occurrences in the developed countries. Recently, studies showed that 3,5-dicarbamoyl derivatives of 1,4-dihydropyridine (DHP) with lipophilic groups have considerable anti-tubercular activity against M. tuberculosis H37Rv (Trivedi et al., 2011; Khoshneviszadeh et al., 2009).
Generally, the basic skeleton of DHP was first discovered by Hantzsch in 1882 (Hantzsch, 1882). Due to the biological importance of these compounds several methods have been reported for the improvement of 1,4-dihydropyridine ring and polyhydroquinoline derivatives. Different approaches for the syntheses of 1,4-dihydropyridine derivatives using various catalysts, such as cellulose sulphuric acid (Safari et al., 2011), triphenylphosphine (Debache et al., 2009), silica supported 12-tungstophosphoric acid (Rafiee et al., 2009), Iron (III) trifluoroacetate (Adibi et al., 2007), ionic liquid [tbmim]Cl2/AlCl3 (Reddy et al., 2011), organo catalyst (Baghbanian et al., 2010), cerric ammonium nitrate (Reddy and Raghu, 2008), nickel nanoparticle (Saikia et al., 2012), aluminium phosphate (Purandhar et al., 2012), bismuth nitrate (Bandyopadhyay et al., 2012), gadolinium triflate (Mansoor et al., 2012a), titanium dioxide nanoparticles (Tajbakhsh et al., 2012), ferric fluoride (Surasani et al., 2012) and silica sulphuric acid (Kolvari et al., 2011), MgO nanoparticles (Mirzaei and Davoodnia, 2012), visible light (Ghosh et al., 2013) and protic pyridinium ionic liquid (Tajbakhsh et al., 2013) have been reported. Many of these reported methods involve the use of expensive reagents, hazardous solvents, long reaction times and tedious workup procedures. Thus, the search for new reagents and methods is still of growing importance.
Melamine trisulfonic acid is effectively used as a catalyst in organic reactions, such as regioselective nitration of aromatic compounds (Albadi et al., 2012), N-formylation of amines (Yang and Zhang, 2012), aryldithienylmethanes (Wu et al., 2012), spiro[pyrazolo[3,4-b]pyridine-4,3′-indoline] derivatives (Yang et al., 2012), acetylation of alcohols, phenols and amines (Shirini et al., 2010a), trimethylsilylation of alcohols and phenols (Wu et al., 2011), solvent free synthesis of coumarins (Shirini et al., 2010b), chemoselective methoxymethylation of alcohols (Shirini et al., 2010c), synthesis of chromen-6-ones (Ma et al., 2011) and synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones (Shirini et al., 2011).
To the best of our knowledge, there are no examples on the use of melamine trisulfonic acid as a catalyst for the synthesis of 1,4-dihydropyridine derivatives. In continuation of our investigation with the one-pot synthesis of biologically active molecules, such as 3,4-dihydropyrimidin-2(1H)-ones/-thiones/imines (Mansoor et al., 2011), β-amino ketone compounds (Mansoor et al., 2012b), amidoalkyl naphthols (Mansoor et al., 2012c), 2-amino-4,6-diphenylpyridine-3-carbonitrile derivatives (Mansoor et al., 2012d) and α-amino nitriles (Mansoor et al., 2012e), herein, we wish to report the one-pot condensation of aldehydes, ethyl/methyl acetoacetate and ammonium acetate under solvent free conditions at 60 °C using melamine trisulfonic acid as a reusable catalyst for the synthesis of 1,4-dihydropyridine derivatives via Hantzsch reaction. Melamine trisulfonic acid is safe, easy to handle and environmentally benign.
2 Experimental
Chemicals were purchased from Merck, Fluka and Aldrich Chemical Companies. The benzaldehydes used were with substituents H, p-OCH3, p-CH3, p-Cl, p-NO2, p-Br, p-OH, m-Cl, m-NO2 and m-OH. Heterocyclic aldehydes like Furfural and 2-Thienal were also used for the synthesis. The solid aldehydes were used as such and the liquid aldehydes were used after vacuum distillation. Ethyl acetoacetate and methyl acetoacetate were used as 1,3-dicarbonyl compounds. Ammonium acetate was used as the nitrogen source. Solvents like THF, methanol, ethanol, dichloromethane, acetonitrile, cyclohexane and benzene were used. Melamine and chlorosulfonic acid were used for the preparation of MTSA. All yields refer to isolated products unless otherwise stated.
2.1 Preparation of melamine trisulfonic acid (MTSA)
Melamine trisulfonic acid was prepared from melamine and chlorosulfonic acid as reported previously in the literature by Shirini et al. (2010a) Scheme 1.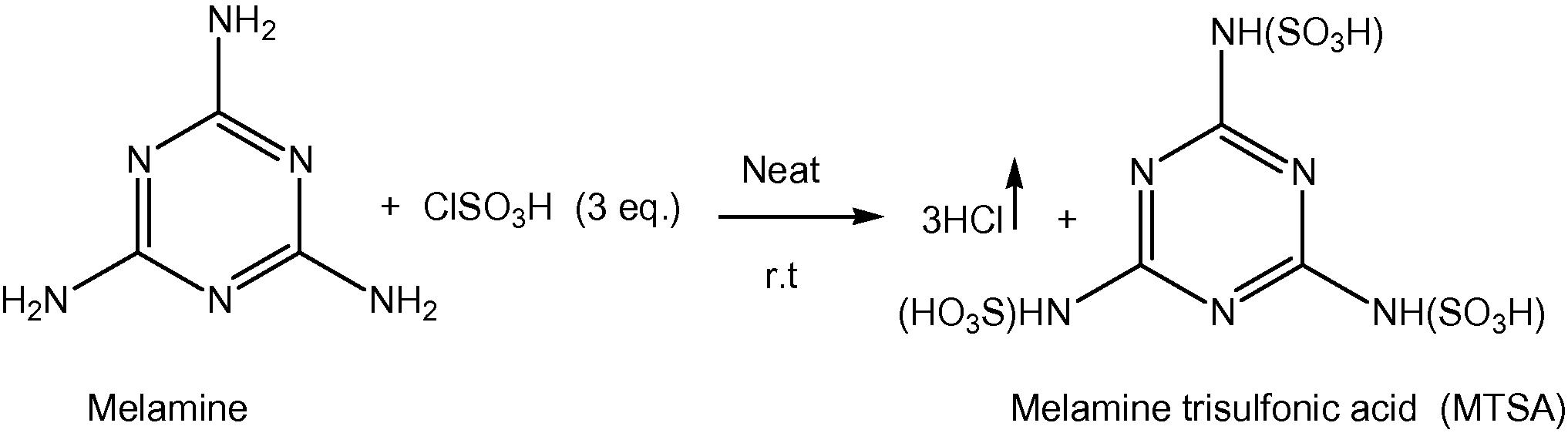
Preparation of melamine trisulfonic acid.
2.2 General experimental procedure for the synthesis (compounds 4a–p)
A mixture of aldehyde 1 (1 mmol), ethyl acetoacetate 2 (2 mmol), ammonium acetate 3 (1.5 mmol) and MTSA (5 mol%) was taken in a 50 ml flask and heated at 60 °C under solvent-free condition for the appropriate time monitored by TLC. The reaction mixture, after being cooled to room temperature was poured into cold water and extracted with ethyl acetate. The organic layer was washed with brine and water and dried over Na2SO4. The crude products were purified by crystallization from ethanol to afford 1,4-dihydropyridines. The catalyst was filtered and washed with methanol for reuse (see Scheme 2).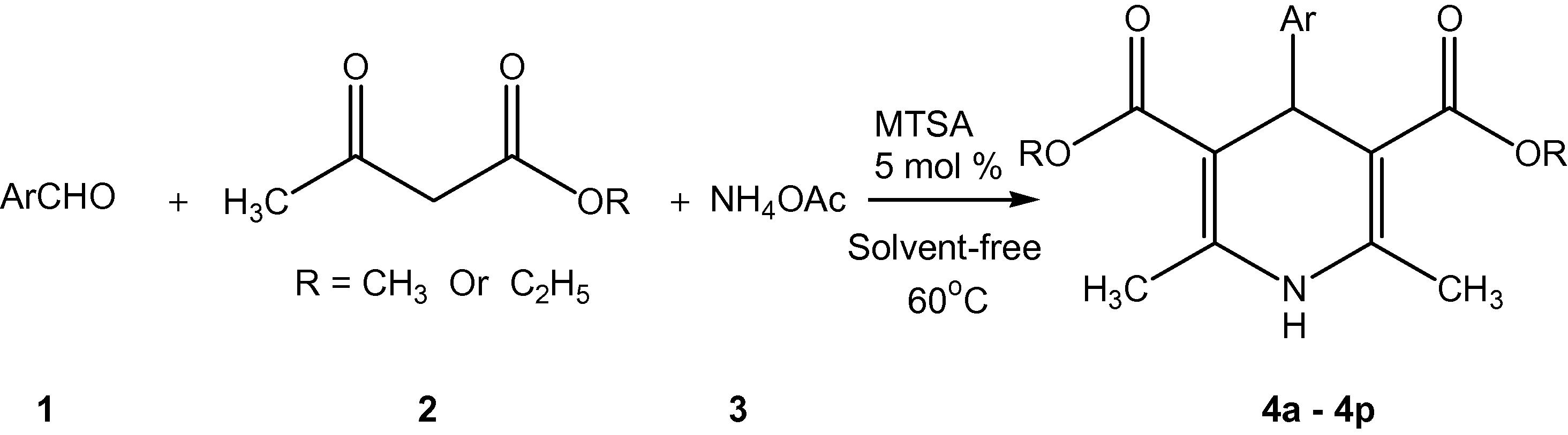
The reaction of aromatic aldehyde, ethyl/methyl acetoacetate and ammonium acetate in the presence of MTSA as catalyst at 60 °C under solvent free condition.
2.3 Spectral data for the synthesized compounds
2.3.1 2,6-Dimethyl-4-phenyl-1,4-dihydropyridine-3,5-diethylcarboxylate (4a)
White solid; mp 157–159 °C; IR (KBr, cm−1): 3342, 1691, 1643, 1489, 1210, 779.
1H NMR (500 MHz, DMSO-d6) δ: 1.19 (t, J = 7.2 Hz, 6H, 2CH3CH2), 2.33 (s, 6H, 2CH3), 4.08 (q, J = 7.0 Hz, 4H, 2CH3CH2), 4.96 (s, 1H, CH), 5.97 (s, 1H, NH), 7.16–7.33 (m, 5H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 14.0, 19.4, 39.6, 59.5, 104.0, 121.8, 129.0, 131.0, 144.4, 146.5, 166.8 ppm; MS (ESI): m/z 330 (M + H)+. Anal. Calcd. for C19H23NO4 (%): C, 69.30; H, 6.99; N, 4.25. Found: C, 69.22; H, 6.94; N, 4.23.
2.3.2 2,6-Dimethyl-4-(4-methylphenyl)-1,4-dihydropyridine-3,5-diethylcarboxylate (4b)
Yellow solid; mp 135–137 °C; IR (KBr, cm−1): 3338, 1698, 1653, 1480, 1200, 789.
1H NMR (500 MHz, DMSO-d6) δ: 1.24 (t, J = 7.4 Hz, 6H, 2CH3CH2), 2.28 (s, 6H, 2CH3), 4.09 (q, J = 7.2 Hz, 4H, 2CH3CH2), 5.00 (s, 1H, CH), 5.90 (s, 1H, NH), 7.10–7.43 (m, 4H, Ar-H), 2.22 (s, 3H, CH3) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 14.3, 19.5, 38.9, 60.0, 103.5, 119.3, 129.8, 131.0, 144.0, 146.4, 167.5 ppm; MS (ESI): m/z 344 (M + H)+. Anal. Calcd. for C20H25NO4 (%): C, 69.97; H, 7.29; N, 4.08. Found: C, 69.91; H, 7.28; N, 4.07.
2.3.3 2,6-Dimethyl-4-(4-methoxyphenyl)-1,4-dihydropyridine-3,5-diethylcarboxylate (4c)
Yellow solid; mp 156–158 °C; IR (KBr, cm−1): 3329, 1700, 1633, 1494, 1214, 783.
1H NMR (500 MHz, DMSO-d6) δ: 1.21 (t, J = 7.4 Hz, 6H, 2CH3CH2), 2.29 (s, 6H, 2CH3), 4.10 (q, J = 7.0 Hz, 4H, 2CH3CH2), 4.99 (s, 1H, CH), 6.07 (s, 1H, NH), 6.96–7.12 (m, 4H, Ar-H), 3.62 (s, 3H, OCH3) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 15.0, 20.0, 40.3, 59.5, 104.4, 118.8, 131.0, 131.5, 144.4, 147.3, 166.5 ppm; MS (ESI): m/z 360 (M + H)+. Anal. Calcd. for C20H25NO5 (%): C, 66.85; H, 6.96; N, 3.90. Found: C, 66.77; H, 6.97; N, 3.88.
2.3.4 2,6-Dimethyl-4-(4-nitrophenyl)-1,4-dihydropyridine-3,5-diethylcarboxylate (4d)
Yellow solid; mp 132–134 °C; IR (KBr, cm−1): 3348, 1688, 1644, 1481, 1207, 782.
1H NMR (500 MHz, DMSO-d6) δ: 1.24 (t, J = 7.4 Hz, 6H, 2CH3CH2), 2.31 (s, 6H, 2CH3), 4.08 (q, J = 7.2 Hz, 4H, 2CH3CH2), 5.06 (s, 1H, CH), 6.00 (s, 1H, NH), 7.14–7.54 (m, 4H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 15.1, 19.6, 39.8, 59.5, 103.9, 119.0, 129.5, 131.4, 143.9, 146.5, 167.0 ppm; MS (ESI): m/z 375 (M + H)+. Anal. Calcd. for C19H22N2O6 (%): C, 60.96; H, 5.88; N, 7.49. Found: C, 60.93; H, 5.86; N, 7.48.
2.3.5 2,6-Dimethyl-4-(4-chlorophenyl)-1,4-dihydropyridine-3,5-diethylcarboxylate (4e)
White solid; mp 144–146 °C; IR (KBr, cm−1): 3335, 1695, 1645, 1499, 1219, 789.
1H NMR (500 MHz, DMSO-d6) δ: 1.19 (t, J = 7.2 Hz, 6H, 2CH3CH2), 2.34 (s, 6H, 2CH3), 4.10 (q, J = 7.2 Hz, 4H, 2CH3CH2), 5.09 (s, 1H, CH), 5.94 (s, 1H, NH), 7.22–7.48 (m, 4H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 14.6, 19.4, 39.8, 59.4, 103.6, 119.0, 130.4, 131.4, 144.5, 146.6, 166.8 ppm; MS (ESI): m/z 364.45 (M + H)+. Anal. Calcd. for C19H22ClNO4 (%): C, 62.73; H, 6.05; N, 3.85. Found: C, 62.64; H, 6.01; N, 3.80.
2.3.6 2,6-Dimethyl-4-(4-bromophenyl)-1,4-dihydropyridine-3,5-diethylcarboxylate (4f)
White solid; mp 160–162 °C; IR (KBr, cm−1): 3332, 1692, 1649, 1491, 1213, 772.
1H NMR (500 MHz, DMSO-d6) δ: 1.25 (t, J = 7.2 Hz, 6H, 2CH3CH2), 2.31 (s, 6H, 2CH3), 4.14 (q, J = 7.2 Hz, 4H, 2CH3CH2), 4.97 (s, 1H, CH), 6.02 (s, 1H, NH), 7.12–7.53 (m, 4H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 14.4, 18.9, 39.7, 59.6, 103.7, 119.5, 130.2, 131.2, 143.9, 145.7, 165.3 ppm; MS (ESI): m/z 408.9 (M + H)+. Anal. Calcd. for C19H22BrNO4 (%): C, 55.90; H, 5.39; N, 3.43. Found: C, 55.82; H, 5.36; N, 3.40.
2.3.7 2,6-Dimethyl-4-(3-chlorophenyl)-1,4-dihydropyridine-3,5-diethylcarboxylate (4g)
White solid; mp 140–142 °C; IR (KBr, cm−1): 3340, 1689, 1652, 1480, 1200, 770.
1H NMR (500 MHz, DMSO-d6) δ: 1.20 (t, J = 7.2 Hz, 6H, 2CH3CH2), 2.29 (s, 6H, 2CH3), 4.09 (q, J = 7.0 Hz, 4H, 2CH3CH2), 5.02 (s, 1H, CH), 5.96 (s, 1H, NH), 7.26–7.61 (m, 4H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 15.1, 19.3, 39.7, 60.1, 104.0, 119.4, 130.0, 131.1, 144.4, 146.4, 165.2 ppm; MS (ESI): m/z 364.45 (M + H)+. Anal. Calcd. for C19H22ClNO4 (%): C, 62.73; H, 6.05; N, 3.85. Found: C, 62.66; H, 6.03; N, 3.84.
2.3.8 2,6-Dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-diethylcarboxylate (4h)
Yellow solid; mp 162–164 °C; IR (KBr, cm−1): 3344, 1701, 1643, 1487, 1217, 777.
1H NMR (500 MHz, DMSO-d6) δ: 1.21 (t, J = 7.2 Hz, 6H, 2CH3CH2), 2.32 (s, 6H, 2CH3), 4.16 (q, J = 7.2 Hz, 4H, 2CH3CH2), 4.96 (s, 1H, CH), 6.04 (s, 1H, NH), 7.06–7.42 (m, 4H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 14.6, 19.7, 39.8, 59.5, 102.9, 119.5, 130.3, 131.3, 143.9, 146.4, 166.6 ppm; MS (ESI): m/z 375 (M + H)+. Anal. Calcd. for C19H22N2O6 (%): C, 60.96; H, 5.88; N, 7.49. Found: C, 60.88; H, 5.88; N, 7.47.
2.3.9 2,6-Dimethyl-4-(4-hydroxyphenyl)-1,4-dihydropyridine-3,5-diethylcarboxylate (4i)
White solid; mp 228–230 °C; IR (KBr, cm−1): 3443, 3341, 1698, 1640, 1493, 1213, 778.
1H NMR (500 MHz, DMSO-d6) δ: 1.23 (t, J = 7.2 Hz, 6H, 2CH3CH2), 2.33 (s, 6H, 2CH3), 4.09 (q, J = 7.0 Hz, 4H, 2CH3CH2), 5.11 (s, 1H, CH), 6.10 (s, 1H, NH), 7.12–7.43 (m, 4H, Ar-H), 9.96 (s, 1H, OH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 14.9, 19.6, 38.9, 59.7, 103.6, 119.9, 129.9, 130.5, 144.0, 146.5, 166.8 ppm; MS (ESI): m/z 346 (M + H)+. Anal. Calcd. for C19H23NO5 (%): C, 66.09; H, 6.67; N, 4.06. Found: C, 66.01; H, 6.65; N, 4.04.
2.3.10 2,6-Dimethyl-4-(3-hydroxyphenyl)-1,4-dihydropyridine-3,5-diethylcarboxylate (4j)
White solid; mp 172–174 °C; IR (KBr, cm−1): 3429, 3336, 1687, 1633, 1487, 1215, 781.
1H NMR (500 MHz, DMSO-d6) δ: 1.25 (t, J = 7.4 Hz, 6H, 2CH3CH2), 2.27 (s, 6H, 2CH3), 4.08 (q, J = 7.2 Hz, 4H, 2CH3CH2), 4.98 (s, 1H, CH), 5.99 (s, 1H, NH), 6.96–7.23 (m, 4H, Ar-H) 9.88 (s, 1H, OH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 15.0, 19.6, 39.6, 59.7, 103.6, 119.7, 130.0, 130.7, 144.6, 147.0, 167.2 ppm; MS (ESI): m/z 346 (M + H)+. Anal. Calcd. for C19H23NO5 (%): C, 66.09; H, 6.67; N, 4.06. Found: C, 66.05; H, 6.66; N, 4.05.
2.3.11 2,6-Dimethyl-4-(2-furyl)-1,4-dihydropyridine-3,5-diethylcarboxylate (4k)
Yellow solid; mp 160–162 °C; IR (KBr, cm−1): 3333, 1704, 1635, 1499, 1213, 772.
1H NMR (500 MHz, DMSO-d6) δ: 1.22 (t, J = 7.4 Hz, 6H, 2CH3CH2), 2.29 (s, 6H, 2CH3), 4.10 (q, J = 7.1 Hz, 4H, 2CH3CH2), 4.96 (s, 1H, CH), 6.03 (s, 1H, NH), 6.32–6.41 (m, 2H, Furyl-H), 7.13 (m, 1H, Furyl-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 14.3, 19.4, 37.3, 59.8, 103.6, 118.8, 132.0, 133.8, 144.5, 147.9, 167.5 ppm; MS (ESI): m/z 320 (M + H)+. Anal. Calcd. for C17H21NO5 (%): C, 63.95; H, 6.58; N, 4.39. Found: C, 63.88; H, 6.55; N, 4.36.
2.3.12 2,6-Dimethyl-4-(2-thienyl)-1,4-dihydropyridine-3,5-diethylcarboxylate (4l)
Yellow solid; mp 172–174 °C; IR (KBr, cm−1): 3347, 1700, 1630, 1486, 1215, 766.
1H NMR (500 MHz, DMSO-d6) δ: 1.19 (t, J = 7.4 Hz, 6H, 2CH3CH2), 2.28 (s, 6H, 2CH3), 4.08 (q, J = 7.2 Hz, 4H, 2CH3CH2), 5.04 (s, 1H, CH), 6.07 (s, 1H, NH), 6.08–6.13 (m, 2H, Thienyl-H), 6.89 (m, 1H, Thienyl-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 14.5, 19.5, 39.4, 59.6, 103.7, 118.9, 131.1, 131.8, 144.6, 146.5, 167.3 ppm; MS (ESI): m/z 336 (M + H)+. Anal. Calcd. for C17H21NO4S (%): C, 60.89; H, 6.27; N, 4.18. Found: C, 60.80; H, 6.28; N, 4.17.
2.3.13 2,6-Dimethyl-4-phenyl-1,4-dihydropyridine-3,5-dimethylcarboxylate (4m)
White solid; mp 116–118 °C; IR (KBr, cm−1): 3317, 1692, 1648, 1477, 1205, 765.
1H NMR (500 MHz, DMSO-d6) δ: 3.72 (s, 6H, 2OCH3), 2.27 (s, 6H, 2CH3), 4.96 (s, 1H, CH), 6.08 (s, 1H, NH), 6.91–7.22 (m, 5H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 20.2, 41.3, 56.8, 105.4, 129.0, 130.8, 143.5, 148.9, 167.6 ppm; MS (ESI): m/z 302 (M + H)+. Anal. Calcd. for C17H19NO4 (%): C, 67.77; H, 6.31; N, 4.65. Found: C, 67.66; H, 6.28; N, 4.62.
2.3.14 2,6-Dimethyl-4-(4-chlorophenyl)-1,4-dihydropyridine-3,5-dimethylcarboxylate (4n)
Yellow solid; mp 194–196 °C; IR (KBr, cm−1): 3315, 1698, 1653, 1487, 1212, 772.
1H NMR (500 MHz, DMSO-d6) δ: 3.72 (s, 6H, 2OCH3), 2.27 (s, 6H, 2CH3), 5.05 (s, 1H, CH), 5.94 (s, 1H, NH), 7.01–7.34 (m, 4H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 20.4, 41.4, 56.5, 105.6, 129.0, 130.4, 143.6, 149.1, 167.4 ppm; MS (ESI): m/z 336.45 (M + H)+. Anal. Calcd. for C17H18ClNO4 (%): C, 60.81; H, 5.36; N, 4.17. Found: C, 60.70; H, 5.34; N, 4.15.
2.3.15 2,6-Dimethyl-4-(4-nitrophenyl)-1,4-dihydropyridine-3,5-dimethylcarboxylate (4o)
Yellow solid; mp 152–154 °C; IR (KBr, cm−1): 3322, 1690, 1650, 1479, 1207, 771.
1H NMR (500 MHz, DMSO-d6) δ: 3.72 (s, 6H, 2OCH3), 2.27 (s, 6H, 2CH3), 4.99 (s, 1H, CH), 6.00 (s, 1H, NH), 7.09–7.47 (m, 4H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 20.1, 41.0, 56.3, 105.7, 129.7, 131.3, 144.0, 149.3, 167.2 ppm; MS (ESI): m/z 347 (M + H)+. Anal. Calcd. for C17H18N2O6 (%): C, 58.96; H, 5.20; N, 8.09. Found: C, 58.90; H, 5.18; N, 8.06.
2.3.16 2,6-Dimethyl-4-(2-furyl)-1,4-dihydropyridine-3,5-dimethylcarboxylate (4p)
Yellow solid; mp 148–150 °C; IR (KBr, cm−1): 3324, 1694, 1654, 1481, 1211, 773.
1H NMR (500 MHz, DMSO-d6) δ: 3.66 (s, 6H, 2OCH3), 2.19 (s, 6H, 2CH3), 4.93 (s, 1H, CH), 6.07 (s, 1H, NH), 6.22–6.34 (m, 2H, Furyl-H), 7.22 (m, 1H, Furyl-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 19.8, 41.0, 56.4, 105.7, 129.3, 130.7, 143.6, 149.0, 167.1 ppm; MS (ESI): m/z 292 (M + H)+. Anal. Calcd. for C15H17NO5 (%): C, 61.85; H, 5.84; N, 4.81. Found: C, 61.75; H, 5.82; N, 4.79.
3 Results and discussion
Initially, 4-nitro benzaldehyde has been used to react with ethyl acetoacetate and ammonium acetate in the presence of 5 mol% melamine trisulfonic acid under various solvents like THF, methanol, ethanol, dichloromethane, acetonitrile, cyclohexane and benzene at 60 °C in order to optimize the reaction conditions (Table 1, entries 1–7). The reaction was studied under solvent-free conditions also. It was found that the best results were obtained with 5 mol% MTSA under solvent-free condition. (Table 1, entry 8). The reaction was completed within 4 h and the expected product was obtained in a 92% yield.
Entry
Solvent
Amount of catalyst (mol %)
Time (h)
Yield (%)b
1
THF
5
6
68
2
Methanol
5
5
78
3
Ethanol
5
5
80
4
Dichloromethane
5
6
73
5
Acetonitrile
5
6
65
6
Cyclohexane
5
6
70
7
Benzene
5
6
55
8
None
5
4
92
Next, we have studied the effect of temperature for the model reaction. The reaction has been studied at various temperatures like room temperature, 40, 50, 60, 70 and 80 °C. The yield of the product increased up to 60 °C. After 60 °C, increasing temperature leads to a decrease in yields. Therefore, our optimized condition is 5 mol% of MTSA under solvent free condition at 60 °C Table 2.
Entry
Temperature (°C)
Time (h)
Yield (%)b
1
r.t
6.5
65
2
40
6.0
74
3
50
5.0
83
4
60
4.0
92
5
70
3.5
86
6
80
3.0
80
The reusability of the catalyst is one of the most important benefits and makes it useful for commercial applications. Thus the recovery and reusability of melamine trisulfonic acid were investigated. The reusability of the catalyst was checked by separating the melamine trisulfonic acid from the reaction mixture and drying in a vacuum oven at 60 °C for 5 h prior to reuse in subsequent reactions. The recovered catalyst can be reused at least three additional times in subsequent reactions without significant loss in product yield Fig. 1 Table 3.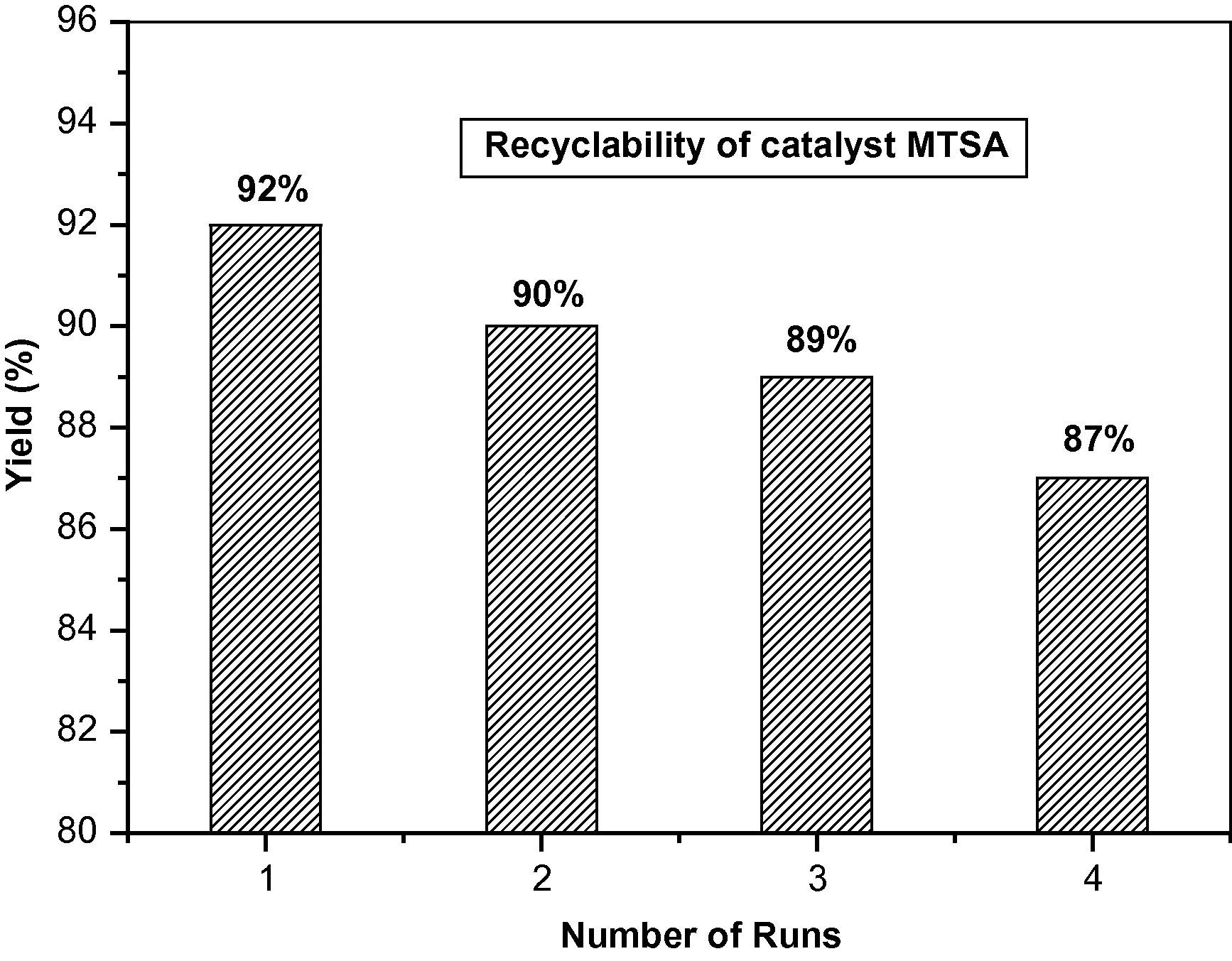
Recyclability of melamine trisulfonic acid for the synthesis of 2,6-dimethyl-4-(4-nitrophenyl)-1,4-dihydropyridine-3,5-diethylcarboxylate.
Entry
Cycle
Time (h)
Yield (%)b
1
0
4.0
92
2
1
4.0
90
3
2
4.0
89
4
3
4.0
87
A series of 1,4-dihydropyridines were synthesized by using diverse aldehydes, 1,3-diketo compounds and ammonium acetate in the presence of MTSA (5 mol%) as catalyst under solvent-free conditions. As shown in Table 4, the reaction proceeded equally well irrespective of the nature of the carbonyl compounds (aromatic, heteroaromatic) to afford the corresponding products in excellent yield (86–94%). The catalytic system worked well. It is noteworthy to mention that, the effect of the nature of the substituents on the aromatic ring showed no obvious effect on this conversion, because they were obtained in high yields in relatively short reaction times.
Entry
Aldehyde Ar
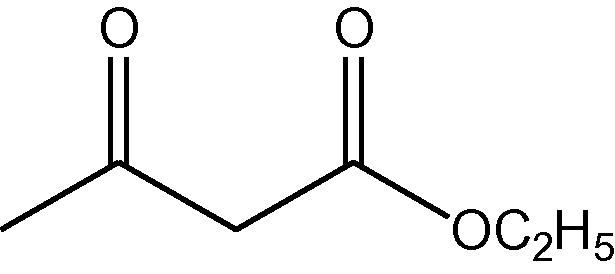
1,3-dicarbonyl compound
Ammonia source
Product
Time (h)
Yieldb (%)
1

NH4OAc
4a
4.0
86
2
NH4OAc
4b
3.5
88
3
NH4OAc
4c
3.5
87
4
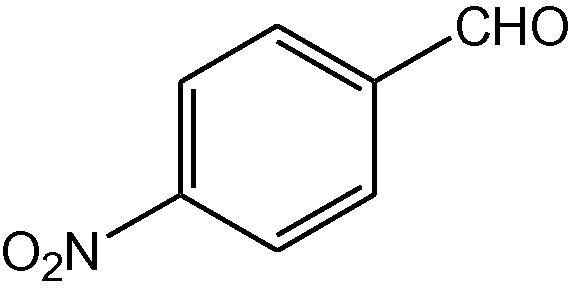

NH4OAc
4d
4.0
92
5
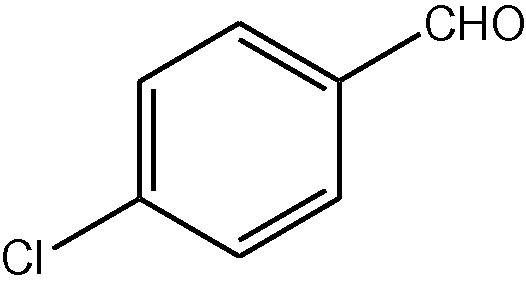
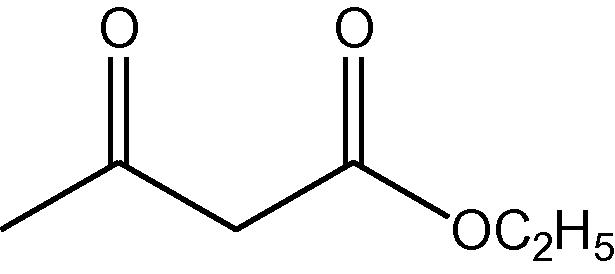
NH4OAc
4e
3.0
91
6
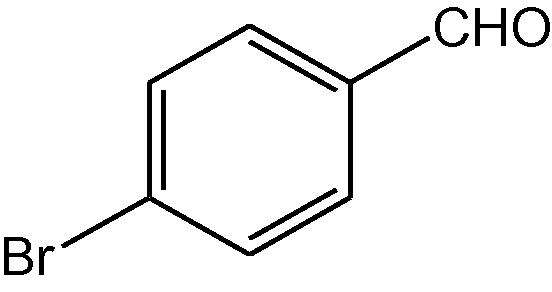
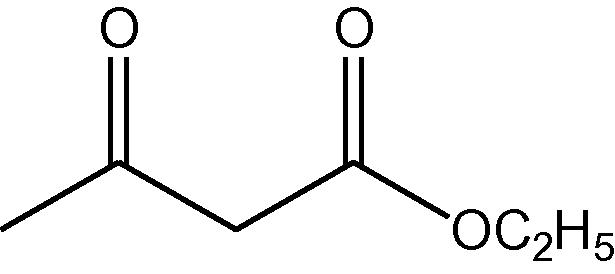
NH4OAc
4f
3.0
92
7
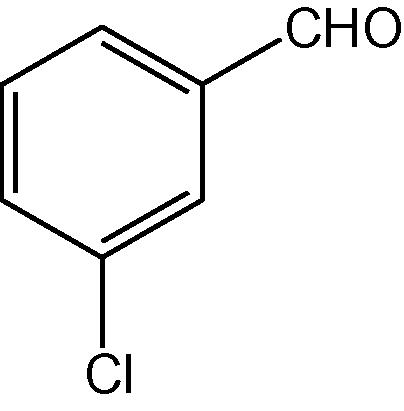
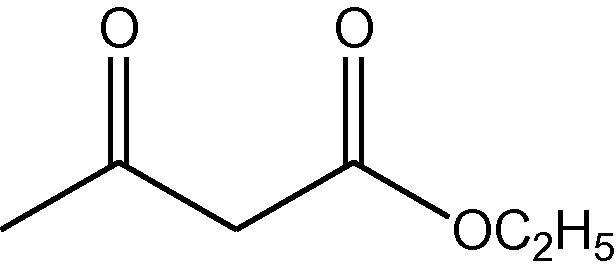
NH4OAc
4g
3.5
90
8
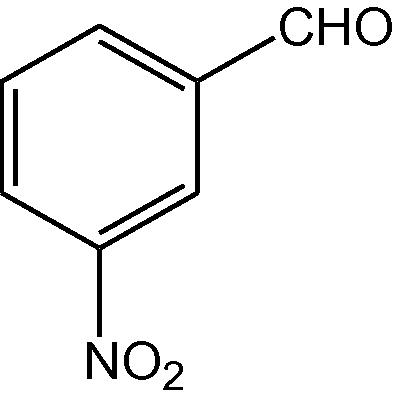
NH4OAc
4h
4.0
91
9
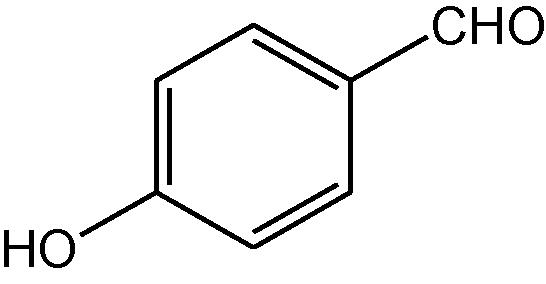
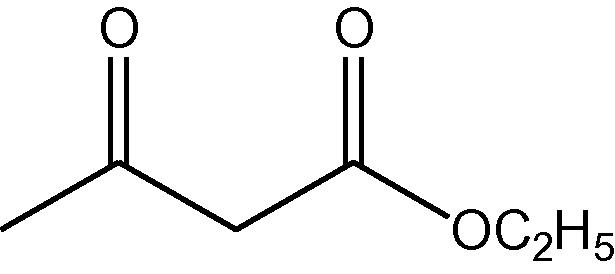
NH4OAc
4i
3.0
89
10
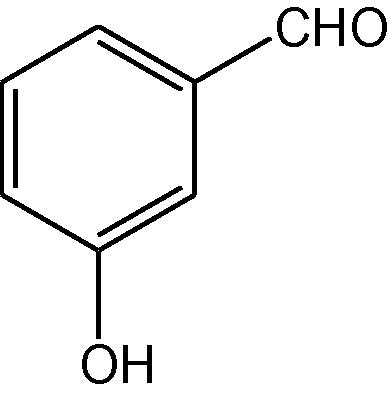
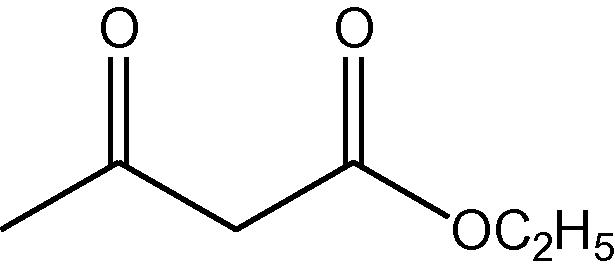
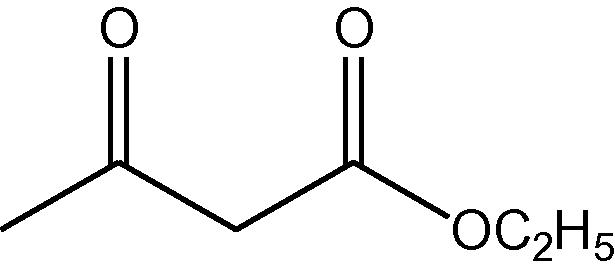
NH4OAc
4j
3.5
87
11
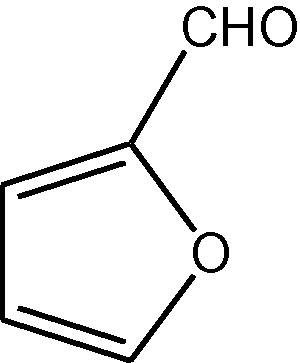
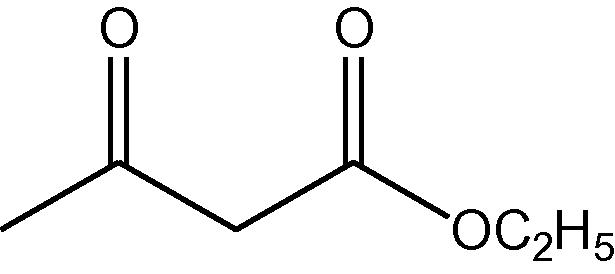
NH4OAc
4k
4.0
91
12
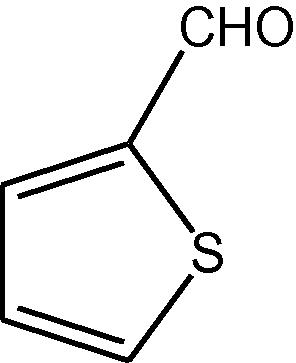
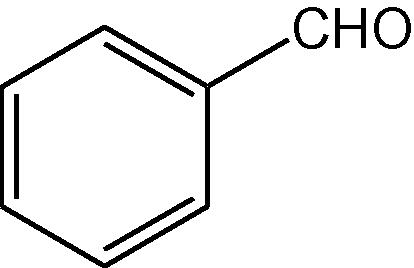
NH4OAc
4l
4.0
90
13
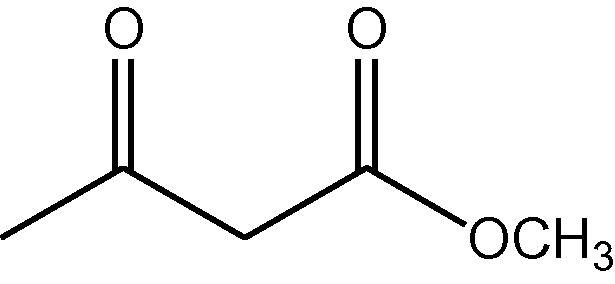
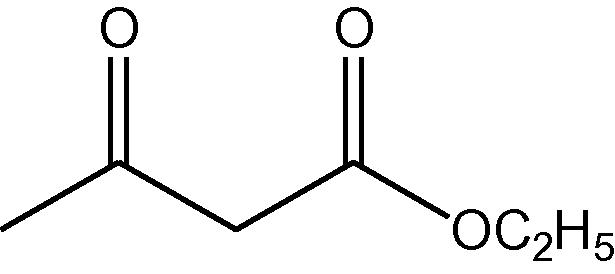
NH4OAc
4m
4.0
91
14
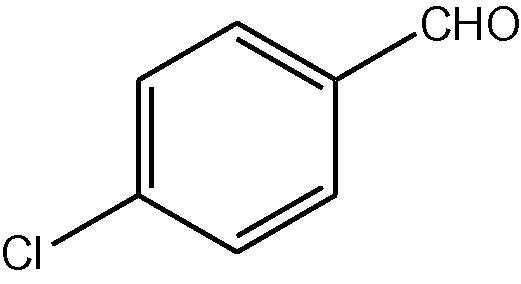
NH4OAc
4n
3.0
93
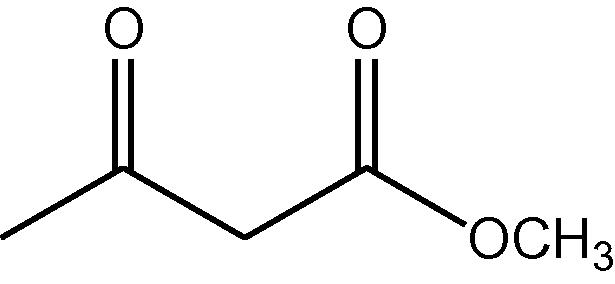
15
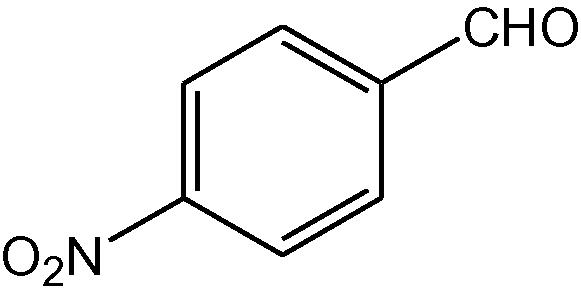
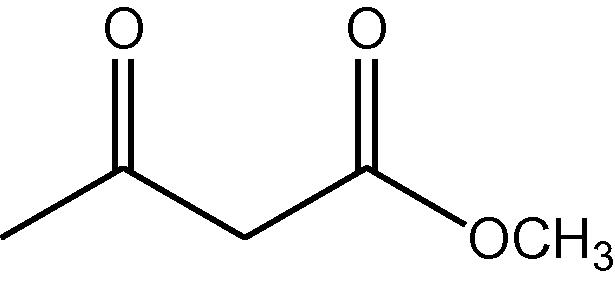
NH4OAc
4o
4.0
94
16
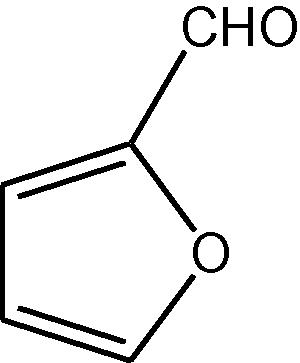
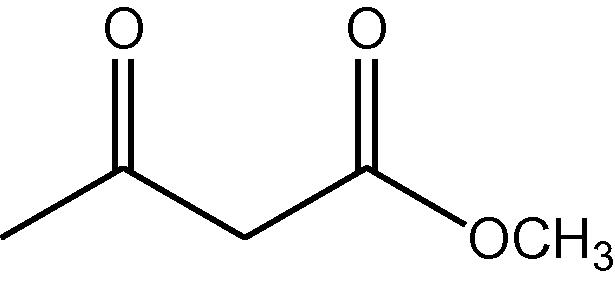
NH4OAc
4p
4.0
91
In order to show the merit of MTSA in comparison with other catalysts, we summarized some of the results for the preparation of 2,6-Dimethyl-4-(4-nitrophenyl)-1,4-dihydropyridine-3,5-diethylcarboxylate (4d) in Table 5. A number of Lewis acid catalysts such as ZnCl2, AlCl3, FeCl3, BiCl3, Bi(OTf)3 and BiBr3 have been screened using the model reaction under solvent-free conditions at 60 °C (Table 5, entry 2–7). The results showed that MTSA (5 mol%) is a more efficient catalyst with respect to reaction temperature, catalyst load, reaction time and yield than other catalysts (Table 5, entry 9). It was found that the reaction without catalyst produced low yield (Table 5, entry 1).
Entry
Catalyst (h)
Amount of catalyst (mol%)
Time
Yieldb (%)
1
None
–
6
32
2
ZnCl2
50
6
42
3
AlCl3
50
64
8
4
FeCl3
50
6
40
5
BiCl3
10
6
70
6
Bi(OTf)3
10
6
76
7
BiBr3
20
6
60
8
MTSA
10
4
88
9
MTSA
5
4
92
10
MTSA
3
4
72
11
MTSA
2
4
66
Recently Debache et al., reported the synthesis of 1,4-dihydropyridines using triphenylphosphine (20 mol%) as a Lewis base in ethanol (Debache et al., 2009). In our method we have synthesized 1,4-dihydropyridines using MTSA (5 mol%) under solvent-free conditions. We have described the reusability of the catalyst also.
4 Conclusions
In conclusion, MTSA was found to be an efficient catalyst in one-pot reaction of aldehydes, ethyl/methyl acetoacetate and ammonium acetate to afford 1,4-dihydropyridines. Synthesis of biologically significant heterocyclic molecules under solvent-free conditions is very promising and challenging. The ultimate aim, of course, is to use no solvent at all and to conduct the reactions under solvent-free conditions. Development of cleaner technologies is a major emphasis in green chemistry. Solvent-free reaction condition is used as an eco-friendly approach for the synthesis of a variety of products and this generally leads to large reductions in reaction times and enhancements of conversions. There is a growing interest in the one-pot three component synthesis of 1,4-dihydropyridines because of the significant importance of this scaffold in preparing a wide variety of biologically and pharmacologically active molecules. On this basis we have developed an extremely rapid, convenient and environmentally benign route for the one-step synthesis of 1,4-dihydropyridines. The present methodology offers attractive features such as shorter reaction times, milder conditions, and simplicity of the reaction as well as excellent yield of the products. This reaction will be applicable to the synthesis of various organic compounds of medicinal interest. Also the catalyst could be successfully recovered and recycled at least for four runs without significant loss in activity. The one-pot nature and the use of reusable and an eco-friendly catalyst make it an interesting alternative to multi-step approaches.
Acknowledgement
The authors gratefully acknowledge the University Grants Commission, Government of India, New Delhi for the financial support (Major Research Project: F. No. 40-44/2011(SR)).
References
- Iron (III) trifluoroacetate and trifluoromethane sulfonate: recyclable Lewis acid catalysts for one-pot synthesis of 3,4-dihydropyrimidinones or their sulfur analogues and 1,4-dihydropyridines via solvent-free Biginelli and Hantzsch condensation protocols. Catal. Commun.. 2007;8:2119-2124.
- [Google Scholar]
- Melamine trisulfonic acid catalyzed regioselevtive nitration of aromatic compounds with sodium nitrate under solvent-free conditions. Arab. J. Chem. 2012 http://dx.doi.org/10.1016/j.arabjc.2012.10.011
- [Google Scholar]
- One-step, synthesis of Hantzsch esters and polyhydroquinoline derivatives using new organocatalyst. Chin. Chem. Lett.. 2010;22:563-567.
- [Google Scholar]
- A microwave-assisted bismuth nitrate-catalyzed unique route toward 1,4-dihydropyridines. Molecules. 2012;17:2643-2662.
- [Google Scholar]
- An efficient one-step synthesis of 1,4-dihydropyridines via a triphenylphosphine-catalyzed three-component Hantzsch reaction under mild conditions. Tetrahedron Lett.. 2009;50:5248-5250.
- [Google Scholar]
- Hantzsch 1,4-dihydropyridine synthesis in aqueous ethanol by visible light. Tetrahedron Lett.. 2013;54(2013):58-62.
- [Google Scholar]
- Jus. Lie. Ann. Chim.. 1882;215:1-82.
- Synthesis and biological evaluation of some new 1,4-dihydropyridines containing different ester substitute and diethyl carbamoyl group as anti-tubercular agents. Bioorg. Med. Chem.. 2009;17:1579-1586.
- [Google Scholar]
- A simple and efficient one-pot synthesis of Hantzsch 1,4-dihydropyridines using silica sulphuric acid as a heterogeneous and reusable catalyst under solvent-free conditions. Chem. Pap.. 2011;65(6):898-902.
- [Google Scholar]
- Derivatives of 3-cyano-6-phenyl-4-(3′-pyridyl)pyridine-2(1H)-thione and their neurotropic activity. Eur. J. Med. Chem.. 1999;34:301-310.
- [Google Scholar]
- Synthesis and anticoagulant activity of a new series of 1,4-dihydropyridine derivatives. Eur. J. Med. Chem.. 2011;46:804-810.
- [Google Scholar]
- Synthesis and antimicrobial activity of a new series of 1,4-dihydropyridine derivatives. J. Serb. Chem. Soc.. 2011;76:1-11.
- [Google Scholar]
- Reusable melamine trisulfonic acid-catalyzed three-component synthesis of 7-alkyl-6H,7H-naphtho[1′,2′:5,6] pyrano-[3,2-c]chromen-6-ones. Monatsh. Chem.. 2011;142:163-167.
- [Google Scholar]
- An efficient one-pot multicomponent synthesis of 3,4-dihydropyrimidine-2-(1H)-ones/thiones/imines via a Lewis base catalyzed Biginelli-type reaction under solvent-free conditions, 2011. Arab. J. Chem 2011
- [CrossRef] [Google Scholar]
- An efficient one-pot multicomponent synthesis of polyhydroquinoline derivatives through Hantzsch reaction catalysed by gadolinium triflate. Arab. J. Chem 2012
- [CrossRef] [Google Scholar]
- An efficient synthesis of β-amino ketone compounds through one-pot three-component Mannich-type reactions using bismuth nitrate as catalyst. J. Saudi Chem. Soc 2012 http://dx.doi.org/10.1016/j.jscs.2012.04.008
- [Google Scholar]
- ZrOCl2·8H2O: an efficient and recyclable catalyst for the three-component synthesis of amidoalkyl naphthols under solvent-free conditions. J. Saudi Chem. Soc 2012 http://dx.doi.org/10.1016/j.jscs.2012.06.003
- [Google Scholar]
- [Bmim] BF4 ionic liquid: an efficient reaction medium for the one-pot multi-component synthesis of 2-amino-4,6-diphenylpyridine-3-carbonitrile derivatives. J. Saudi Chem. Soc 2012 http://dx.doi.org/10.1016/j.jscs.2012.07.011
- [Google Scholar]
- An efficient one-pot three-component synthesis of α-amino nitriles via strecker reaction catalysed by bismuth (III) nitrate. J. Saudi Chem. Soc 2012 http://dx.doi.org/10.1016/j.jscs.2012.10.009
- [Google Scholar]
- Synthesis, study of 3D structurs, and pharmacological activities of lipophilic nitroimidazolyl-1,4-dihydropyridines as calcium channel antagonist. Bioorg. Med. Chem.. 2006;14:4842-4849.
- [Google Scholar]
- Microwave assisted sol–gel synthesis of MgO nanoparticles and their catalytic activity in the synthesis of Hantzsch 1,4-dihydropyridines. Chin. J. Catal.. 2012;33:1502-1507.
- [Google Scholar]
- Aluminum phosphate [AlPO4(H)]: a mild and efficient recyclable catalyst for one-pot synthesis of polyhydroquinoline via the Hantzsch reaction under solvent-free conditions. J. Heterocycl. Chem.. 2012;49:232-236.
- [Google Scholar]
- Silica supported 12-tungstophosphoric acid catalysts for synthesis of 1,4-dihydropyridines under solvent-free conditions. Inorg. Chim. Acta. 2009;362:3555-3562.
- [Google Scholar]
- Ionic liquid [tbmim]Cl2/AlCl3 under ultrasonic irradiation towards synthesis of 1,4-DHP’s. Arab. J. Chem.. 2011;11
- [CrossRef] [Google Scholar]
- Cerium(IV) ammonium nitrate catalysed facile and efficient synthesis of polyhydroquinoline derivatives through Hantzsch multicomponent condensation. Chin. Chem. Lett.. 2008;19:775-779.
- [Google Scholar]
- Cellulose sulfuric acid catalyzed multicomponent reaction for efficient synthesis of 1,4-dihydropyridines via unsymmetrical Hantzsch reaction in aqueous media. J. Mol. Catal. A: Chem.. 2011;335:46-50.
- [Google Scholar]
- Efficient clay supported Ni nanoparticles as heterogeneous catalyst for solvent-free synthesis of Hantzsch polyhydroquinoline. Catal. Commun.. 2012;19:1-4.
- [Google Scholar]
- Efficient acetylation of alcohols, phenols, and amines catalyzed by melamine trisulfonic acid (MTSA) Synth. Commun.. 2010;40(7):1022-1028.
- [Google Scholar]
- Melamine trisulfonic acid as a new, efficient and reusable catalyst for the solvent free synthesis of coumarins. J. Iran. Chem. Soc.. 2010;7(4):895-899.
- [Google Scholar]
- Melamine trisulfonic acid (MTSA): a new efficient catalyst for the chemoselective methoxymethylation of alcohols. Synth. Commun.. 2010;40(6):910-914.
- [Google Scholar]
- Melamine trisulfonic acid: a new, efficient and recyclable catalyst for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones in the absence of solvent. Chin. Chem. Lett.. 2011;22:318-321.
- [Google Scholar]
- FeF3 as a novel catalyst for the synthesis of polyhydroquinoline derivatives via unsymmetrical Hantzsch reaction. J. Fluo. Chem.. 2012;135:91-96.
- [Google Scholar]
- Titanium dioxide nanoparticles catalyzed synthesis of Hantzsch esters and polyhydroquinoline derivatives. Chin. J. Catal.. 2012;33:1517-1522.
- [Google Scholar]
- Protic pyridinium ionic liquid as a green and highly efficient catalyst for the synthesis of polyhydroquinoline derivatives via Hantzsch condensation in water. J. Mol. Liq.. 2013;177:44-48.
- [Google Scholar]
- Newly synthesized dihydropyridine derivatives as modulatore of P-Glycoproteion-mediated multidrug resistance. Bioorg. Med. Chem.. 1998;6:2219-2227.
- [Google Scholar]
- Synthesis and structure-activity analysis of novel dihydropyridine derivatives to overcome multidrug resistance. Bioorg. Med. Chem. Lett.. 2001;11:275-277.
- [Google Scholar]
- Tetrabutylammonium hydrogen sulfate catalyzed eco-friendly and efficient synthesis of glycosyl 1,4-dihydropyridines. Tetrahedron Lett.. 2004;45:9011-9014.
- [Google Scholar]
- Synthesis and biological evaluation of some novel N-aryl-1,4-dihydropyridines as potential antitubercular agents. Bioorg. Med. Chem. Lett.. 2011;21:5181-5183.
- [Google Scholar]
- Circumvention of vincristine and adriamycin resistance in vitro and in vivo by calcium influx blockers. Cancer Res.. 1983;43:2905-2910.
- [Google Scholar]
- Hantzsch reaction: synthesis and characterization of some new 1,4-dihydropyridine derivatives as potent antimicrobial and antioxidant agents. Euro. J. Med. Chem.. 2011;46:5591-5597.
- [Google Scholar]
- Melamine-trisulfonic-acid-catalyzed trimethylsilylation of alcohols and phenols. Phosphorus, Sulfur Silicon Relat. Elem.. 2011;186(10):2055-2060.
- [Google Scholar]
- Melamine trisulfonic acid: a new efficient catalyst for the synthesis of aryldithienylmethanes. Phosphorus, Sulfur Silicon Relat. Elem.. 2012;187(2):149-154.
- [Google Scholar]
- Melamine trisulfonic acid-catalyzed N-formylation of amines under solvent-free conditions. Res. Chem. Intermed. 2012 10.1007/s11164-012-0803-7
- [Google Scholar]
- One-pot three-component synthesis of spiro[pyrazolo[3,4-b]pyridine-4,3′-indoline] derivatives catalyzed by melamine trisulfonic acid. J. Chin. Chem. Soc 2012
- [CrossRef] [Google Scholar]
- Antagonist binding sites of voltage-dependent calcium channels. Drug Dev. Res.. 1998;42:131-143.
- [Google Scholar]
- New 4-aryl-1,4-dihydropyridines and 4-arylpyridines as P-glycoprotein inhibitors. Drug Metab. Dispos.. 2005;33:321-328.
- [Google Scholar]







