Translate this page into:
Mechanistic understanding of PtyroneTM: A plant based natural anti diabetic product
⁎Corresponding authors. yogesh_dound@yahoo.com (Yogesh Arun Dound), alamprez@gmail.com (Pravej Alam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
To investigate the combinatorial effect of hydroalcoholic extracts of Andrographis paniculata Nees. and Pterocarpus marsupium Roxb. plants prospective to diabetes management.
Methods
Taking a lead from the scientific literature, in silico studies have also been designed for the screening of anti-diabetic targets against andrographolide and pterostilbene compounds followed by in vivo studies from Andrographis paniculata Nees. and Pterocarpus marsupium Roxb. Furthermore, the diabetes was induced by STZ model and the impact of Andrographis paniculata Nees. and Pterocarpus marsupium Roxb. have been conformed by relative expression studies by qPCR.
Results
Our results have shown that andrographolide and pterostilbene are SGLT2 inhibitors and selective PPARγ agonists in in silico studies. Later, during in vivo mRNA expression studies confirming the same pattern. The findings of the study has shown to overcome the common knowledge of the only C–glycoside based molecules inhibiting the SGLT2.
Conclusions
The possible mechanism for PtyroneTM in the management of diabetes could be a selective PPARγ agonist, GLUT4 translocation and SGLT2 inhibition molecule.
Keywords
PtyroneTM
Diabetes mellitus type 2
Andrographis paniculata Nees.
Pterocarpus marsupium Roxb.
Andrographolide
Pterostilbene
- PPARγ
-
Peroxisome proliferator-activated receptor gamma
- DPPIV
-
Dipeptidyl peptidase-4 inhibitor
- GPR40
-
G-protein-coupled receptor 40
- SGLT2
-
Sodium-glucose co-transporter-2
- GLUT4
-
Glucose transporter type 4
- AMP
-
adenosine monophosphate-activated protein kinase
- GSK3β
-
Glycogen synthase kinase 3 beta
- PPARα
-
Peroxisome proliferator-activated receptor
Abbreviations
1 Introduction
The statistics of Type 2 diabetes mellitus (T2DM) is prevailing as a main non-communicable disease wondering ∼366 million of world population. One of the major studies led by diabetic researchers, by 2030, it is estimated as a high rate reaching 439–552 million (Whiting et al., 2011). Risk associated with the T2DM is metabolic syndromes and micro–macro vascular complications increased the risk of cardiovascular indications (Tahrani et al., 2011). The majority of patients required different types of medications to balance their blood-glucose levels worldwide. The catastrophe of use of metformin, sulphonylureas and/or gliptins is often followed by the introduction of insulins. Though productive, this strategy had several limitations, which eventually lead to therapeutic inertia and compromise the targets for glycemic control. Hence an urge of safe and efficient modality for its management is urgently needed (Lankatillake et al., 2019; Dias et al., 2012).
Plants like Pterocarpus marsupium Roxb. and Andrographis paniculata Nees are few such candidates who have high possibilities in administering diabetes. Pterocarpus marsupium Roxb. extensively used to treat Type 2 diabetes mellitus for thousands of years (Jung et al., 2006). Similarly, Andrographis paniculata Nees. has also been used as an anti-diabetic plants with other activities such as anti-analgesic, antioxidants, and hepatoprotector (Jayakumar et al., 2013). These plants biosynthesized a major class of phytoactive compounds, pterostilbene (1) and andrographolide (2) respeticvely, have been known as anti-diabetic (Titi and Sabtanti, 2015; Perera, 2016; Fig. 1). More recently, Chakraborty et al. (2010) and Islam (2017) has studied Andrographis paniculata Nees. and Pterocarpus marsupium Roxb. impact against diabetes and evaluated their interactions with GLUT4, PPARα and PPARγ.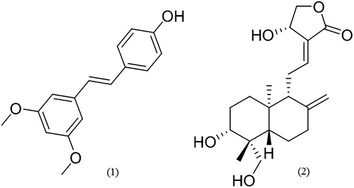
Structures of pterostilbene (1) and andrographolide (2).
The development of a new agent of plant-based origin that assures safety with moderate but definite efficacy is the need of the time. Several clinical and pre-clinical studies on the traditional medicinal plants have shown their antidiabetic potential. Various attempts are being made to prepare formulations with optimized contents of phytoactive compounds of the plant by other (Lankatillake et al., 2019). The combined activity of these phytoactive materials, in a defined reference window, against multiple selected targets can be pooled together as a complementary agent with specific actions for the management of T2DM.
2 Material and methods
2.1 Plant material and extraction
Based on previous research data, a blend of PtyroneTM, containing combination of hydroalcoholic extract of Andrographis paniculata Nees. and Pterocarpus marsupium Roxb. in specific proportion was developed and provided by Shreepad Shree Vallabh (SSV) Phytopharmaceuticals, Mumbai for studying its impact against type 2 diabetes. Before sending the material, the percentage of pterostilbene and andrographolide in the formulation PtyroneTM was ensured to be NLT 3% and NLT 30%, respectively. The requisite quantity of the blend (0.9 g) was weighed and transferred to mortar. The needful volume of 0.25% CMC was added in aliquots, and the mixture were triturated to get a final uniform suspension. This formulation was prepared fresh on the daily basis before dosing.
2.2 Animal
The approval for test facilities was given by the Institutional Animals Ethics Committee (IAEC, ECR/1036/Inst/MH/2018). Animal selection and other vital parameters are given in Table S1 (supplementary files).
2.3 Induction of diabetes mellitus
Streptozotocin (STZ; Sigma Aldrich, St. Louis, USA) was taken to induce diabetes in the test animals at a dose of 60 mg/kg body. Dose and pH were adjusted to 4.5 and maintained on ice before use. After 7 days of injecting STZ, confirmation of diabetes was given by determining the concentration level of FBG and animals exhibiting FBG levels more than 200 mg/dl were taken for the experiment.
2.4 Study design
Three groups of rats were defined before inducing diabetes. Each group contains 3 animals.
Diabetic group: This group of rats was administered water (distilled) orally for 7 days at a dose of 1 mL/kg/d body weight.
PtyroneTM treated diabetic group: This group of diabetic rats was given PtyroneTM at the dose of b.i.d., 450 mg/10 mL distilled water/kg of body weight/d for 7 days at fasting state.
Metformin treated diabetic group: This group of diabetic rats was given with metformin at the dose of 180 mg/kg body weight/d orally for 7 days.
2.5 Screening of antihyperglycemic activity
2.5.1 Evaluation of fasting blood glucose
In all experimental rats, the collection of samples of blood was taken by tail prick method and glucose level in blood was measured by Glucometer (Make: Accu Check). Every time animals were checked for fasting blood glucose (approx. 8–12 h overnight fasting) at day 1 and on day 8.
2.6 RNA extraction and qPCR
From each group of experimental rats, hepatic tissue was dissected out and frozen immediately in liquid nitrogen until further use. Liver tissues were homogenized in sterile PBS. RNA extraction was carried out from the liver tissue samples obtained from all the animals. Following the instructions mentioned in the manual, RNA extraction was carried out using commercial columns (Qiagen, USA). Briefly, 560 μl of lysis buffer was aliquoted to a tube. To this, 5.6 μl of carrier RNA was added along with the homogenized tissue sample (140 μl). The suspension was vortexed and incubated at room temperature for 10 min. RNA was precipitated by adding 560 μl of chilled ethanol. The required number of columns were labeled and placed on the rack. 630 μl of the suspension from each tube was added into the corresponding columns and centrifuged at 8000 rpm for 1 min. To the same columns the remaining sample suspension was added and centrifuged at 8000 rpm for 1 min. The bound RNA was washed with the wash buffers provided in the kit. Finally, the columns were placed in a fresh RNase free tube and elution buffer (60 μl) was added to each column and incubated for 60 s at room temperature. The columns were centrifuged at 8000 rpm for 1 min. The eluted RNA was further used for cDNA conversion.
Taking a commercial cDNA Archive Kit (ABI, USA), reverse transcription of total RNA extracted from the tissue homogenate samples was carried out. cDNA synthesis was carried out in an RNase free tube. To the labeled tubes 2 μl of 10X RT buffer, 0.8 μl of Multi-scribe reverse transcriptase, 0.8 μl of dNTP mix (provided in the kit) was aliquoted. To each tube 500 nM concentration of gene-specific primers was aliquoted. 8 μl of the extracted RNA was added to appropriate tubes and incubated at 45 °C for 30 min to facilitate cDNA synthesis. Further, the cDNA thus synthesized was used for amplification.
PCR primers of the following biomarkers were evaluated: Glucose transporter (GLUT4) (Mendes et al., 2009), Sodium/Glucose co-transporter (SGLT2) (Dietrich et al., 2016), glycogen synthase K (GSK3β) (Lawrence and Roach, 1997) and both peroxisome proliferator-activated receptors (PPARα and PPARγ; Im et al., 2006; Table S2; supplimentry file) were used for cDNA and qPCR assay.
Nucleic acid amplification was carried out in a qPCR machine (Quant-Studio 3, Applied Biosystems). All amplifications were carried out in a 20 μl reaction volume using Powerup SYBR Green master mix, ABI. Amplification was carried out using 500 nM concentration of primers. All the amplifications were carried out in triplicates. Briefly, the required quantity of the SYBR Green mix was aliquoted into two specific tubes and labeled appropriately. To one tube primer specific to GAPDH was added and to the second marker, specific primers were added and mixed. The volume was adjusted to 18 μl by adding sterile water. The mix was then aliquoted to each well. To each well, 2 μl of the prepared cDNA was added and the amplification was carried out for 40 cycles at 95 °C and 60 °C. The calculation of the double ΔCt was done by calculating the Ct values for both the genes (diabetic marker gene and the housekeeping gene) in both the experimental and the control groups. Amplification for each of the marker along with the house-keeping gene had be carried out in triplicate. The inbuilt software of Quant Studio calculated the 2^-ΔΔCt values at the end of the reaction.
2.7 DPPIV inhibition assay
DPPIV inhibition assay was carried out using ENZO DPPIV/CD26 Assay kit for Biological samples. Through cardiac puncture, the collection of terminal blood samples was done. The collected samples were put in tubes containing 10% K2EDTA. The plasma was harvested and frozen at −80 °C until further use. Inhibition assay was carried out using the colorimetric substrate and the absorbance was measured at 405 nm as per the kit instruction.
2.8 Statistical analysis
The glucose values (mg/dl), relative expression values of GLUT4, SGLT2, GSK3β, PPARα and PPARγ were estimated in each group. Significant differences between group means and control were analyzed by one way ANOVA, followed by Dunnett’s multiple comparison test, using Graphpad Prism at 95% confidence levels. Differences were measured remarkable when P value were<0.05 (p < 0.05).
2.9 Structural retrieval for target proteins
The structures of human proteins-GSK3β, PPARα, PPARγ and DPPIV were retrieved form Protein Data Bank or PDB on the basis of PDB search and structures containing the required binding and active sites. The crystal structure of targets are as follows-GSK3β (PDB Id:1Q5K), PPARγ (PDB Id: 1I7I), PPARα (PDB Id:1I7G) and DPPIV (PDB Id: 1R9N).
2.10 Homology modelling and dynamic studies of target proteins
Human structures of proteins targets-GPR40, GLUT4, SGLT2, AMPKγ suitable for interaction studies were not available in the database of PDB; hence, protein structure was predicted using MODELLER (Šali and Blundell, 1993) based on homology. The sequence of protein targets was taken from UNIPROT (UniProt Consortium, 2019) database. The identity percentage of selected templates and the target protein was above 30%, as shown in Table S3. The homology models were then validated using PDBsum and PROCHECK. Visualization of the predicted models was done using discovery studio (https://www.3dsbiovia.com/products/collaborative-science/biovia-discovery-studio/visualization-download.php). All the generated homology models were energy minimized, equilibrated and simulated for 1 ns using GROMACS 2018.1 version.
2.10.1 Ligand structure
The structures of pterostilbene (https://pubchem.ncbi.nlm.nih.gov/compound/5281727) and andrographolide (https://pubchem.ncbi.nlm.nih.gov/compound/5318517https://pubchem.ncbi.nlm.nih.gov/compound/5318517) were obtained in the structure data file format. The structure was energy minimized and converted into Tripos Mol2 format using MarvinView.
2.10.2 Molecular docking
In molecular docking studies, prediction of the binding poses of the ligand to our proteins (targets) was analyzed. AutoDockTools 4.2 was used to perform and analyze the docking of ligands and proteins (Morris et al., 2009). The obtained structures of protein and ligand were as input and processed as per AutoDockTools Package. For docking studies, dimensions of grid and spacing were set as 60 and 0.375 Å, respectively. Sixty docking confirmations were performed for each docking set of protein and ligand. Active site prediction required for docking was done using available literature or server-MetaPocket (Zhang et al., 2011; Huang, 2009).
3 Results
In this study, we have observed that there is significant decrease in fasting blood glucose (FBG) level the fasting blood glucose (FBG) in PtyroneTM treated diabetic group (184 ± 44.54 mg/dl) in comparison with a diabetic group (344.3 ± 56.62 mg/dl) (Table 1) with an increase in PPARγ expression in PtyroneTM treated diabetic group (2.25 ± 1) in comparison with the diabetic control group (1 ± 0.01) (Table 1). The study of SGLT2 and PPARα expression was shown a reduction in SGLT 2 (0.90 ± 0.02) but does not affect PPARα expression in PtyroneTM treated diabetic group (0.97 ± 0.13) in comparison with control diabetic group (1 ± 0.0.01), evocative the PPARγ activity (Table 2). Interestingly, for the first-time metformin has also shown statistically significant SGLT2 inhibitory activity in this study. Similarly, the GSK3β and GLUT4 expression level, (0.9 ± 0.1) and (1.1 ± 0.15) in PtyroneTM treated diabetic group as compared to the GSK3β and GLUT4 expression level (1 ± 0.01) and (1 ± 0.01) in the diabetic control group (Table 2). The PtyroneTM treated diabetic group (Table 3) did not exhibit significant DPP4 inhibition (39.01 ± 4.54) in comparison with the control diabetic group (0 ± 0). Values expressed in Mean ± SD; SD- Standard Deviation Values expressed in Mean ± SD; SD- Standard Deviation, *- Statistically significant (P < 0.05).
S. No.
Group
Fasting Blood glucose (mg/dL)
1.
Diabetic (control))
344.3 ± 56.62
2.
PtyroneTM
184 ± 44.54*
3.
Metformin
108.7 ± 8.33*
Group
Relative mRNA Expression with respect to control
SGLT2
GLUT4
PPARα
GSK3β
PPARγ
Diabetic (control)
1 ± 0.01
1 ± 0.01
1 ± 0.01
1 ± 0.01
1 ± 0.01
PtyroneTM
0.90 ± 0.02
1.1 ± 0.15
0.97 ± 0.13
0.9 ± 0.1
2.25 ± 1
Metformin
0.51 ± 0.03*
0.33 ± 0.04
0.54 ± 0.06
5.56 ± 0.41
41.64 ± 7.22*
Group
Percent Inhibition in comparison to control
Diabetic (control)
0 ± 0
PtyroneTM
39.01 ± 4.54
Metformin
10.46 ± 2.32
3.1 Molecular interactions
In this study, we predicted homology models for the four protein targets namely GPR40, GLUT4, SGLT2, and AMPKγ. Ramachandran plot (RC Plot) was used to study the torsion angles of the models built. Ramachandran plot for all the four homology models is shown in Fig. 2. Nanosecond dynamic simulations were executed using GROMACS 2018.1 version. Before simulations, all the homology models were energy minimized and potential energy was checked (for stable structure the average potential energy should be in the scale of −1*105 kcal/mol) shown in Table S4. As per the RC Plots, potential energy, and the RMSD, these homology models are reasonably good for further in silico studies. For molecular docking studies, ligands are directed to the active site as predicted. The active site prediction for our target proteins is described in Table S5. The images showing the actives site are given in Fig. 3. The ligands pterostilbene and andrographolide were docked with 3D structures of protein targets using AutodockTools (Table S6). Binding modes for pterostilbene and andrographolide with all the protein targets are shown in Fig. 3. Molecular docking studies and predicted activity with minimum energy interactions concerning their active sites are shown in Table S7 for pterostilbene and andrographolide.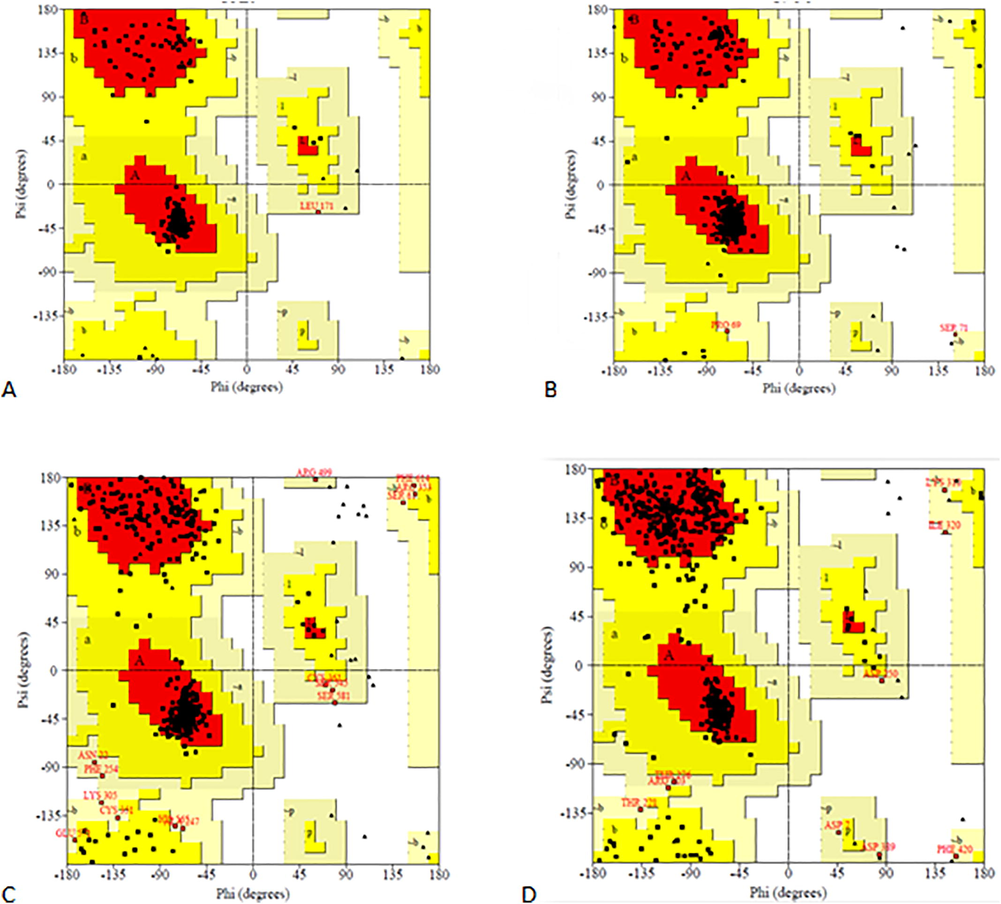
RC Plots of GPR40(A), GLUT4(B), SGLT2(C) & AMPKγ(D).
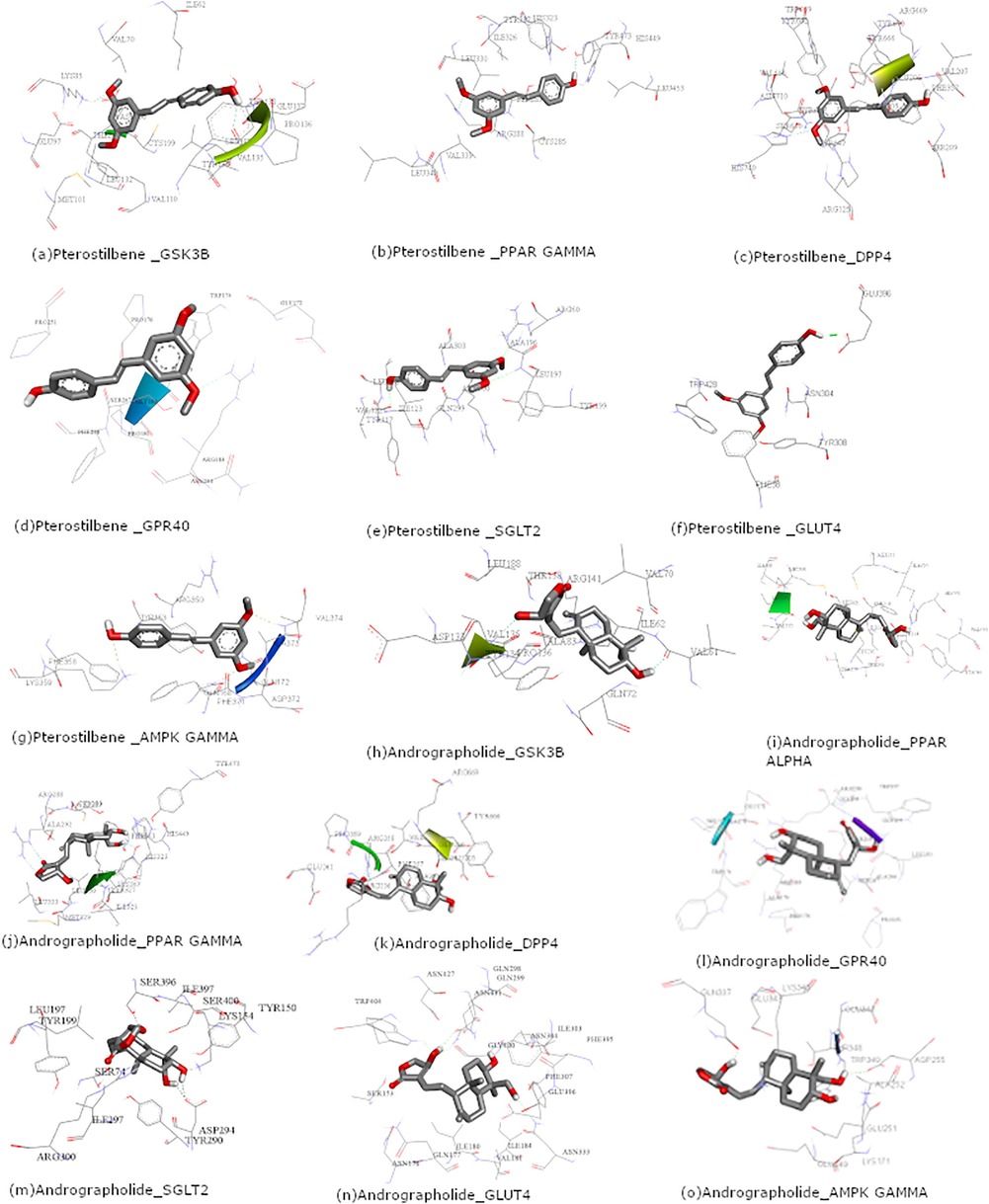
Images showing binding mode of Pterostilbene with (a) GSK3β, (b) PPARγ, (c), DPPIV (d) GPR40, (e) SGLT2, (f) GLUT4, (g) AMP; binding mode of Andrographolide (h) GSK3β, (i) PPARα, (j) PPARγ, (k) DPPIV , (l) GPR40, (m) SGLT2, (n) GLUT4 & (o) AMPK.
4 Discussion
Type 2 diabetes mellitus (DM), is a metabolic disorder wherein the currently used therapeutic modalities for the management of Type 2 DM have limitations and side effects. Hence, there is a need for the development of natural multi-targeted agents for the comprehensive management of diabetes. In the current article, for the first time, we have used hydroalcoholic extracts of Andrographis paniculata Nees. and Pterocarpus marsupium Roxb. plants combined in the certain proportion for the diabetes management. The diabetic rat was induced by a low-dose STZ to assess the outcome of PtyroneTM (hydroalcoholic extracts of Pterocarpus marsupium Roxb. and Andrographis paniculata Nees.) and evaluating the FBG and mRNA expression levels of PPARα, PPARγ, GLUT4, SGLT2, GSK-3β and DPPIV to check the inhibition of Type 2 diabetes in diabetic rats as compared to metformin. Afterwards, we have confirmed the anti-diabetic nature of pterostilbene and andrographolide and their binding interctions with GSK3β, PPARγ, DPPIV, GPR40, SGLT2, GLUT4 and AMPK through in silico studies (Fig. 3).
PPARs are known for nuclear hormone receptors that acts as transcription factors which regulates the genetic expression of both lipid metabolism as well as insulin resistance (Lamichane et al., 2018). Notably, PPARγ plays an imperative role in adipocyte differentiation, glucolipid metabolism and insulin resistance (Jia et al., 2015), which is why PPARγ regulates the usual physiological processes of cells. Also, PPARγ is linked with obesity, fat differentiation and insulin resistance as PPARγ is a type of ligand-activated nuclear transcription factor (Wasik et al., 2017). Metformin treatment group has shown prominent PPARγ upregulation but also a significant downregulation in PPARα mRNA expression. This could also be one of the possible reason for the cardiovascular side effects of metformin. Similar results were also observed with PPARγ agonist glitazones such as pioglitazones and rosiglitazones. One of the major concern with PPAR is their downregulated expression in diabetic patient is by the disease itself. If a drug has an antagonist activity or downregulates the expression of PPARα, it may lead to water retention and CHF (Schernthaner et al., 2013). However, with the use of PtyroneTM formulation, it has been observed that no significant decrease in PPARα was heeded with a slight upregulation in PPARγ expression while comparing the diabetic control group. This further establishes the safety of the PtyroneTM formulation. The beneficial effects of increased production of enzymes via PPARγ activiation is increased glucose tolerance and improved insulin sensitivity followed by, improved energy expenditure in white adipose tissue (Kaupang and Hansen, 2020). So, the need of the hour is that of selective PPARγ agonist and with no effect on PPARα wherein our formulation suffices this issue.
GLUT4 is a transmembrane transport protein which boosts glucose transport to insulin-sensitive tissues for intracellular exploitation (Irudayaraj et al., 2016) which is not only found in skeletal muscle and adipocytes, but also in the brain and heart (Wang et al., 2020). Our research reveals that in T2DM rats, the expression level of GLUT4 were increased by PtyroneTM. Its upregulation can increase the translocation into the cell membranes which thereby increases the glucose uptake in the cell which will be helpful in achieving the glucose homeostasis (Wan et a., 2020). It was also monitored that the same formulation also downregulated the SGLT2 expression, suggesting the inhibitory affect of SGLT2 activity. Ferrannini et al. (2013) reported that the renal SGLT2 threshold levels for glucose reabsorption is paradoxically augmented in hyperglycemia in an experiment on diabetic rodents and humans. Also, its inhibitors exhibit glucose-dependent as well as -independent renoprotective effects (Kawanami et al., 2017). Since the inhibitors of SGLT2 shows pleiotropic effects, hence, they may exert beneficial effects on peripheral nerves, thereby addressing diabetic peripheral neuropathy.
The role of a serine/threonine kinase based glycogen synthase kinase-3 (GSK-3) is significant in the regulation of glycogen metabolism together with other numerous cellular activities. Along with this, obese people suffering from type 2 diabetic are also related to defective capability of insulin to stimulate the dumping of glucose and glycogen synthase due to overexpression and over-activity of GSK-3 in skeletal muscles. A supplementary imperative accomplishment of these serine/threonine kinase inhibitors in the framework of obese-associated diabetes (type 2) caused the decrease of hepatic glucose level may be due to the downregulation mechanism of genes related to gluconeogenesis (Henriksen and Dokken, 2006). It is also advocated that during the treatment of CNS neuropathy of diabetes mellitus, GSK-3 might be a significant target since it has shown a vital role in the pathogenesis (Qu et al., 2014). GSK-3β expression in our study was downregulated in comparison to the diabetic control group. Its inhibition will further improve the insulin production and glucose metabolism in human skeletal muscle (Nikoulina et al., 2020) which will further improve the diabetes patient health.
Dipeptidyl peptidase IV (DPPIV) is generally a scattered multifunctional transmembrane glycoprotein in organs and tissues of humans that selectively dissects dipeptides after proline or alanine residues (Yang et al., 2007; Longenecker et al., 2006). It is acclaimed for its deactivation of incretin hormones engaged in glucose homeostasis, specifically, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) (Green et al., 2006), which stops the secretion of glucagon and activates insulin secretion. DPPIV inhibitors are involved in lowering glucose levels and hence they stall the active time of GLP-1 and GIP as their elementary pharmacological action. In this study, after STZ treatment, the PtyroneTM group showed DPPIV inhibition.
Molecular docking performed for the ligands (pterostilbene and andrographolide) with protein targets (GSK-3β, PPARα, PPARγ, DPPIV, GPR40, SGLT2, GLUT4 and AMPK) showing good mode of binding or interactions (in terms of hydrogen bond formation) with each ligand with protein. Interacting residues, binding energy for all the protein–ligand and their activity is shown in Tables S6 and S7.
5 Conclusion
The authors for the first time developed a formulation (PtyroneTM) of two major hydroalcoholic extracts based phytoactives each from both plants for its action against type 2 diabetes mellitus. The in vivo studies have shown that this formulation significantly reduced fasting blood glucose, along with an increase in PPARγ and GLUT4 expressions with a reduction in SGLT2 and GSK-3β expressions. The formulation did not even exhibit significant DPPIV inhibition in comparison with the diabetic control group. These findings are further confirmed with in silico studies. The docking studies were excuted to better understand the interaction between the ligands-pterostilbene, andrographolide along with eight protein targets. Both the ligands formed hydrogen bonds with all the target proteins except pterostilbene which showed no interaction with PPARα. Thus, it can be concluded that PtyroneTM formulation with the phytoactives from two plants in specific proportions is a potential antidiabetic candidate with its multi-targeted effects. This is for the first time such a plant-based product derived from natural origin has emerged with so many multiple targets in diabetes.
Acknowledgements
Logistic and technical supports provided by RASA Life Science Informatics, Pune, India and Prince Sattam bin Abdulaziz university are highly acknowledge. We are also thankful to Dr. Ramesh Jayaraman, of TheraIndex Life Science Pvt. Bangalore, India for provided laboratory facilities for this study.
Author contributions
YAD, SC, MMA, and PA designed the work, YAD performed the experiments in the laboratory while SSC, SR SC, MMA, MHG, and PA were performed the bioinformatics analyses.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- In vitro evaluation of the cytotoxic, anti-proliferative and anti-oxidant properties of pterostilbene isolated from Pterocarpus marsupium. Toxicol. In Vitro. 2010;24:1215-1228.
- [Google Scholar]
- historical overview of natural products in drug discovery. Metabolites. 2012;2(2):303-336.
- [Google Scholar]
- The DPP4 inhibitor linagliptin protects from experimental diabetic retinopathy. PLoS One. 2016;11:e0167853
- [Google Scholar]
- Renal glucose handling: impact of chronic kidney disease and sodium glucose co-transporter 2 inhibition in patients with type 2 diabetes. Diabetes Care. 2013;36:1260-1265.
- [Google Scholar]
- Dipeptidyl peptidase IV (DPP IV) inhibitors: A newly emerging drug class for the treatment of type 2 diabetes. Diabetes Vasc. Dis. Res. 2006;3:159-165.
- [Google Scholar]
- Role of glycogen synthase kinase-3 in insulin resistance and type 2 diabetes. Curr. Drug Targets. 2006;7:1435-1441.
- [Google Scholar]
- MetaPocket: A Meta approach to improve protein ligand binding site prediction. OMICS. 2009;13:325-330.
- [Google Scholar]
- Regulation of GLUT4 gene expression by SREBP-1c in adipocytes. Biochem. J. 2006;399:131-139.
- [Google Scholar]
- Antioxidant, antilipidemic and antidiabetic effects of ficusin with their effects on GLUT4 translocation and PPARγ expression in type 2 diabetic rats. Chem. Biol. Interact. 2016;256:8593.
- [Google Scholar]
- Andrographolide, a new hope in the prevention and treatment of metabolic syndrome. Front. Pharmacol.. 2017;8:571.
- [Google Scholar]
- Experimental and clinical pharmacology of Andrographis paniculata and its major bioactive phytoconstituent andrographolide. Med: Evid. Based Complement Alternat; 2013. p. :846740.
- PPARγ links maternal malnutrition and abnormal glucose and lipid metabolism in the offspring of mice. Yi Chuan.. 2015;37:7076.
- [Google Scholar]
- Kaupang, Å., Hansen, T.V., 2020. The PPAR Ω Pocket: Renewed Opportunities for Drug Development. PPAR Res 2020, Article ID 9657380. https://doi.org/10.1155/2020/9657380
- SGLT2 inhibitors as a therapeutic option for diabetic nephropathy. Int. J. Mol. Sci.. 2017;18:E1083.
- [Google Scholar]
- Pivotal roles of peroxisome proliferator-activated receptors (PPARs) and their signal cascade for cellular and whole-body energy homeostasis. Int. J. Mol. Sci.. 2018;19:949.
- [Google Scholar]
- Understanding glycaemic control and current approaches for screening antidiabetic natural products from evidence-based medicinal plants. Plant Methods. 2019;15:105.
- [CrossRef] [Google Scholar]
- New insights into the role and mechanism of glycogen synthase activation by insulin. Diabetes. 1997;46:541-547.
- [Google Scholar]
- Crystal structures of DPP-IV (CD26) from rat kidney exhibit flexible accommodation of peptidase-selective inhibitors. Biochemistry. 2006;45:7474-7482.
- [Google Scholar]
- Lithium reduces Gsk3b mRNA Levels: Implications for Alzheimer disease. Eur. Arch. Psychiatry Clin. Neurosci.. 2009;259:16-22.
- [Google Scholar]
- AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem.. 2009;30:2785-2791.
- [Google Scholar]
- Inhibition of glycogen synthase kinase 3 improves insulin action and glucose metabolism in human skeletal muscle. Diabetes. 2020;51:2190-2198.
- [Google Scholar]
- Glycogen synthase kinase-3 regulates production of amyloid-β peptides and tau phosphorylation in diabetic rat brain. Scientific World J.. 2014;2014:878123
- [Google Scholar]
- Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol.. 1993;234:779-815.
- [Google Scholar]
- Do we still need pioglitazone for the treatment of type 2 diabetes? A risk-benefit critique in 2013. Diabetes Care. 2013;36:S155-S161.
- [Google Scholar]
- Management of type 2 diabetes: New and future developments in treatment. Lancet. 2011;378:182-197.
- [Google Scholar]
- A review on the anti-diabetic activity of Andrographis paniculata (Burm. f.) Nees based in-vivo study. IJPHS. 2015;4:256-263.
- [Google Scholar]
- UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res.. 2019;47:D506-D515.
- [Google Scholar]
- Current understanding of glucose transporter 4 expression and functional mechanisms. World J. Biol. Chem. 2020;11:76-98.
- [Google Scholar]
- Septin 7 reduces nonmuscle myosin IIA activity in the SNAP23 complex and hinders GLUT4 storage vesicle docking and fusion. Exp. Cell Res.. 2017;350:336348
- [Google Scholar]
- IDF Diabetes Atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract.. 2011;94:311-321.
- [Google Scholar]
- Increase in DPP-IV in the intestine, liver and kidney of the rat treated with high fat diet and streptozotocin. Life Sci.. 2007;81:272-279.
- [Google Scholar]
- Identification of cavities on protein surface using multiple computational approaches for drug binding site prediction. Bioinformatics. 2011;27:2083-2088.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2021.101454.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







